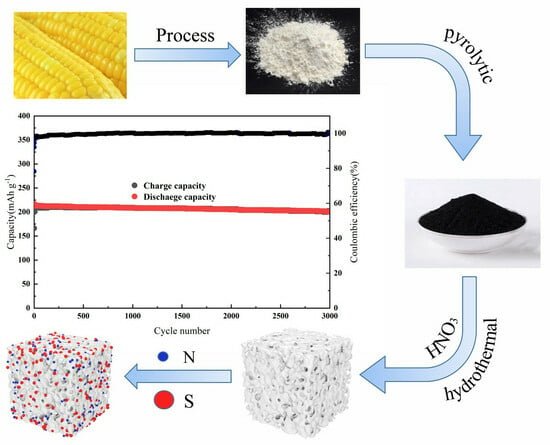
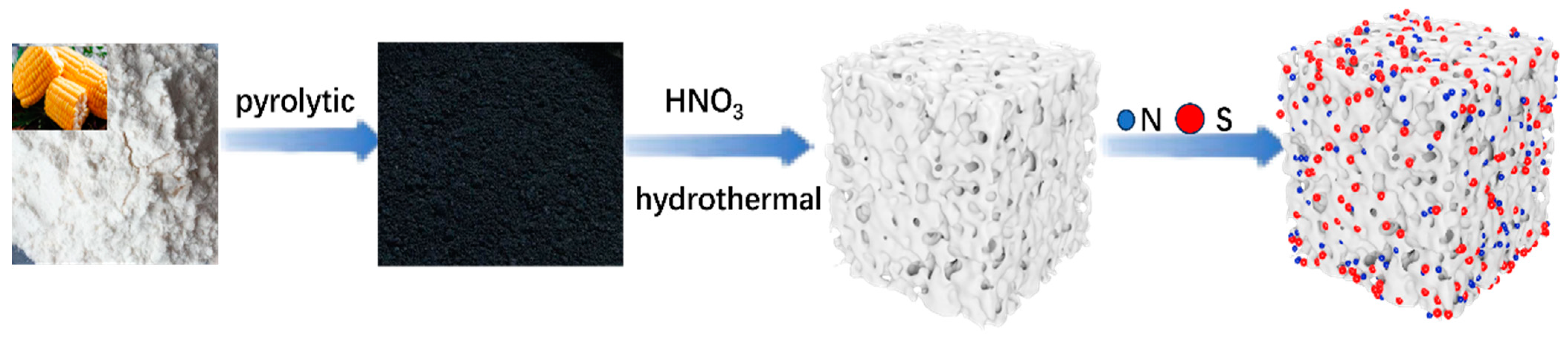
Synthesis of Low-Cost and High-Performance Dual-Atom Doped Carbon-Based Materials with a Simple Green Route as Anodes for Sodium-Ion Batteries
Abstract
:1. Introduction
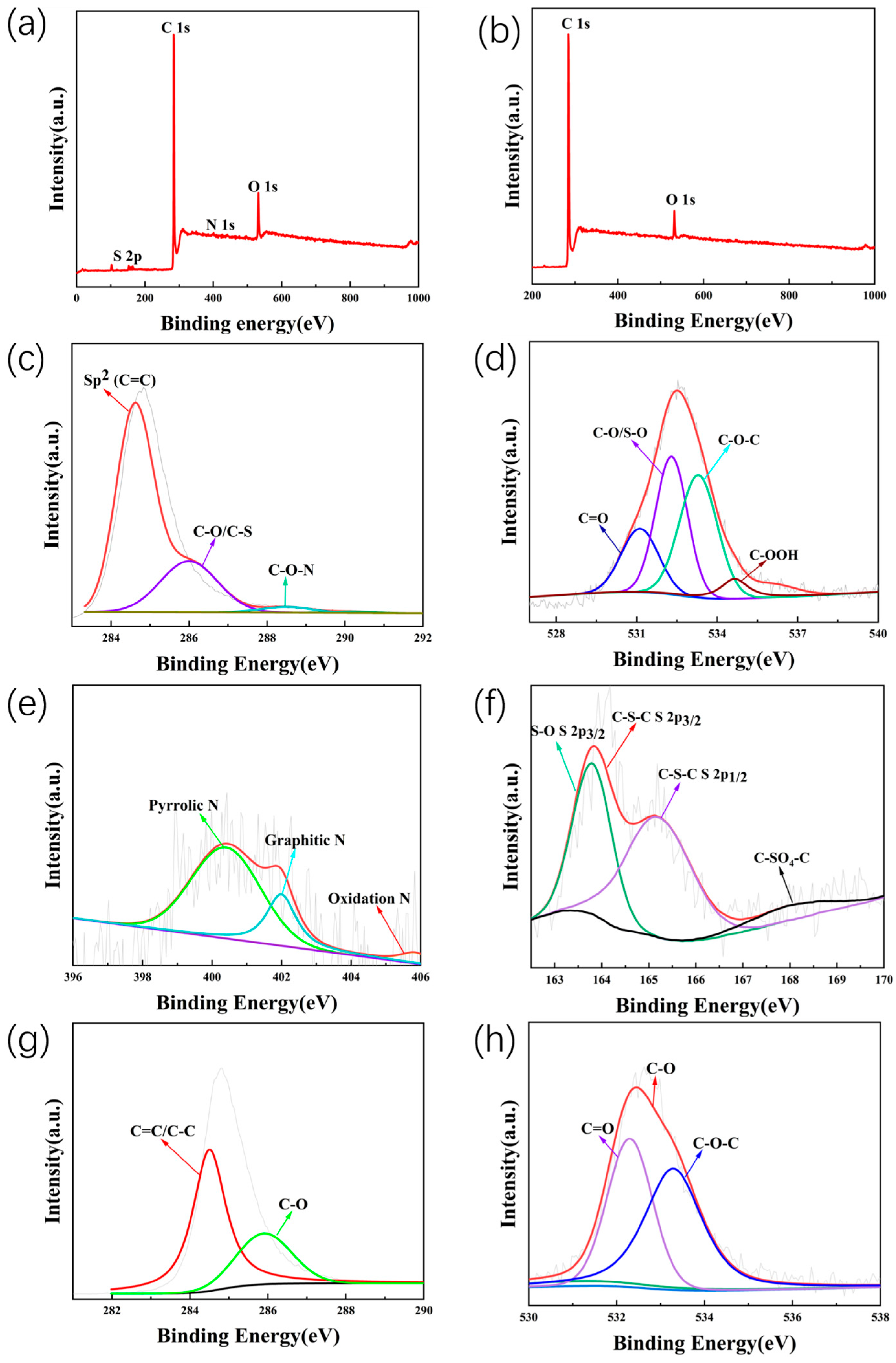
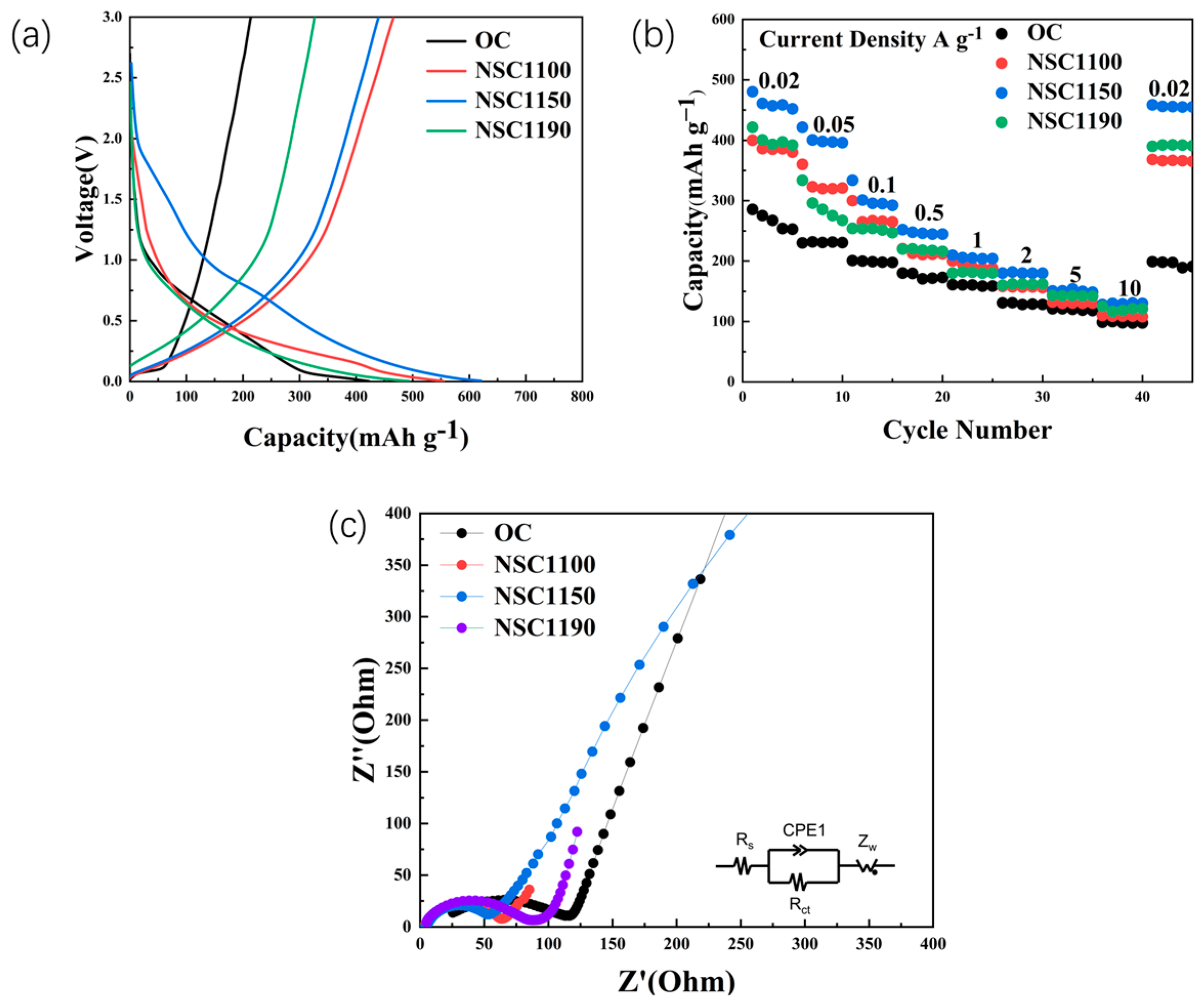
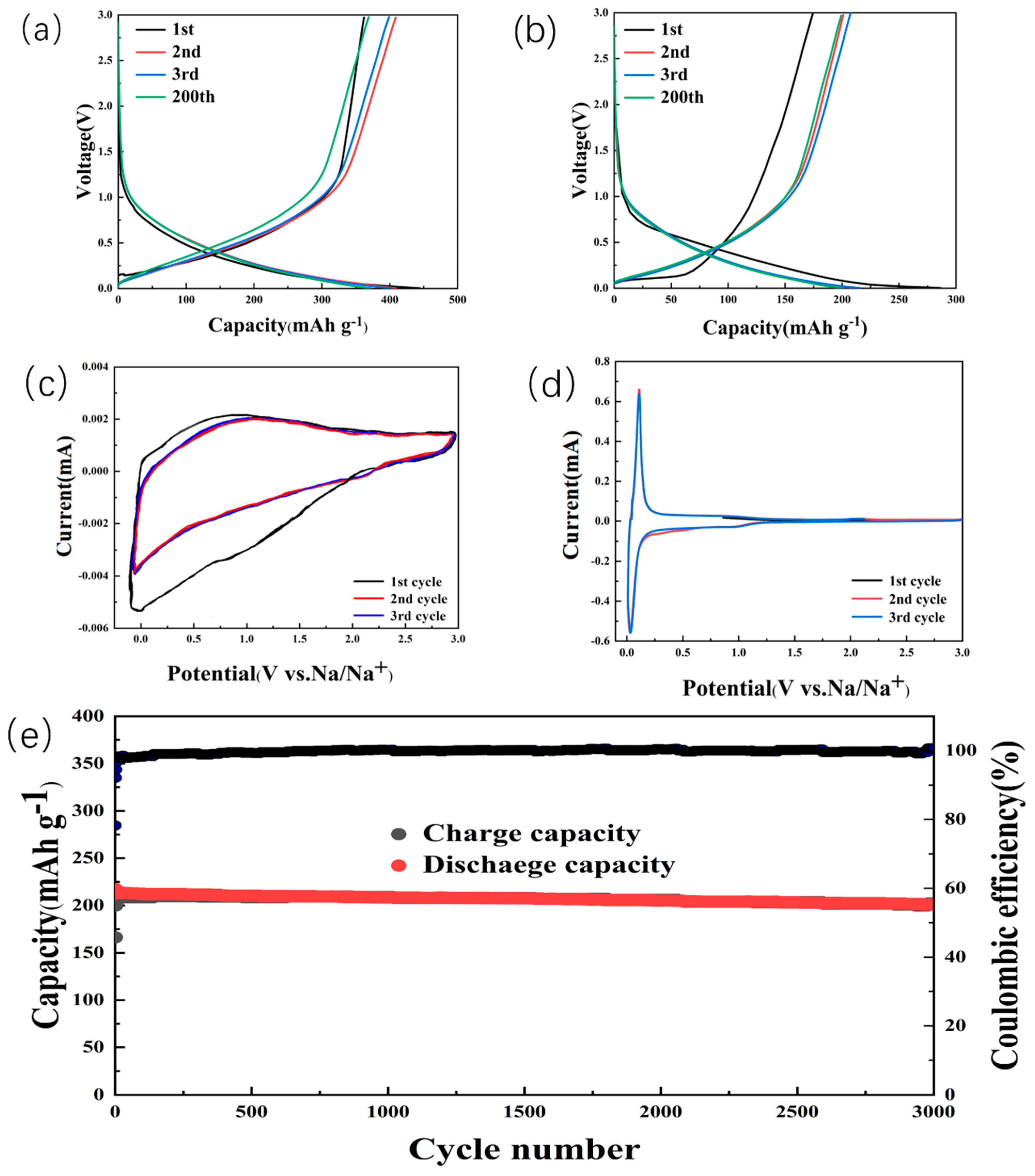
2. Results and Discussion
3. Experimental Section
3.1. Production of Origin Carbon (OC) and (N, S)-C
3.2. Sample Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Q.; Xu, R.; Mu, D.; Tan, G.; Gao, H.; Li, N.; Wu, F. Progress in electrolyte and interface of hard carbon and graphite anode for sodium-ion battery. Carbon Energy 2022, 4, 458–479. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-Solid-State Garnet Type Sulfurized Polyacrylonitrile/Lithium-Metal Battery Enabled by an Inorganic Lithium Conductive Salt and a Bilayer Electrolyte Architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Mitlin, D. Carbon nanosheet frameworks derived from peat moss as high performance sodium ion battery anodes. ACS Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef] [PubMed]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-Gonzalez, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, e5884–e5901. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Liu, Y.; Wang, C. Electrochemical performance of porous carbon/tin composite anodes for sodium-ion and lithium-ion batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, J.; Yang, Y.; Zhao, J.; Rong, J.; Li, H.; Niu, L. Design advanced porous polyaniline-PEDOT: PSS composite as high- performance cathode for sodium ion batteries. Compos. Commun. 2021, 24, 100674. [Google Scholar] [CrossRef]
