Theoretical Calculations Facilitating Catalysis for Advanced Lithium-Sulfur Batteries
Abstract
:1. Introduction
2. Electronic Structures
2.1. Band Structures
2.2. Densities of States
2.2.1. Conductivity Analyses
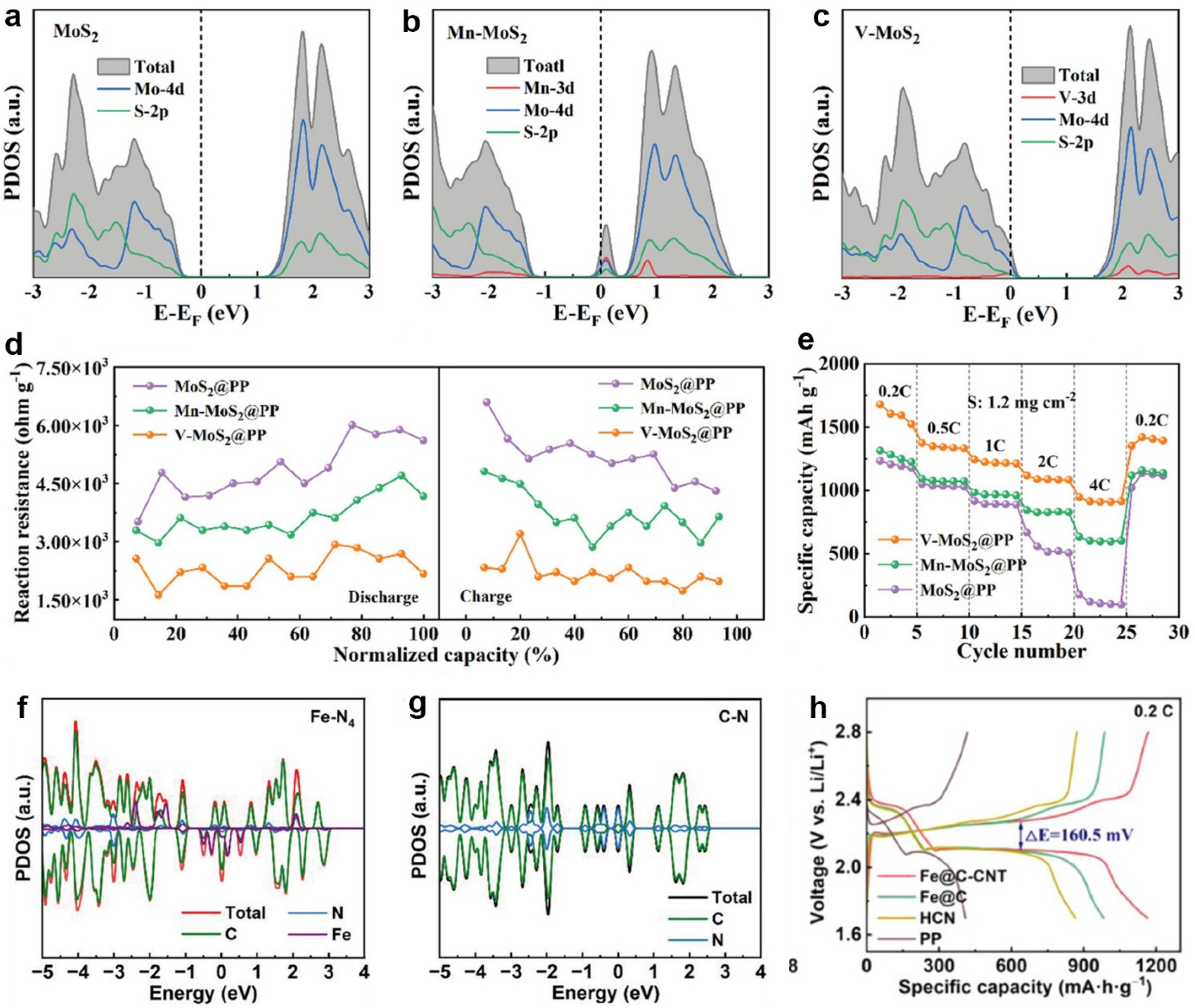
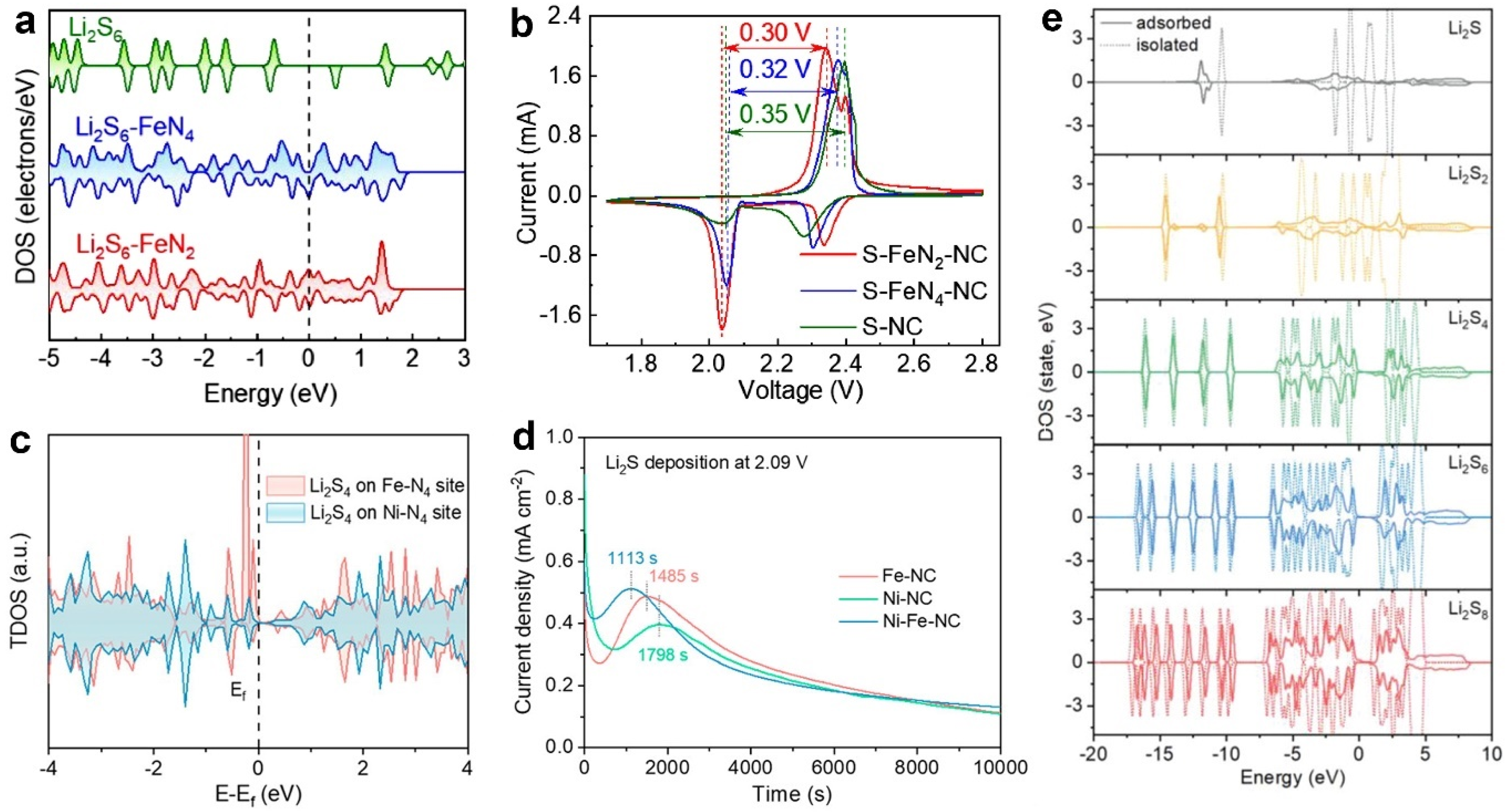
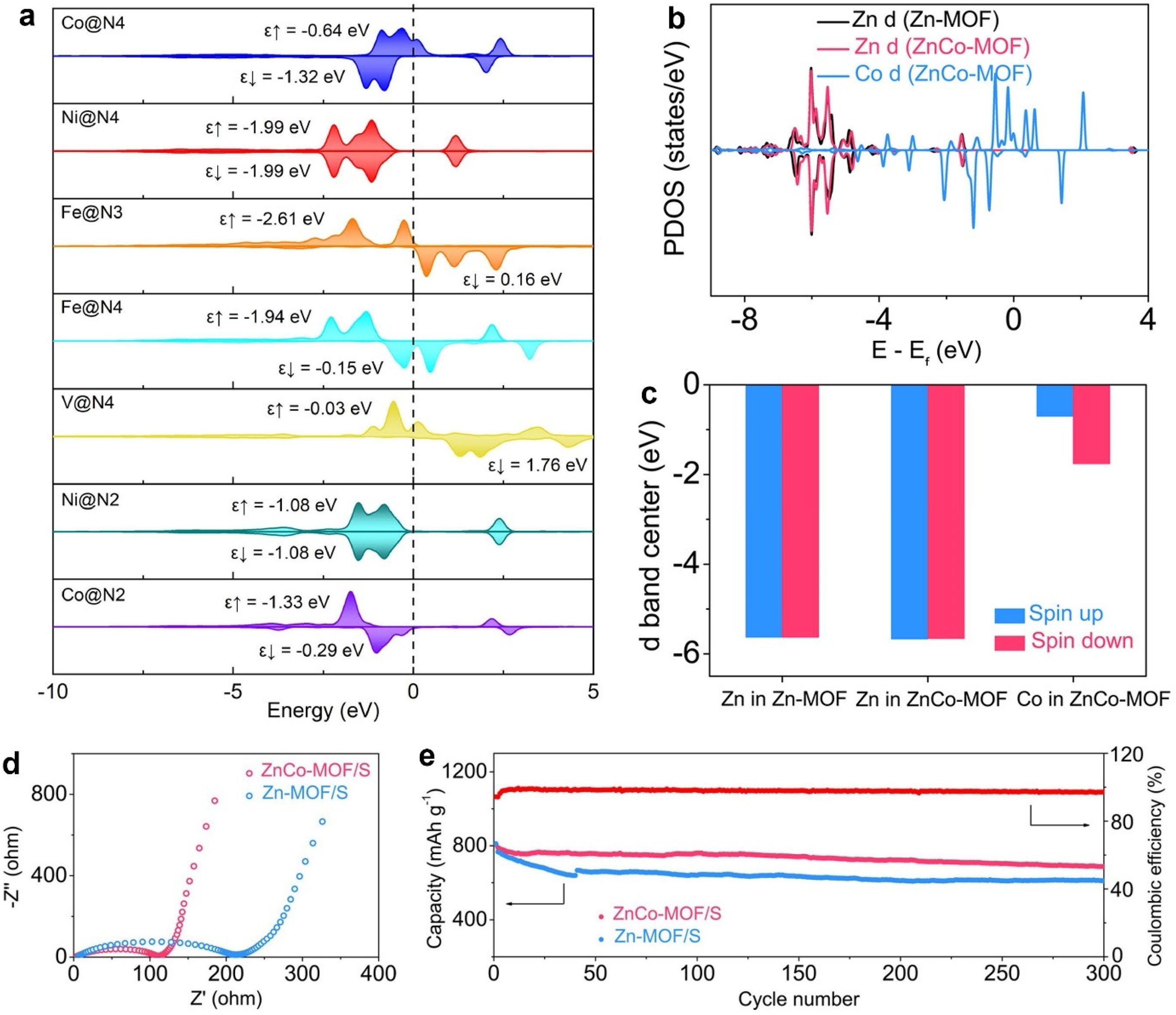
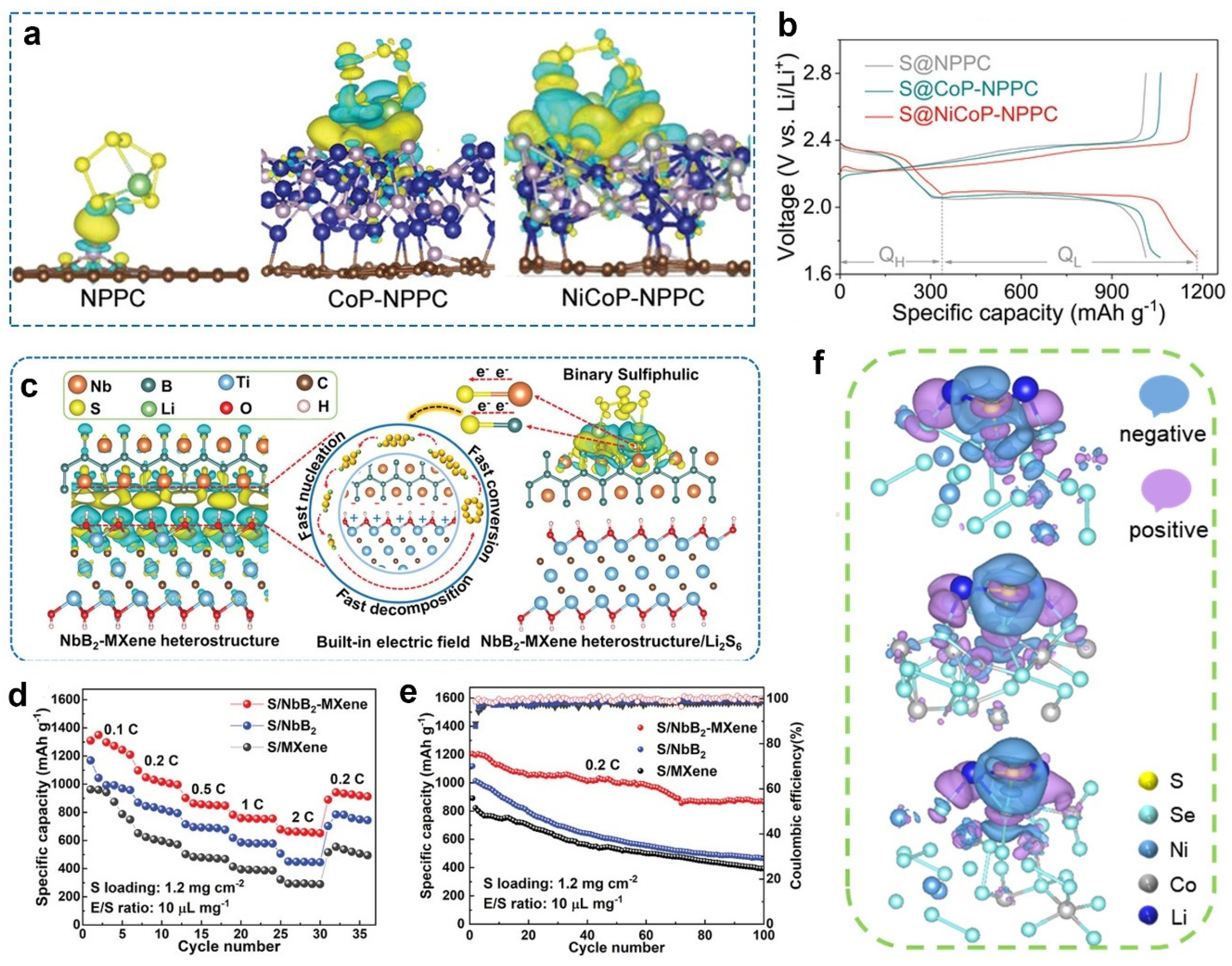
2.2.2. Interaction between Catalysts and Polysulfides
2.2.3. d-Band Center Calculations
2.3. Charge Distribution
3. Binding Energy between Sulfur Species and Catalysts
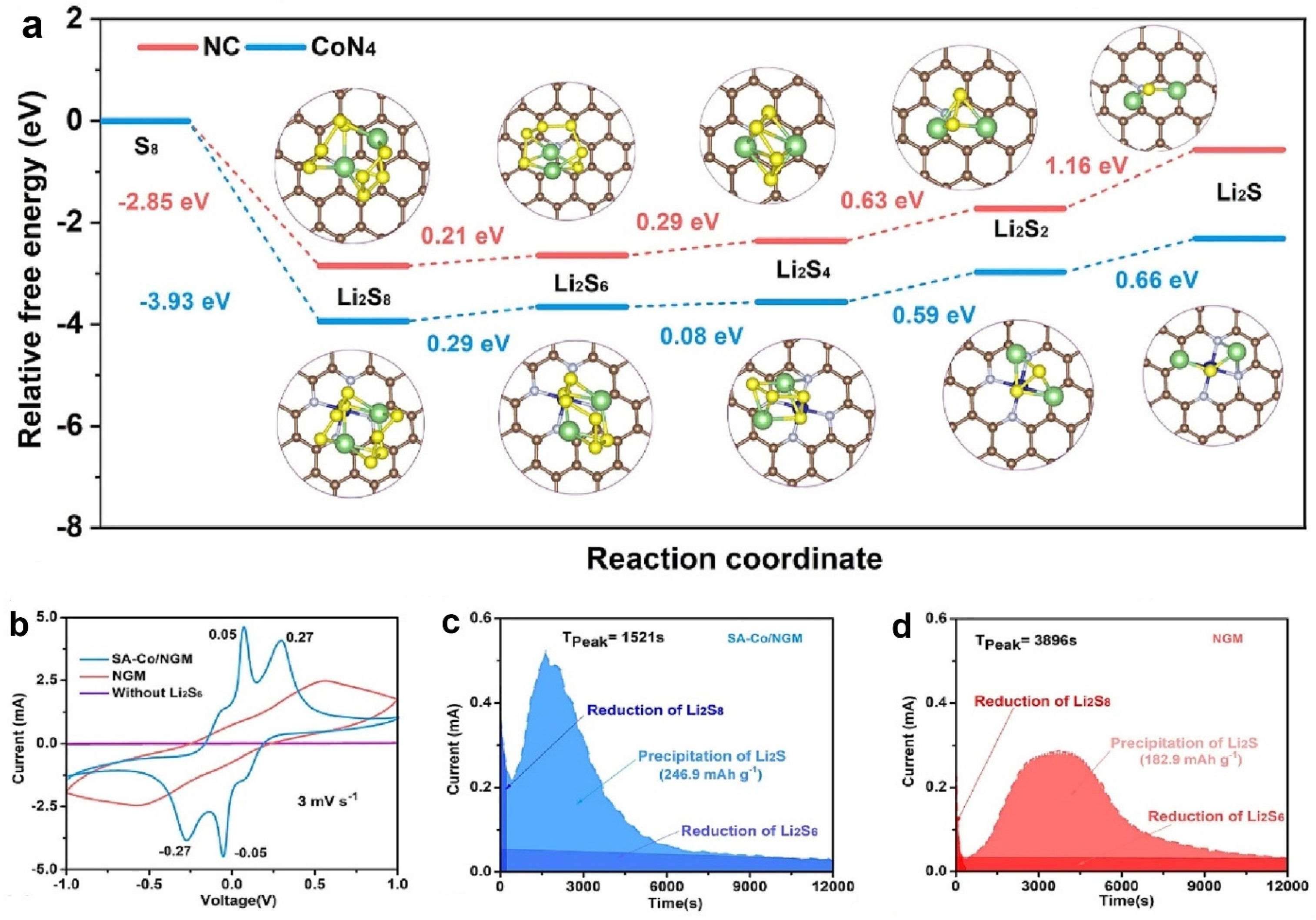
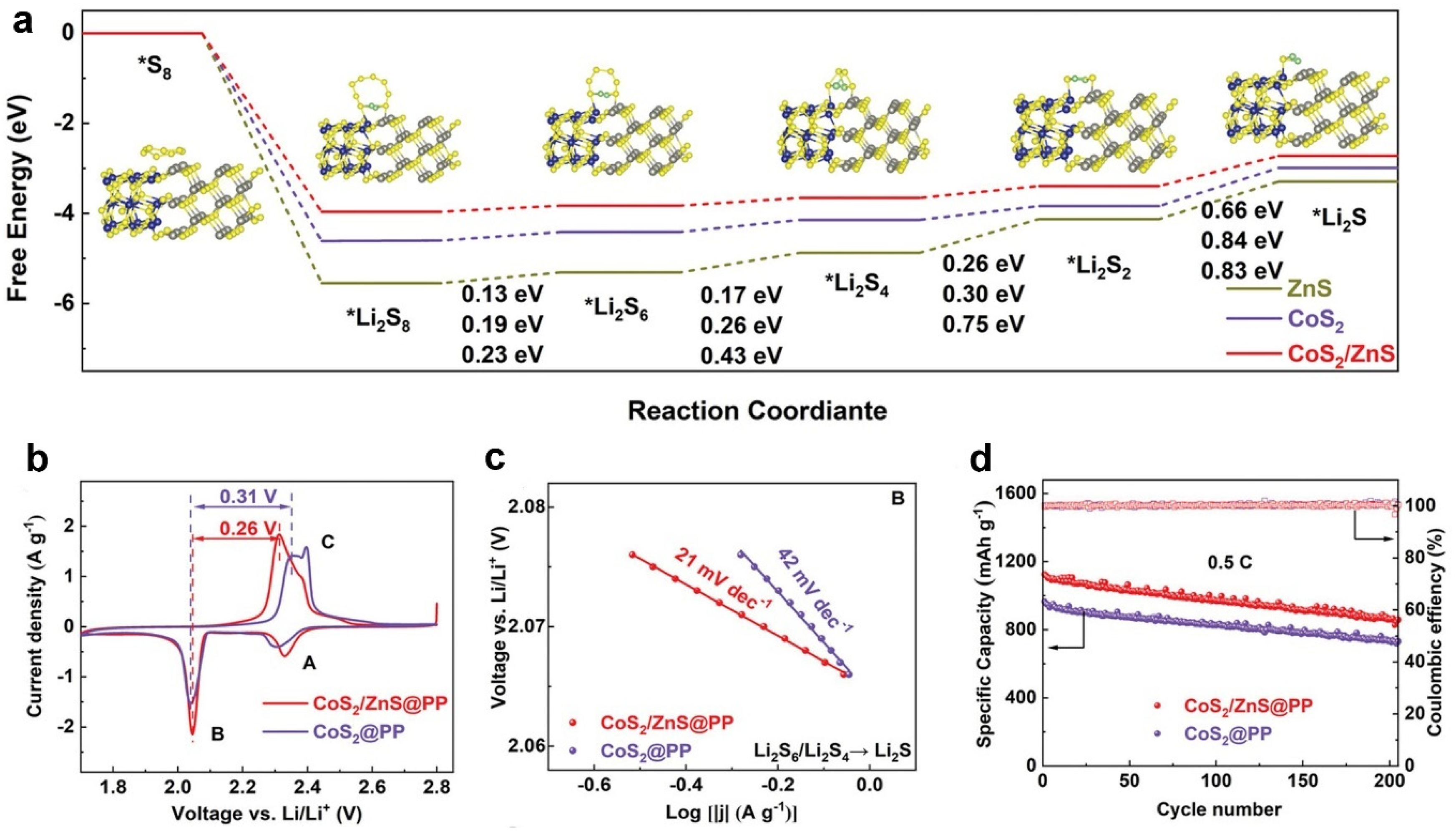
4. Gibbs Free Energy
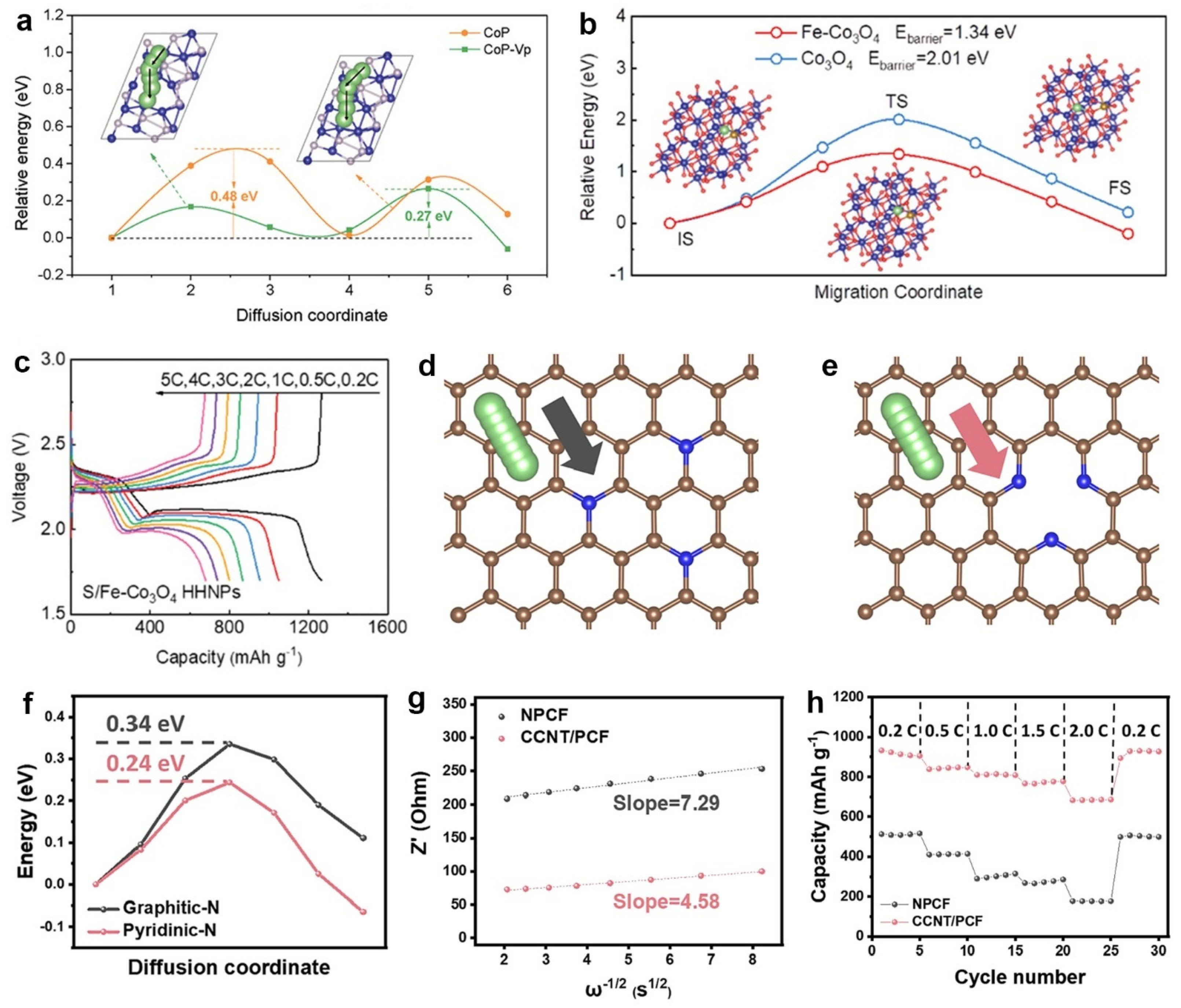
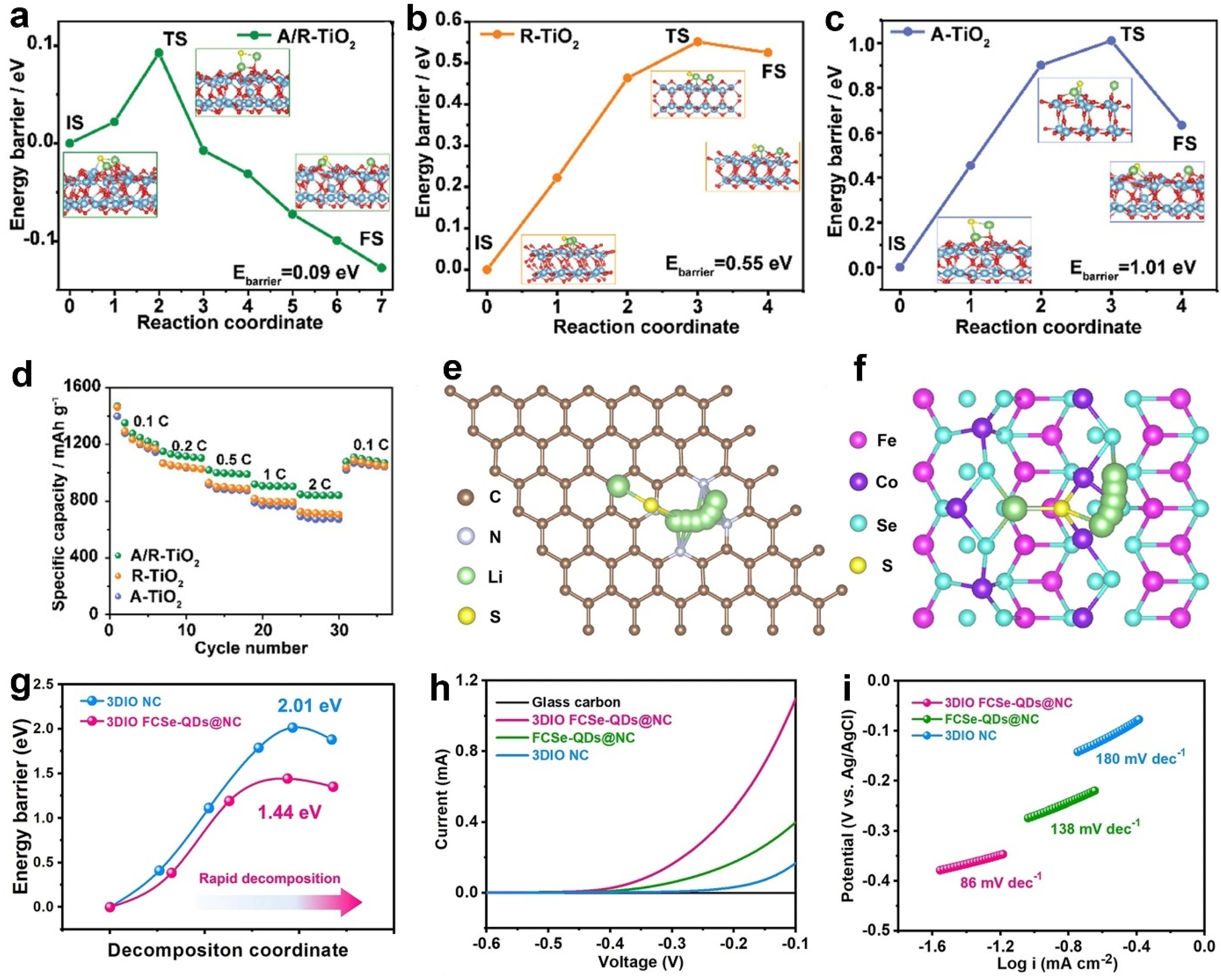
5. Lithium-Ion Diffusion Energy Barriers
6. Li2S Decomposition Energy Barriers
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable batteries for grid scale energy storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ma, L. Recent progress in hydrogen storage alloys for nickel/metal hydride secondary batteries. Int. J. Hydrogen Energy 2009, 34, 4788–4796. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.-G.; Yang, J.; Xue, S.-C.; Gao, H.-L.; Yan, X.-H.; Huo, Q.-Y.; Wang, S.-W.; Cao, Y.; Yan, J.; et al. Advances and challenges in improvement of the electrochemical performance for lead-acid batteries: A comprehensive review. J. Power Sources 2022, 520, 230800. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. Host materials anchoring polysulfides in Li-S batteries reviewed. Adv. Energy Mater. 2021, 11, 2001304. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y. Challenges and prospects of lithium-sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Wu, X.; Zhang, Y.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Double-shelled Co3O4/C nanocages enabling polysulfides adsorption for high-performance Lithium-sulfur batteries. ACS Appl. Energy Mater. 2019, 2, 8153–8162. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Qiao, F.; Eichel, R.-A.; Notten, P.H.L. ZnFe2O4 hollow rods enabling accelerated polysulfide conversion for advanced lithium-sulfur batteries. Electrochim. Acta 2022, 414, 140231. [Google Scholar] [CrossRef]
- Dong, H.; Qi, S.; Wang, L.; Chen, X.; Xiao, Y.; Wang, Y.; Sun, B.; Wang, G.; Chen, S. Conductive polymer coated layered double hydroxide as a novel sulfur reservoir for flexible lithium-sulfur batteries. Small 2023, 19, 2300843. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Mo, Y.; Li, S.; Yu, J. Nitrogen-doped porous carbon fiber/vertical graphene as an efficient polysulfide conversion catalyst for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2022, 10, 690–698. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, Y.; Chen, C.; Li, B.; Li, W.; Lu, H.; Yu, L.; Zheng, S.; Wang, B. Low-temperature Li-S battery enabled by CoFe bimetallic catalysts. J. Mater. Chem. A 2022, 10, 8378–8389. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Z.; Wen, Y.; He, Q.; Yao, J.; Cheng, H.; Gao, T.; Wang, X.; Zhang, H.; Jiao, H. CrP Nanocatalyst within porous mof architecture to accelerate polysulfide conversion in lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2023, 15, 21040–21048. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, J.; Zhao, Y.; Wang, Y.; Lu, X.; Zhou, M.; Zhang, G.; Liu, W.; Xu, H.; Sun, X. Regulating electronic structure of Fe-N4 single atomic catalyst via neighboring sulfur doping for high performance lithium-sulfur batteries. Adv. Funct. Mater. 2023, 33, 2210509. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Qiao, F.; Wang, J.; Li, H.; Eichel, R.A.; Notten, P.H.L. Sulfur reduction reaction in lithium-sulfur batteries: Mechanisms, catalysts, and characterization. Adv. Energy Mater. 