Abstract
Coordination polymers (CPs) are an assorted class of coordination complexes that are gaining attention for the safe and sustainable removal of organic dyes from wastewater discharge by either adsorption or photocatalytic degradation. Herein, three different coordination polymers with compositions [Ni(HL)(H2O)2·1.9H2O] (1), [Mn3(HL)(L)(μ3-OH)(H2O)(phen)2·2H2O] (2), and [Cd(HL)4(H2O)]·H2O (3) (H3L = 2-(3,5-dicarboxyphenyl)-6-carboxybenzimidazole; phen = 1,10-phenanthroline) have been synthesized and characterized spectroscopically and by single crystal X-ray diffraction. Single crystal X-ray diffraction results indicated that 1 forms a 2D layer-like framework, while 2 exhibits a 3-connected net with the Schläfli symbol of (44.6), and 3 displays a 3D supramolecular network in which two adjacent 2D layers are held by π···π interactions. All three compounds have been used as photocatalysts to catalyze the photodegradation of antibiotic dinitrozole (DTZ) and rhodamine B (RhB). The photocatalytic results suggested that the Mn-based CP 2 exhibited better photodecomposition of DTZ (91.1%) and RhB (95.0%) than the other two CPs in the time span of 45 min. The observed photocatalytic mechanisms have been addressed using Hirshfeld surface analyses.
1. Introduction
Release of a significant quantity of organic compounds, especially aromatic dyes and antibiotics, in water bodies from industries and residential areas has resulted in considerable water pollution, which not only destroys the aquatic environment but also poses safety problems for humans and animals [1,2,3,4,5,6,7,8,9,10]. Thus, developing advanced methods and materials to safely eliminate these classes of compounds from wastewater must be taken into consideration by chemists and environmentalists. Coordination polymers (CPs) are an assorted class of coordination complexes that are designed and synthesized by coordinating rationally selected polydentate ligands with targeted metal cation centers. Such strategy gives rise to varied types of two-dimensional and three-dimensional frameworks in this class of crystalline materials that find applications as adsorbents [11,12,13,14,15,16,17,18,19], as supercapacitors [20,21], in drug delivery [22,23,24,25,26,27,28,29,30,31], as sensors [32,33,34,35,36,37,38,39,40,41,42], and in catalysis [43,44,45,46] and drug delivery [47,48]. Because of fascinating characteristics such as peculiar architectures and homogenous distribution of active sites, this type of material has received significant attention as photocatalytic materials [49,50,51,52,53]. Wang et al., for example, reported Fe-based MOFs (Fe-MIL-101) for tetracycline (TC) removal under visible light illumination, with a TC removal rate of 57.4% [54,55].
The selection of suitable ligands is critical in order to construct tailored coordination polymers. Among the many classes of ligands, aromatic multicarboxylic acids have been extensively employed as building blocks for designing and fabricating the desired CPs [56,57,58,59,60,61,62]. Among the widely used group of multicarboxylic acid ligands, the rigid blocks having at least two aromatic rings and several COOH groups arranged in different positions are capable of forming fascinating structures. Moreover, such ligands induce functionally active characteristics in the CPs [63,64,65,66]. Additionally, the use of ancillary ligands (heterocyclic N- or N,N-donor) can also be employed to stabilize and extend the structures for designing newer frameworks along with these multicarboxylate building blocks [67,68,69,70,71,72].
These characteristics of the multicarboxylate ligands therefore motivated us to investigate the design and synthesis of new CP-based photocatalysts. Hence, in the presented investigation, three new transition metal-based CPs have been synthesized using 2-(3,5-dicarboxyphenyl)-6-carboxybenzimidazole as the main ligand. This ligand was selected due to its semi-rigid nature and the presence of three carboxyl groups, which can generate multiple nodes and enhance thermal stability and overall rigidity of the frameworks. In view of this, three new Ni(II)/Mn(II)/Cd(II)-CPs with the aforementioned ligand have been synthesized, characterized, and used as photocatalytic materials to catalyze the photodegradation of dinitrazole antibiotic and rhodamine B dyes. The outcomes of these studies are presented herewith.
2. Results and Discussion
2.1. Structural Description of 1[Ni(HL)(H2O)2·1.9H2O]
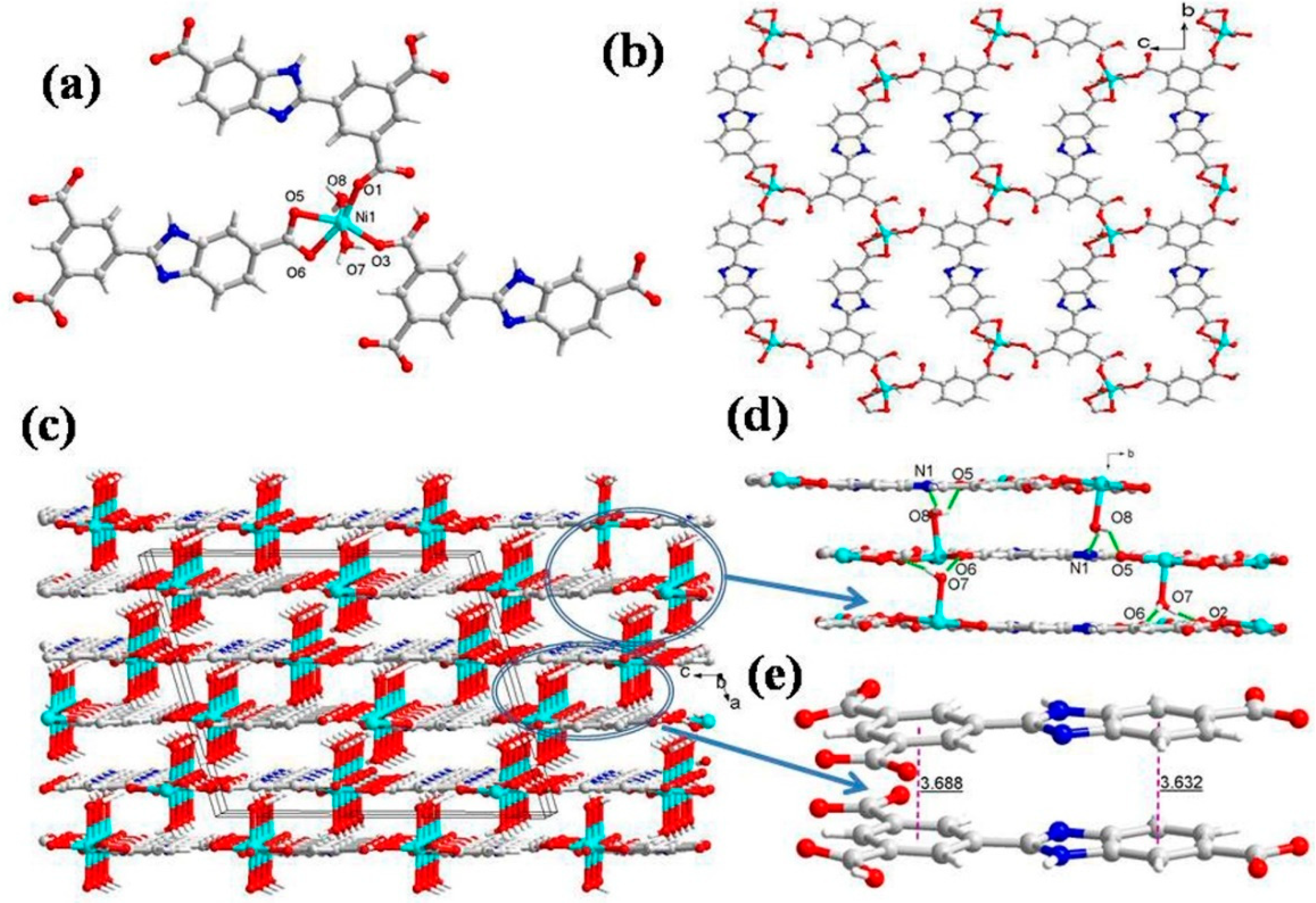
The single crystal X-ray diffraction analysis revealed that CP 1 crystallizes in the monoclinic unit cell with a C2/c space group. The asymmetric unit of 1 consists of one Ni(II), one HL2− ligand, two coordinated water molecules, and 1.9 free water molecules (Figure 1a). The immediate geometry around the Ni(II) is distorted octahedral, wherein the equatorial plane is occupied by four O atoms (O1, O3, O5 and O6) from three HL2− ligands (Ni1-O1 = 1.980(3) Å; Ni1-O3 = 1.981(3) Å; Ni1-O5 = 2.126(3) Å; and Ni1-O6 = 2.103(3) Å), while the axial positions are occupied by two O atoms (O7, O8) of aqua ligands that are transverse with respect to each other (Ni1-O7 = 2.090(4) Å and Ni1-O8 = 2.059(4) Å) with ∠O7-Ni1-O8 of 175.12(14)° (Table S2). Interestingly, the two HL2− ligands exhibit monodentate coordination and the third ligand displays bidentate chelating coordination with the Ni(II) center. In CP 1, each HL2− connects three Ni2+ centers with a linkage angle of 5.1°; in addition, the two aromatic rings are almost parallel, with a dihedral angle of 7.2°. The three carboxylic groups in HL2− show (κ1 − κ0)-μ1–COO− monodentate mode and (κ1 − κ1)-μ1–COO− chelating mode, and one remains as –COOH. These coordination modes of HL2− linkers join the octahedral Ni(II) to form a 2D layer (Figure 1b). The framework robustness in the 2D layer is provided by four types of weak interactions (Figure 1d): (i) the aqua ligand hydrogen (H7B) forms the intermolecular hydrogen bond with neighboring uncoordinated carboxylate oxygen (O2), with a O7-H7B···O2 interaction distance of 2.21 Å; (ii) the carboxylate oxygen (O6) hydrogen bonds with the H7A hydrogen of the coordinated aqua ligand O7 of the adjacent molecule with a O6···H7A interaction distance of 1.97 Å; (iii) the coordinated water molecule (O8) hydrogen H8B forms hydrogen bonding with the carboxylic O atom (O5) of the neighboring moiety with O8-H8B···O5 separation of 2.14 Å; and (iv) the coordinated water molecule (O8) hydrogen (H8A) forms another hydrogen bond with the adjacent nitrogen N1 of the imidazole ring, having O8-H8A···N1 interaction distance of 2.06 Å (Table S5). Additionally, two 2D layers are connected by the hydrogen bonds in face-to-face mode to engender a 3D supramolecular network using π···π interactions between the HL2− ligand and two adjacent 2D layers (Figure 1e). From a topological point of view, each HL2− acts as a 3-connected node, and hence each Ni2+ center can be regarded as a 3-connected node. Thus, the resulting structure of CP 1 is a 3-connected hexagonal-type topology with a Schläfli symbol of (63) (Figure S1a).
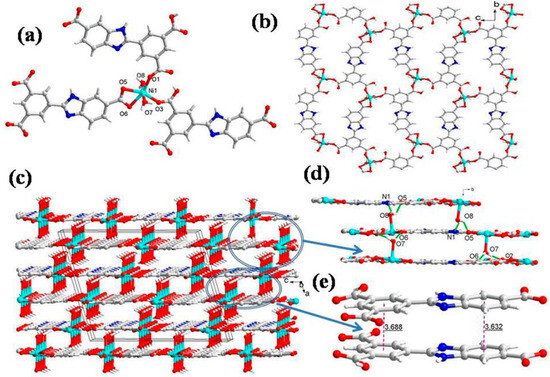
Figure 1.
(a) Asymmetric unit displaying coordination environment around Ni(II) in CP 1; (b) 2D layer viewed along the bc plane; (c) 3D supramolecular network; (d) the different hydrogen bonding interactions (e) π···π stacking within 3D supramolecular net for CP 1.