- Song, K.; Liu, C.; Mi, L.; Chou, S.; Chen, W.; Shen, C. Recent progress on the alloy-based anode for sodium-ion batteries and potassium-ion batteries. Small 2021, 17, 1903194. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Bresser, D.; Passerini, S. Transition metal oxide anodes for electrochemical energy storage in lithium-and sodium-ion batteries. Trans. Metal Oxides Electrochem. Energy Storage 2022, 10, 55–99. [Google Scholar]
- Zhao, Q.; Xia, Z.; Qian, T.; Rong, X.; Zhang, M.; Dong, Y.; Wu, M. PVP-assisted synthesis of ultrafine transition metal oxides encapsulated in nitrogen-doped carbon nanofibers as robust and flexible anodes for sodium-ion batteries. Carbon 2021, 174, 325–334. [Google Scholar] [CrossRef]
- Li, Y.; Lin, W.; Xue, L.; Xie, J.; Wei, B.; Chen, G.; Chen, D. Facile preparation of V2O3/black fungus-derived carbon composite with hierarchical porosity as a promising electrode for lithium/sodium ion batteries. J. Alloys Compd. 2022, 905, 164258. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, S.H.; Sun, Y.K. The application of metal sulfides in sodium ion batteries. Adv. Energy Mater. 2017, 7, 1601329. [Google Scholar] [CrossRef]
- Xiao, B.; Rojo, T.; Li, X. Hard carbon as sodium-ion battery anodes: Progress and challenges. ChemSusChem 2019, 12, 133–144. [Google Scholar] [CrossRef]
- Honma, T.; Togashi, T.; Ito, N.; Komatsu, T. Fabrication of Na2FeP2O7 glass-ceramics for sodium ion battery. J. Ceram. Soc. JPN 2012, 120, 344–346. [Google Scholar] [CrossRef]