2022, 12, 2202094. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Zhang, Y.; Jiang, M.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Enhanced sulfur utilization in lithium-sulfur batteries by hybrid modified separators. Mater. Today Comm. 2021, 26, 102133. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Moore, J.S. Toward a molecular understanding of energetics in Li-S batteries using nonaqueous electrolytes: A high-level quantum chemical study. J. Phys. Chem. C 2014, 118, 11545–11558. [Google Scholar] [CrossRef]
- Feng, S.; Fu, Z.H.; Chen, X.; Zhang, Q. A review on theoretical models for Lithium-sulfur battery cathodes. InfoMat 2022, 4, e12304. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z.; Xu, X.; Liu, Z.; Zhang, D.; Li, F.; Li, Y.; Zeng, L.; Liu, J. Surface/interface structure and chemistry of lithium-sulfur batteries: From density functional theory calculations’ perspective. Adv. Energy Sustain. Res. 2021, 2, 2100007. [Google Scholar] [CrossRef]
- He, Q.; Yu, B.; Li, Z.; Zhao, Y. Density functional theory for battery materials. Energy Environ. Mater. 2019, 2, 264–279. [Google Scholar] [CrossRef]
- Han, X.; Xu, X. Indirect modulation of Cu atoms supported on black phosphorus for fast kinetic Li-S batteries: A theoretical study. ACS Mater. Lett. 2023, 5, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Shi, P.; Wang, L.; Yan, T.; Guo, T.; Xia, X.; Chen, C.; Mao, J.; Sun, D.; Zhang, L. Cooperative catalysis of polysulfides in lithium-sulfur batteries through adsorption competition by tuning cationic geometric configuration of dual-active sites in spinel oxides. Angew. Chem. Int. Ed. 2023, 62, e2022162. [Google Scholar]
- Shen, Z.; Cao, M.; Wen, Y.; Li, J.; Zhang, X.; Hui, J.; Zhu, Q.; Zhang, H. Tuning the local coordination of CoP1–xSx between NiAs- and MnP-type structures to catalyze lithium-sulfur batteries. ACS Nano 2023, 17, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, S.; Yao, Y.; Dong, P.; Song, H. Transition metal compounds family for Li-S batteries: The DFT-guide for suppressing polysulfides shuttle. Adv. Funct. Mater. 2023, 33, 230082. [Google Scholar] [CrossRef]
- Fang, M.; Han, J.; He, S.; Ren, J.-C.; Li, S.; Liu, W. Effective screening descriptor for mxenes to enhance sulfur reduction in lithium-sulfur batteries. J. Am. Chem. Soc. 2023, 145, 12601–12608. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Kong, Y.; Wang, F.; Jing, Z.; Yang, X.; Qian, Y.; Chen, M.; Xu, L. Hafnium diboride spherical superstructure born of 5d-Metal Hf-MOF-induced p orbital activity of B atom and enhanced kinetics of sulfur cathode reaction. Adv. Energy Mater. 2023, 13, 2300590. [Google Scholar] [CrossRef]
- Tahir, M.N.; Mirza, S.H.; Khalid, M.; Ali, A.; Khan, M.U.; Braga, A.A.C. Synthesis, single crystal analysis and DFT based computational studies of 2,4-diamino-5-(4-chlorophenyl)-6-ethylpyrim idin-1-ium 3,4,5-trihydroxybenzoate -methanol (DETM). J. Mol. Struct. 2019, 1180, 119–126. [Google Scholar] [CrossRef]
- Shafiq, I.; Amanat, I.; Khalid, M.; Asghar, M.A.; Baby, R.; Ahmed, S.; Alshehri, S.M. Influence of azo-based donor modifications on nonlinear optical amplitude of D-π-A based organic chromophores: A DFT/TD-DFT exploration. Synth. Met. 2023, 297, 117410. [Google Scholar] [CrossRef]
