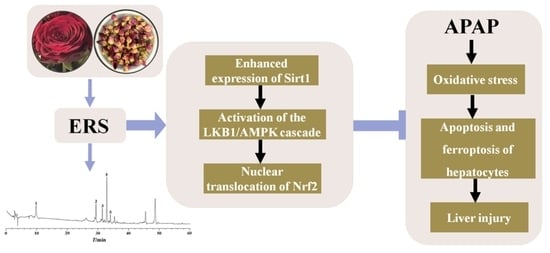
Ethanol Extract of Rosa rugosa Ameliorates Acetaminophen-Induced Liver Injury via Upregulating Sirt1 and Subsequent Potentiation of LKB1/AMPK/Nrf2 Cascade in Hepatocytes
Abstract
:1. Introduction
2. Results
2.1. The Chemical Profile of ERS
2.2. ERS Ameliorates APAP-Provoked Apoptosis and Ferroptosis in AML-12 Hepatocytes
2.3. ERS Elevates Sirt1 Expression and Activates LKB1/AMPK/Nrf2 Axis in AML-12 Cells
2.4. Inhibition of Sirt1 Eliminates ERS-Mediated Potentiation of LKB1/AMPK/Nrf2 Axis
2.5. Knockdown of LKB1 Abrogates ERS-Mediated Activation of AMPK/Nrf2 Pathway
2.6. ERS Alleviates APAP-Caused Liver Injury in Mice
2.7. ERS Reduces Hepatocyte Apoptosis and Ferroptosis in APAP-Intoxicated Mice
2.8. ERS Modulates Sirt1/LKB1/AMPK/Nrf2 Cascade in Mouse Livers
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of ERS
4.3. Cells and Transfection
4.4. Cytotoxicity Detection and ROS Quantification
4.5. Detection of Apoptosis and Ferroptosis
4.6. Immunoblotting, Immunohistochemistry and Immunofluorescence
4.7. Quantification of Gene Transcription
4.8. Animal Experiments
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| ALT | alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| APAP | acetaminophen |
| AST | aspartate aminotransferase |
| Bax | BCL2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| CAT | catalase |
| COX2 | cyclooxygenase-2 |
| ERS | ethanol extract of Rosa rugosa |
| EX527 | 6-Chloro-2,3,4,9-tetrahydro-1H-Carbazole-1-carboxamide |
| GCLC | glutamate-cysteine ligase |
| GPX | glutathione peroxidase |
| GPX4 | glutathione peroxidase 4 |
| GSH | glutathione |
| HE | hematoxylin-eosin |
| HO-1 | heme oxygenase 1 |
| HPLC | high-performance liquid chromatography |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| iNOS | inducible nitric oxide synthase |
| Keap1 | kelch-like ECH-associated protein 1 |
| LDH | lactate dehydrogenase |
| LKB1 | liver kinase B1 |
| LPO | lipid peroxidation |
| MDA | malondialdehyde |
| NAC | N-acetyl cysteine |
| NAM | nicotinamide |
| NO | nitric oxide |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| p-AMPK | phosphorylated AMPK |
| p-LKB1 | phosphorylated LKB1 |
| PEG2 | prostaglandin E2 |
| ROS | reactive oxygen species |
| Sirt1 | sirtuin 1 |
| SOD | superoxide dismutase |
| T-AOC | total antioxidant capacity |
| TNF-α | tumor necrosis factor-α |
References
- Ramachandran, A.; Jaeschke, H. Acetaminophen Hepatotoxicity. Semin. Liver Dis. 2019, 39, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, C.I.; Pérez, M.J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef]
- Chiew, A.L.; Gluud, C.; Brok, J.; Buckley, N.A. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst. Rev. 2018, 2, CD003328. [Google Scholar] [CrossRef] [PubMed]
- Smilkstein, M.J.; Knapp, G.L.; Kulig, K.W.; Rumack, B.H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 1988, 319, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Akakpo, J.Y.; Umbaugh, D.S.; Ramachandran, A. Novel therapeutic approaches against acetaminophen-induced liver injury and acute liver failure. Toxicol. Sci. 2020, 174, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Gores, G.J. Apoptosis: A mechanism of acute and chronic liver injury. Gut 2005, 54, 1024–1033. [Google Scholar] [CrossRef]