- Sridhar, V.; Lee, I.; Park, H. Metal organic frameworks derived Fe-NC nanostructures as high-performance electrodes for sodium ion batteries and electromagnetic interference (EMI) shielding. Molecules 2021, 26, 1018. [Google Scholar] [CrossRef] [PubMed]
- Muruganantham, R.; Gu, Y.J.; Song, Y.D.; Kung, C.W.; Liu, W.R. Ce-MOF derived ceria: Insights into the Na-ion storage mechanism as a high-rate performance anode material. Appl. Mater. Today 2021, 22, 100935. [Google Scholar] [CrossRef]
- Zhu, J.; Roscow, J.; Chandrasekaran, S.; Deng, L.; Zhang, P.; He, T.; Huang, L. Biomass-derived carbons for sodium-ion batteries and sodium-ion capacitors. ChemSusChem 2020, 13, 1275–1295. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Hwang, J.Y.; Shin, H.J.; Jeong, M.G.; Chung, K.Y.; Sun, Y.K.; Jung, H.G. Nano/microstructured silicon–carbon hybrid composite particles fabricated with corn starch biowaste as anode materials for li-ion batteries. Nano Lett. 2019, 20, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liang, F.; Zhou, Z.; Zeng, X.; Wang, D.; Dong, P.; Li, X. Expanded biomass-derived hard carbon with ultra-stable performance in sodium-ion batteries. J. Mater. Chem. A 2018, 6, 1513–1522. [Google Scholar] [CrossRef]
- Hong, K.L.; Qie, L.; Zeng, R.; Yi, Z.Q.; Zhang, W.; Wang, D.; Huang, Y.H. Biomass derived hard carbon used as a high performance anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Yu, H.; Zhang, X.; Liu, T.; Xia, M.; Shu, J. Heteroatom-doped carbon-based materials for lithium and sodium ion batteries. Energy Storage Mater. 2020, 32, 65–90. [Google Scholar]
