The Influence of Chirality on the β-Amino-Acid Naphthalenediimides/G-Quadruplex DNA Interaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Naphthalenediimide Design
2.2. G-Quadruplex Topology Analysis
2.3. Circular Dichroism Variable Temperature Studies
2.4. GNINA Modelling
2.5. NDI-DNA Interactions
2.5.1. c-KIT1
2.5.2. h-TELO
2.5.3. TBA
2.5.4. dsDNA
2.6. NDI-DNA Titration Studies
3. Materials and Methods
- c-KIT1: GGG AGG GCG CTG GGA GGA GGG;
- h-TELO: AGG GTT AGG GTT AGG GTT AGG GT;
- TBA: GGT TGG TGT GGT TGG;
- dsDNA: TAT AGC TAT A HEG T ATA GCT ATA.
3.1. Naphthalenediimide Synthesis
General Synthetic Procedure
3.2. Variable-Temperature CD Studies
3.3. Titration Studies
3.4. GNINA Modelling
3.5. HPLC Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Rentsch, K.M. The Importance of Stereoselective Determination of Drugs in the Clinical Laboratory. J. Biochem. Biophys. Methods 2002, 54, 1–9. [Google Scholar] [CrossRef]
- Caldwell, J. Stereochemical Determinants of the Nature and Consequences of Drug Metabolism. J. Chromatogr. A 1995, 694, 39–48. [Google Scholar] [CrossRef]
- Eichelbaum, M. Pharmacokinetic and Pharmacodynamic Consequences of Stereoselective Drug Metabolism in Man. Biochem. Pharmacol. 1988, 37, 93–96. [Google Scholar] [CrossRef]
- McConathy, J.; Owens, M.J. Stereochemistry in Drug Action. Prim. Care Companion CNS Disord. 2003, 5, v05n0202. [Google Scholar] [CrossRef] [PubMed]
- Răsădean, D.M.; Harrison, S.W.O.; Owens, I.R.; Miramont, A.; Bromley, F.M.; Pantoș, G.D. Importance of Chiral Recognition in Designing Metal-Free Ligands for G-Quadruplex DNA. Molecules 2019, 24, 1473–1490. [Google Scholar] [CrossRef] [PubMed]
- Răsădean, D.M.; Sheng, B.; Dash, J.; Pantoş, G.D. Amino-Acid-Derived Naphthalenediimides as Versatile G-Quadruplex Binders. Chem. Eur. J. 2017, 23, 8491–8499. [Google Scholar] [CrossRef]
- Gao, F.; Sun, M.; Zhang, J.; Chang, Y.; Gao, W.; Ma, G.; Ma, X.; Guo, Y. Fenton-like Reaction and Glutathione Depletion by Chiral Manganese Dioxide Nanoparticles for Enhanced Chemodynamic Therapy and Chemotherapy. J. Colloid Interface Sci. 2022, 616, 369–378. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Zhang, Q.; Zhang, P. Chirality in Metal-Based Anticancer Agents. Dalton Trans. 2018, 47, 4017–4026. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-Quadruplexes in Gene Promoters: A Novel Anticancer Strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef]
- Bončina, M.; Podlipnik, Č.; Piantanida, I.; Eilmes, J.; Teulade-Fichou, M.-P.; Vesnaver, G.; Lah, J. Thermodynamic Fingerprints of Ligand Binding to Human Telomeric G-Quadruplexes. Nucleic Acids Res. 2015, 43, 10376–10386. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. Quantitative Visualization of DNA G-Quadruplex Structures in Human Cells. Nat. Chem. 2013, 5, 182–186. [Google Scholar] [CrossRef]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting Chromosomes against Genome Instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hänsel-Hertsch, R.; Beraldi, D.; Lensing, S.V.; Marsico, G.; Zyner, K.; Parry, A.; Di Antonio, M.; Pike, J.; Kimura, H.; Narita, M.; et al. G-Quadruplex Structures Mark Human Regulatory Chromatin. Nat. Genet. 2016, 48, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Bochman, M.L.; Paeschke, K.; Zakian, V.A. DNA Secondary Structures: Stability and Function of G-Quadruplex Structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef]