- Wang, B.; Ren, Y.; Zhu, Y.; Chen, S.; Chang, S.; Zhou, X.; Wang, P.; Sun, H.; Meng, X.; Tang, S. Construction of Co3O4/ZnO Heterojunctions in hollow n-doped carbon nanocages as microreactors for lithium-sulfur full batteries. Adv. Sci. 2023, 10, 2300860. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, X.; Wang, X.; Zhen, M. Dual-conductive CoSe2@TiSe2-C heterostructures promoting overall sulfur redox kinetics under high sulfur loading and lean electrolyte. Small 2023, 19, 2300089. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, Y.; Wang, B.; Chang, S.; Zhai, Q.; Wu, H.; Dai, Y.; Yang, Y.; Tang, S.; Meng, X. Furnishing continuous efficient bidirectional polysulfide conversion for long-life and high-loading lithium-sulfur batteries via the built-in electric field. Small 2023, 19, 2300065. [Google Scholar] [CrossRef] [PubMed]
- Schütze, Y.; Gayen, D.; Palczynski, K.; de Oliveira Silva, R.; Lu, Y.; Tovar, M.; Partovi-Azar, P.; Bande, A.; Dzubiella, J. How regiochemistry influences aggregation behavior and charge transport in conjugated organosulfur polymer cathodes for lithium-sulfur batteries. ACS Nano 2023, 17, 7889–7900. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Shen, S.; Wang, S.; Shi, L.; Liu, D.; Fu, Y.; He, D. Multi-perspective synergistic construction of dual-functional heterostructures for high-temperature Li-S batteries. Chem. Eng. J. 2023, 468, 143562. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, Q.; Tian, S.; Tao, K.; Xie, E.; Zhang, Z. Deciphering the electrocatalysis essence of cobalt diselenide in lithium-sulfur electrochemistry from crystal-phase engineering. Chem. Eng. J. 2023, 463, 142416. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, J.; Guo, C.; Wang, H.; Li, H.; Xue, P.; Lee, J.-M. Hierarchically constructed ZnO/Co3O4 nanoheterostructures synergizing dendrite inhibition and polysulfide conversion in Lithium-sulfur battery. ACS Mater. Lett. 2022, 4, 1358–1367. [Google Scholar] [CrossRef]
- Wan, T.; He, Y.; He, Z.; Han, W.; Zhang, Y.; Liu, G. Integrated host configuration of flexibly fibrous skeleton towards efficient polysulfide conversion and dendrite-free behavior in stable lithium-sulfur pouch cells. J. Energy Chem. 2023, 83, 43–52. [Google Scholar] [CrossRef]
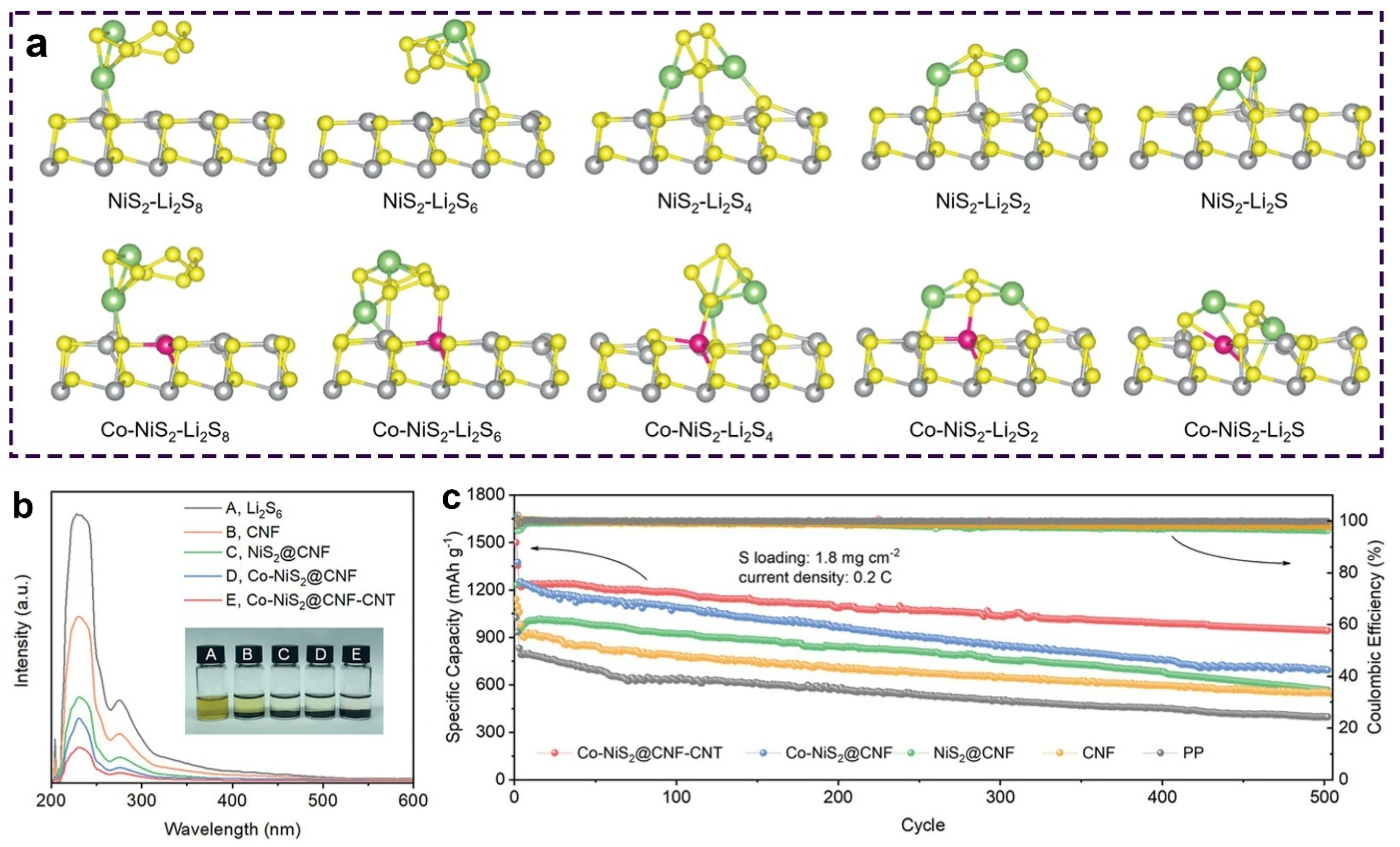
- Dai, X.; Lv, G.; Wu, Z.; Wang, X.; Liu, Y.; Sun, J.; Wang, Q.; Xiong, X.; Liu, Y.; Zhang, C.; et al. Flexible hierarchical Co-doped NiS2@CNF-CNT electron deficient interlayer with grass-roots structure for Li-S batteries. Adv. Energy Mater. 2023, 13, 2300452. [Google Scholar] [CrossRef]
- Dai, X.; Wang, X.; Lv, G.; Wu, Z.; Liu, Y.; Sun, J.; Liu, Y.; Chen, Y. Defect-engineered sulfur vacancy modified NiCo2S4-x nanosheet anchoring polysulfide for improved lithium sulfur batteries. Small 2023, 19, 2302267. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, Q.; Sui, X.; Tian, S.; Sun, X.; Wu, Q.; Li, X.; Zhang, Y.; Tao, K.; Xie, E.; et al. Modulating d-band electronic structures of molybdenum disulfide via p/n doping to boost polysulfide conversion in lithium-sulfur batteries. Small 2023, 19, 2301085. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yue, L.; Wang, X.; Wu, S.; Wang, W.; Lu, D.; Liu, X.; Zhou, W.; Li, Y. Synergistically accelerating adsorption-electrocataysis of sulfur species via interfacial built-in electric field of SnS2-mxene mott-schottky heterojunction in Li-S batteries. Small 2023, 19, 220646. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jiang, H.; Wang, K.; Yu, M.; Jiang, X.; He, G.; Li, X. Enhancing electron conductivity and electron density of single atom based core-shell nanoboxes for high redox activity in lithium sulfur batteries. Small 2023, 19, 2301849. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, R.; Yuan, D.; Zhang, L.; Wu, C.; Ma, T.; Yan, W.; Wang, R.; Liu, L.; Jiang, X.; et al. Single-atomic tungsten-doped Co3O4 nanosheets for enhanced electrochemical kinetics in lithium-sulfur batteries. Carbon Energy 2023, 5, e329. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Y.; Zhang, S.; Wang, L.; Zhang, C.; Chen, Y.; Wu, Q.; Chen, L.; Zhou, L.; Wei, W. Integrating energy band alignment and oxygen vacancies engineering of TiO2 anatase/rutile homojunction for kinetics-enhanced Li-S batteries. Adv. Funct. Mater. 2023, 2305788. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, Y.; Pei, Z.; Luan, D.; Wang, X.; Lou, X.W. Bimetal-organic framework nanoboxes enable accelerated redox kinetics and polysulfide trapping for lithium-sulfur batteries. Angew. Chem. Int. Ed. 2023, 62, e2023058. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, W.; Li, G.; Liu, J.; Luo, D.; Zhang, Y.; Zhao, Y.; Zhou, G.; Shui, L.; Wang, X.; et al. Coordinatively deficient single-atom Fe-N-C electrocatalyst with optimized electronic structure for high-performance lithium-sulfur batteries. Energy Storage Mater. 2022, 46, 269–277. [Google Scholar] [CrossRef]
- Yang, J.-L.; Yang, P.; Cai, D.-Q.; Wang, Z.; Fan, H.J. Atomically dispersed Fe–N4 and Ni–N4 independent sites enable bidirectional sulfur redox electrocatalysis. Nano Lett. 2023, 23, 4000–4007. [Google Scholar] [CrossRef]
- Yi, Z.; Su, F.; Dai, L.; Wang, Z.; Xie, L.; Zuo, Z.; Chen, X.; Liu, Y.; Chen, C.-M. Uncovering electrocatalytic conversion mechanisms from Li2S2 to Li2S: Generalization of computational hydrogen electrode. Energy Storage Mater. 2022, 47, 327–335. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Luo, D.; Li, J.; Zhang, X.; Li, Q.; Li, H.; Zhang, Y.; Chen, Z. Incorporation of heteroatomic Fe activates rapid catalytic behaviors of Co3O4 hollow nanoplates toward advanced lithium-sulfur batteries. Adv. Funct. Mater. 2023, 2303357. [Google Scholar] [CrossRef]
- Liu, J.; Gao, W.; Zhang, X.; Li, J.; Li, Q.; Li, G.; Zhang, Y.; Chen, Z. Rational construction of rich coordination-unsaturated Zr-BTB electrocatalyst towards advanced lithium-sulfur batteries. Chem. Eng. J. 2023, 471, 144238. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Zhang, Y.; Chen, Y.; Peng, X.; Wang, X.; Zhao, W.; Qin, C.; Liu, Q.; Liu, X.; et al. Single-atomic Co-B2N2 sites anchored on carbon nanotube arrays promote lithium polysulfide conversion in lithium-sulfur batteries. Carbon Energy 2023, e306. [Google Scholar] [CrossRef]
- Zhou, C.; Hong, M.; Hu, N.; Yang, J.; Zhu, W.; Kong, L.; Li, M. Bi-metallic coupling-induced electronic-state modulation of metal phosphides for kinetics-enhanced and dendrite-free Li-S batteries. Adv. Funct. Mater. 2023, 33, 2213310. [Google Scholar] [CrossRef]
- Lu, D.; Wang, X.; Hu, Y.; Yue, L.; Shao, Z.; Zhou, W.; Chen, L.; Wang, W.; Li, Y. Expediting stepwise sulfur conversion via spontaneous built-in electric field and binary sulfiphilic effect of conductive NbB2-mxene heterostructure in lithium-sulfur batteries. Adv. Funct. Mater. 2023, 33, 2212689. [Google Scholar] [CrossRef]
- Sun, G.W.; Liu, Q.Y.; Zhang, C.Y.; Jin, M.J.; Pan, J.L.; Wang, Y.C.; Hou, X.Y.; Wang, J.T.; Gao, X.P.; Sun, G.Z.; et al. Revealing the enhancement mechanism of carbon-encapsulated surface-strained MoNi4 bimetallic nanoalloys toward high-stability polysulfide conversion with a wide temperature range. Energy Storage Mater. 2023, 60, 102842. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, C.; Wu, M.; Yu, Y.; Yu, K.; Yan, K.; Shen, C.; Gu, J.; Yan, M.; Sun, C.; et al. Boosting bi-directional redox of sulfur with dual metal single atom pairs in carbon spheres toward high-rate and long-cycling lithium-sulfur battery. Adv. Energy Mater. 2023, 13, 2301505. [Google Scholar] [CrossRef]
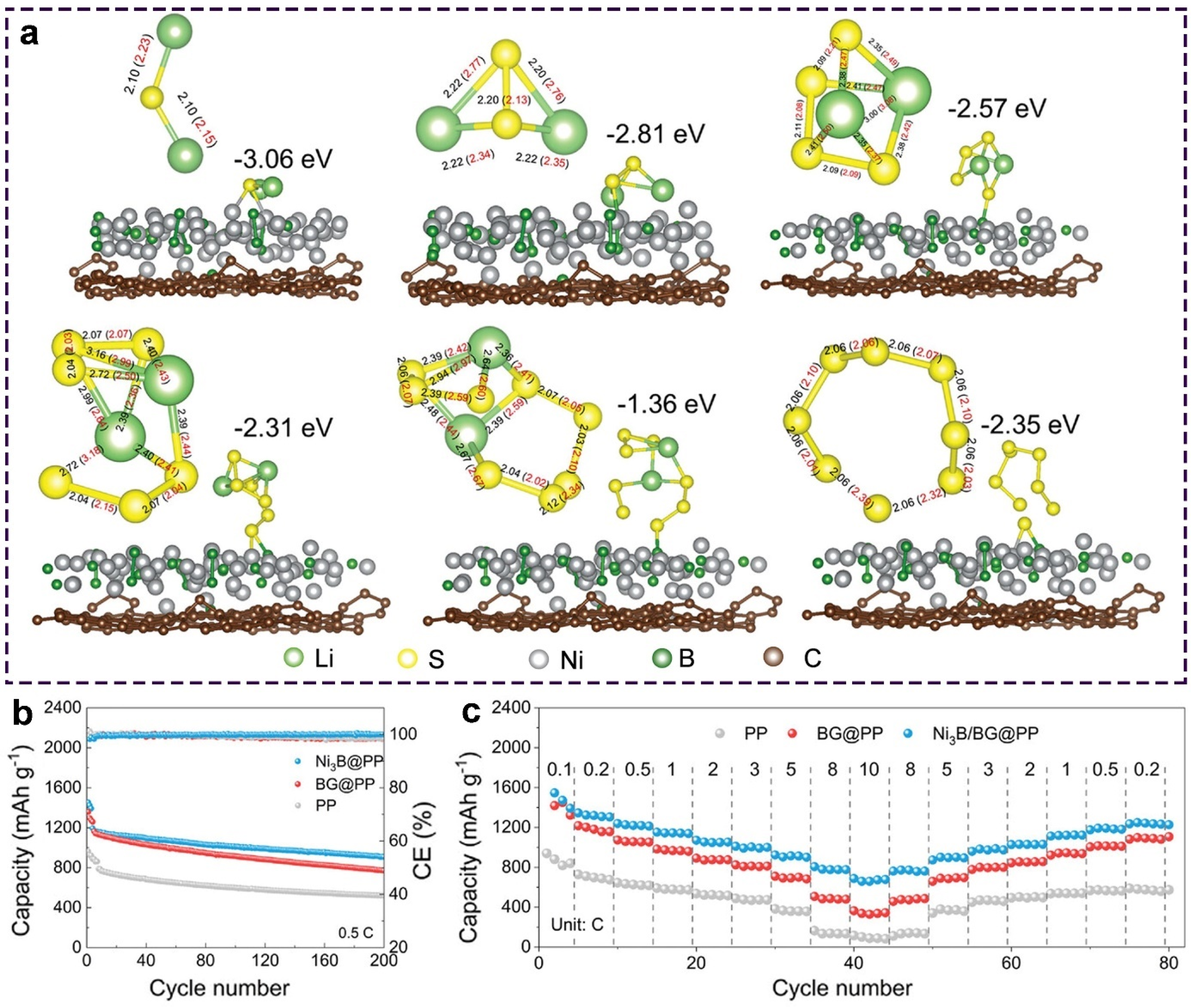