- Xu, J.; Wang, M.; Wickramaratne, N.P.; Jaroniec, M.; Dou, S.; Dai, L. High-performance sodium ion batteries based on a 3D anode from nitrogen-doped graphene foams. Adv. Mater. 2015, 27, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Dong, R.; Bai, Y.; Li, Y.; Chen, G.; Wang, Z.; Wu, C. Phosphorus-doped hard carbon nanofibers prepared by electrospinning as an anode in sodium-ion batteries. ACS Appl. Mater. Interface 2018, 10, 21335–21342. [Google Scholar] [CrossRef] [PubMed]
- Quan, B.; Jin, A.; Yu, S.H.; Kang, S.M.; Jeong, J.; Abruña, H.D.; Sung, Y.E. Solvothermal-derived S-doped graphene as an anode material for sodium-ion batteries. Adv. Sci. 2018, 5, 1700880. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Feng, W. Fluorine-doped hard carbon as the advanced performance anode material of sodium-ion batteries. Trans. Tianjin Univ. 2022, 28, 123–131. [Google Scholar] [CrossRef]
- Zhang, F.; Qin, D.; Xu, J.; Liu, Z.; Zhao, Y.; Zhang, X. Nitrogen and oxygen co-doping carbon microspheres by a sustainable route for fast sodium-ion batteries. Electrochim. Acta 2019, 303, 140–147. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, H.; Zhan, W.; Lee, Y.S.; Shin, H.J.; Wei, X.; Yang, X. Integrated N, P co-doped and dense carbon networks produced by a chemical crosslinking strategy: Facilitating high gravimetric/volumetric performance sodium ion batteries. Carbon 2020, 165, 204–215. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Li, Y.; Fan, P.; Wu, M. Revisiting N, S co-doped carbon materials with boosted electrochemical performance in sodium-ion capacitors: The manipulation of internal electric field. Nano Res. Energy 2023, 3, e9120098. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Liu, J. Low-defect and low-porosity hard carbon with high coulombic efficiency and high capacity for practical sodium ion battery anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Zhang, T.; Mao, J.; Liu, X.; Xuan, M.; Bi, K.; Zhang, L.X.; Hu, J.; Fan, J.; Chen, S.; Shao, G. Pinecone biomass-derived hard carbon anodes for high-performance sodium-ion batteries. RSC Adv. 2017, 7, 41504–41511. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Wu, D.; Zhao, X.; Zhou, Z. S-doped N-rich carbon nanosheets with expanded interlayer distance as anode materials for sodium-ion batteries. Adv. Mater. 2017, 29, 1604108. [Google Scholar] [CrossRef]
- Shaji, N.; Ho, C.W.; Nanthagopal, M.; Santhoshkumar, P.; Sim, G.S.; Lee, C.W. Biowaste-derived heteroatoms-doped carbon for sustainable sodium-ion storage. J. Alloy Compd. J. 2021, 872, 159670. [Google Scholar] [CrossRef]
- Zhou, C.; Li, A.; Cao, B.; Chen, X.; Jia, M.; Song, H. The non-ignorable impact of surface oxygen groups on the electrochemical performance of N/O dual-doped carbon anodes for sodium ion batteries. J. ELectrochem. Soc. 2018, 165, A1447. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Yang, W.; Liang, D.; Liu, L.; Liang, S.; Deng, C. N/S-Co-doped porous carbon sheets derived from bagasse as high-performance anode materials for sodium-ion batteries. Nanomaterials 2019, 9, 1203. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Liu, X.; Xiao, L.; Cao, Y. A green route to synthesize low-cost and high-performance hard carbon as promising sodium-ion battery anodes from sorghum stalk waste. Green Energy Environ. 2017, 2, 310–315. [Google Scholar] [CrossRef]