2.2. Structural Description of 2
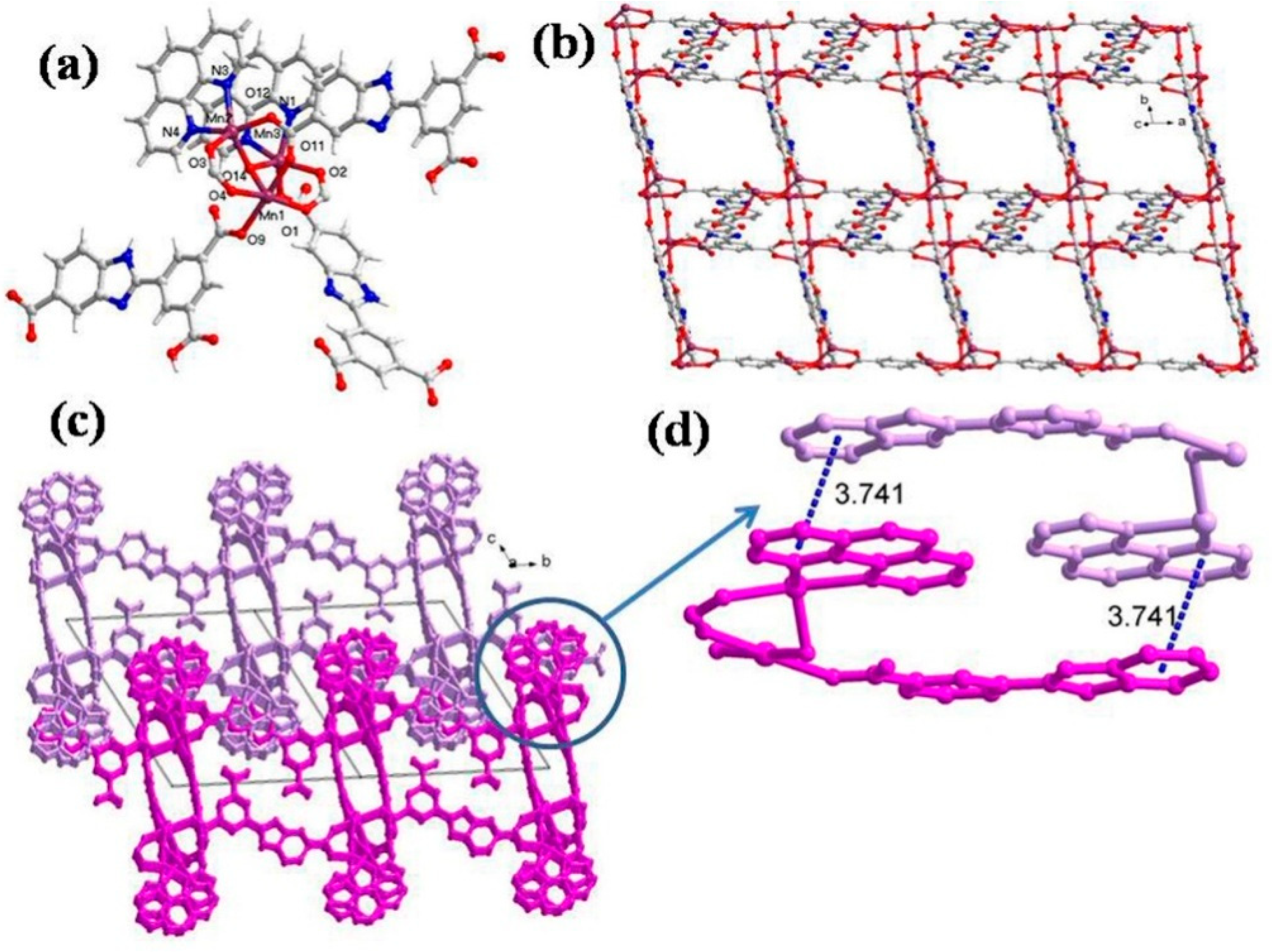
The single crystal X-ray analysis reveals that CP 2 crystallizes in the triclinic unit cell with a P-1 space group. The asymmetric unit of 2 possesses three Mn(II), one HL2− ligand, one L3−, one coordinated aqua ligand, and one hydroxyl group along with two chelating phen ligands and two free water molecules (Figure 2a). The immediate geometry around Mn1 is distorted octahedral with four oxygen center atoms coming from carboxylate ligands HL2− and L3−, one bridging O of the OH group and one aqua ligand. The O1, O4, O9, and O11 are located at the equatorial plain, while the O13 and O14 oxygen atoms occupy the axial positions (Mn1-O9 = 2.303(4) (3) Å; Mn1-O11 = 2.164(4) Å) (Table S3). In addition, the bond angles around Mn1 vary between 81.87(16)°–178.14(15)°, and the distortion around Mn1 can be ascribed to the Jahn-Teller effect. Apart from this, Mn2 and Mn3 display similar trigonal bipyramidal geometry (maximum atomic deviation of 0.018 Å), wherein Mn2 and Mn3 are displaced by 0.251 Å from the trigonal plane toward the strongly coordinated axial ligand. Moreover, 2, the carboxylate groups of HL2−, adopt two different coordination modes viz. (κ1 − κ1)-μ2-COO− and (κ1 − κ0)-μ1-COO−, respectively. The two differently coordinated HL2−/L3− ligands link Mn(II) centers to form a 2D layer along the ab plane (Figure 2b), but this 2D layer framework is different from 1. The phen ligands protrude from the 2D layer and are distributed regularly on the same side of the layer (Figure 2c). Furthermore, the phen and HL2− ligands form weak π···π interactions with a separation of 3.741 Å (Figure 2d). From a topological point of view, each HL2− acts as a 3-connected node, and hence each Mn2+ center can be regarded as a 3-connected node. Thus, the resulting structure of CP 2 is a 3-connected net with a Schläfli symbol of (44.6) (Figure S1b).
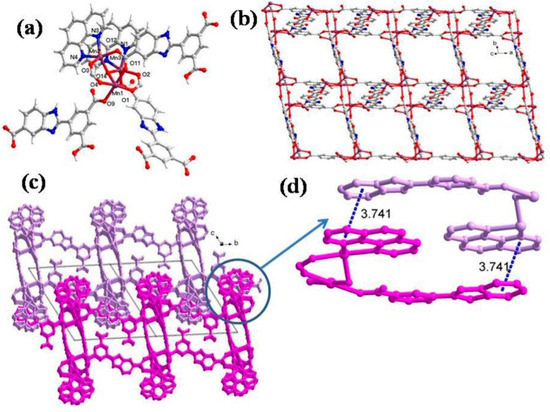
Figure 2.
(a) Asymmetric unit displaying coordination environment around Mn(II) in CP 2; (b) 2D layer viewed along the ac plane; (c) 3D supramolecular network; (d) π···π stacking mode.
2.3. Structural Description of 3
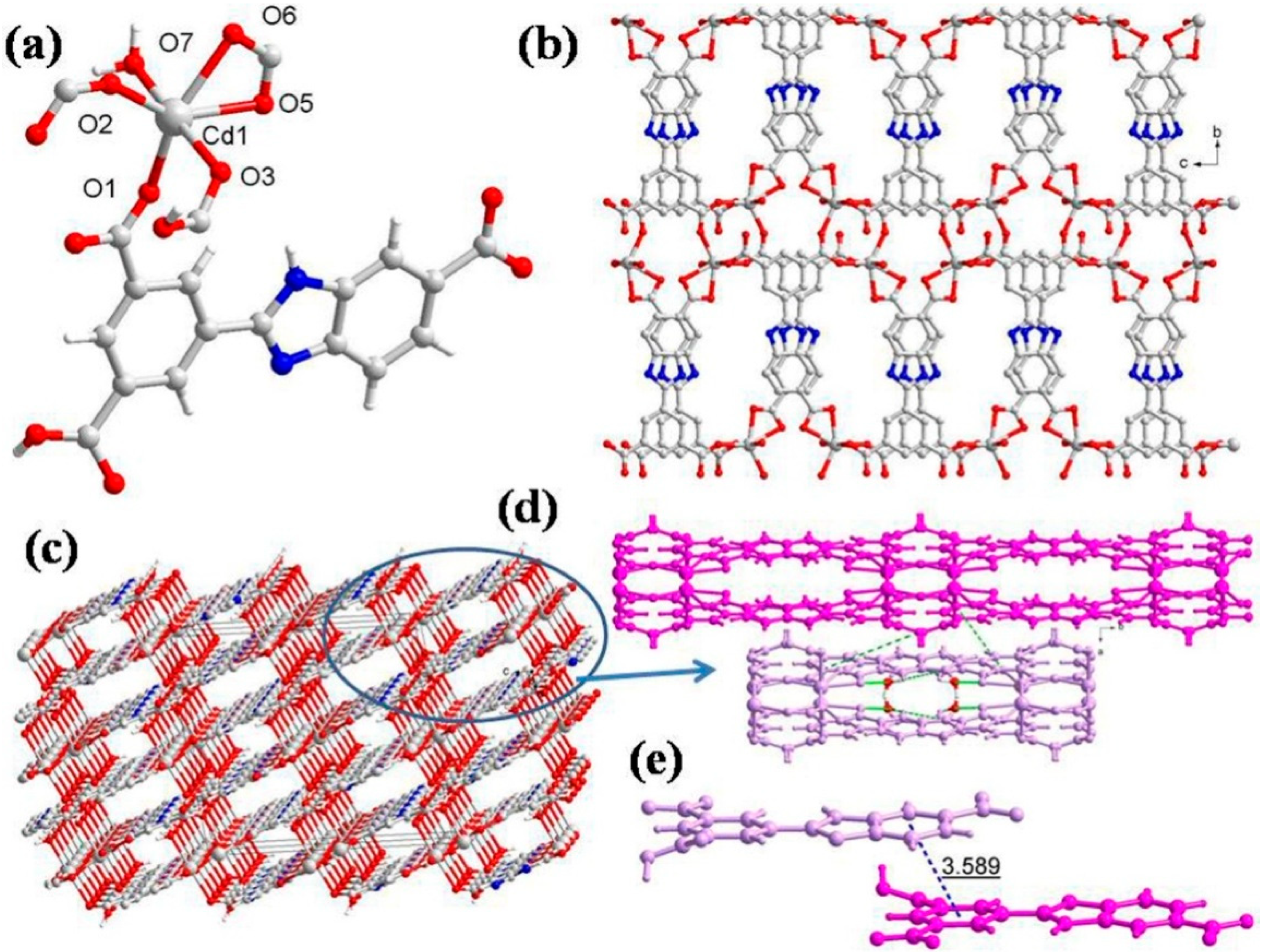
The CP 3 crystallizes in the monoclinic unit cell with the C2/c space group, and the asymmetric unit consists of one independent Cd(II) ion, one HL2- ligand, one aqua ligand, and one free water molecule (Figure 3a). The geometry around Cd(II) is distorted octahedral, wherein the equatorial plane is occupied by four O atoms (O1, O2, O5, and O6) from three HL2− ligands, in which one HL2− ligand coordinates in bidentate chelating mode and the other two in monodentate bridging mode between two Cd(II) centers, while the two trans axial positions are adopted by two O atoms (O3, O7) from the carboxylate oxygen of HL2− and aqua ligands, respectively (Cd1-O3 = 2.367(8) Å and Cd1-O7 = 2.337(8) Å) (Table S2). The ∠O3-Cd1-O7 is 175.7(3)° (Table S4), indicating that Cd1 lies at the equatorial plane of the octahedron. In CP 3, each HL2− connects four Cd(II) centers with a bond angle of 2.9°, and the two aromatic rings are almost parallel, with a dihedral angle of 3.1°. The carboxylic groups in HL2− exhibit (κ1 − κ0)-μ1–COO− monodentate mode, (κ1 − κ1)-μ1–COO− chelating mode, and (κ1 − κ1)-μ2–COO− bridging mode. These coordination modes of HL2− linkers join the octahedral Cd(II) to produce a layer (Figure 3b). The 2D layer is held by bifurcated hydrogen-binding interactions (Figure 3d): (i) The coordinated aqua ligand (O7) forms the intermolecular hydrogen bond to the neighboring uncoordinated carboxylic O atom (O4) with O7-H7A···O4 separation of 1.90 Å. (ii) The same aqua ligand is involved in hydrogen bonding with another carboxylate oxygen atom (O6) with O7-H7B···O6 distance of 2.07 Å (Table S7). Further, the two face-to-face 2D layers are connected by the hydrogen bonds to generate a 3D supramolecular network (Figure 3c). Additionally, the π···π interaction distance between the HL2− ligands is 3.589 Å, and two adjacent 2D layers propagated into a 3D supramolecular network (Figure 3e). From a topological point of view, each HL2− acts as a 3-connected node, and hence each Cd2+ center can be regarded as a 6-connected node. Thus, the resulting structure of CP 3 is a 3,6-connected net with a Schläfli symbol of {42.6}2{44.69.82} (Figure S1c).
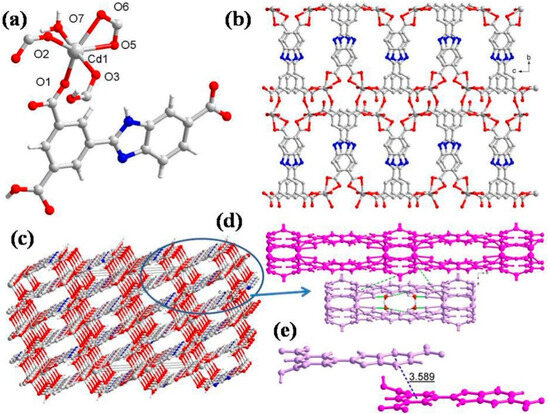
Figure 3.
(a) Asymmetric unit displaying coordination environment around Cd(II) in CP 3; (b) 2D layer viewed along the bc plane; (c) 3D supramolecular network; (d) different hydrogen-bonding interactions; (e) π···π stacking within 3D supramolecular net for CP 3.
