Synergistic Remediation of Organic Dye by Titanium Dioxide/Reduced Graphene Oxide Nanocomposite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Materials
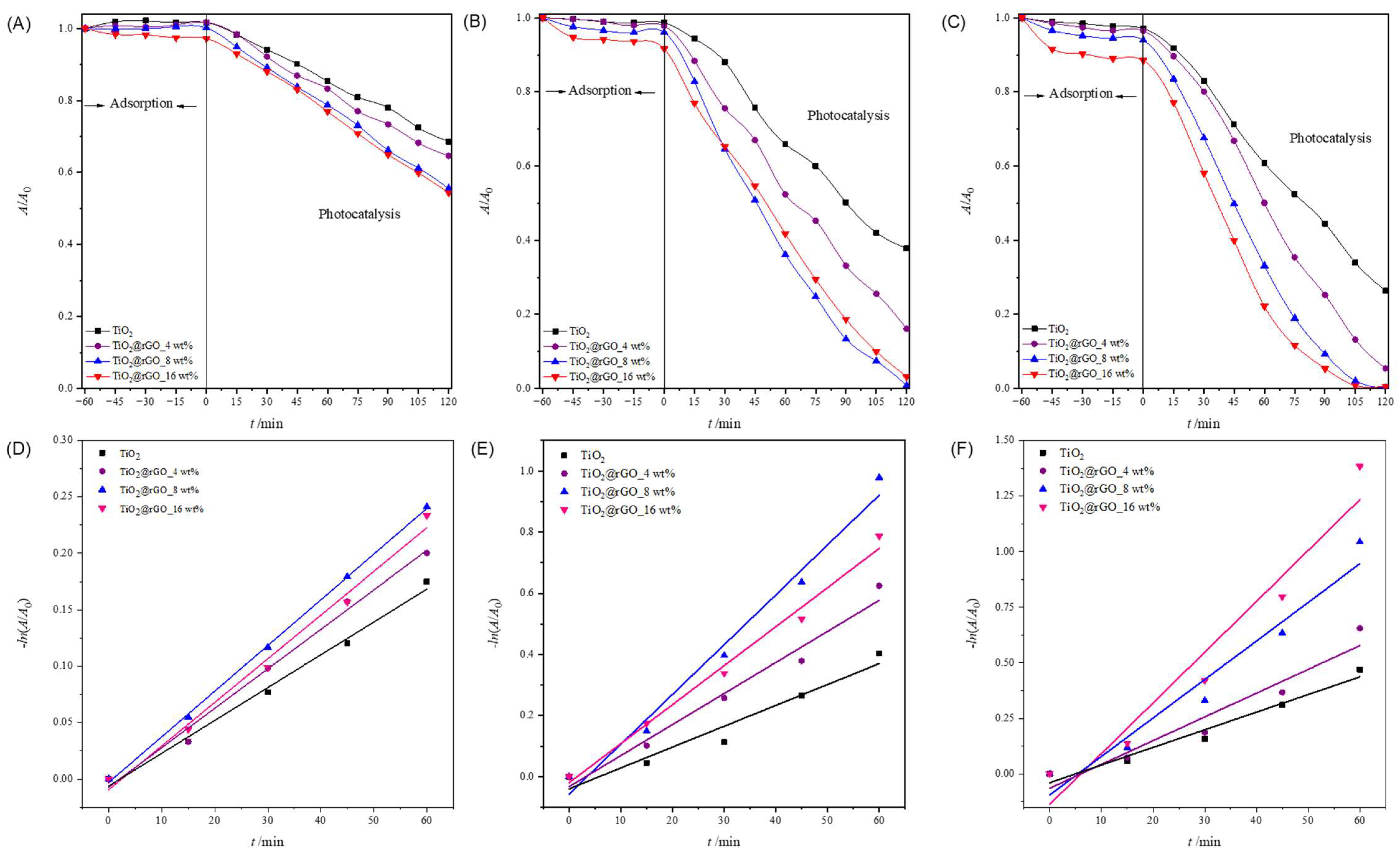
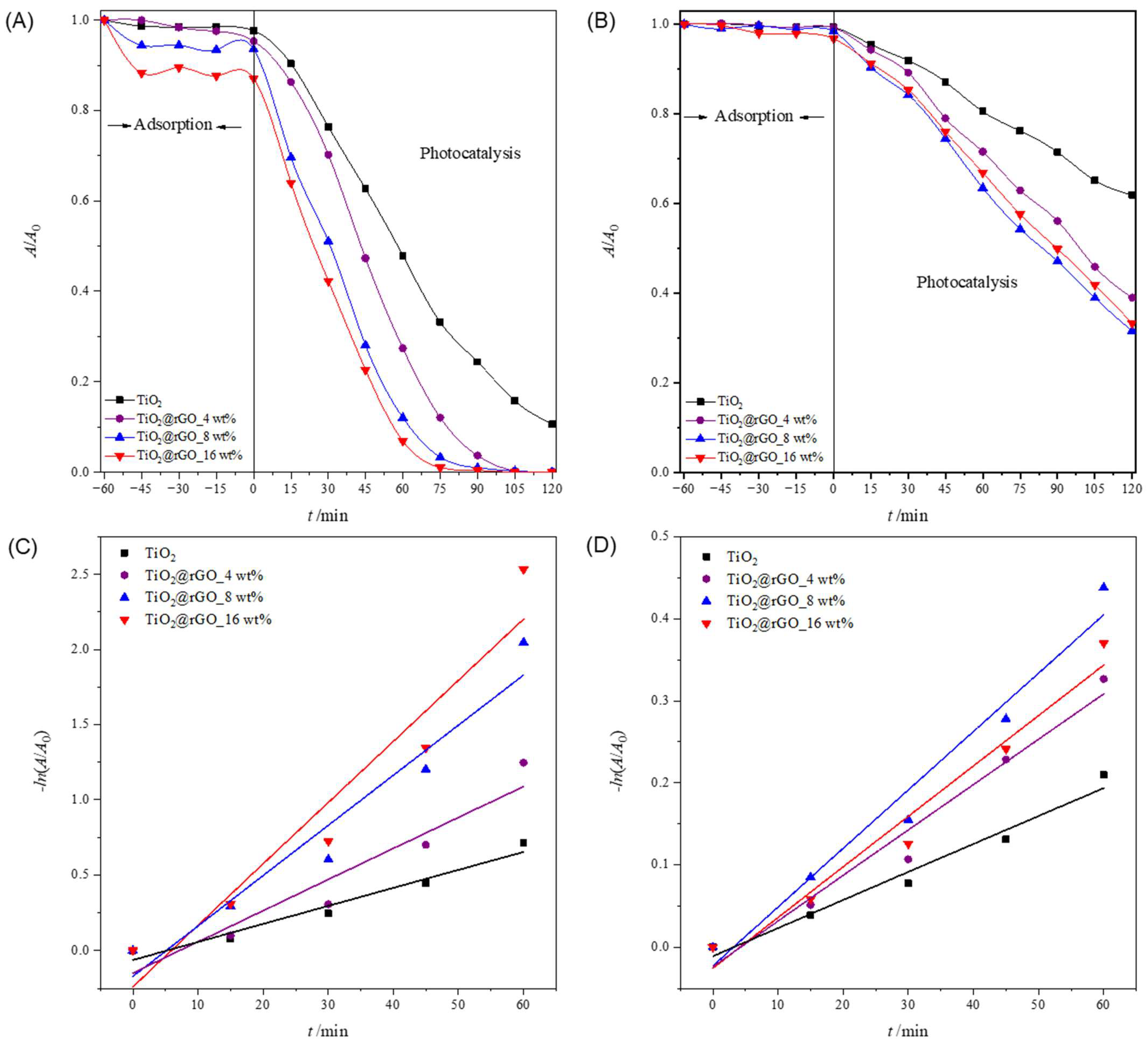
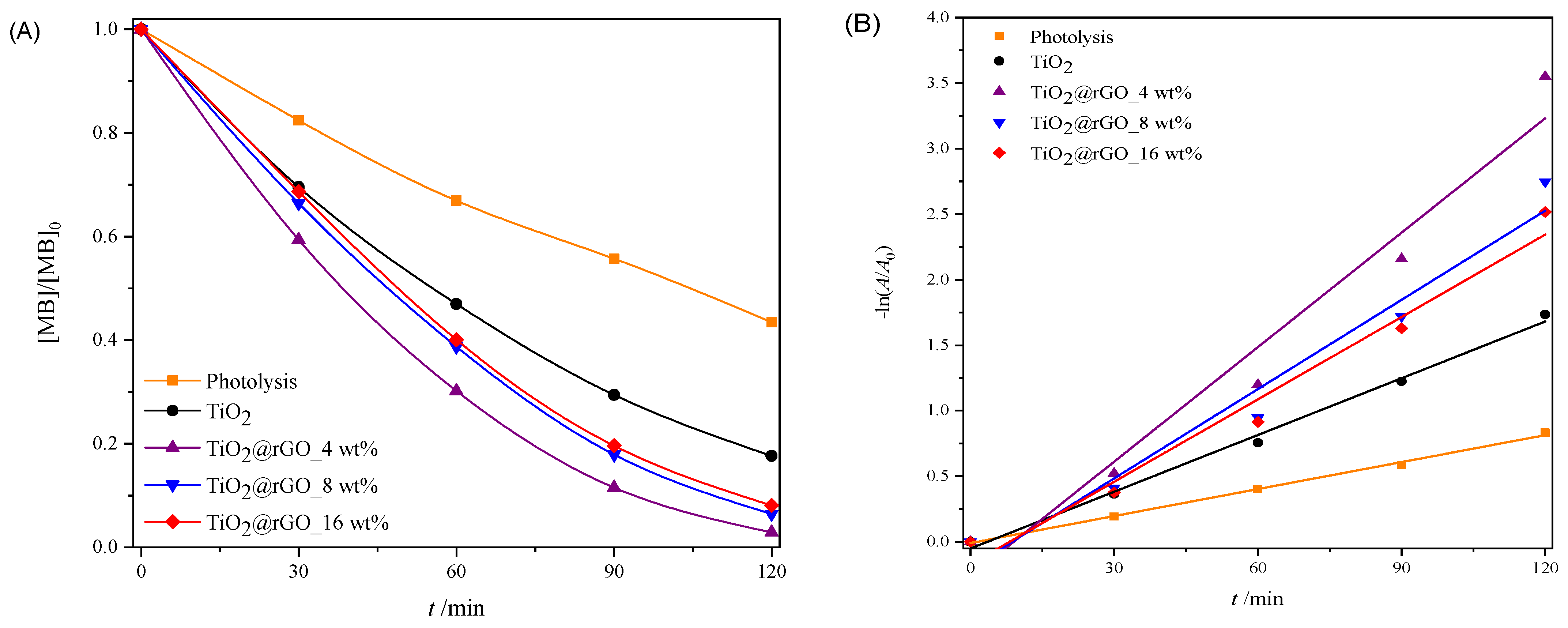
2.2. Photocatalytic Performance
2.2.1. Effect of Photocatalyst Concentration
2.2.2. The Influence of the Initial Dye Concentration
2.2.3. Sunlight Photocatalytic Activity
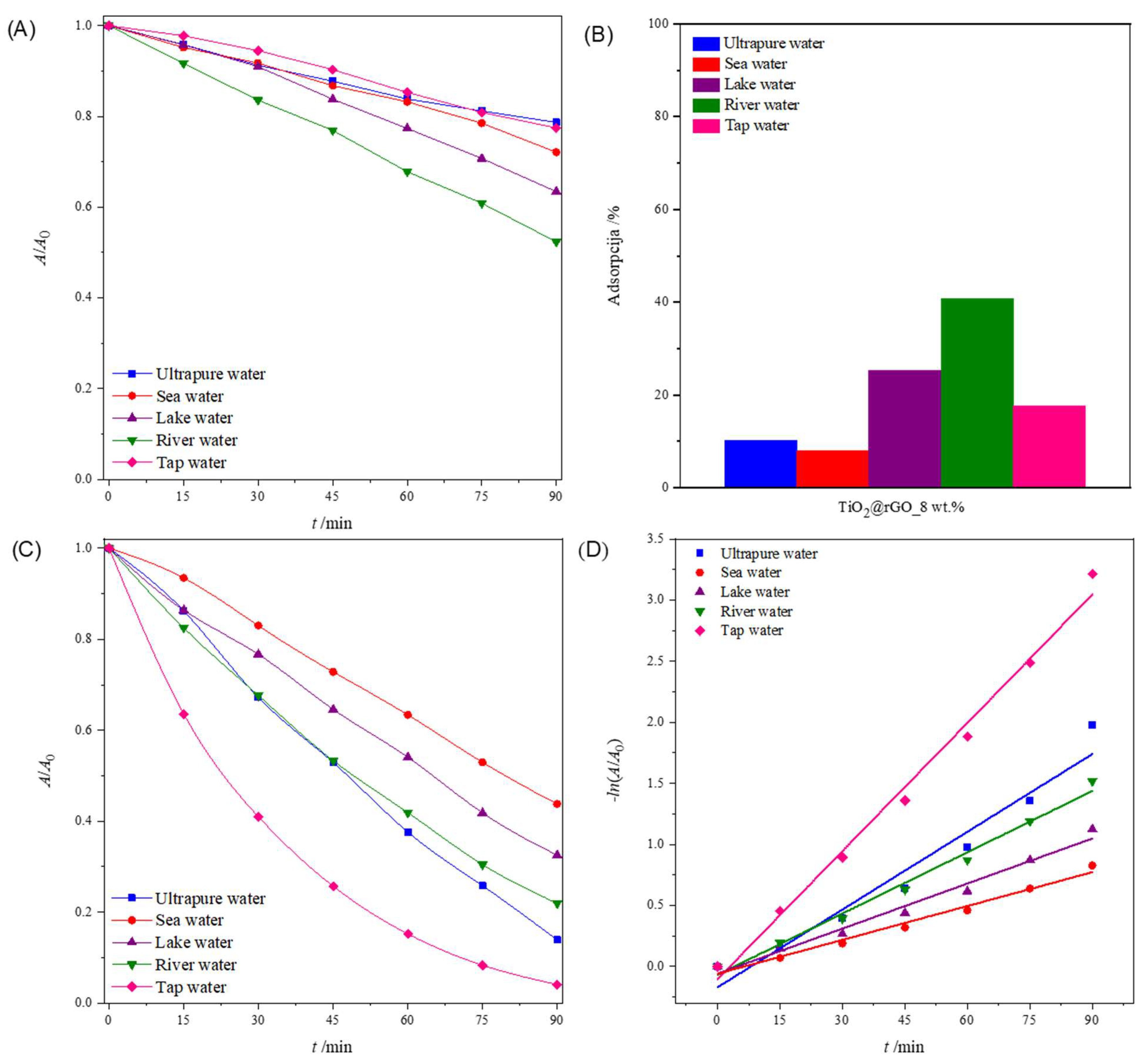
2.2.4. Influence of the Water Matrix
2.2.5. Reuse of Photocatalysts