- Li, Q.; Wu, M.; Wang, Y.; Wang, G.; Zhuo, L.; Li, Z. Untra-high pseudocapacitance enhanced anode of N, P dual-doped carbon nanosheet derived from biomass toward high performance sodium ion battery. Nano Select. 2021, 2, 129–139. [Google Scholar] [CrossRef]
- Xia, J.; Jiang, K.; Xie, J.; Guo, S.; Liu, L.; Zhang, Y.; Wang, X. Tin disulfide embedded in N-, S-doped carbon nanofibers as anode material for sodium-ion batteries. Chem. Eng. J. 2019, 359, 1244–1251. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Liu, J. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Shi, J.; Kong, X.; Cao, X.; Liang, S.; Pan, A. Tin sulfide nanoparticles embedded in sulfur and nitrogen dual-doped mesoporous carbon fibers as high-performance anodes with battery-capacitive sodium storage. Energy Storage Mater. 2019, 18, 366–374. [Google Scholar] [CrossRef]
- Lu, M.; Yu, W.; Shi, J.; Liu, W.; Chen, S.; Wang, X.; Wang, H. Self-doped carbon architectures with heteroatoms containing nitrogen, oxygen and sulfur as high-performance anodes for lithium-and sodium-ion batteries. Electrochim. Acta 2017, 251, 396–406. [Google Scholar] [CrossRef]
- Miao, X.; Sun, D.; Zhou, X.; Lei, Z. Designed formation of nitrogen and sulfur dual-doped hierarchically porous carbon for long-life lithium and sodium ion batteries. Chem. Eng. J. 2019, 364, 208–216. [Google Scholar] [CrossRef]