2.4. FTIR Spectroscopy, Thermogravimetric Analyses, PXRD, and BET Surface Area Analysis
In the FTIR spectra of all three CPs, the relatively weak band appearing at ~3000 cm−1 is attributed to νC-H of the aromatic ring. The broad absorption bands in the range of 3400–3200 cm−1 may be assigned to the characteristic peaks of O–H stretching vibrations from coordinated or lattice water molecules. The appearance of a band ~1700 cm−1 suggested partial deprotonation of H3L ligands. In addition, bands between 1550–1600 cm−1 and 1340–1380 cm−1 can be assigned to νasymm and νsymm, respectively, of the COO− groups of the coordinated ligand (Figure S2).
Thermogravimetric studies (TGA) were also carried out to learn more about the thermal stabilities of all three CPs. CP 1 shows two distinct weight loss regions in the TGA curve. The first sharp weight loss occurred at ca. 238 °C due to the loss of aqua ligands (obsd. 8.2%, calcd. 8.6%). Furthermore, the TGA of CP 1 revealed weight loss occurring between 210 and 755 °C, corresponding to the decomposition of the organic moiety with the formation of Ni2O3 (obsd. 37.9%, calcd. 39.4%). In CP 2, the first sharp weight loss occurred until ca. 194 °C due to the release of the coordinated aqua ligand and co-crystallized water molecules (obsd. 5.3%, calcd. 5.7%). Moreover, it is worthy to mention here that the co-crystallized water molecules were release from the lattice of 2 at ca. 89 °C. Further, weight loss between 390–855 °C corresponded to the decomposition of the organic group to engender Mn2O3 (obsd. 39.8%, calcd. 37.9%). CP 3 exhibits two weight loss regions in its TGA curve. The first weight loss between 45–212 °C corresponds to the loss of the coordinated aqua ligand and co-crystallized water molecules (obsd. 7.9%, calcd. 7.6%). Similarly, in the second step, the framework of 3 collapsed with the loss of organic species quickly from temperature range 450–890 °C to form CdO (obsd. 26.9%, calcd. 27.1%). Additionally, powder X-ray diffraction (PXRD) experiments were conducted to evaluate the phase purity of all three CPs. The experimental PXRD profiles of all three CPs match satisfactorily with simulated PXRD profiles, indicating their bulk phase purity (Figures S4–S6). Additionally, UV-Vis spectroscopy was used to evaluate the optical band gaps of MOFs that revealed absorption edges at 322 nm. On this basis, the optical band gap energies were calculated by the Kubelka-Munk function: αhν = A(hν − Eg)n/2, in which α stands for the absorption coefficient, hν stands for the photon energy, A is a constant, and Eg is the band gap. The band gaps for 1, 2, and 3 were calculated to be 2.10, 2.82, and 2.23 eV, respectively (Figure S7), indicating their semiconducting properties. Hence, CPs 1–3 can be utilized as photocatalysts for nitrophenol photodegradation.
The textural properties of 1–3 were explored using the BET N2 adsorption isotherm method performed at 77 K. It was observed that the isotherms displayed a continuous increase at low relative pressure (P/P0 < 0.05) and displayed a small hysteresis loop at high relative pressure (0.45 < P/P0 < 1), which indicated the existence of micropores in these CPs. In addition, CP 2 possesses relatively larger BET surface area and pore volume than CPs 1 and 3, which might be arising because of the dense structural packing in later CPs. Furthermore, the DTZ pore size distribution in all the materials is smaller than 0.50 nm (Figure S10).
2.5. Photocatalytic Applications
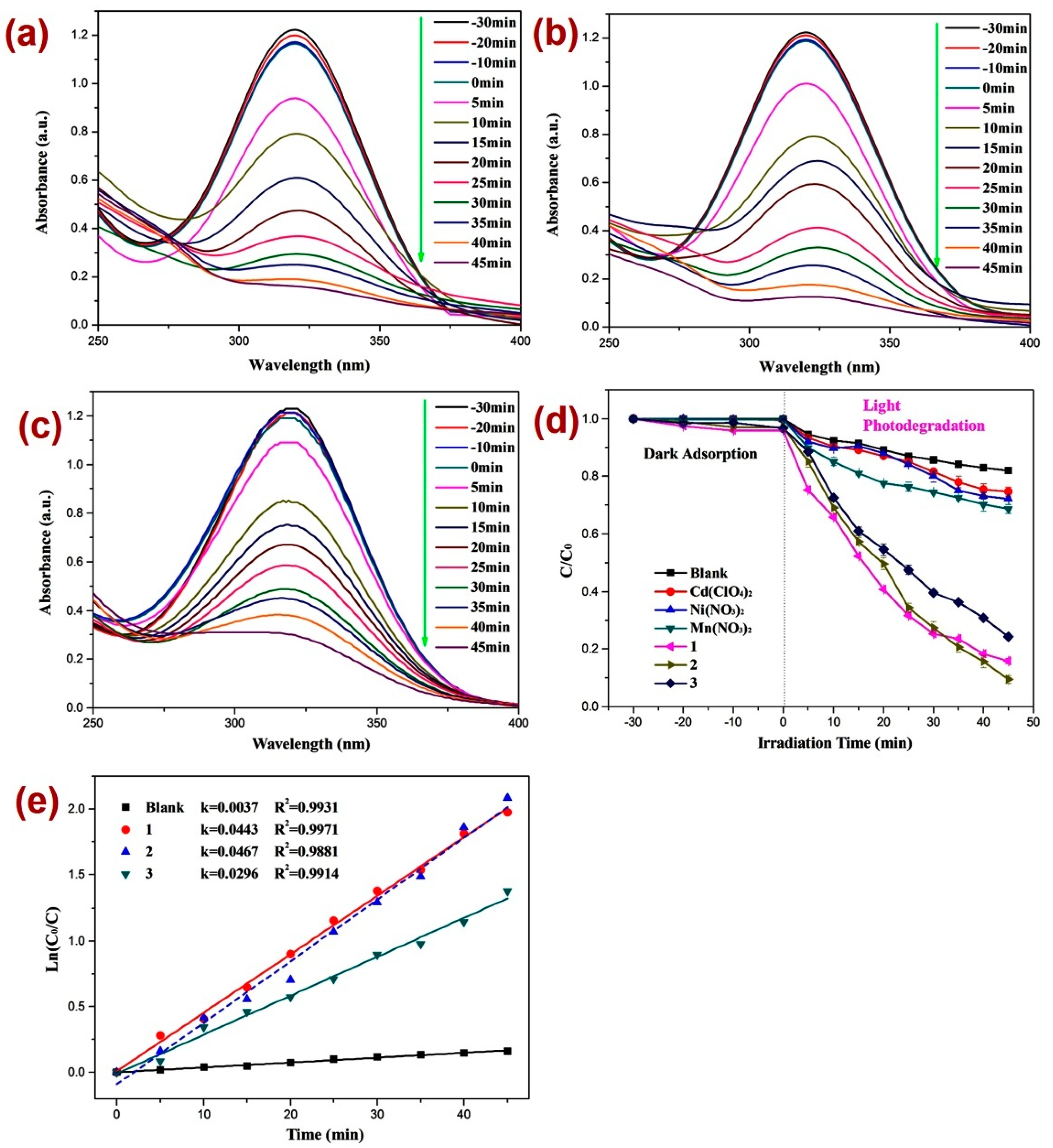
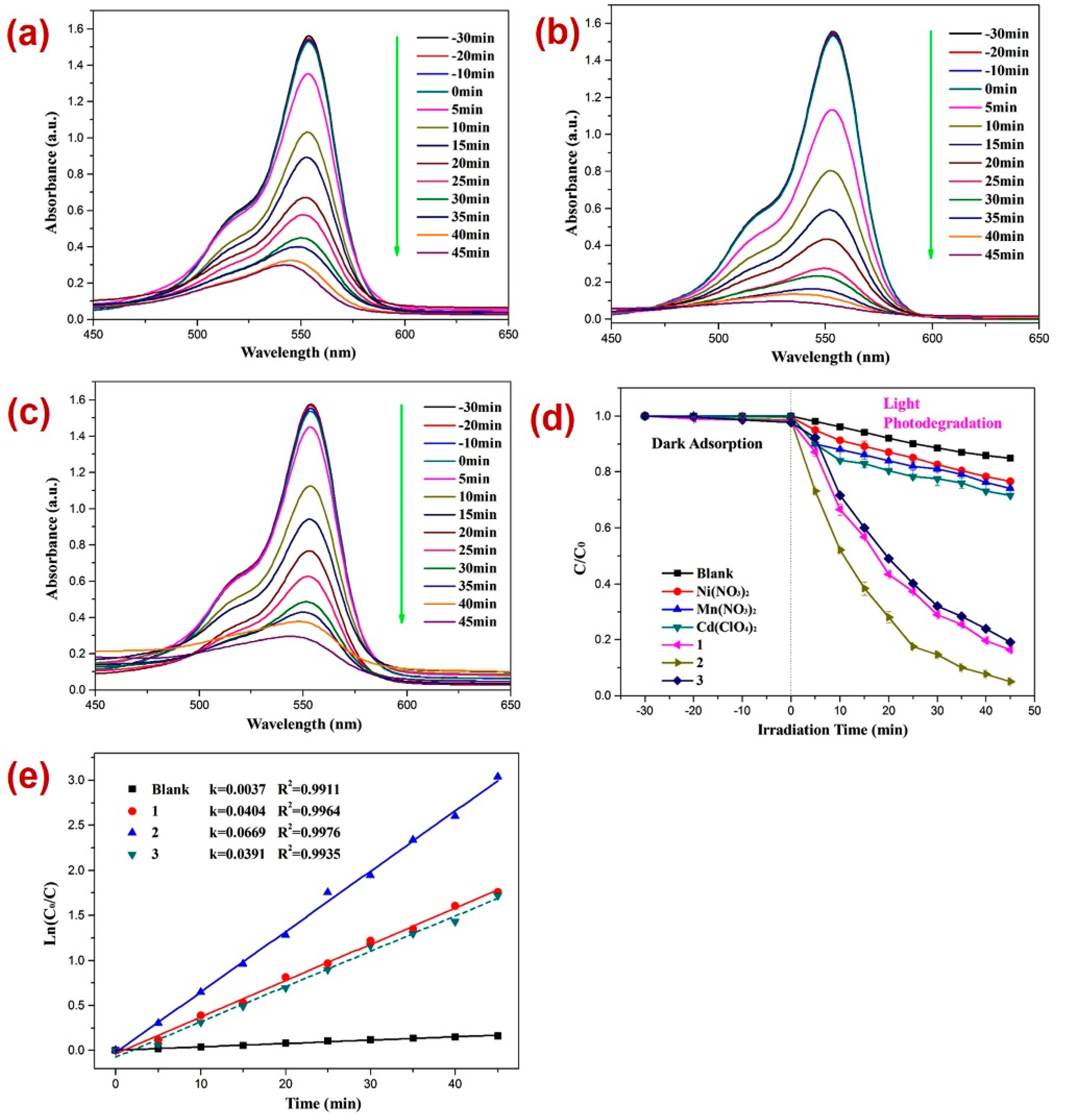
After firm structural characterization of all three CPs, their applicability as photocatalysts were assessed by employing previously reported methods [73,74,75,76] against the decomposition of the dinitrazole (DTZ) antibiotic, which was assessed under UV light irradiation at a mean wavelength of 365 nm (Figure 4). Before performing the photocatalysis, attempts were made to establish the adsorption–desorption equilibrium by keeping the reaction mixture in the dark for 30 min and monitoring the UV-Vis spectra for all three reaction mixtures (Figure 4a–c) and also by constructing a graph of C/C0 vs. time plot (Figure 4d). Such investigation revealed little alternation in the absorption intensity of DTZ within this time span, thereby indicating the establishment of the adsorption–desorption between DTZ and CPs 1–3. Further, the photocatalytic experiments indicated that the electronic absorption intensity of the DTZ solution decreased with time (Figure 4). In addition, in the timeframe of 45 min, the percentage decomposition of DTZ in the presence of photocatalysts 1, 2, and 3 was 84.2%, 91.1%, and 75.1%, respectively (Figure 4a–c). However, in the absence of these photocatalysts, the percentage photodegradation of DTZ was merely 18.2% (Figure 4d). Moreover, the percentage photodegradation of DTZ in the presence of the metal salts Ni(NO3)2·6H2O, Mn(NO3)2·4H2O, and Cd(ClO4)2·6H2O was 28.1%, 31.1%, and 25.3%, respectively (Figure S8). Hence, the photocatalytic performance of pristine metal salts revealed that the photocatalysis is not only arising because of the metal centers of the CPs 1–3; rather, the frameworks of CPs are collectively playing a role in their photocatalytic activities, photodegrading DTZ. Furthermore, the photodegradation of DTZ in the presence of these CP-based photocatalysts displayed pseudo-first-order kinetics (Figure 4e) and the rate constants for all three CPs calculated by using the plot of ln(Ct/C0) vs. time (Table 1) suggested that the rate constant k (min−1) was highest in the presence of CP 2. Hence, the percentage photodegradation of DTZ and the rate constant parameters further suggested that amongst all three CPs, the Mn(II) CP 2 displayed the best photocatalytic efficiency. These differences in the catalytic activities of the CPs might be arising because of the variation in the structural environments around metal centers [77,78,79].