- Marchetti, C.; Minarini, A.; Tumiatti, V.; Moraca, F.; Parrotta, L.; Alcaro, S.; Rigo, R.; Sissi, C.; Gunaratnam, M.; Ohnmacht, S.A.; et al. Macrocyclic Naphthalene Diimides as G-Quadruplex Binders. Bioorg. Med. Chem. 2015, 23, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Zyner, K.G.; Ohnmacht, S.A.; Robson, M.; Haider, S.M.; Morton, J.P.; Marsico, G.; Vo, T.; Laughlin-Toth, S.; Ahmed, A.A.; et al. Targeting Multiple Effector Pathways in Pancreatic Ductal Adenocarcinoma with a G-Quadruplex-Binding Small Molecule. J. Med. Chem. 2018, 61, 2500–2517. [Google Scholar] [CrossRef]
- Collie, G.W.; Promontorio, R.; Hampel, S.M.; Micco, M.; Neidle, S.; Parkinson, G.N. Structural Basis for Telomeric G-Quadruplex Targeting by Naphthalene Diimide Ligands. J. Am. Chem. Soc. 2012, 134, 2723–2731. [Google Scholar] [CrossRef]
- Pirota, V.; Nadai, M.; Doria, F.; Richter, S. Naphthalene Diimides as Multimodal G-Quadruplex-Selective Ligands. Molecules 2019, 24, 426–452. [Google Scholar] [CrossRef]
- Di Antonio, M.; Doria, F.; Richter, S.N.; Bertipaglia, C.; Mella, M.; Sissi, C.; Palumbo, M.; Freccero, M. Quinone Methides Tethered to Naphthalene Diimides as Selective G-Quadruplex Alkylating Agents. J. Am. Chem. Soc. 2009, 131, 13132–13141. [Google Scholar] [CrossRef]
- Xu, Y.; Yamazaki, S.; Osuga, H.; Sugiyama, H. The Recognition of Higher-Order G–Quadruplex by Chiral Cyclic-Helicene Molecules. Nucleic Acids Symp. Ser. 2006, 50, 183–184. [Google Scholar] [CrossRef]
- Hu, X.; Yang, D.; Yao, T.; Gao, R.; Wumaier, M.; Shi, S. Regulation of Multi-Factors (Tail/Loop/Link/Ions) for G-Quadruplex Enantioselectivity of Δ- and Λ- [Ru(Bpy)2(Dppz-Idzo)]2+. Dalton Trans. 2018, 47, 5422–5430. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Ren, J.; Zhao, C.; Qu, X. G-Quadruplex Binding Enantiomers Show Chiral Selective Interactions with Human Telomere. Nucleic Acids Res. 2014, 42, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Ouyang, C.; Liu, J.; Karges, J.; Lin, X.; Chen, X.; Chen, Y.; Wan, J.; Ji, L.; Chao, H. Chiral RuII-PtII Complexes Inducing Telomere Dysfunction against Cisplatin-Resistant Cancer Cells. Angew. Chem. Int. Ed. 2022, 61, e202204866. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.N.; Rosenfeldt, L.; Frederick, M.; Miller, W.; Waltz, D.; Kombrinck, K.; McElhinney, K.E.; Flick, M.J.; Monia, B.P.; Revenko, A.S.; et al. Colon Cancer Growth and Dissemination Relies upon Thrombin, Stromal PAR-1, and Fibrinogen. Cancer Res. 2015, 75, 4235–4243. [Google Scholar] [CrossRef]
- Zhou, S.; Xiao, W.; Pan, X.; Zhu, M.; Yang, Z.; Zhang, F.; Zheng, C. Thrombin Promotes Proliferation of Human Lung Fibroblasts via Protease Activated Receptor-1-Dependent and NF-ΚB-Independent Pathways. Cell Biol. Int. 2014, 38, 747–756. [Google Scholar] [CrossRef]
- Gholamjani Moghaddam, K.; Majid Hashemianzadeh, S. The Effect of Amino Substituents on the Interactions of Quinazolone Derivatives with C-KIT G-Quadruplex: Insight from Molecular Dynamics Simulation Study for Rational Design of Ligands. RSC Adv. 2015, 5, 76642–76650. [Google Scholar] [CrossRef]
- Rankin, S.; Reszka, A.P.; Huppert, J.; Zloh, M.; Parkinson, G.N.; Todd, A.K.; Ladame, S.; Balasubramanian, S.; Neidle, S. Putative DNA Quadruplex Formation within the Human C-Kit Oncogene. J. Am. Chem. Soc. 2005, 127, 10584–10589. [Google Scholar] [CrossRef]
- Ji, X.; Sun, H.; Zhou, H.; Xiang, J.; Tang, Y.; Zhao, C. The Interaction of Telomeric DNA and C-Myc22 G-Quadruplex with 11 Natural Alkaloids. Nucleic Acid Ther. 2012, 22, 127–136. [Google Scholar] [CrossRef]