- Cao, P.; Sun, J.; Sullivan, M.A.; Huang, X.; Wang, H.; Zhang, Y.; Wang, N.; Wang, K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 1133–1139. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Galle, P.R.; Schuchmann, M. Apoptosis in liver disease. Liver Int. 2006, 26, 904–911. [Google Scholar] [CrossRef]
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998, 17, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Bio. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. BBA-Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef]
- Dang, Q.; Sun, Z.; Wang, Y.; Wang, L.; Liu, Z.; Han, X. Ferroptosis: A double-edged sword mediating immune tolerance of cancer. Cell Death Dis. 2022, 13, 925. [Google Scholar] [CrossRef]
- Niu, B.; Lei, X.; Xu, Q.; Ju, Y.; Xu, D.; Mao, L.; Li, J.; Zheng, Y.; Sun, N.; Zhang, X.; et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol. Toxicol. 2022, 38, 505–530. [Google Scholar] [CrossRef]
- Yamada, N.; Karasawa, T.; Kimura, H.; Watanabe, S.; Komada, T.; Kamata, R.; Sampilvanjil, A.; Ito, J.; Nakagawa, K.; Kuwata, H.; et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020, 11, 144. [Google Scholar] [CrossRef]
- Lőrincz, T.; Jemnitz, K.; Kardon, T.; Mandl, J.; Szarka, A. Ferroptosis is Involved in Acetaminophen Induced Cell Death. Pathol. Oncol. Res. 2015, 21, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, T.; Tong, Y.; Cui, R.; Qu, K.; Liu, C.; Zhang, J. Ulinastatin protects against acetaminophen-induced liver injury by alleviating ferroptosis via the SIRT1/NRF2/HO-1 pathway. Am. J. Transl. Res. 2021, 13, 6031–6042. [Google Scholar]
- Cai, X.; Hua, S.; Deng, J.; Du, Z.; Zhang, D.; Liu, Z.; Khan, N.U.; Zhou, M.; Chen, Z. Astaxanthin Activated the Nrf2/HO-1 Pathway to Enhance Autophagy and Inhibit Ferroptosis, Ameliorating Acetaminophen-Induced Liver Injury. ACS Appl. Mater. Interfaces 2022, 14, 42887–42903. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Weng, Q.; Gong, S.; Zhang, W.; Wang, J.; Huang, Y.; Li, Y.; Guo, J.; Lan, T. Kaempferol prevents acetaminophen-induced liver injury by suppressing hepatocyte ferroptosis via Nrf2 pathway activation. Food Funct. 2023, 14, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Peace, C.G.; O’Neill, L.A. The role of itaconate in host defense and inflammation. J. Clin. Investig. 2022, 132, e148548. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Lv, H.; Hong, L.; Tian, Y.; Yin, C.; Zhu, C.; Feng, H. Corilagin alleviates acetaminophen-induced hepatotoxicity via enhancing the AMPK/GSK3β-Nrf2 signaling pathway. Cell Commun. Signal. 2019, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, K.C.; Lu, Y.F.; Ekuase, E.; Klaassen, C.D. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid. Med. Cell. Longev. 2013, 2013, 305861. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.J.; Woodard, K.T.; Weaver, M.A.; Gaylor, J.P.; Weiss, E.R.; Samulski, R.J. AAV-Nrf2 Promotes Protection and Recovery in Animal Models of Oxidative Stress. Mol. Ther. 2017, 25, 765–779. [Google Scholar] [CrossRef]