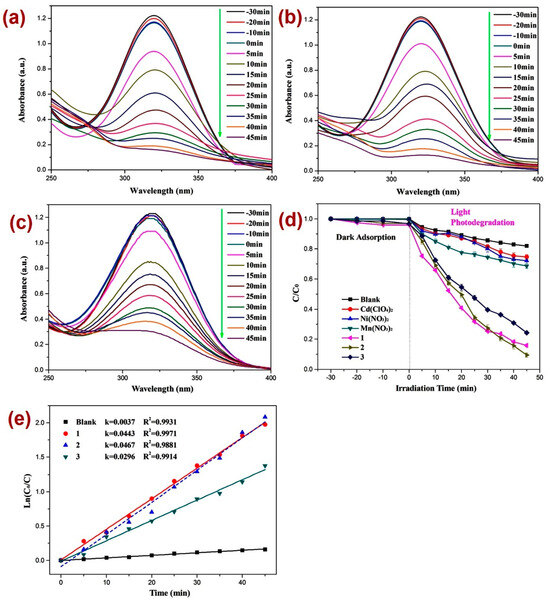
Figure 4.
Photodegradation of DTZ in the presence of photocatalysts (a) 1; (b) 2; (c) 3; (d) graph representing the plot of C/C0 vs. irradiation time, where C/C0 represents the concentration ratio of DTZ in the presence of metal salts and their corresponding CPs; (e) results of kinetic studies displaying pseudo-first-order kinetics.

Table 1.
The different rate constants (k) obtained by fitting of the pseudo-first-order kinetics curves.
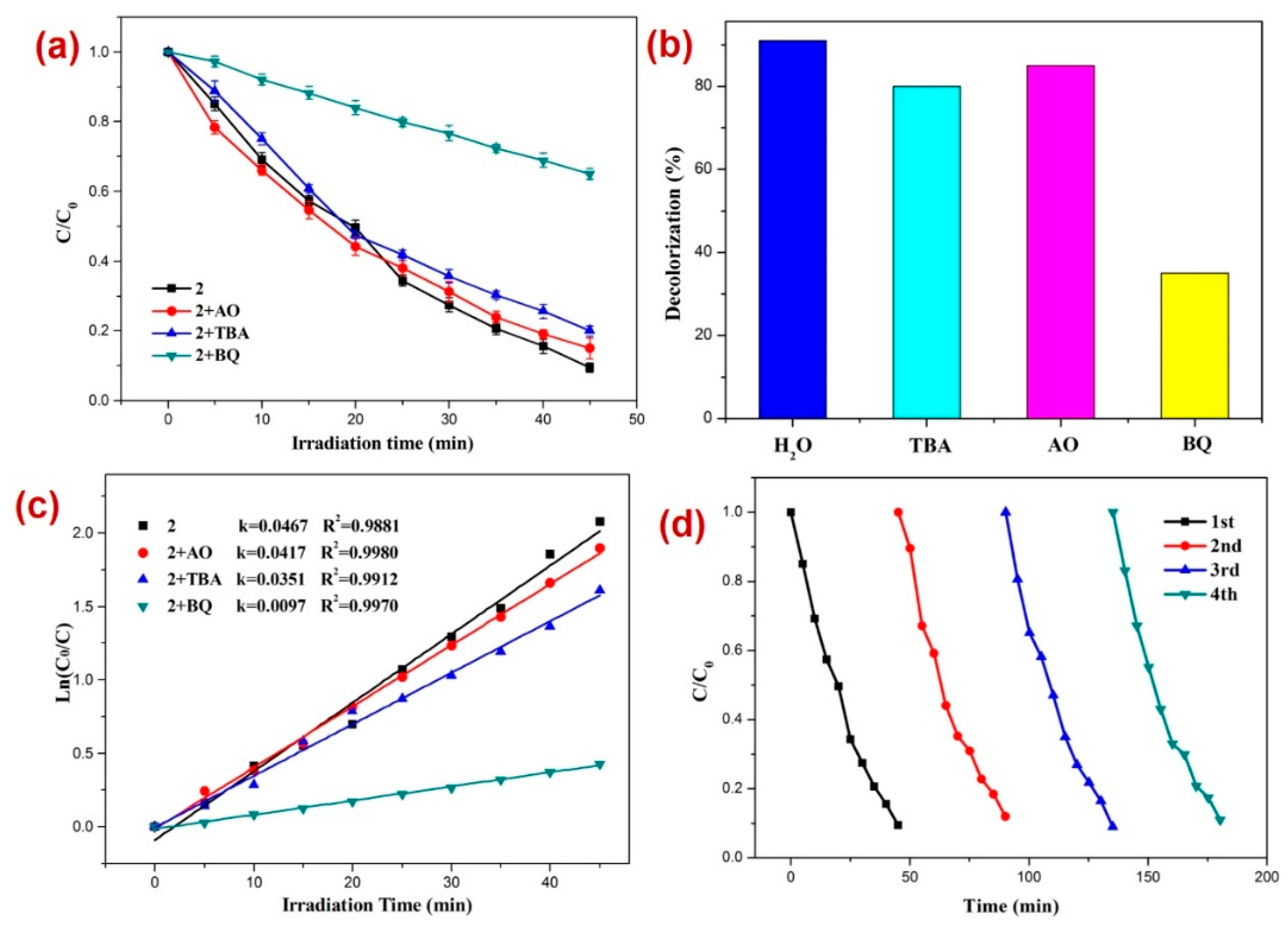
Moreover, to assess the nature of possible active species generated in situ that are responsible for the photodecomposition of DTZ in the presence of photocatalyst 2, radical scavenging experiments were performed. For this purpose, excess tertiary butyl alcohol (TBA) as the •OH scavenger, benzoquinone (BQ) as the superoxide (O2˙−) scavenger, and ammonium oxalate (AO) holes (h+) scavenger were added in three separate reaction mediums comprising DTZ and CP 2 under similar reaction conditions [80,81,82,83,84,85,86,87,88,89] (Figure 5). The experiments suggested that the order of photodecomposition as evident by the percentage decolorization of DTZ in the presence of different scavengers as well as CP 2 follows the order AO > TBA > BQ (Figure 5b,c). This was also evident by the significant decrease in the pseudo-first-order rate constant (k) from 0.0467 min−1 to 0.0097 min−1 when BQ was used as the scavenger (Figure 5c). This suggested that superoxide (O2˙−) is the main active species responsible for the decomposition of DTZ in the course of photocatalysis [90,91,92]. In addition, to appraise the stability and reusability of 2, the photocatalytic performance of CP 2 was tested for its recycled sample (Figure 5d), which indicated no perceptible decline in the photocatalytic activity after four catalytic cycles.
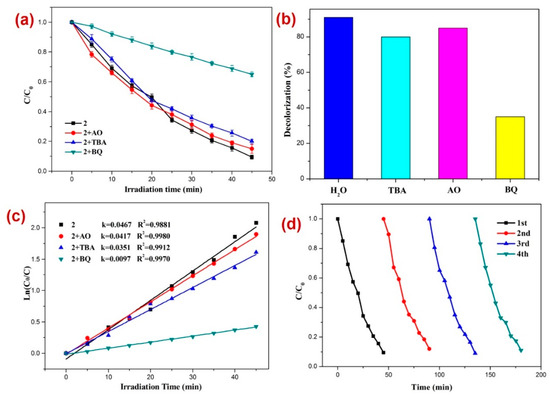
Figure 5.
Results of the radical scavenging experiments displaying (a) the plot of C/C0 vs. irradiation time for the decomposition of DTZ in the presence of photocatalyst 2 and different radical scavengers; (b) bar graphs presenting percentage decolorization of DTZ in the presence of different radical scavengers and 2; (c) results of the kinetic studies; and (d) recycle experiments.
Apart from DTZ, the photocatalytic performances of all three CPs were tested against the photodecomposition of rhodamine B (RhB). In this case also, before performing the photocatalysis, the adsorption–desorption equilibrium was established by keeping the reaction mixture in the dark for 30 min and monitoring the UV-Vis spectra for all three reaction mixtures (Figure 6a–c) and constructing a graph of C/C0 vs. time plot (Figure 6d). Such investigation revealed little alternation in the absorption intensity of EhB within this timespan, thereby indicating the establishment of the adsorption–desorption between RhB and CPs 1–3. In these experiments too, the electronic absorption intensity of the RhB solution deteriorated with time (Figure 6) in the presence of all three photocatalysts 1–3. Moreover, in the timespan of 45 min, the percentage decomposition of RhB in the presence of photocatalysts 1, 2, and 3 was 83.7%, 95.0%, and 81.1%, respectively (Figure 6a–c). However, without these photocatalysts, the percentage photodegradation of RhB under similar reaction conditions was merely 15.1%, which evinced that the CPs can photocatalyze the degradation of RhB as well. Moreover, the percentage photodegradation of RhB in the presence of the metal salts Ni(NO3)2·6H2O, Mn(NO3)2·4H2O, and Cd(ClO4)2·6H2O was 23.3%, 26.1%, and 28.4%, respectively (Figure S9). Hence, the photocatalytic performance pristine metal salts revealed that the photocatalysis is not only arising because of the metal centers of the CPs 1–3; rather, the frameworks of CPs are collectively playing a role in their photocatalytic activities, degrading RhB. In addition, like DTZ photodecomposition, the photodegradation of RhB also followed pseudo-first-order kinetics (Figure 6d), and the rate constants for all three CPs calculated by using the plot of ln(Ct/C0) vs. time (Table 1) suggested that the rate constant k (min−1) for RhB degradation was highest in the presence of CP 2. Hence, the percentage photodegradation of RhB and the rate constant parameters further suggested that amongst all three CPs, Mn(II) CP 2 displayed the best photocatalytic efficiency, which might be because of the variation in the structural environments around the metal centers [77,78,79].
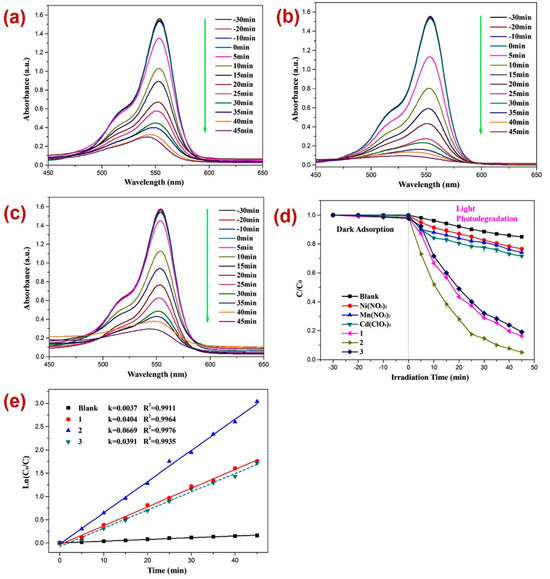
Figure 6.
Photodegradation of RhB in the presence of photocatalysts (a) 1; (b) 2; (c) 3; (d) graph representing the plot of C/C0 vs. irradiation time, where C/C0 represents the concentration ratio of RhB in the presence of metal salts and their corresponding CPs; (e) results of kinetic studies displaying pseudo-first-order kinetics.