- Liu, X.; Yu, C.; Hou, H.; Xu, Z.; Meng, K.; Zhu, J.; Wang, L. The recycling of the expired donkey-hide gelatin pulp for N/S co-doped hollow carbon nano-spheres anode in sodium ion battery. Environ. Sci. Pollut. Res. 2020, 27, 13467. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, K.; Feng, P.; Zhang, Z.; Cheng, S.; Jiang, K. Surface-dominated storage of heteroatoms-doping hard carbon for sodium-ion batteries. Energy Storage Materials. 2020, 27, 43. [Google Scholar] [CrossRef]
- Muruganantham, R.; Wang, F.M.; Liu, W.R. A green route N, S-doped hard carbon derived from fruit-peel biomass waste as an anode material for rechargeable sodium-ion storage applications. Electrochimica. Acta 2022, 424, 140573. [Google Scholar] [CrossRef]
- Wan, H.; Shen, X.; Jiang, H.; Zhang, C.; Jiang, K.; Chen, T.; Chen, Y. Biomass-derived N/S dual-doped porous hard-carbon as high-capacity anodes for lithium/sodium ions batteries. Energy 2021, 231, 121102. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Wei, G.; Li, S.; Lu, Z.; Wang, X.; Lou, X. Sodium storage mechanism of N, S co-doped nanoporous carbon: Experimental design and theoretical evaluation. Energy Storage Mater. 2018, 11, 274–281. [Google Scholar] [CrossRef]
- Kumaresan, T.K.; Masilamani, S.A.; Raman, K.; Karazhanov, S.Z.; Subashchandrabose, R. High performance sodium-ion battery anode using biomass derived hard carbon with engineered defective sites. Electrochim. Acta 2021, 368, 137574. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Hu, Y.S.; Li, H.; Chen, L.; Huang, X. A waste biomass derived hard carbon as a high-performance anode material for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 13046–13052. [Google Scholar] [CrossRef]
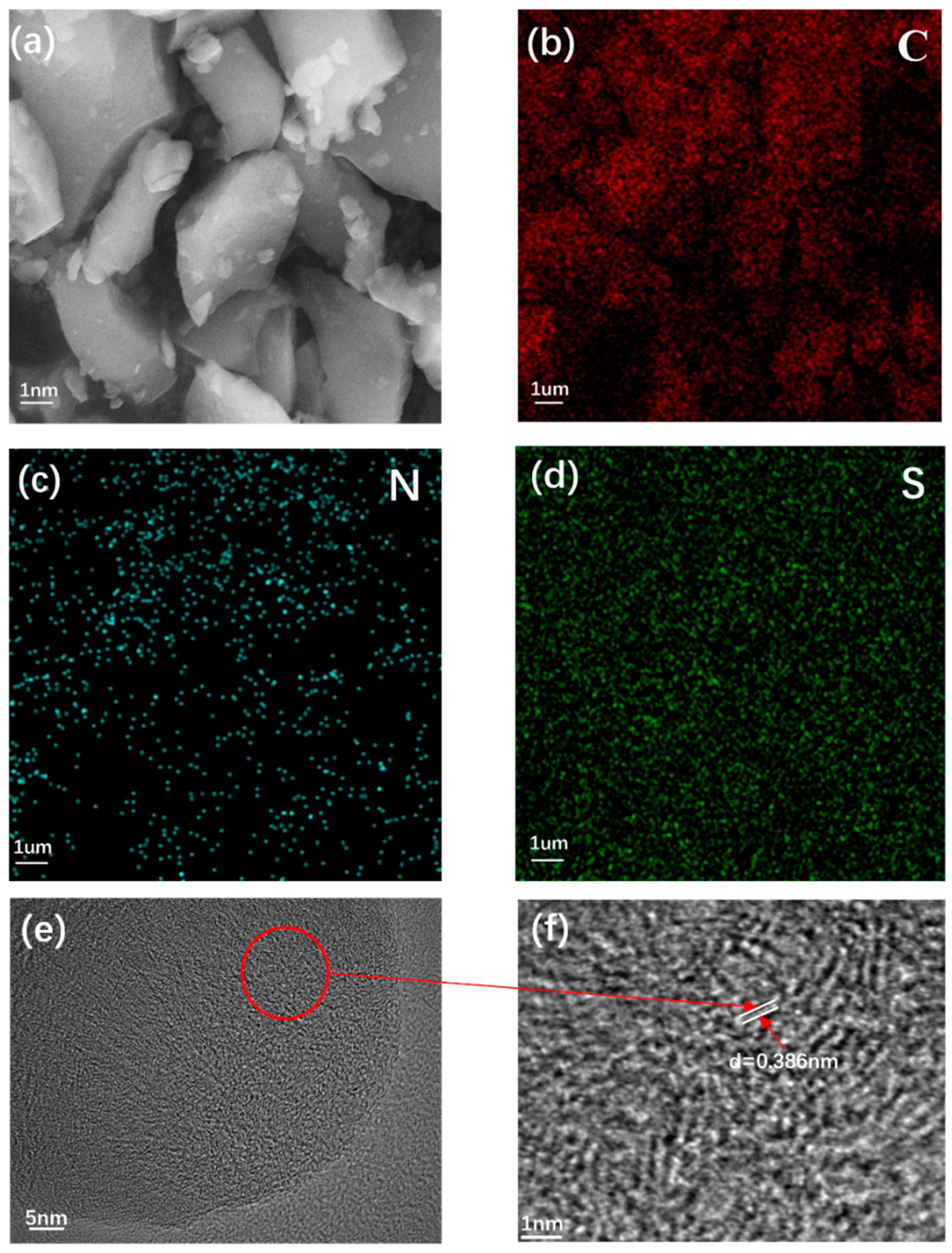
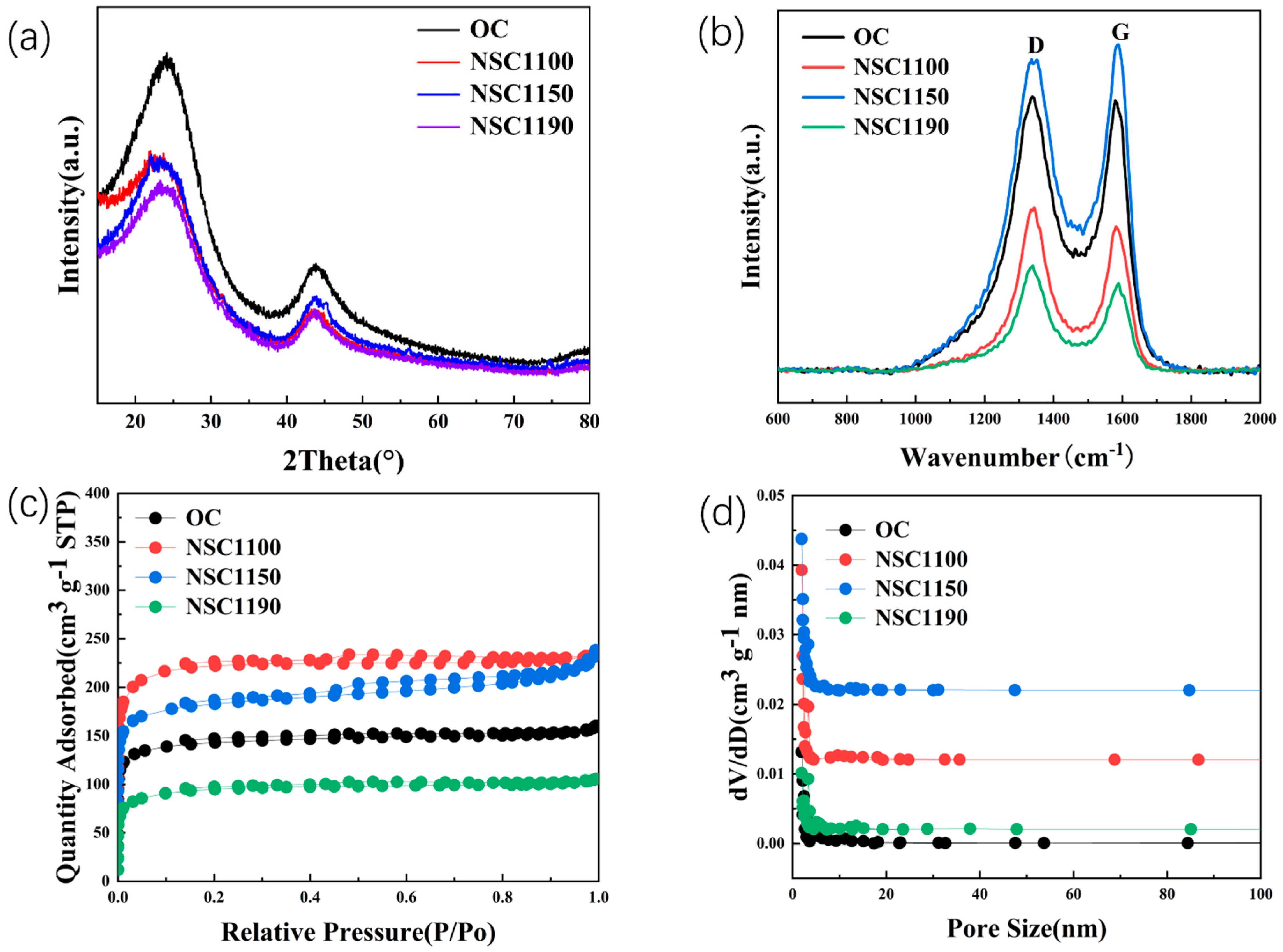
| Sample | ID/IG | SBET(m2 g−1) | d002(nm) | DP(nm) |
|---|---|---|---|---|