- Gao, Z.; Yi, W.; Tang, J.; Sun, Y.; Huang, J.; Lan, T.; Dai, X.; Xu, S.; Jin, Z.G.; Wu, X. Urolithin A protects against acetaminophen-induced liver injury in mice via sustained activation of Nrf2. Int. J. Biol. Sci. 2022, 18, 2146–2162. [Google Scholar] [CrossRef] [PubMed]
- Didamoony, M.A.; Atwa, A.M.; Abd El-Haleim, E.A.; Ahmed, L.A. Bromelain ameliorates D-galactosamine-induced acute liver injury: Role of SIRT1/LKB1/AMPK, GSK3β/Nrf2 and NF-κB p65/TNF-α/caspase-8, -9 signalling pathways. J. Pharm. Pharmacol. 2022, 74, 1765–1775. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef]
- Lee, E.H.; Baek, S.Y.; Park, J.Y.; Kim, Y.W. Rifampicin activates AMPK and alleviates oxidative stress in the liver as mediated with Nrf2 signaling. Chem.-Biol. Interact. 2020, 315, 108889. [Google Scholar] [CrossRef]
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm. Biol. 2021, 59, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, R.; Pu, Q.; Jiang, S.; Yu, F.; Yang, Z.; Han, T. Investigation of nephrotoxicity on mice exposed to polystyrene nanoplastics and the potential amelioration effects of DHA-enriched phosphatidylserine. Sci. Total Environ. 2023, 892, 164808. [Google Scholar] [CrossRef]
- Li, S.N.; Yu, Y.L.; Wang, B.Y.; Qiao, S.Y.; Hu, M.M.; Wang, H.; Fu, C.N.; Dong, B. Overexpression of G Protein-Coupled Receptor 40 Protects Obesity-Induced Cardiomyopathy Through the SIRT1/LKB1/AMPK Pathway. Hum. Gene Ther. 2022, 33, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, S.; Li, X.; Kou, R.; Wang, Q.; Wang, X.; Zhao, N.; Zeng, T.; Xie, K. Diallyl sulfide treatment protects against acetaminophen-/carbon tetrachloride-induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. Toxicol. Res. 2018, 8, 67–76. [Google Scholar] [CrossRef]
- Li, X.; Park, S.J.; Jin, F.; Deng, Y.; Yang, J.H.; Chang, J.H.; Kim, D.Y.; Kim, J.A.; Lee, Y.J.; Murakami, M.; et al. Tanshinone IIA suppresses FcεRI-mediated mast cell signaling and anaphylaxis by activation of the Sirt1/LKB1/AMPK pathway. Biochem. Pharmacol. 2018, 152, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhou, M.; Li, J.; Zong, R.; Yan, Y.; Kong, L.; Zhu, Q.; Li, C. Notch-activated mesenchymal stromal/stem cells enhance the protective effect against acetaminophen-induced acute liver injury by activating AMPK/SIRT1 pathway. Stem Cell Res. Ther. 2022, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, H.; Luo, C.; Du, D.; Huang, J.; Ming, Q.; Jin, F.; Wang, D.; Huang, W. Acetaminophen Responsive miR-19b Modulates SIRT1/Nrf2 Signaling Pathway in Drug-Induced Hepatotoxicity. Toxicol. Sci. 2019, 170, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Gao, W.; Li, L.; Niu, S.M.; Zhao, L.; Liu, J.; Shi, L.S.; Fu, M.; Liu, F. Rose (Rosa rugosa)-flower extract increases the activities of antioxidant enzymes and their gene expression and reduces lipid peroxidation. Biochem. Cell Biol. 2005, 83, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yasen, M.; Tang, D.; Ye, J.; Aisa, H.A.; Xin, X. Polyphenol-enriched extract of Rosa rugosa Thunb regulates lipid metabolism in diabetic rats by activation of AMPK pathway. Biomed. Pharmacother. 