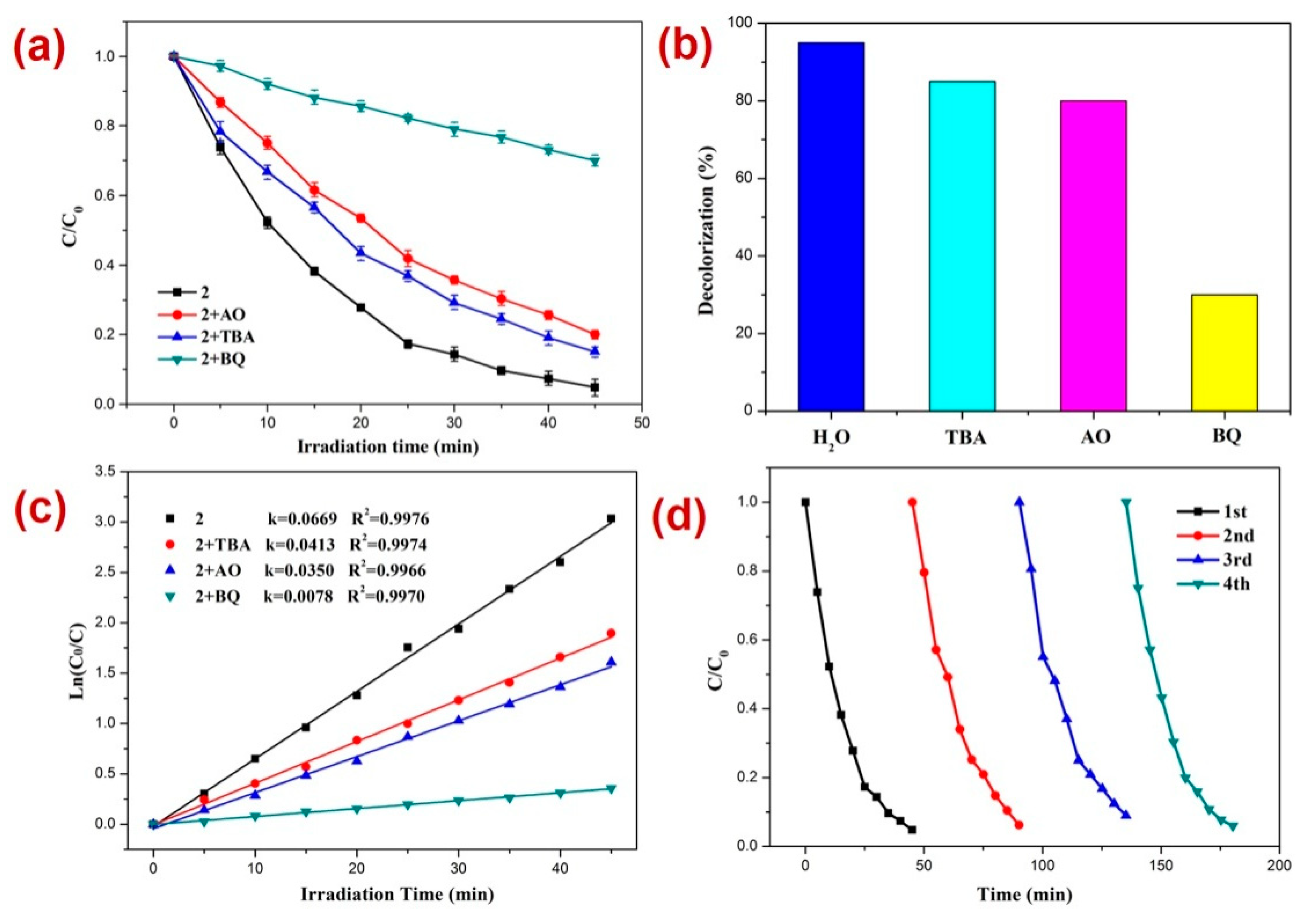
Furthermore, for RhB photodegradation, 2 radical scavenging experiments in the presence of a photocatalyst were performed [80,81,82,83,84,85,86,87,88,89] (Figure 7). The experiments suggested that the order of photodecomposition as evident by the percentage decolorization of DTZ in the presence of different scavengers as well as CP 2 follows the order TBA > AO > BQ (Figure 7b,c). This was also made evident by the significant decrease in the pseudo-first-order rate constant (k) from 0.0669 min−1 to 0.0078 min−1 in the presence of BQ (Figure 7c). Hence, these scavenging experiments indicated that in this case also, the superoxide (O2˙−) radicals are the main active species responsible for RhB decomposition during photocatalysis [90,91,92]. Further, it was observed that similar to the photocatalysis of RhB, in this case also, the CP 2 indicated no perceptible decline in the photocatalytic activity after four catalytic cycles (Figure 7d).
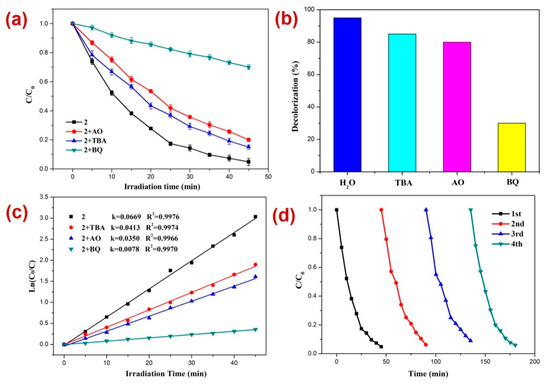
Figure 7.
Results of the radical scavenging experiments displaying (a) the plot of C/C0 vs. irradiation time for the decomposition of RhB in the presence of photocatalyst 2 and different radical scavengers; (b) bar graphs presenting percentage decolorization of RhB in the presence of different radical scavengers and 2; (c) results of the kinetic studies; and (d) recycle experiments.
Furthermore, the PXRD experiments performed for all three recovered CPs after photocatalysis after DTZ and RhB decomposition indicated no significant changes in 2θ positions when compared to the pristine batches of these CPs (Figures S2–S5). However, slight broadening in the peaks in the recycled samples as compared to pristine batches may be attributed to a decrease in the particle size of the CPs during photocatalysis. This suggested good stability of all three photocatalysts during the photodegradation process and authenticated the structural framework integrity of CPs during the photocatalysis process.
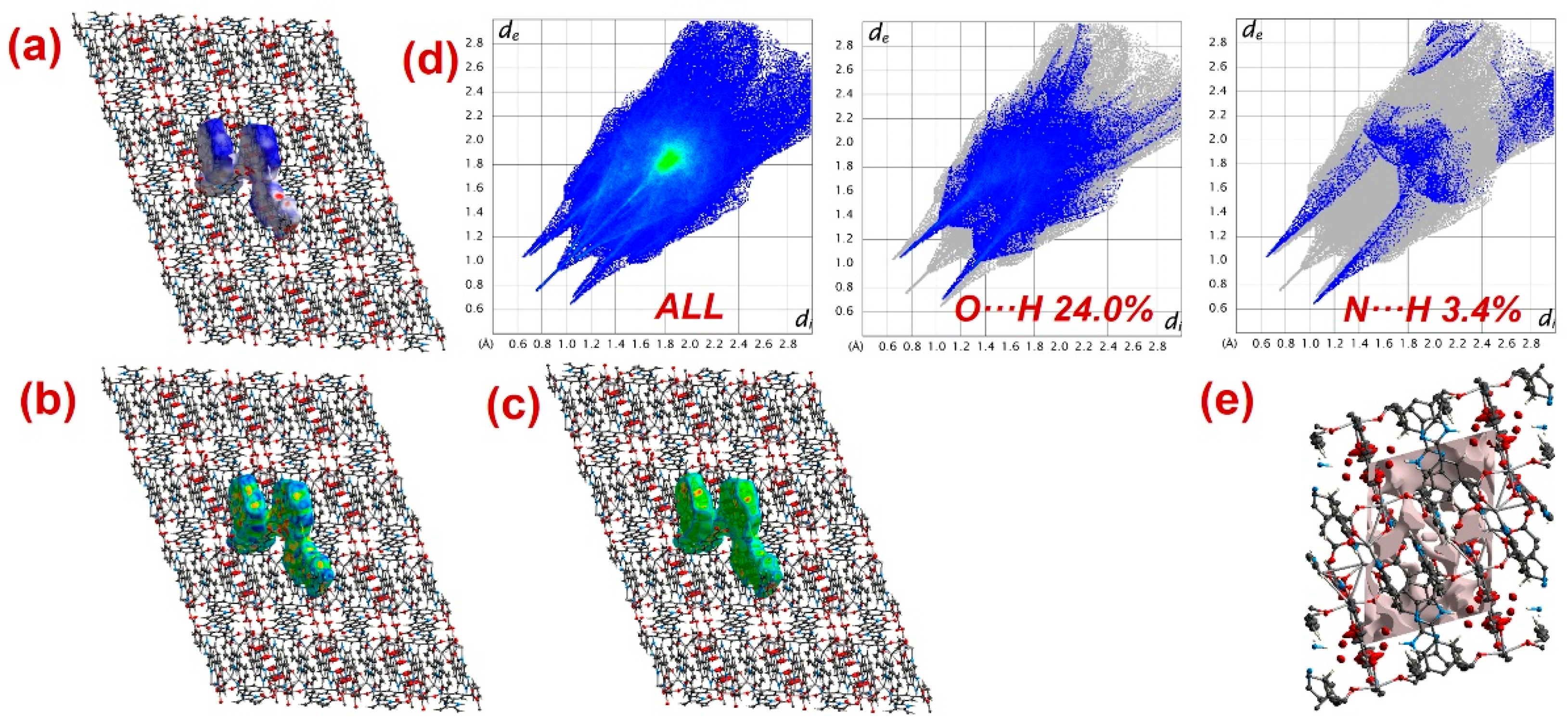
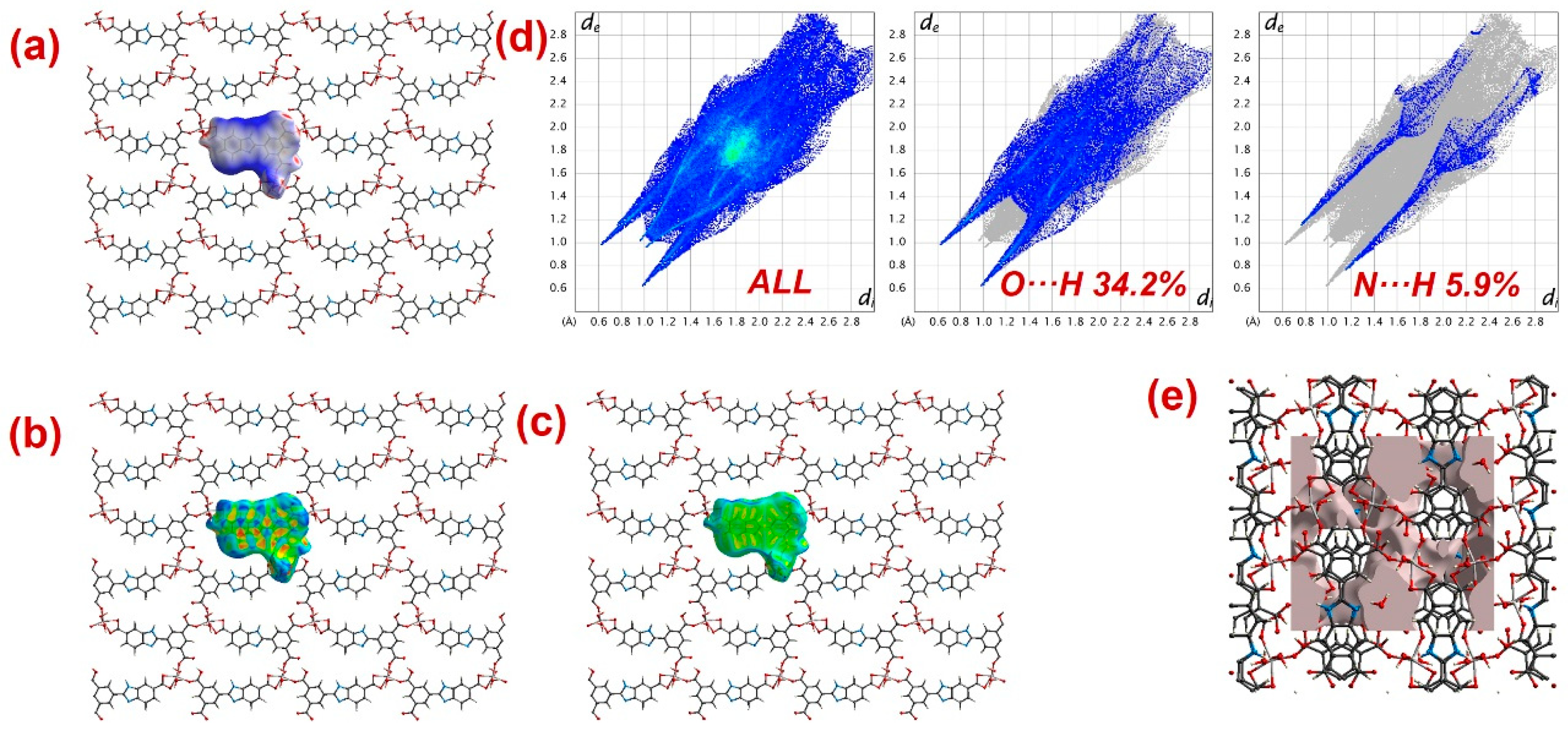
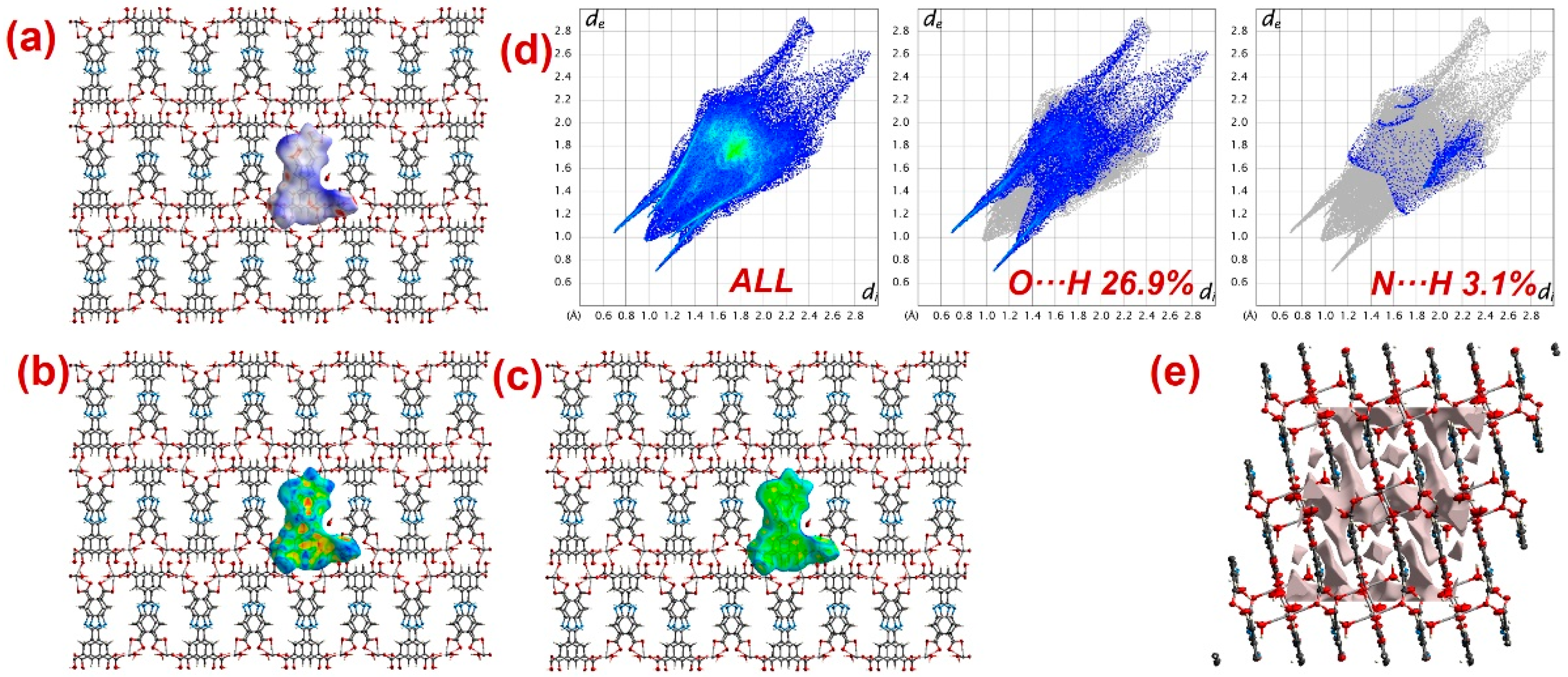
Furthermore, to have information about the interaction between CPs and DTZ/RhB molecules, the molecular Hirshfeld surfaces in the crystal structures of 1–3 were constructed by using the procedure mentioned previously (Figure 8, Figure 9 and Figure 10) [93,94,95]. In all three CPs, the dnorm plots revealed prominent red circular depressions, indicating strong interaction zones, and the comparatively weaker interactions are represented by diffuse red areas (Figure 8a, Figure 9a, and Figure 10a). Additionally, the fingerprint plots (Figure 8d, Figure 9d, and Figure 10d) indicated that amongst all three CPs, CP 2 exhibits maximum O···H and N···H interactions. This indicates that 2 possesses the best capability to form weak interactions with DTZ/RhB molecules for their concomitant photodegradation. In addition, the crystal lattice void spaces for all three CPs were calculated (Figure 8e, Figure 9e, and Figure 10e) within a crystal radius of 10.0 Å. The calculation revealed lattice void volumes of 367.32 Å3, 682.64 Å3, and 304.17 Å3 in 1, 2, and 3, respectively, with void areas of 75.31 Å2, 1032.63 Å2, and 847.93 Å2, in CPs 1, 2, and 3, respectively. Hence, these calculations authenticated the microporous nature of these CPs. Furthermore, the calculated void area and volume indicate that DTZ/RhB molecules in 2 could be best absorbed and undergo decomposition. This might be the reason for the superior performance of CP 2 as a photocatalyst for the decomposition of DTZ/RhB pollutants. Furthermore, another reason for the better performance of 2 is the presence of the co-ligand 1,10-phenanthroline, which can attune the electron communication of this CP in an opposite manner than the other two CPs devoid of any co-ligand.
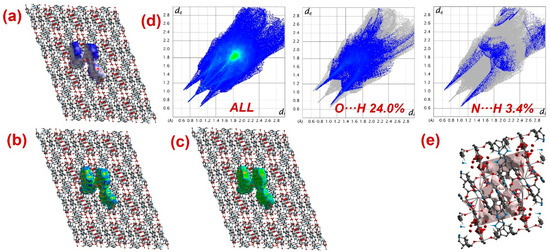
Figure 8.
Results of the Hirshfeld surface analysis for 1 presented as (a) dnorm; (b) shape index; (c) curvedness; (d) full and partial fingerprint plots; (e) void volume calculation results.
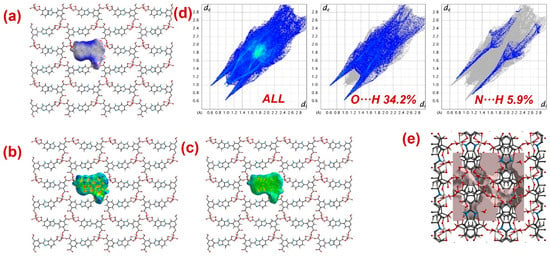
Figure 9.
Results of the Hirshfeld surface analysis for 2 presented as (a) dnorm; (b) shape index; (c) curvedness; (d) full and partial fingerprint plots; (e) void volume calculation results.
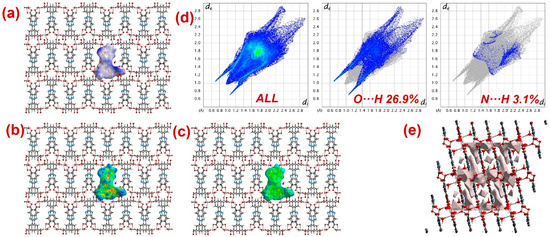
Figure 10.
Results of the Hirshfeld surface analysis for 3 presented as (a) dnorm; (b) shape index; (c) curvedness; (d) full and partial fingerprint plots; (e) void volume calculation results.
Hence, the plausible photocatalysis takes place as follows: On irradiation, the electrons (e−) in these catalysts get photo-excited from the valence band (VB) → conduction band (CB) with concomitant generation of an equivalent number of holes (h+) in VB. The superoxide radicals (O2•−) thereafter are produced by the reaction of molecular oxygen with e− and also by oxidizing hydroxide ions by h+. These reactive oxygen species collectively decompose DTZ/RhB molecules in the aqueous medium.
3. Conclusions