- Moye, A.L.; Porter, K.C.; Cohen, S.B.; Phan, T.; Zyner, K.G.; Sasaki, N.; Lovrecz, G.O.; Beck, J.L.; Bryan, T.M. Telomeric G-Quadruplexes Are a Substrate and Site of Localization for Human Telomerase. Nat. Commun. 2015, 6, 7643–7655. [Google Scholar] [CrossRef]
- Song, M. Synthesis and DNA-Binding Analysis of Naphthalenediimides for the Stabilization of G-quadruplex DNA. Marster’s Thesis, University of Bath, Bath, UK, 2016. [Google Scholar]
- Pengo, P.; Pantoş, G.D.; Otto, S.; Sanders, J.K.M. Efficient and Mild Microwave-Assisted Stepwise Functionalization of Naphthalenediimide with α-Amino Acids. J. Org. Chem. 2006, 71, 7063–7066. [Google Scholar] [CrossRef]
- Lousen, B.; Pedersen, S.K.; Răsădean, D.M.; Pantoş, G.D.; Pittelkow, M. Triggering G-Quadruplex Conformation Switching with [7]Helicenes. Chem. Eur. J. 2021, 27, 6064–6069. [Google Scholar] [CrossRef]
- Vo, T.; Oxenford, S.; Angell, R.; Marchetti, C.; Ohnmacht, S.A.; Wilson, W.D.; Neidle, S. Substituted Naphthalenediimide Compounds Bind Selectively to Two Human Quadruplex Structures with Parallel Topology. ACS Med. Chem. Lett. 2020, 11, 991–999. [Google Scholar] [CrossRef]
- Wen, L.-N.; Xie, M.-X. Evidence of Different G-Quadruplex DNA Binding with Biogenic Polyamines Probed by Electrospray Ionization-Quadrupole Time of Flight Mass Spectrometry, Circular Dichroism and Atomic Force Microscopy. Biochimie 2013, 95, 1185–1195. [Google Scholar] [CrossRef]
- Monchaud, D.; Teulade-Fichou, M.-P. A Hitchhiker’s Guide to G-Quadruplex Ligands. Org. Biomol. Chem. 2008, 6, 627–636. [Google Scholar] [CrossRef]
- Ambrus, A.; Chen, D.; Dai, J.; Bialis, T.; Jones, R.A.; Yang, D. Human Telomeric Sequence Forms a Hybrid-Type Intramolecular G-Quadruplex Structure with Mixed Parallel/Antiparallel Strands in Potassium Solution. Nucleic Acids Res. 2006, 34, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Carver, M.; Punchihewa, C.; Jones, R.A.; Yang, D. Structure of the Hybrid-2 Type Intramolecular Human Telomeric G-Quadruplex in K+ Solution: Insights into Structure Polymorphism of the Human Telomeric Sequence. Nucleic Acids Res. 2007, 35, 4927–4940. [Google Scholar] [CrossRef]
- Zhu, B.-C.; He, J.; Xia, X.-Y.; Jiang, J.; Liu, W.; Liu, L.-Y.; Liang, B.-B.; Yao, H.-G.; Ke, Z.; Xia, W.; et al. Solution Structure of a Thrombin Binding Aptamer Complex with a Non-Planar Platinum(II) Compound. Chem. Sci. 2022, 13, 8371–8379. [Google Scholar] [CrossRef]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of Single-Stranded DNA Molecules That Bind and Inhibit Human Thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef]
- Chaires, J.B. Human Telomeric G-Quadruplex: Thermodynamic and Kinetic Studies of Telomeric Quadruplex Stability. FEBS J. 2010, 277, 1098–1106. [Google Scholar] [CrossRef]
- Chen, M.; Song, G.; Wang, C.; Hu, D.; Ren, J.; Qu, X. Small-Molecule Selectively Recognizes Human Telomeric G-Quadruplex DNA and Regulates Its Conformational Switch. Biophys. J. 2009, 97, 2014–2023. [Google Scholar] [CrossRef]
- McNutt, A.T.; Francoeur, P.; Aggarwal, R.; Masuda, T.; Meli, R.; Ragoza, M.; Sunseri, J.; Koes, D.R. GNINA 1.0: Molecular Docking with Deep Learning. J. Cheminformatics 2021, 13, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Ragoza, M.; Hochuli, J.; Idrobo, E.; Sunseri, J.; Koes, D.R. Protein–Ligand Scoring with Convolutional Neural Networks. J. Chem. Inf. Model. 2017, 57, 942–957. [Google Scholar] [CrossRef]
- James, J.P. Stewart, Stewart Computational Chemistry, Colorado Springs, CO, USA, MOPAC2016. 2016. Available online: http://OpenMOPAC.net (accessed on 23 October 2023).