2018, 100, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, L.; Liu, B.; Xiao, J.; Cheng, K.W.; Chen, F.; Wang, M. Tricoumaroylspermidine from rose exhibits inhibitory activity against ethanol-induced apoptosis in HepG2 cells. Food Funct. 2021, 12, 5892–5902. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Chen, J.; Song, H.; Yang, C. Anti-hyperplasia effects of Rosa rugosa polyphenols in rats with hyperplasia of mammary gland. Environ. Toxicol. Pharmacol. 2015, 39, 990–996. [Google Scholar] [CrossRef]
- Shu, G.; Lei, X.; Lei, Y.; Zhang, T.; Sun, H.; Wang, C.; Song, A.; Deng, X. A characterized ethanol extract of Rosa rugosa inhibits hepatic stellate cell activation through elevating Hint1 and subsequent upregulation of Smad7. J. Funct. Foods 2023, 107, 105634. [Google Scholar] [CrossRef]
- Nijat, D.; Lu, C.F.; Lu, J.J.; Abdulla, R.; Hasan, A.; Aidarhan, N.; Aisa, H.A. Spectrum-effect relationship between UPLC fingerprints and antidiabetic and antioxidant activities of Rosa rugosa. J. Chromatogr. B 2021, 1179, 122843. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Ahmad, S.; Tang, A.; Wang, J.; Cheng, T.; Pan, H.; Zhang, Q. Flavonols and Carotenoids in Yellow Petals of Rose Cultivar (Rosa ‘Sun City’): A Possible Rich Source of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Qiu, Y.; Hao, J.; Fu, Q.; Deng, X. γ-Oryzanol alleviates acetaminophen-induced liver injury: Roles of modulating AMPK/GSK3β/Nrf2 and NF-κB signaling pathways. Food Funct. 2019, 10, 6858–6872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Sun, R.; Sun, Y.; Liu, D.; Lin, M.; Chen, Z.; Zhou, J.; Lv, L.; Tian, X.; et al. circ-CBFB upregulates p66Shc to perturb mitochondrial dynamics in APAP-induced liver injury. Cell Death Dis. 2020, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar]
- Yang, R.; Song, C.; Chen, J.; Zhou, L.; Jiang, X.; Cao, X.; Sun, Y.; Zhang, Q. Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-κB inflammatory response via upregulating Sirt1. Phytomedicine 2020, 69, 153211. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, J.; Xie, H.; Liu, Y.; Bai, Y.; Che, Q.; Cao, H.; Huang, G.; Guo, J.; Su, Z. Protective effect and mechanism of chitooligosaccharides on acetaminophen-induced liver injury. Food Funct. 2021, 12, 9979–9993. [Google Scholar] [CrossRef]
- Papackova, Z.; Heczkova, M.; Dankova, H.; Sticova, E.; Lodererova, A.; Bartonova, L.; Poruba, M.; Cahova, M. Silymarin prevents acetaminophen-induced hepatotoxicity in mice. PLoS ONE 2018, 13, e0191353. [Google Scholar] [CrossRef]
- Piotrowicz, Z.; Tabisz, Ł.; Waligórska, M.; Pankiewicz, R.; Łęska, B. Phenol-rich alternatives for Rosa x damascena Mill. Efficient phytochemical profiling using different extraction methods and colorimetric assays. Sci. Rep. 2021, 11, 23883. [Google Scholar] [CrossRef]