- Wang, Y.; Wang, P.; Yuan, J.; Song, N.; An, X.; Ma, X.; Feng, J.; Xi, B.; Xiong, S. Binary sulfiphilic nickel boride on boron-doped graphene with beneficial interfacial charge for accelerated Li-S dynamics. Small 2023, 19, 2208281. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Nazar, L.F. A graphene-like metallic cathode host for long-life and high-loading Lithium-sulfur batteries. Mater. Horiz. 2016, 3, 130–136. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Seh, Z.W.; Fu, Z.; Zhang, R.; Cui, Y. Understanding the anchoring effect of two-dimensional layered materials for lithium-sulfur batteries. Nano Lett. 2015, 15, 3780–3786. [Google Scholar] [CrossRef]
- Pu, J.; Tan, Y.; Wang, Z.; Fang, Z.; Xue, P.; Yao, Y. Fe3P electrocatalysts assisted carbon based sandwich sulfur cathode “top–bottom” strategy for high rate and high temperature lithium-sulfur batteries. Chem. Eng. J. 2023, 471, 144374. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Xu, R.; Li, A.; Feng, X.; Lv, S.; Wang, T.; Song, L.; Li, J.; Wei, Z. Establishing transition metal phosphides as effective sulfur hosts in Lithium-sulfur batteries through the triple effect of “confinement–adsorption–catalysis”. Small 2023, 19, 2303599. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhang, Y.; Li, X.; Guan, Q.; Wang, J.; Zhuang, Z.; Zhuang, Q.; Cheng, X.; Liu, H.; et al. Accelerated Li+ desolvation for diffusion booster enabling low-temperature sulfur redox kinetics via electrocatalytic carbon-grazfted-CoP porous nanosheets. Adv. Funct. Mater. 2023, 33, 2302624. [Google Scholar] [CrossRef]
- Zou, K.; Jing, W.; Dai, X.; Chen, X.; Shi, M.; Yao, Z.; Zhu, T.; Sun, J.; Chen, Y.; Liu, Y.; et al. A highly efficient sulfur host enabled by nitrogen/oxygen dual-doped honeycomb-like carbon for advanced lithium-sulfur batteries. Small 2022, 18, 210738. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wei, Z.; Wan, C.; Li, J.; Chen, Z.; Zhu, D.; Baumann, D.; Liu, H.; Allen, C.S.; Xu, X.; et al. A fundamental look at electrocatalytic sulfur reduction reaction. Nat. Catal. 2020, 3, 762–770. [Google Scholar] [CrossRef]
- Yang, J.; Lee, D.; Yun, W.C.; Kang, D.W.; Kim, Y.; Lee, J.W. Efficient utilization of lithium polysulfides in CO2-derived CNT free-standing electrode of Li-S batteries. Chem. Eng. J. 2023, 470, 144337. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, X.; Tian, J.; Li, M.; Yuan, Y.; Zhang, S.; Fang, S.; Fan, X.; Xu, W.; Lu, H.; et al. Cation-doped ZnS catalysts for polysulfide conversion in Lithium-sulfur batteries. Nat. Catal. 2022, 5, 555–563. [Google Scholar] [CrossRef]
- Hua, W.; Shang, T.; Li, H.; Sun, Y.; Guo, Y.; Xia, J.; Geng, C.; Hu, Z.; Peng, L.; Han, Z.; et al. Optimizing the p charge of S in p-block metal sulfides for sulfur reduction electrocatalysis. Nat. Catal. 2023, 6, 174–184. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Park, J.S.; Shim, H.C.; Yuk, J.M.; Kim, J.H.; Kim, D.; Lee, S.M. Accelerated sulfur evolution reactions by TiS2/TiO2@MXene host for high-volumetric-energy-density lithium-sulfur batteries. Adv. Funct. Mater. 2023, 33, 2303503. [Google Scholar] [CrossRef]
- Tian, J.; Shen, Z.; Cheng, H.; Jin, X.; Li, Z.; Wen, Y.; Hui, J.; Guo, S.; Zhang, H.; Zhu, Q. In-depth understanding of catalytic and adsorbing effects in polysulfides conversion and rationally designing coaxial nanofibers for Li-S batteries. Chem. Eng. J. 2023, 464, 142541. [Google Scholar] [CrossRef]
- Zeng, P.; Zou, H.; Cheng, C.; Wang, L.; Yuan, C.; Liu, G.; Mao, J.; Chan, T.S.; Wang, Q.; Zhang, L. In situ non-topotactic reconstruction-induced synergistic active centers for polysulfide cascade catalysis. Adv. Funct. Mater. 2023, 33, 2214770. [Google Scholar] [CrossRef]