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- del Villar-Guerra, R.; Trent, J.O.; Chaires, J.B. G-Quadruplex Secondary Structure Obtained from Circular Dichroism Spectroscopy. Angew. Chem. Int. Ed. 2018, 57, 7171–7175. [Google Scholar] [CrossRef]
- Nallagatla, S.R.; Heuberger, B.; Haque, A.; Switzer, C. Combinatorial Synthesis of Thrombin-Binding Aptamers Containing Iso-Guanine. J. Comb. Chem. 2009, 11, 364–369. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Merlino, A.; Giancola, C.; Randazzo, A.; Mazzarella, L.; Sica, F. Thrombin–Aptamer Recognition: A Revealed Ambiguity. Nucleic Acids Res. 2011, 39, 7858–7867. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-Y.; Wang, X.-N.; Cheng, S.-Q.; Su, X.-X.; Ou, T.-M. Developing Novel G-Quadruplex Ligands: From Interaction with Nucleic Acids to Interfering with Nucleic Acid–Protein Interaction. Molecules 2019, 24, 396. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Salgado, G.F.; Cabrita, E.J.; Cruz, C. G-Quadruplexes and Their Ligands: Biophysical Methods to Unravel G-Quadruplex/Ligand Interactions. Pharmaceuticals 2021, 14, 769. [Google Scholar] [CrossRef] [PubMed]