In the presented work, three new coordination polymers comprising Ni(II), Mn(II), and Cd(II) metal centers have been synthesized using 2-(3,5-dicarboxyphenyl)-6-carboxybenzimidazole and 1,10-phenanthroline as co-ligands in a Mn(II)-based coordination polymer. These CPs were used as photocatalysts against the photodecomposition of dinitrazole (DTZ) antibiotics and rhodamine B (RhB) dye. The photocatalytic experiments indicated that amongst all three CPs, the Mn(II)-based CP displayed the best photocatalytic performance and degraded 91.1% of DTZ and 95.0% of RhB within 45 min. The plausible photocatalytic degradation mechanism has been proposed with the help of trapping experiments, which suggested that the presence of superoxide radicals play a pivotal role in the decomposition of Mn(II)-CP and assisted decomposition of DTZ and RhB. In addition, better performance of Mn(II)-CP was accredited to the presence of the co-ligand 1,10-phenanthroline, which can attune the electron communication of this CP in an opposite manner than the other two CPs devoid of any co-ligand. Hence, it can be concluded that the mixed ligand strategy engenders CPs having attuned electron communication with better antibiotic/dye adsorption and radical generation properties. Such CPs could be exploited as photocatalysts for the safe and sustainable degradation of aromatic dyes and antibiotics in an aqueous medium.
4. Experimental
4.1. Materials and Method
All chemicals were of analytical grade and used without additional purification. PXRD measurements were performed on a Bruker ADVANCE X-ray diffractometer employing Cu-Kα radiation (λ = 1.5418 Å,) (Bruker, Billerica, MA, USA), while FT-IR spectral data in KBr discs were collected on a Nicolet Impact 750 FTIR spectrometer.
4.2. Syntheses
4.2.1. Synthesis of 1
A mixture of Ni(NO3)2·6H2O (0.0291 g, 0.1 mmol), 2-(3,5-dicarboxyphenyl)-6-carboxybenzimidazole (0.0326 g, 0.1 mmol), and deionized water (10 mL) was stirred for 30 min in air. The resulting solution was placed in a Teflon-lined stainless-steel vessel (25 mL) and heated to 180 °C for 72 h. The solution was then cooled to room temperature at a rate of 5 °C h−1, and green block crystals of 1 were obtained. (Yield 45% based on Ni). Anal. (%) calcd for 1: C, 42.36; H, 3.49; N, 6.18. Found: C, 42.33; H, 3.45; N, 6.20. IR(cm−1): 3350 m, 2361 w, 1680 v, 1500 v, 1400 v, 1296 w, 1375 s, 1235 s, 841 m, 757 m, 709 w.
4.2.2. Synthesis of 2
A mixture of Mn(NO3)2·4H2O (0.0251 g, 0.1 mmol), 2-(3,5-dicarboxyphenyl)-6-carboxybenzimidazole (0.0326 g, 0.1 mmol), 1,10-phenanthroline (0.0180 g, 0.1 mmol), and deionized water (10 mL) was stirred for 30 min in air. The resulting solution was placed in a Teflon-lined stainless-steel vessel (25 mL) and heated to 180 °C for 72 h, and pink block crystals of 2 were obtained. (Yield 55% based on Mn). Anal. (%) calcd for 2: C, 54.12; H, 3.00; N, 9.02. Found: C, 54.20; H, 2.96; N, 9.08. IR(cm−1): 3432 m, 3077 w, 1625 v, 1565 v, 1513 s, 1384 s, 1165 m, 1090 s, 787 w, 721 w.
4.2.3. Synthesis of 3
A mixture of Cd(ClO4)2·6H2O (0.0420 g, 0.1 mmol), 2-(3,5-dicarboxyphenyl)-6-carboxybenzimidazole (0.0326 g, 0.1 mmol), and deionized water (10 mL) was stirred for 30 min in air. The resulting solution was placed in a Teflon-lined stainless-steel vessel (25 mL) and heated to 160 °C for 72 h, and yellow block crystals of 3 were obtained. (Yield 55% based on Cd). Anal. (%) calcd for 3: C, 40.66; H, 2.56; N, 5.93. Found: C, 40.70; H, 2.60; N, 5.89. IR (cm−1): 3420 m, 1621 v, 1568 v, 1392 s, 1110 s, 787 m, 710 s.
4.3. X-ray Crystallography
The single crystal X-ray diffraction data were recorded on a Bruker SMART APEX diffractometer (Billerica, MA, USA) using monochromatic Mo-Kα radiation (λ = 0.71073 Å) using the ɷ-scan method. The structure solutions were performed employing a direct method (SHLEXS-2014), and refinement was executed using a full-matrix least-square procedure based on F2 (SHELXL-2014) [96]. Using a riding model, all hydrogen atoms were geometrically created and refined isotropically, while anisotropic displacement parameters were used for the refinement of all non-hydrogen atoms. The crystallographic information and relevant geometrical parameters for 1–3 are shown in Tables S1–S7. CCDC numbers: 2289725 (1); 2289726 (2); and 2289727 (3)
4.4. Photocatalytic Method
For assessment of the photocatalytic properties, the reaction mixture was prepared by adding finely powdered CP (25 mg) to 50 mL aqueous solution of DTZ/RhB (20 μmol/L). This mixture was agitated in the dark for 30 min to attain adsorption–desorption equilibrium. Thereafter, the reaction mixture was placed in a UV-400 type photochemical reactor equipped with a 400 W mercury lamp (λ = 365 nm) (Shanghai Libi Vacuum Technology Co., Shanghai, China) for photocatalysis. During photocatalysis, batches of 5.0 mL were isolated after every 5 min and the DTZ/RhB solution separated from CP by centrifugation, and then the UV-Vis spectra were recorded. Furthermore, control experiments were executed under similar reaction conditions but without incorporation of photocatalysts in the solutions of DTZ/RhB.
4.5. Computational Details
Molecular Hirshfeld surfaces in the crystal structures of all three CPs were constructed by using the procedure mentioned previously [93,94,95].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28217318/s1.