- Izcara, S.; Perestrelo, R.; Morante-Zarcero, S.; Câmara, J.S.; Sierra, I. High throughput analytical approach based on μQuEChERS combined with UHPLC-PDA for analysis of bioactive secondary metabolites in edible flowers. Food Chem. 2022, 393, 133371. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, J.B.; Wang, C.; Xu, Z.; Nie, H.; Qin, X.Y.; Chen, X.M.; Gong, Q. Curcumin protects against acetaminophen-induced apoptosis in hepatic injury. World J. Gastroenterol. 2013, 19, 7440–7446. [Google Scholar] [CrossRef]
- Hussain, S.; Ashafaq, M.; Alshahrani, S.; Siddiqui, R.; Ahmed, R.A.; Khuwaja, G.; Islam, F. Cinnamon oil against acetaminophen-induced acute liver toxicity by attenuating inflammation, oxidative stress and apoptosis. Toxicol. Rep. 2020, 7, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; AlQudsy, L.H.H.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef]
- Etemadi, Y.; Akakpo, J.Y.; Ramachandran, A.; Jaeschke, H. Nrf2 as a therapeutic target in acetaminophen hepatotoxicity: A case study with sulforaphane. J. Biochem. Mol. Toxicol. 2023, 20, e23505. [Google Scholar] [CrossRef]
- Dina, E.; Sklirou, A.D.; Chatzigeorgiou, S.; Manola, M.S.; Cheilari, A.; Louka, X.P.; Argyropoulou, A.; Xynos, N.; Skaltsounis, A.L.; Aligiannis, N.; et al. An enriched polyphenolic extract obtained from the by-product of Rosa damascena hydrodistillation activates antioxidant and proteostatic modules. Phytomedicine 2021, 93, 153757. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Wu, Y.; Zuo, Y.; Li, J.; Lv, H.; Ma, L.; Ren, B.; Zhao, L.; Li, W.; et al. Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway. Biomed. Pharmacother. 2019, 110, 85–94. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Z.; Tu, J.; Wang, Z.; Gao, X.; Deng, K.; El-Samahy, M.A.; You, P.; Fan, Y.; Wang, F. γ-Linolenic Acid Prevents Lipid Metabolism Disorder in Palmitic Acid-Treated Alpha Mouse Liver-12 Cells by Balancing Autophagy and Apoptosis via the LKB1-AMPK-mTOR Pathway. J. Agric. Food Chem. 2021, 69, 8257–8267. [Google Scholar] [CrossRef]
- Maillet, V.; Boussetta, N.; Leclerc, J.; Fauveau, V.; Foretz, M.; Viollet, B.; Couty, J.P.; Celton-Morizur, S.; Perret, C.; Desdouets, C. LKB1 as a Gatekeeper of Hepatocyte Proliferation and Genomic Integrity during Liver Regeneration. Cell Rep. 2018, 22, 1994–2005. [Google Scholar] [CrossRef]
- Li, B.; Lee, D.S.; Kang, Y.; Yao, N.Q.; An, R.B.; Kim, Y.C. Protective effect of ganodermanondiol isolated from the Lingzhi mushroom against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 2013, 53, 317–324. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.W.; Kim, S.G. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem. Pharmacol. 2010, 79, 1352–1362. [Google Scholar] [CrossRef]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef]