- Linder, J.; Garner, T.P.; Williams, H.E.L.; Searle, M.S.; Moody, C.J. Telomestatin: Formal Total Synthesis and Cation-Mediated Interaction of Its Seco-Derivatives with G-Quadruplexes. J. Am. Chem. Soc. 2011, 133, 1044–1051. [Google Scholar] [CrossRef]
- Xiong, Y.-X.; Huang, Z.-S.; Tan, J.-H. Targeting G-Quadruplex Nucleic Acids with Heterocyclic Alkaloids and Their Derivatives. Eur. J. Med. Chem. 2015, 97, 538–551. [Google Scholar] [CrossRef]
- Arnott, S.; Campbell-Smith, P.J.; Chandrasekaran, R. Handbook of Biochemistry and Molecular Biology—Nucleic Acids; CRC Press: Boca Raton, FL, USA, 1976; Volume 2, pp. 411–422. [Google Scholar]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
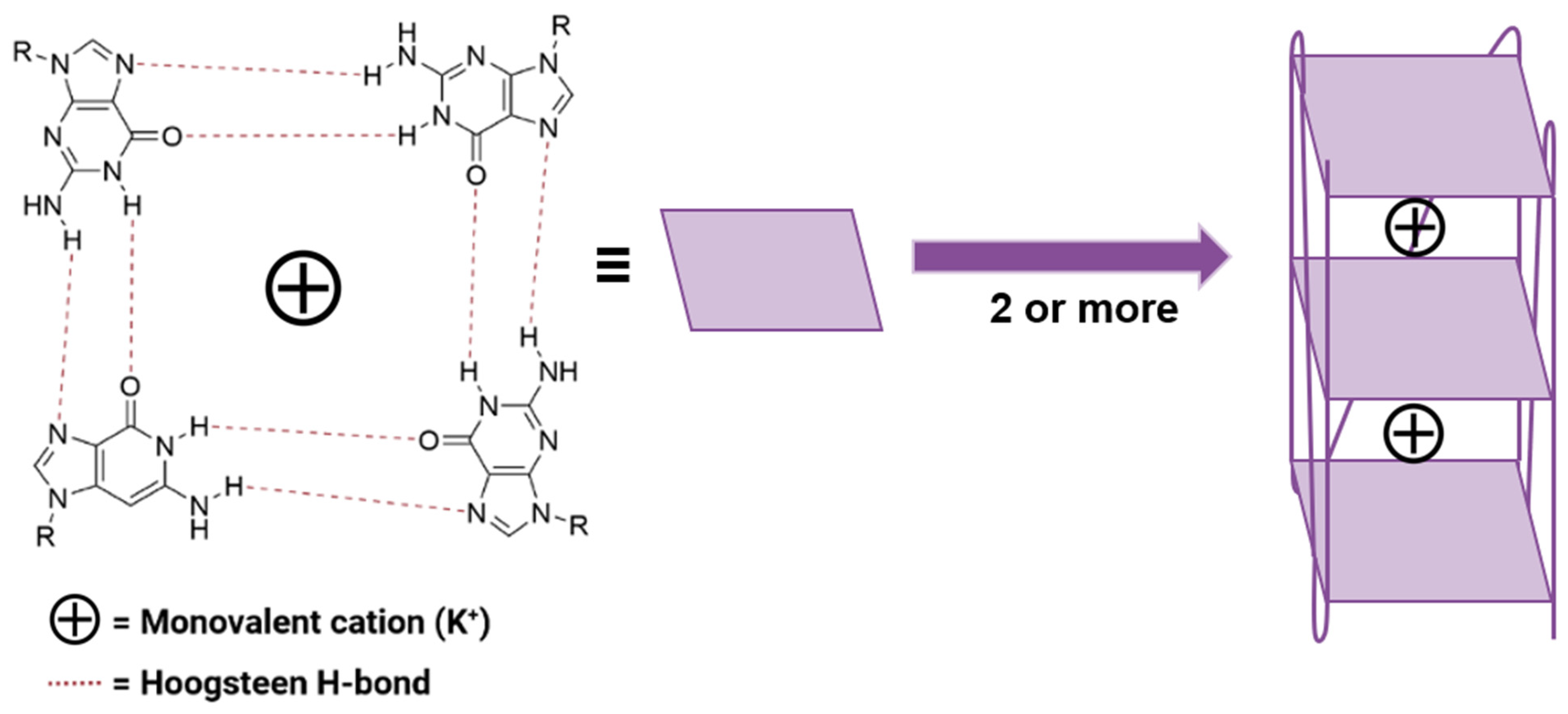
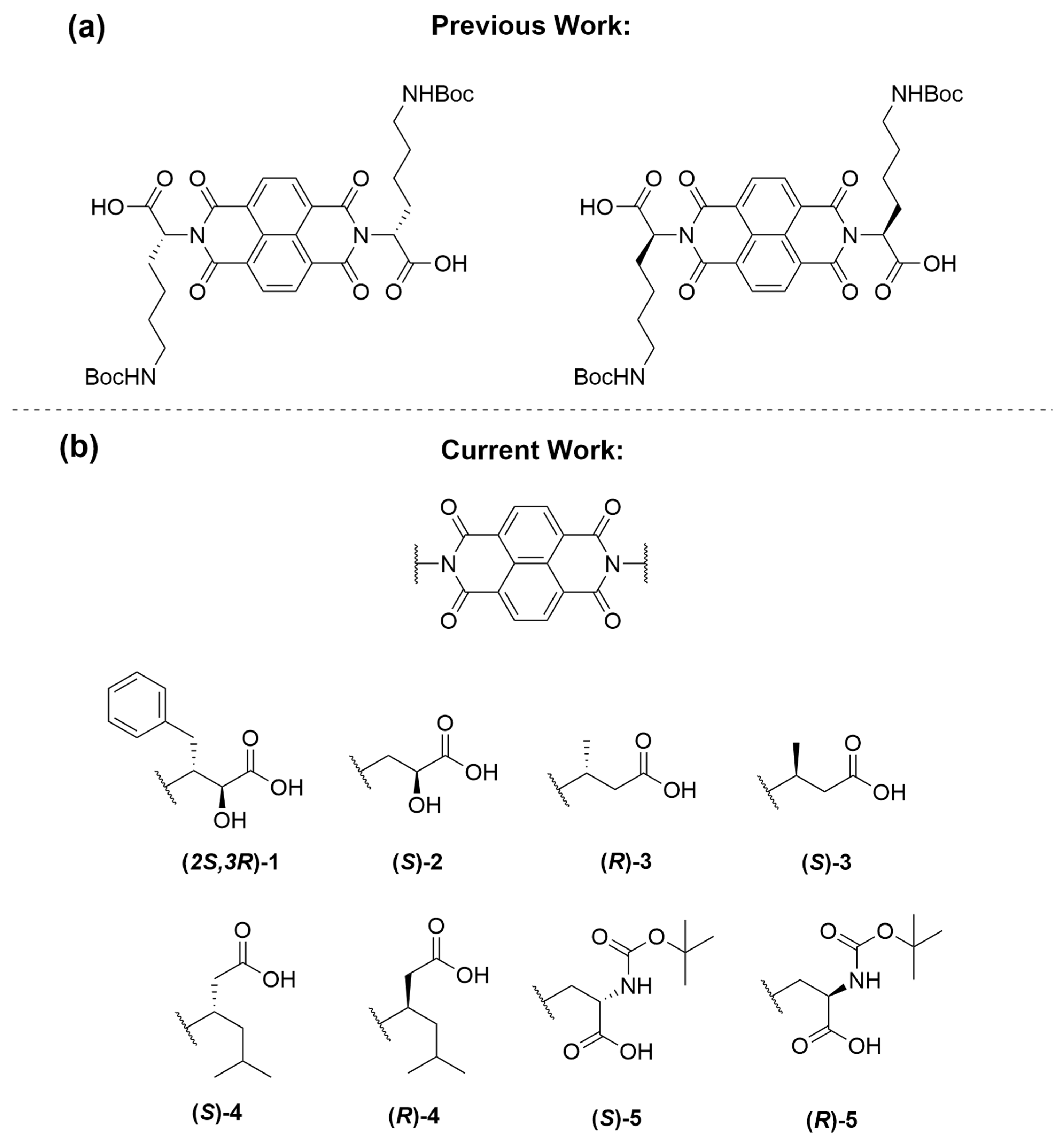


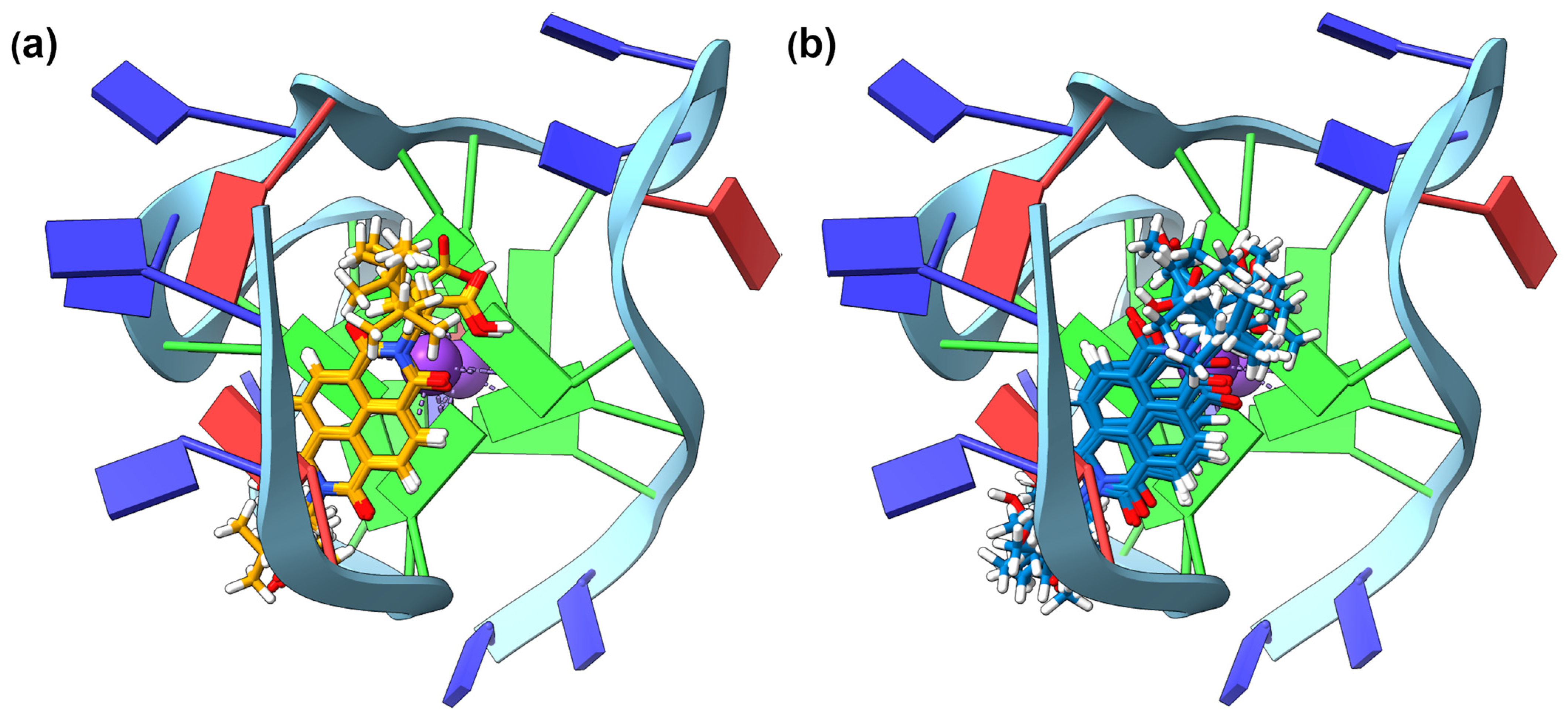
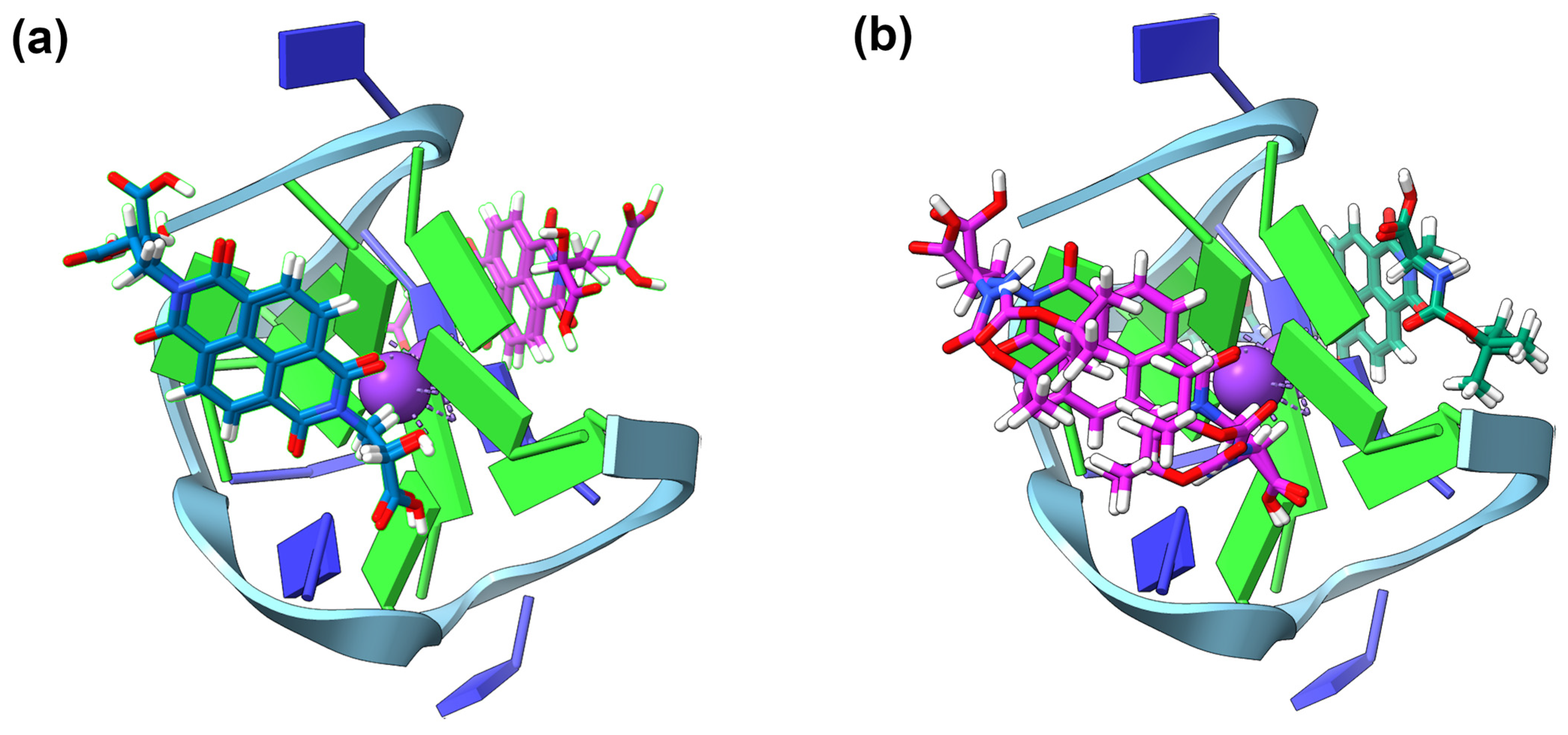

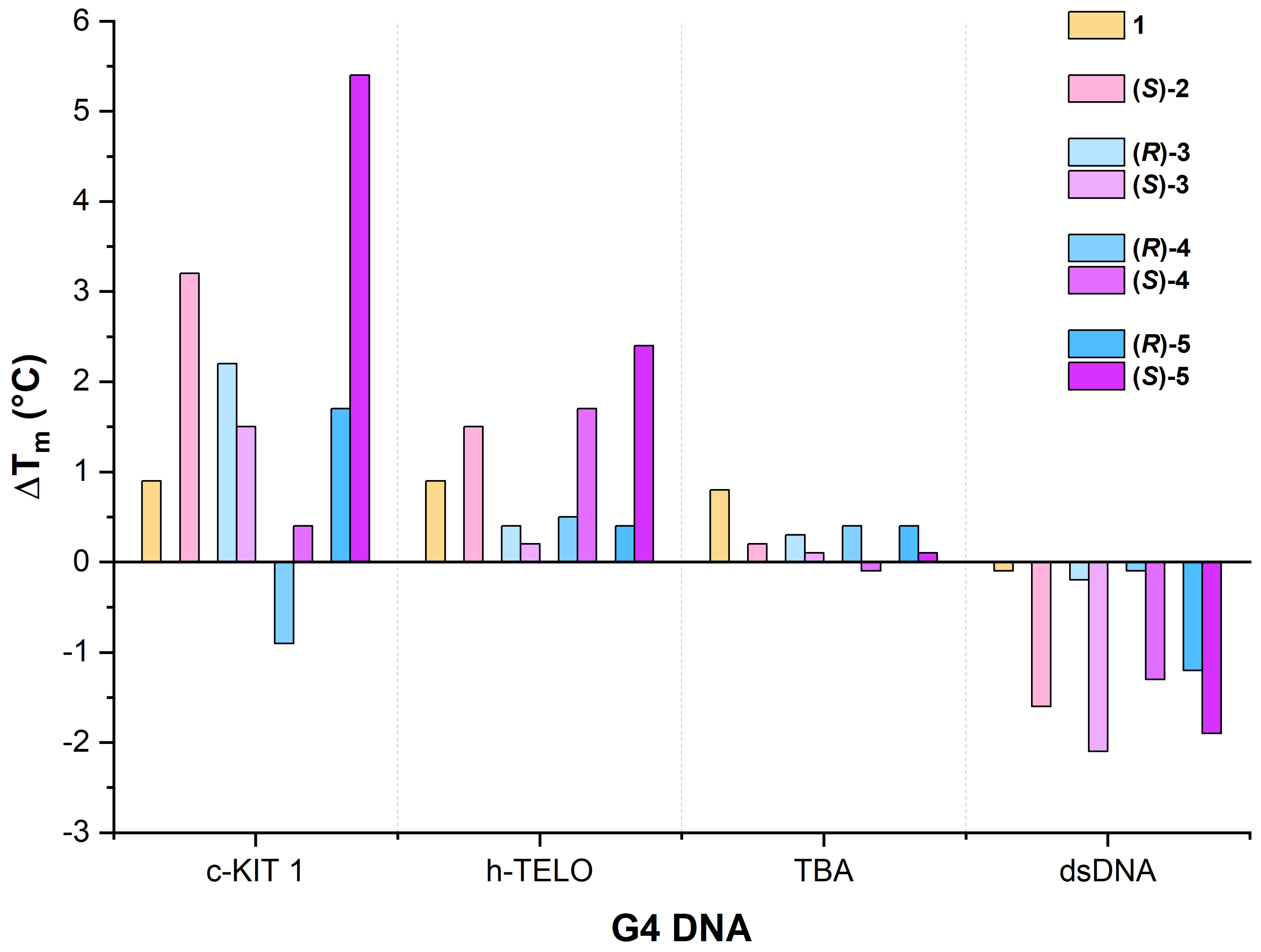
| Sequence/Assembly | Average Tm (°C) | ΔTm (°C) 1 |