Author Contributions
W.Z. and X.W.: methodology, investigation, visualization, writing—original draft. W.W., A.M. and Z.D.: methodology, investigation, visualization. O.D. and M.R.J.: visualization, investigation, review and editing. M.M.: fund and investigation. Y.W., O.P. and Y.P.: supervision, writing–review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge financial assistance from the Natural Science Foundation of Sichuan Province (2022NSFSC1241), the Scientific Research Foundation for Returned Scholars from Sichuan University of Science and Engineering (No. 2020RC41), Student’s Platform for Innovation and Entrepreneurship Training Program (CX2023051), and the Opening Project of Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education (LYJ2101). Mohd. Muddassir is grateful to Researchers Supporting Project number (RSP2023R141), King Saud University, Riyadh, Saudi Arabia, for financial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- He, X.; Wu, M.; Ao, Z.; Lai, B.; Zhou, Y.; An, T.; Wang, S. Metal–organic frameworks derived C/TiO2 for visible light photocatalysis: Simple synthesis and contribution of carbon species. J. Hazard. Mater. 2020, 403, 124048. [Google Scholar] [CrossRef] [PubMed]
- Ryoji, A.; Takeshi, M.; Hiroshi, I.; Takeshi, O. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Kansal, S.K. Synthesis of ZnS/CQDs nanocomposite and its application as a photocatalyst for the degradation of an anionic dye, ARS. Superlattices Microstruct. 2016, 98, 86–95. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Ma, Z. Flower-like Ag2O/Bi2MoO6 p-n heterojunction with enhanced photocatalytic activity under visible light irradiation. J. Mol. Catal. A Chem. 2016, 424, 37–44. [Google Scholar] [CrossRef]
- Wang, B.; Feng, W.; Zhang, L.; Zhang, Y.; Huang, X.; Fang, Z.; Liu, P. In situ construction of a novel Bi/CdS nanocomposite with enhanced visible light photocatalytic performance. Appl. Catal. B Environ. 2017, 206, 510–519. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, X.; Zhang, N.; Zhang, J.; Zhang, R.; Liu, Y.; Fang, Y. A visible-light driven Bi2S3@ ZIF-8 core–shell heterostructure and synergistic photocatalysis mechanism. Dalton Trans. 2018, 47, 684–692. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; O’keeffe, M.; Yaghi, O.M. Topological Analysis of Metal–Organic Frameworks with Polytopic Linkers and/or Multiple Building Units and the Minimal Transitivity Principle. Chem. Rev. 2013, 114, 1343–1370. [Google Scholar] [CrossRef]
- Huang, L.; Liu, B. Synthesis of a novel and stable reduced graphene oxide/MOF hybrid nanocomposite and photocatalytic performance for the degradation of dyes. RSC Adv. 2016, 6, 17873–17879. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Liu, S.; Sun, Y.; Xu, Y.-J. Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Gómez, V.; Larrechi, M.; Callao, M. Kinetic and adsorption study of acid dye removal using activated carbon. Chemosphere 2007, 69, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Z. Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution. J. Hazard. Mater. 2006, 136, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.; Rauf, N. Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 2006, 63, 1842–1848. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Shao, Q.; Peng, X.; Guo, Z. Magnetic Nanocarbon Adsorbents with Enhanced Hexavalent Chromium Removal: Morphology Dependence of Fibrillar vs. Particulate Structures. Ind. Eng. Chem. Res. 2017, 56, 10689–10701. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Dong, H.; Chen, X.; Leng, L.; Wu, Z.; Peng, L. In situ synthesis of In2S3@MIL-125(Ti) core–shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl. Catal. B Environ. 2016, 186, 19–29. [Google Scholar] [CrossRef]
- Ma, D.Y.; Li, Z.; Zhu, J.X.; Zhou, Y.P.; Chen, L.L.; Mai, X.F.; Liufu, M.L.; Wu, Y.B.; Li, Y.W. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Ke, F.; Pan, A.; Liu, J.; Liu, X.; Yuan, T.; Zhang, C.; Fu, G.; Peng, C.; Zhu, J.; Wan, X. Hierarchical camellia-like metal–organic frameworks via a bimetal competitive coordination combined with alkaline-assisted strategy for boosting selective fluoride removal from brick tea. J. Colloid Interface Sci. 2023, 642, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Atsushi, K.; Hiroshi, K.; Hiroshi, N.; Lucia, C.; Davide, M.P.; Gianfranco, C.; Kenichi, K.; Masaki, T.; Hiroko, S.; Masami, S.; et al. Super flexibility of a 2D Cu-based porous coordination framework on gas adsorption in comparison with a 3D framework of identical composition: Framework dimensionality-dependent gas adsorptivities. J. Am. Chem. Soc. 2011, 133, 10512–10522. [Google Scholar]
- Liu, K.-G.; Rouhani, F.; Moghanni-Bavil-Olyaei, H.; Wei, X.-W.; Gao, X.-M.; Li, J.-Z.; Yan, X.-W.; Hu, M.-L.; Morsali, A. A conductive 1D high-nucleus silver polymer as a brilliant non-hybrid supercapacitor electrode. J. Mater. Chem. A 2020, 8, 12975–12983. [Google Scholar] [CrossRef]
- Sakhapov, I.F.; Zagidullin, A.A.; Dobrynin, A.B.; Litvinov, I.A.; Yakhvarov, D.G.; Bondarenko, M.A.; Novikov, A.S.; Fedin, V.P.; Adonin, S.A. Crystal Structures of 3, 3′, 5, 5′-Tetrabromo-4, 4′-bipyridine and Co(II) Coordination Polymer Based Thereon. Crystals 2023, 13, 704. [Google Scholar] [CrossRef]
- Zhao, X.; He, X.; Hou, A.; Cheng, C.; Wang, X.; Yue, Y.; Wu, Z.; Wu, H.; Liu, B.; Li, H.; et al. Growth of Cu2O nanoparticles on two-dimensional Zr–ferrocene–metal–organic framework nanosheets for photothermally enhanced chemodynamic antibacterial therapy. Inorg. Chem. 2022, 61, 9328–9338. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.T.; Xu, Z.J.; Liao, D.H.; Jiang, Y.H.; Pu, H.J.; Wu, Z.Y.; Xu, X.T.; Zhao, Z.; Liu, J.Q.; Lu, X.W.; et al. An H2S-BMP6 Dual-Loading System with Regulating Yap/Taz and Jun Pathway for Synergistic Critical Limb Ischemia Salvaging Therap. Adv. Healthc. Mater. 2023, e2301316. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Wang, H.; Liu, X.; Zhou, X.; Wang, F. DNAzyme-loaded metaleorganic frameworks (MOFs) for self-sufficient gene therapy. Angew. Chem. Int. Ed. 2019, 58, 7380–7384. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.B.; Wang, S.Y.; Jiang, C.J.; Zheng, M.B.; Bai, Z.; Srivastava, D.; Kumar, A.; Liu, J.Q. Synthesis and characterized three Zn(II)-based mixed geometry coordination polymers and photocatalytic activity against dyes. Dye. Pigment. 2023, 219, 111596. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, W.; Lian, D.; Wang, Y.; Han, J.; Wang, Y. HRP@ZIF-8/DNA hybrids: Functionality integration of ZIF-8 via biomineralization and surface absorption. ACS Sustain. Chem. Eng. 2019, 7, 14611–14620. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.; Huang, S.; Ye, K.; Jiang, Y.; Liu, J.; Liu, J.; Lu, X.; Li, B. A metal-organic framework-based immunomodulatory nanoplatform for anti-atherosclerosis treatment. J. Control. Release 2023, 354, 615–625. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Peng, Z.X.; Peng, Y.Q.; Li, B.; Pan, Y.; Ouyang, Q.; Sakiyama, H.; Muddassir, M.; Liu, J.Q. Construction of Fe-doped ZIF-8/DOX nanocomposites for ferroptosis strategy in the treatment of breast cancer. J. Mater. Chem. B 2023, 11, 6335–6345. [Google Scholar] [CrossRef]
- Qin, J.-H.; Zhang, H.; Sun, P.; Huang, Y.-D.; Shen, Q.; Yang, X.-G.; Ma, L.-F. Ionic liquid induced highly dense assembly of porphyrin in MOF nanosheets for photodynamic therapy. Dalton Trans. 2020, 49, 17772–17778. [Google Scholar] [CrossRef]
- Feng, J.; Xu, Z.; Dong, P.; Yu, W.; Liu, F.; Jiang, Q.; Wang, F.; Liu, X. Stimuliresponsive multifunctional metaleorganic framework nanoparticles for enhanced chemo-photothermal therapy. J. Mater. Chem. B 2019, 7, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qu, X.; Chai, J.; Tong, C.; Fan, Y.; Wang, L. Stable coordination polymers with linear dependence color tuning and luminescent properties for detection of metal ions and explosives. Dye. Pigment. 2019, 170, 107583. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Zhao, Y.; Chen, K.; Guo, J.-H.; Wang, P.; Wu, H.; Sun, W.-Y. Cucurbit[6]uril-based supramolecular assemblies incorporating metal complexes with multiaromatic ligands as structure-directing agent for detection of aromatic amines and nitroaromatic compounds. Sens. Actuators B Chem. 2018, 282, 844–853. [Google Scholar] [CrossRef]
- Qin, J.-H.; Qin, W.-J.; Xiao, Z.; Yang, J.-K.; Wang, H.-R.; Yang, X.-G.; Li, D.-S.; Ma, L.-F. Efficient energy-transfer-induced high photoelectric conversion in a dye-encapsulated ionic pyrene-based metal–organic framework. Inorg. Chem. 2021, 60, 18593–18597. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, Y.; Shi, X.; Lu, Z.; Ren, J.; Wang, Z.; Xu, J.; Fan, Y.; Wang, L. Eu-MOF and its mixed-matrix membranes as a fluorescent sensor for quantitative ratiometric pH and folic acid detection, and visible fingerprint identifying. Inorg. Chem. Front. 2021, 8, 4924–4932. [Google Scholar] [CrossRef]
- Wang, S.; Ouyang, L.; Deng, G.; Deng, Z.; Wang, S. DNA adsorption on nanoscale zeolitic imidazolate framework-8 enabling rational design of a DNA-based nanoprobe for gene detection and regulation in living cells. RSC Adv. 2020, 10, 31012–31021. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Feng, Y.; Zhang, K.; Chen, N.; Fang, H.; Li, Z. Series of d10 complexes based on sulfamethoxazole: Auxiliary ligand induces structure diversity, luminescence and antibacterial properties. J. Solid State Chem. 2021, 302, 122351. [Google Scholar] [CrossRef]
- Feng, X.; Feng, Y.Q.; Chen, J.J.; Wang, L.Y.; Guo, J.Z. Reticular three-dimensional 3d–4f frameworks constructed through substituted imidazole-dicarboxylate: Syntheses, luminescence and magnetic properties study. Dalton Trans. 2015, 44, 804–816. [Google Scholar] [CrossRef]
- Hu, M.-L.; Joharian, M.; Razavi, S.A.A.; Morsali, A.; Wu, D.-Z.; Tehrani, A.A.; Wang, J.; Junk, P.C.; Guo, Z.-F. Phenolic nitroaromatics detection by fluorinated metal-organic frameworks: Barrier elimination for selective sensing of specific group of nitroaromatics. J. Hazard. Mater. 2020, 406, 124501. [Google Scholar] [CrossRef]
- Qin, J.-H.; Zhang, J.-R.; Xiao, Z.; Wu, Y.-P.; Xu, H.-M.; Yang, X.-G.; Ma, L.-F.; Li, D.-S. Topology- and Guest-Dependent Photoelectric Conversion of 2D Anionic Pyrene-Based Metal–Organic Framework. Cryst. Growth Des. 2022, 22, 4018–4024. [Google Scholar] [CrossRef]
- Li, G.; Wang, T.; Zhou, S.; Wang, J.; Lv, H.; Han, M.; Singh, D.P.; Kumar, A.; Jin, J. New highly luminescent 3D Tb(III)-MOF as selective sensor for antibiotics. Inorg. Chem. Commun. 2021, 130, 108756. [Google Scholar] [CrossRef]
- Llabrés i Xamena, F.X.; Corma, A.; Garcia, H. Applications for metal-organic frameworks (MOFs) as quantum dot semiconductors. J. Phys. Chem. C 2007, 111, 80–85. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, X.; Qu, K.; Huang, Z.; Liu, S.; Dong, M.; Guo, Z. Bimetallic metal-organic frameworks anchored corncob-derived porous carbon photocatalysts for synergistic degradation of organic pollutants. Chemosphere 2020, 259, 127389. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Sigalat, J.; Bradshaw, D. Synthesis and applications of metal-organic framework–quantum dot (QD@MOF) composites. Co-ord. Chem. Rev. 2016, 307, 267–291. [Google Scholar] [CrossRef]