- Gautam, S.; Zhang, L.; Lee, C.; Arnaoutova, I.; Chen, H.D.; Resaz, R.; Eva, A.; Mansfield, B.C.; Chou, J.Y. Molecular mechanism underlying impaired hepatic autophagy in glycogen storage disease type Ib. Hum. Mol. Genet. 2023, 32, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Huang, J.; Nisar, M.F.; Wan, C.; Huang, W. The Beneficial Roles of SIRT1 in Drug-Induced Liver Injury. Oxid. Med. Cell. Longev. 2019, 2019, 8506195. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Pardo, V.; Mobasher, M.A.; García-Martínez, I.; Ruiz, L.; González-Rodríguez, Á.; Sanchez-Ramos, C.; Muntané, J.; Alemany, S.; James, L.P.; et al. SIRT1 Controls Acetaminophen Hepatotoxicity by Modulating Inflammation and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 1187–1208. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Liu, Y.; Sun, Y.; Li, Y.; Yao, Q.; Li, J.; Zhang, Q.; Gao, Y.; Gao, L.; et al. Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J. Nutr. Biochem. 2014, 25, 1207–1217. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple polyphenol extract alleviates lipid accumulation in free-fatty-acid-exposed HepG2 cells via activating autophagy mediated by SIRT1/AMPK signaling. Phytother. Res. 2021, 35, 1416–1431. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, L.; Jiang, R.; Chen, W.; Zheng, W.; Chen, L.; Tang, L.; Li, L.; Li, L.; Tang, W.; et al. Protective effects of nicotinamide against acetaminophen-induced acute liver injury. Int. Immunopharmacol. 2012, 14, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, L.; Jiang, R.; Hu, K.; Hu, D.; Liao, C.; Jiang, S.; Yang, Y.; Huang, J.; Tang, L.; et al. Nicotinamide improves NAD+ levels to protect against acetaminophen-induced acute liver injury in mice. Hum. Exp. Toxicol. 2021, 40, 1938–1946. [Google Scholar] [CrossRef]
- Guo, H.; Sun, J.; Li, D.; Hu, Y.; Yu, X.; Hua, H.; Jing, X.; Chen, F.; Jia, Z.; Xu, J. Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2019, 112, 108704. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Sun, H.; Zhang, T.; Zhu, A.; Lei, X.; Wang, C.; Song, A.; Deng, X. Theaflavine inhibits hepatic stellate cell activation by modulating the PKA/LKB1/AMPK/GSK3β cascade and subsequently enhancing Nrf2 signaling. Eur. J. Pharmacol. 2023, 956, 175964. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Li, X.; Lu, Y.; Jin, Y.; Jeong, Y.T.; Kim, Y.D.; Lee, I.K.; Taketomi, Y.; Sato, H.; Cho, Y.S.; et al. AMP-activated protein kinase negatively regulates FcεRI-mediated mast cell signaling and anaphylaxis in mice. J. Allergy Clin. Immunol. 2013, 132, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Baiyisaiti, A.; Wang, Y.; Zhang, X.; Chen, W.; Qi, R. Rosa rugosa flavonoids exhibited PPARα agonist-like effects on genetic severe hypertriglyceridemia of mice. J. Ethnopharmacol. 2019, 240, 111952. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Gustavsson, K.E.; Oredsson, S.; Głąb, B.; Yilmaz, J.L.; Olsson, M.E. Determination of free and esterified carotenoid composition in rose hip fruit by HPLC-DAD-APCI(+)-MS. Food Chem. 2016, 210, 541–550. [Google Scholar] [CrossRef] [PubMed]








| Group | ALT (U·L−1) | AST (U·L−1) | LDH (U·L−1) | Liver Index (%) |
|---|---|---|---|---|
| Normal | 26 ± 5.2 | 38 ± 6.9 | 60 ± 10 | 5.0 ± 0.24 |
| APAP | 199 ± 58 ## | 150 ± 23 ## | 150 ± 27 ## | 6.9 ± 0.28 ## |