|---|---|---|
| c-KIT1 only | 61.2 ± 0.3 | N/A |
| 1 | 62.1 ± 0.2 | 0.9 |
| (S)-2 | 64.4 ± 0.2 | 3.2 |
| (R)-3 | 63.4 ± 0.2 | 2.2 |
| (S)-3 | 62.7 ± 0.6 | 1.5 |
| (R)-4 | 60.3 ± 0.9 | −0.9 |
| (S)-4 | 61.6 ± 0.6 | 0.4 |
| (R)-5 | 62.9 ± 1.0 | 1.7 |
| (S)-5 | 66.6 ± 1.3 | 5.4 |
| Sequence/Assembly | Average Tm (°C) | ΔTm (°C) 1 |
|---|---|---|
| h-TELO only | 63.7 ± 0.8 | N/A |
| 1 | 64.6 ± 0.4 | 0.9 |
| (S)-2 | 65.2 ± 0.7 | 1.5 |
| (R)-3 | 64.1 ± 0.5 | 0.4 |
| (S)-3 | 63.9 ± 0.2 | 0.2 |
| (R)-4 | 64.3 ± 0.4 | 0.5 |
| (S)-4 | 65.4 ± 1.7 | 1.7 |
| (R)-5 | 64.2 ± 0.5 | 0.4 |
| (S)-5 | 66.2 ± 2.0 | 2.4 |
| Sequence/Assembly | Average Tm (°C) | ΔTm (°C) 1 |
|---|---|---|
| TBA only | 50.2 ± 0.4 | N/A |
| 1 | 51.0 ± 0.1 | 0.8 |
| (S)-2 | 50.4 ± 0.1 | 0.2 |
| (R)-3 | 50.5 ± 0.2 | 0.3 |
| (S)-3 | 50.3 ± 0.1 | 0.1 |
| (R)-4 | 50.6 ± 0.3 | 0.4 |
| (S)-4 | 50.1 ± 0.1 | −0.1 |
| (R)-5 | 50.6 ± 0.2 | 0.4 |
| (S)-5 | 50.3 ± 0.1 | 0.1 |
| Sequence/Assembly | Average Tm (°C) | ΔTm (°C) 1 |
|---|---|---|