- Jin, J.-C.; Yang, M.; Zhang, Y.-L.; Dutta, A.; Xie, C.-G.; Kumar, A. Integration of mixed ligand into a multivariate metal-organic framework for enhanced UV-light photocatalytic degradation of Rhodamine B. J. Taiwan Inst. Chem. Eng. 2021, 129, 410–417. [Google Scholar] [CrossRef]
- Zhuang, J.; Kuo, C.H.; Chou, L.Y.; Liu, D.Y.; Weerapana, E.; Tsung, C.K. Optimized Metal-organic framework nanospheres for drug delivery: Evaluation of small-molecule encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Lin, Z.; Liao, D.; Jiang, C.; Song, H.; Nezamzadeh-Ejhieh, A.; Zheng, M.; Yuan, H.; Lu, C. Current status and prospect of MIL-based MOF materials for biomedicine applications. RSC Med. Chem. 2023, 14, 1914–1933. [Google Scholar]
- Zhang, X.; Zhang, W.; Xiang, R.; Lan, L.; Dong, X.; Sakiyama, H.; Muddassir, M. Auxiliary linkers-induced assembly of two 2D Co(II)-based coordination polymers with different interpenetrating fashion: Structure and magnetism. Polyhedron 2023, 244, 116625. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloy. Compd. 2021, 897, 163178. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, C.J.; Feng, Q.Z.; Zhai, J.J.; Qi, S.S.; Zhong, A.G.; Chu, M.M.; Xu, D.Q. Copper-catalyzed asymmetric 1, 6-conjugate addition of in situ generated para-quinone methides with β-ketoesters. Chem. Commun. 2022, 58, 6653–6656. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, J.; Lou, Y.; Wu, H.; Qi, X.; Yang, J.; Zhong, A. Chemoselective hydroborative reduction of nitro motifs using a transition-metal-free catalyst. Org. Chem. Front. 2021, 8, 4554–4559. [Google Scholar] [CrossRef]
- Wang, L.-H.; Chen, X.-J.; Ye, D.-N.; Liu, H.; Chen, Y.; Zhong, A.-G.; Li, C.-Z.; Liu, S.-Y. Pot- and atom-economic synthesis of oligomeric non-fullerene acceptors via C–H direct arylation. Polym. Chem. 2022, 13, 2351–2361. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhang, X.; Li, D.; Dong, X.; Qi, N.; Shang, Y.; Sakiyamad, H.; Afzal, M.; Alarifi, A. Temperature modulation on functional coordination polymers with tetracarboxylate linker: Syntheses, structural traits, and magnetism. J. Mol. Struct. 2023, 1291, 136074. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Wu, J.; Han, Y.; Li, B.; Cai, S.; Huang, H.; Singh, A.; Kumar, A.; Liu, J. Rational synthesis of a luminescent uncommon (3, 4, 6)-c connected Zn (II) MOF: A dual channel sensor for the detection of nitroaromatics and ferric ions. Dalton Trans. 2018, 47, 9627–9633. [Google Scholar] [CrossRef]
- Jin, J.-C.; Wu, J.; Liu, W.-C.; Ma, A.-Q.; Liu, J.-Q.; Singh, A.; Kumar, A. A new Zn(ii) metal–organic framework having 3D CdSO4 topology as luminescent sensor and photocatalyst for degradation of organic dyes. New J. Chem. 2018, 42, 2767–2775. [Google Scholar] [CrossRef]
- Jin, J.-C.; Wu, J.; He, Y.-X.; Li, B.-H.; Liu, J.-Q.; Prasad, R.; Kumar, A.; Batten, S.R. A 3D luminescent Zn(ii) MOF for the detection of high explosives and the degradation of organic dyes: An experimental and computational study. CrystEngComm 2017, 19, 6464–6472. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qin, T.R.; Xiang, R.F.; Dong, X.Y.; Muddassir, H.S.M.; Pan, Y. Impact of N-donor auxiliary ligands on three new Co(II)-based coordination polymers with symmetrical tetracarboxylate ligands: Magnetism study. New J. Chem. 2023, 46, 11623–11631. [Google Scholar]
- Gu, J.Z.; Cai, Y.; Wen, M.; Shi, Z.F.; Alexander, M.K. A new series of Cd (II) metal–organic architectures driven by soft ether-bridged tricarboxylate spacers: Synthesis, structural and topological versatility, and photocatalytic properties. Dalton Trans. 2018, 47, 14327–14339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Qin, T.R.; Xiang, R.F.; Dong, X.Y.; Muddassir, H.S.M.; Afzal, M.; Alarifi, A. Structures and magnetic studies of four new Ni(II) coordination polymers built by symmetrical tetracarboxylate and N-donor linkers. New J. Chem. 2023. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Liang, X.-X.; Cai, Y.; Wu, J.; Shi, Z.-F.; Kirillov, A.M. Hydrothermal assembly, structures, topologies, luminescence, and magnetism of a novel series of coordination polymers driven by a trifunctional nicotinic acid building block. Dalton Trans. 2017, 46, 10908–10925. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.E.; Gang, C.; Zeller, M.; Fabini, D.H.; Oertel, C.M. Ligand-Induced Variations in Symmetry and Structural Dimensionality of Lead Oxide Carboxylates. Cryst. Growth Des. 2017, 17, 1574–1582. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Y.; Bai, C.; Guan, Y.; Pan, Y.; Hu, H.-M. Series of coordination polymers with multifunctional properties for nitroaromatic compounds and CuII sensing. J. Solid State Chem. 2020, 288, 121381. [Google Scholar] [CrossRef]
- Biçer, F.A.; Yeşilel, O.Z. Synthesis, Characterization and the Effect of the Auxiliary Ligands on the Dimensionality of Two Cobalt(II)-Fumarate Coordination Polymers with Bis(Imidazole) Ligands. J. Struct. Chem. 2023, 64, 1423–1434. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Z.; He, C.; Tong, X.; Li, Y.; Niu, X.; Zhang, H. UiO-66(Zr) coupled with Bi2MoO6 as photocatalyst for visible-light promoted dye degradation. J. Colloid Interface Sci. 2017, 497, 126–133. [Google Scholar] [CrossRef]
- Brechin, E.K.; Gould, R.O.; Harris, S.G.; Parsons, S.; Winpenny, R.E.P. Four cubes and an octahedron: A nickel-sodium supracage assembly. J. Am. Chem. Soc. 1996, 118, 11293–11294. [Google Scholar] [CrossRef]
- Zaguzin, A.S.; Mahmoudi, G.; Zubkov, F.I.; Bondarenko, M.A.; Zherebtsov, D.A.; Val’chuk, K.S.; Abramov, P.A.; Fedin, V.P.; Adonin, S.A. Heteroligand Zn (II) Metal-Organic Frameworks Based on 4-Substituted 4, 2′: 6′, 4″-Terpyridine Derivatives and Terephthalates. Russ. J. Coord. Chem. 2023, 49, 414–419. [Google Scholar] [CrossRef]
- Lin, C.-L.; Chen, Y.-F.; Qiu, L.-J.; Zhu, B.; Wang, X.; Luo, S.-P.; Shi, W.; Yang, T.-H.; Lei, W. Synthesis, structure and photocatalytic properties of coordination polymers based on pyrazole carboxylic acid ligands. CrystEngComm 2020, 22, 6847–6855. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Gu, J.; Kirillova, M.V.; Kirillov, A.M. Introducing a flexible tetracarboxylic acid linker into functional coordination polymers: Synthesis, structural traits, and photocatalytic dye degradation. New J. Chem. 2020, 44, 16082–16091. [Google Scholar] [CrossRef]
- Yunxia, Y.; Shiying, X.; Haixia, Z.; Huihui, N.; Wenjing, D.; Xiangxiang, W. Synthesis, structure, and properties of complexes based on 3, 5-di-(Triazole-1-yl)-Benzoic acid ligands. J. Solid State Chem. 2020, 284, 121180. [Google Scholar] [CrossRef]
- Wei, X.-J.; Liu, D.; Li, Y.-H.; Cui, G.-H. New 2D and 3D Cd(II) coordination polymers from aromatic dicarboxylate and 1,3-bis(5,6-dimethylbenzimidazol-1-yl)-2-propanol ligands: Syntheses, structures, photocatalytic, and luminescence sensing properties. J. Sol. State Chem. 2019, 272, 138–147. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. Multifunctional NH2-mediated zirconium metal–organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr(vi). Dalton Trans. 2013, 42, 13649–13657. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Li, C.X.; Song, M.; Ren, Y.L.; Huang, R.D. The electrochemical properties, nitrogen adsorption, and photocatalytic activities of three 3D metal–organic frameworks bearing the rigid terphenyl tetracarboxylates ligands. CrystEngComm 2016, 18, 3086–3094. [Google Scholar] [CrossRef]
- Wang, Z.X.; Tian, H.X.; Ding, J.G.; Li, B.L.; Wu, B. A Co-MOF with a (4,4)-connected binodal two-dimensional topology: Synthesis, structure and photocatalytic properties. Acta Crystallogr C Struct Chem. 2020, 76, 23–29. [Google Scholar] [CrossRef]
- Yang, H.; Liu, T.; Cao, M.; Li, H.; Gao, S.; Cao, R. A water-insoluble and visible light induced polyoxometalate-based photocatalyst. Chem. Commun. 2010, 46, 2429–2431. [Google Scholar] [CrossRef]
- Meng, J.-X.; Lu, Y.; Li, Y.-G.; Fu, H.; Wang, E.-B. Controllable self-assembly of four new metal–organic frameworks based on different phosphomolybdate clusters by altering the molar ratio of H3PO4 and Na2MoO4. CrystEngComm 2011, 13, 2479–2486. [Google Scholar] [CrossRef]
- Guo, J.; Yang, J.; Liu, Y.Y.; Ma, J.F. Two novel 3D metal–organic frameworks based on two tetrahedral ligands: Syntheses, structures, photoluminescence and photocatalytic properties. CrystEngComm 2012, 14, 6609–6617. [Google Scholar] [CrossRef]
- Zhu, S.-R.; Liu, P.-F.; Wu, M.-K.; Zhao, W.-N.; Li, G.-C.; Tao, K.; Yi, F.-Y.; Han, L. Enhanced photocatalytic performance of BiOBr/NH2-MIL-125(Ti) composite for dye degradation under visible light. Dalton Trans. 2016, 45, 17521–17529. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; He, X.; Zhong, A.; Liu, S.; Zhao, D. What dictates alkane isomerization? A combined density functional theory and information-theoretic approach study. Theor. Chem. Accounts 2023, 142, 78. [Google Scholar] [CrossRef]
- Cao, X.; Rong, C.; Zhong, A.; Lu, T.; Liu, S. Molecular acidity: An accurate description with information-theoretic approach in density functional reactivity theory. J. Comput. Chem. 2017, 39, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ding, J.; Huang, C.; Li, M.; Hou, H.; Fan, Y. Polynuclear CdII Polymers: Crystal Structures, Topologies, and the Photodegradation for Organic Dye Contaminants. Cryst. Growth Des. 2014, 14, 3035–3043. [Google Scholar] [CrossRef]
- Wen, L.; Zhao, J.; Lv, K.; Wu, Y.; Deng, K.; Leng, X.; Li, D. Visible-Light-Driven Photocatalysts of Metal–Organic Frameworks Derived from Multi-Carboxylic Acid and Imidazole-Based Spacer. Cryst. Growth Des. 2012, 12, 1603–1612. [Google Scholar] [CrossRef]
- Hao, J.M.; Yu, B.Y.; Hecke, K.V.; Cui, G.H. A series of d 10 metal coordination polymers based on a flexible bis (2-methylbenzimidazole) ligand and different carboxylates: Synthesis, structures, photoluminescence and catalytic properties. CrystEngComm 2015, 17, 2279–2293. [Google Scholar] [CrossRef]
- Yeber, M.C.; Díaz, L.; Fernández, J. Catalytic activity of the SO4−radical for photodegradation of the azo dye Cibacron Brilliant Yellow 3 and 3, 4-dichlorophenol: Optimization by application of response surface methodology. J. Photochem. Photobiol. A 2010, 215, 90–95. [Google Scholar] [CrossRef]
- Cui, J.W.; An, W.J.; Van Hecke, K.; Cui, G.H. Two copper (I) cyanide coordination polymers modified by semi rigid bis (benzimidazole) ligands: Syntheses, crystal structures, and electrochemical and photocatalytic properties. Dalton Trans. 2016, 45, 17474–17484. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.; He, C.; Duan, C. Metal–Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Hao, S.Y.; Hou, S.X.; Van Hecke, K.; Cui, G.H. Construction of noninterpenetrating and interpenetrating Co (II) networks with halogenated carboxylate modulated by auxiliary N-donor co-ligands: Structural diversity, electrochemical and photocatalytic properties. Dalton Trans. 2017, 46, 1951–1964. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Visualisation and characterisation of voids in crystalline materials. CrystEngComm 2011, 13, 1804–1813. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer, Version 3.1; University of Western Australia: Perth, Australia, 2012. [Google Scholar]
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the origin and nature of internal methyl rotation barriers: An information-theoretic approach study. Theor. Chem. Accounts 2022, 141, 68. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).