- Li, F.; Zhang, M.; Chen, W.; Cai, X.; Rao, H.; Chang, J.; Fang, Y.; Zhong, X.; Yang, Y.; Yang, Z.; et al. Vanadium nitride quantum dots/holey graphene matrix boosting adsorption and conversion reaction kinetics for high-performance lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2021, 13, 30746–30755. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Z.; Xie, P.; Luo, Q.; Li, Z.W.; Zhou, C.Y.; Yin, Y.H.; Liu, X.B.; Li, Y.S.; Wu, Z.P. Binder-free ω-Li3V2O5 catalytic network with multi-polarization centers assists lithium-sulfur batteries for enhanced kinetics behavior. Adv. Funct. Mater. 2022, 32, 2110665. [Google Scholar] [CrossRef]
- Zhou, C.; Li, M.; Hong, M.; Hu, N.; Yang, Z.; Zhang, L.; Zhang, Y. Laser-induced micro-explosion to construct hierarchical structure as efficient polysulfide mediators for high-performance lithium-sulfur batteries. Chem. Eng. J. 2021, 421, 129707. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, Y.; Huang, X.; Wu, X.; Sun, H.; Tang, S. Dual-defect engineering of bidirectional catalyst for high-performing lithium-sulfur batteries. Small 2023, 19, 2301545. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, X.; Hu, W.; Chuang, C.; Xie, S.; Hu, A.; Yan, W.; Kong, X.; Wu, X.; Ji, H.; et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulphur content lithium-sulphur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, C.; Zhao, W.; Song, Y.; Zhu, J.; Huo, H.; Ma, Y.; Du, C.; Zuo, P.; Lou, S.; et al. d-p Hybridization-induced “trapping-coupling-conversion” enables high-efficiency nb single-atom catalysis for Li-S batteries. J. Am. Chem. Soc. 2023, 145, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wang, Z.; Li, R.; Wu, Z.; Feng, P.; Zhao, L.; Wang, T.; Hou, W.; Bai, Y.; Wang, G.; et al. Engineering triple-phase interfaces enabled by layered double perovskite oxide for boosting polysulfide redox conversion. Nano Lett. 2023, 23, 4908–4915. [Google Scholar] [CrossRef]
- Chu, R.; Nguyen, T.T.; Song, H.; P, M.A.; Bai, Y.; Kim, D.H.; Lee, J.H.; Kim, N.H. Crystal transformation engineering for effective polysulfides blocking layer for excellent energy density lithium-sulfur batteries. Energy Storage Mater. 2023, 61, 102877. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, X.; Zhang, X.; Chen, N.; Qian, L.; Zhang, Y.; Wang, X.; Chen, Z. CoFe2O4@rGO as a separator coating for advanced lithium-sulfur batteries. Small. Sci. 2023, 3, 300045. [Google Scholar] [CrossRef]
- Cheng, H.; Shen, Z.; Liu, W.; Luo, M.; Huo, F.; Hui, J.; Zhu, Q.; Zhang, H. Vanadium intercalation into niobium disulfide to enhance the catalytic activity for Lithium-sulfur batteries. ACS Nano 2023, 17, 14695–14705. [Google Scholar] [CrossRef]
- Ma, F.; Chen, Z.; Srinivas, K.; Liu, D.; Zhang, Z.; Wu, Y.; Zhu, M.-Q.; Wu, Q.; Chen, Y. VN quantum dots anchored N-doped carbon nanosheets as bifunctional interlayer for high-performance lithium-metal and lithium-sulfur batteries. Chem. Eng. J. 2023, 459, 141526. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Wang, Z.; Wang, J.; Cheng, Y.; Wu, J.; Peng, B.; Xu, J.; Zhang, W.; Jin, Z. Wide-temperature operation of Lithium-sulfur batteries enabled by multi-branched vanadium nitride electrocatalyst. ACS Nano 2023, 17, 11527–11536. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-T.; Wang, X.-Y.; Cai, D.-Q.; Zhou, S.-Y.; Zhao, S.-X. Enhanced polysulfide trapping and conversion by amorphous–crystalline heterostructured MnO2 interlayers for Li-S batteries. ACS Appl. Mater. Interfaces 2023, 15, 30152–30160. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yu, J.; Li, C.; Cui, Z.; Zhang, C.; Zhang, C.; Nan, B.; Li, J.; Arbiol, J.; Cabot, A. Combined defect and heterojunction engineering in ZnTe/CoTe2@NC sulfur hosts toward robust Lithium-sulfur batteries. Adv. Funct. Mater. 2023, 2305624. [Google Scholar] [CrossRef]