| dsDNA only | 61.9 ± 1.5 | N/A |
| 1 | 61.8 ± 0.5 | −0.1 |
| (S)-2 | 60.2 ± 0.8 | −1.6 |
| (R)-3 | 61.7 ± 1.2 | −0.2 |
| (S)-3 | 59.8 ± 0.1 | −2.1 |
| (R)-4 | 61.8 ± 0.4 | −0.1 |
| (S)-4 | 60.6 ± 0.4 | −1.3 |
| (R)-5 | 60.7 ± 1.0 | −1.2 |
| (S)-5 | 60.0 ± 1.1 | −1.9 |
| Ligand + DNA | KD (μM) | R2 |
|---|---|---|
| (R)-5 + c-KIT1 | 8.0 ± 2.5 | 0.974 |
| (S)-5 + c-KIT1 | 2.6 ± 0.4 | 0.953 |
| (R)-4 + h-TELO | 13.6 ± 3.4 | 0.992 |
| (S)-4 + h-TELO | 11.0 ± 3.1 | 0.971 |
| (R)-5 + dsDNA | 30.7 ± 6.9 | 0.997 |
| (S)-5 + dsDNA | 65.2 ± 35.9 | 0.995 |
| (R)-4 + dsDNA | 50.2 ± 15.4 | 0.992 |
| (S)-4 + dsDNA | 23.6 ± 8.4 | 0.992 |
| Sequence Name | Sequence Composition (5′-3′) |
|---|---|
| c-KIT1 (used experimentally) | GGG AGG GCG CTG GGA GGA GGG |
| Amended PDB ID: 3QXR (c-KIT1) | GGG AGG GCG CUG GGA GGA GGG |
| h-TELO (used experimentally) | AGG GTT AGG GTT AGG GTT AGG GT |
| Amended PDB ID: 6CCW (h-TELO hybrid) | AGG GTT AGG GTT AGG GTT AGG GT |
| TBA (used experimentally) | GGT TGG TGT GGT TGG |