| APAP + ERS (50 mg/kg) | 90 ± 10 ** | 92 ± 15 * | 110 ± 14 ** | 5.8 ± 0.56 * |
| APAP + ERS (100 mg/kg) | 55 ± 8.7 ** | 56 ± 12 ** | 73 ± 14 ** | 5.2 ± 0.23 ** |
| Group | TNF-α (pg/mg Prot) | IL-1β (pg/mg Prot) | IL-6 (pg/mg Prot) | PGE2 (pg/mg Prot) | NO (μM/mg Prot) |
|---|---|---|---|---|---|
| Normal | 155.7 ± 11.6 | 204.4 ± 17.5 | 123.5 ± 17.5 | 231.6 ± 31.3 | 69.4 ± 12.2 |
| APAP | 475.5 ± 24.8 ## | 307.4 ± 29.4 ## | 301.5 ± 22.8 ## | 586.3 ± 75.6 ## | 142.7 ± 23.7 ## |
| APAP + ERS (50 mg/kg) | 250.1 ± 17.1 ** | 260.4 ± 32.4 ** | 242.7 ± 10.3 ** | 386.7 ± 54.3 ** | 74.5 ± 9.8 ** |
| APAP + ERS (100 mg/kg) | 183.7 ± 10.5 ** | 215.3 ± 36.7 ** | 141.4 ± 12.4 ** | 268.2 ± 23.4 ** | 49.1 ± 8.7 ** |
| Group | Iron (μM/μg Tissue) | LPO (nM/mg Prot) | 4-HNE (nM/mg Prot) | MDA (nM/mg Prot) |
|---|---|---|---|---|
| Normal | 1.6 ± 0.18 | 1.8 ± 0.18 | 1.8 ± 0.54 | 0.8 ± 0.36 |
| APAP | 5.4 ± 0.37 ## | 2.9 ± 0.16 ## | 7.8 ± 0.96 ## | 1.3 ± 0.55 ## |
| APAP + ERS (50 mg/kg) | 3.3 ± 0.23 * | 2.2 ± 0.12 * | 3.7 ± 0.28 ** | 1.04 ± 0.22 * |
| APAP + ERS (100 mg/kg) | 2.6 ± 0.17 ** | 2.0 ± 0.11 ** | 2.7 ± 0.71 ** | 0.96 ± 0.47 ** |
| Group | T-AOC (mM/g Prot) | SOD (U/mg Prot) | GSH (μM/g Prot) | GPX-P (μM/mg Prot) | CAT (U/mg Prot) |
|---|---|---|---|---|---|
| Normal | 1.2 ± 0.15 | 20 ± 1.4 | 15 ± 1.54 | 77 ± 7.2 | 43 ± 4.3 |
| APAP | 0.32 ± 0.09 ## | 15 ± 1.1 ## | 6 ± 0.89 ## | 54 ± 10 ## | 15 ± 1.8 ## |
| APAP + ERS (50 mg/kg) | 0.53 ± 0.18 * | 17 ± 1.5 ** | 10 ± 1.24 * | 73 ± 14 ** | 18 ± 2.1 ** |
| APAP + ERS (100 mg/kg) | 0.77 ± 0.19 ** | 19 ± 1.7 ** | 13 ± 1.66 ** | 82 ± 12 ** | 34 ± 2.5 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Lei, X.; Zhu, A.; Xie, S.; Zhang, T.; Wang, C.; Song, A.; Wang, X.; Shu, G.; Deng, X. Ethanol Extract of Rosa rugosa Ameliorates Acetaminophen-Induced Liver Injury via Upregulating Sirt1 and Subsequent Potentiation of LKB1/AMPK/Nrf2 Cascade in Hepatocytes. Molecules 2023, 28, 7307. https://doi.org/10.3390/molecules28217307
Lei Y, Lei X, Zhu A, Xie S, Zhang T, Wang C, Song A, Wang X, Shu G, Deng X. Ethanol Extract of Rosa rugosa Ameliorates Acetaminophen-Induced Liver Injury via Upregulating Sirt1 and Subsequent Potentiation of LKB1/AMPK/Nrf2 Cascade in Hepatocytes. Molecules. 2023; 28(21):7307. https://doi.org/10.3390/molecules28217307
Chicago/Turabian StyleLei, Yecheng, Xiao Lei, Anqi Zhu, Shijie Xie, Tiantian Zhang, Chuo Wang, Anning Song, Xiaoming Wang, Guangwen Shu, and Xukun Deng. 2023. "Ethanol Extract of Rosa rugosa Ameliorates Acetaminophen-Induced Liver Injury via Upregulating Sirt1 and Subsequent Potentiation of LKB1/AMPK/Nrf2 Cascade in Hepatocytes" Molecules 28, no. 21: 7307. https://doi.org/10.3390/molecules28217307
APA StyleLei, Y., Lei, X., Zhu, A., Xie, S., Zhang, T., Wang, C., Song, A., Wang, X., Shu, G., & Deng, X. (2023). Ethanol Extract of Rosa rugosa Ameliorates Acetaminophen-Induced Liver Injury via Upregulating Sirt1 and Subsequent Potentiation of LKB1/AMPK/Nrf2 Cascade in Hepatocytes. Molecules, 28(21), 7307. https://doi.org/10.3390/molecules28217307