- Liu, X.; He, Q.; Liu, J.; Yu, R.; Zhang, Y.; Zhao, Y.; Xu, X.; Mai, L.; Zhou, L. Dual single-atom moieties anchored on n-doped multilayer graphene as a catalytic host for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2023, 15, 9439–9446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ao, X.; Huang, J.; Wei, B.; Zhai, Y.; Zhai, D.; Deng, W.; Su, C.; Wang, D.; Li, Y. Isolated single-atom Ni-N5 catalytic site in hollow porous carbon capsules for efficient lithium-sulfur batteries. Nano Lett. 2021, 21, 9691–9698. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yue, W.; Hu, J.; Niu, X.; Tang, H.; Qin, F.; Li, Y.; Yan, Q.; Liu, X.; Xu, W.; et al. Asymmetrically coordinated Cu-N1C2 single-atom catalyst immobilized on Ti3C2Tx MXene as separator coating for lithium-sulfur batteries. Adv. Energy Mater. 2023, 13, 2204014. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, Z.; Zheng, L.; Zhang, T.; Xu, M.; Xiao, H.; Zhuang, H.; Han, P.; Gao, Q. Edge-distributed iron single-atom moiety with efficient “trapping-conversion” for polysulfides driving high-performance of Li-S battery. Appl. Catal. B 2023, 334, 122876. [Google Scholar] [CrossRef]
- Xiao, H.; Li, K.; Zhang, T.; Liang, X.; Zhang, F.; Zhuang, H.; Zheng, L.; Gao, Q. High loading atomically distributed Fe asymmetrically coordinated with pyridinic and pyrrolic N on porous N-rich carbon matrix driving high performance of Li-S battery. Chem. Eng. J. 2023, 471, 144553. [Google Scholar] [CrossRef]
- Fang, D.; Sun, P.; Huang, S.; Shang, Y.; Li, X.; Yan, D.; Lim, Y.V.; Su, C.-Y.; Su, B.-J.; Juang, J.-Y.; et al. An exfoliation–evaporation strategy to regulate N coordination number of Co single-atom catalysts for high-performance Lithium-sulfur batteries. ACS Mater. Lett. 2022, 4, 1–10. [Google Scholar] [CrossRef]
- Huang, T.; Sun, Y.; Wu, J.; Jin, J.; Wei, C.; Shi, Z.; Wang, M.; Cai, J.; An, X.T.; Wang, P.; et al. A dual-functional fibrous skeleton implanted with single-atomic Co-Nx dispersions for longevous Li-S full batteries. ACS Nano 2021, 15, 14105–14115. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, K.; Hao, J.; Zhang, W.; Shi, H.; Wang, C.; Xiong, Z.; Bai, Z.; Chen, F.-R.; Guo, J.; et al. Engineering single-atom catalysts as multifunctional polysulfide and lithium regulators toward kinetically accelerated and durable lithium-sulfur batteries. Chem. Eng. J. 2023, 466, 143182. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, X.; Zeng, Q.; Liang, F.; Ning, S.; Wang, G.; Shen, P.K.; Ma, S. MnO-Mo2C heterogeneous particles supported on porous carbon: Accelerating the catalytic conversion of polysulfides. Chem. Eng. J. 2023, 460, 141811. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, Y.; Kong, Y.; Wang, Z.; He, K.; Qin, J.; Zhang, Q.; Su, C.; Zhong, Y.L.; Chen, H. Efficient synergism of chemisorption and wackenroder reaction via heterostructured La2O3-Ti3C2Tx-embedded carbon nanofiber for high-energy lithium-sulfur pouch cells. Adv. Funct. Mater. 2023, 33, 2303422. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Knibbe, R.; Luo, B.; Ahad, S.A.; Sun, D.; Wang, L. Cyclic voltammetry in lithium-sulfur batteries-challenges and opportunities. Energy Technol. 2019, 7, 1801001. [Google Scholar] [CrossRef]
- Zhou, G.; Tian, H.; Jin, Y.; Tao, X.; Liu, B.; Zhang, R.; Seh, Z.W.; Zhuo, D.; Liu, Y.; Sun, J.; et al. Catalytic oxidation of Li2S on the surface of metal sulfides for Li-S batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Bai, Y.; Bai, Z.; Peng, L.; Luo, M.; Qu, M.; Gao, Y.; Wang, Z.; Sun, W.; Sun, K. Phosphorus vacancies as effective polysulfide promoter for high-energy-density lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2102739. [Google Scholar] [CrossRef]