| PDB ID: 5MJX (TBA) | GGT TGG TGT GGT TGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clowes, S.R.; Ali, Y.; Astley, O.R.; Răsădean, D.M.; Pantoş, G.D. The Influence of Chirality on the β-Amino-Acid Naphthalenediimides/G-Quadruplex DNA Interaction. Molecules 2023, 28, 7291. https://doi.org/10.3390/molecules28217291
Clowes SR, Ali Y, Astley OR, Răsădean DM, Pantoş GD. The Influence of Chirality on the β-Amino-Acid Naphthalenediimides/G-Quadruplex DNA Interaction. Molecules. 2023; 28(21):7291. https://doi.org/10.3390/molecules28217291
Chicago/Turabian StyleClowes, Samuel R., Yusuf Ali, Olivia R. Astley, Dora M. Răsădean, and G. Dan Pantoş. 2023. "The Influence of Chirality on the β-Amino-Acid Naphthalenediimides/G-Quadruplex DNA Interaction" Molecules 28, no. 21: 7291. https://doi.org/10.3390/molecules28217291
APA StyleClowes, S. R., Ali, Y., Astley, O. R., Răsădean, D. M., & Pantoş, G. D. (2023). The Influence of Chirality on the β-Amino-Acid Naphthalenediimides/G-Quadruplex DNA Interaction. Molecules, 28(21), 7291. https://doi.org/10.3390/molecules28217291