| NSC1100 | 1.03 | 563.45 | 0.381 | 6.25 |
| NSC1150 | 1.21 | 683.85 | 0.386 | 3.99 |
| NSC1190 | 0.94 | 296.17 | 0.376 | 3.64 |
| OC | 0.86 | 315.86 | 0.360 | 7.75 |
| Material | Rs | Rct |
|---|---|---|
| NSC-1100 | 3.48 | 70.53 |
| NSC-1150 | 3.32 | 47.6 |
| NSC-1190 | 6.35 | 95.7 |
| OC | 8.92 | 120.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.; Zhang, C.; Deng, D.-R.; Weng, J.-C.; Song, J.-X.; Fan, X.-H.; Li, G.-F.; Li, Y.; Wu, Q.-H. Synthesis of Low-Cost and High-Performance Dual-Atom Doped Carbon-Based Materials with a Simple Green Route as Anodes for Sodium-Ion Batteries. Molecules 2023, 28, 7314. https://doi.org/10.3390/molecules28217314
Lu B, Zhang C, Deng D-R, Weng J-C, Song J-X, Fan X-H, Li G-F, Li Y, Wu Q-H. Synthesis of Low-Cost and High-Performance Dual-Atom Doped Carbon-Based Materials with a Simple Green Route as Anodes for Sodium-Ion Batteries. Molecules. 2023; 28(21):7314. https://doi.org/10.3390/molecules28217314
Chicago/Turabian StyleLu, Bin, Chi Zhang, Ding-Rong Deng, Jian-Chun Weng, Jia-Xi Song, Xiao-Hong Fan, Gui-Fang Li, Yi Li, and Qi-Hui Wu. 2023. "Synthesis of Low-Cost and High-Performance Dual-Atom Doped Carbon-Based Materials with a Simple Green Route as Anodes for Sodium-Ion Batteries" Molecules 28, no. 21: 7314. https://doi.org/10.3390/molecules28217314
APA StyleLu, B., Zhang, C., Deng, D.-R., Weng, J.-C., Song, J.-X., Fan, X.-H., Li, G.-F., Li, Y., & Wu, Q.-H. (2023). Synthesis of Low-Cost and High-Performance Dual-Atom Doped Carbon-Based Materials with a Simple Green Route as Anodes for Sodium-Ion Batteries. Molecules, 28(21), 7314. https://doi.org/10.3390/molecules28217314