- Sun, R.; Qu, M.; Peng, L.; Yang, W.; Wang, Z.; Bai, Y.; Sun, K. Regulating electrochemical kinetics of CoP by incorporating oxygen on surface for high-performance Li-S batteries. Small 2023, 19, 2302092. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, L.; Zhao, D.; Yang Lee, J. Fast polysulfide conversion catalysis and reversible anode operation by a single cathode modifier in Li-metal anode-free lithium-sulfur batteries. Angew. Chem. Int. Ed. 2023, 62, e2023089. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Misra, S.; Nelson, J.; Toney, M.F.; Cui, Y. High-capacity micrometer-sized Li2S particles as cathode materials for advanced rechargeable lithium-ion batteries. J. Am. Chem. Soc. 2012, 134, 15387–15394. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Tian, D.; Qiu, Y.; Song, X.; Zhang, Y.; Sun, X.; Huang, H.; Zhao, C.; Guo, Z.; Fan, L.; et al. High-index faceted nanocrystals as highly efficient bifunctional electrocatalysts for high-performance lithium-sulfur batteries. Nano-Micro Lett. 2022, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, L.; Zhang, Y.; Liu, L.; Sa, B.; Lin, J.; Wang, L.; Peng, D.-L.; Xie, Q. Dual-functional lithiophilic/sulfiphilic binary-metal selenide quantum dots toward high-performance Li-S full batteries. Nano-Micro Lett. 2023, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-L.; He, Q.-T.; Zeng, Z.; Chen, J.-Y.; Wen, T.; Huang, Y.-X.; Zhuang, L.-C.; Yi, W.; Cai, Y.-P.; Hong, X.-J. Isolated diatomic Zn-Co metal–nitrogen/oxygen sites with synergistic effect on fast catalytic kinetics of sulfur species in Li-S battery. J. Energy Chem. 2023, 79, 505–514. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, B.; Zhang, L.; Li, W.; Li, S.; Zhang, J.; Jiang, G.; Cui, Z.; Song, H.; Grundish, N.; et al. Co4N-Decorated 3D wood-derived carbon host enables enhanced cathodic electrocatalysis and homogeneous lithium deposition for lithium-sulfur full cells. Small 2022, 18, 2105664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ni, Z.; Bai, X.; Shen, H.; Wang, Z.; Wei, C.; Tian, K.; Xi, B.; Xiong, S.; Feng, J. Hierarchical porous N-doped carbon encapsulated fluorine-free MXene with tunable coordination chemistry by one-pot etching strategy for lithium-sulfur batteries. Adv. Energy Mater. 2023, 13, 2301349. [Google Scholar] [CrossRef]
- Yang, D.; Liang, Z.; Zhang, C.; Biendicho, J.J.; Botifoll, M.; Spadaro, M.C.; Chen, Q.; Li, M.; Ramon, A.; Moghaddam, A.O.; et al. NbSe2 Meets C2N: A 2D-2D heterostructure catalysts as multifunctional polysulfide mediator in ultra-long-life lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2101250. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.-T.; Zhou, L.; Chen, C.; Danilov, D.L.; Qiao, F.; Li, H.; Notten, P.H.L. Theoretical Calculations Facilitating Catalysis for Advanced Lithium-Sulfur Batteries. Molecules 2023, 28, 7304. https://doi.org/10.3390/molecules28217304
Fang X-T, Zhou L, Chen C, Danilov DL, Qiao F, Li H, Notten PHL. Theoretical Calculations Facilitating Catalysis for Advanced Lithium-Sulfur Batteries. Molecules. 2023; 28(21):7304. https://doi.org/10.3390/molecules28217304
Chicago/Turabian StyleFang, Xue-Ting, Lei Zhou, Chunguang Chen, Dmitri L. Danilov, Fen Qiao, Haitao Li, and Peter H. L. Notten. 2023. "Theoretical Calculations Facilitating Catalysis for Advanced Lithium-Sulfur Batteries" Molecules 28, no. 21: 7304. https://doi.org/10.3390/molecules28217304