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of TiO2@rGO
3.3. Characterization of TiO2@rGO Photocatalyst
3.4. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzoni, F.; Alvisi, S.; Blokker, M.; Buchberger, S.G.; Castelletti, A.; Cominola, A.; Gross, M.-P.; Jacobs, H.E.; Mayer, P.; Steffelbauer, D.B.; et al. Investigating the Characteristics of Residential End Uses of Water: A Worldwide Review. Water Res. 2023, 230, 119500. [Google Scholar] [CrossRef] [PubMed]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Recent Advances and Prospects of Catalytic Advanced Oxidation Process in Treating Textile Effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile Finishing Dyes and Their Impact on Aquatic Environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Chandra, R.; Bharagava, R.N.; Kapley, A.; Purohit, H.J. Bacterial Diversity, Organic Pollutants and Their Metabolites in Two Aeration Lagoons of Common Effluent Treatment Plant (CETP) during the Degradation and Detoxification of Tannery Wastewater. Bioresour. Technol. 2011, 102, 2333–2341. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent Advances Based on the Synergetic Effect of Adsorption for Removal of Dyes from Waste Water Using Photocatalytic Process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced Oxidation Process-Mediated Removal of Pharmaceuticals from Water: A Review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Kurian, M. Advanced Oxidation Processes and Nanomaterials—A Review. Clean. Eng. Technol. 2021, 2, 100090. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent Advances in Persulfate-Based Advanced Oxidation Processes for Organic Wastewater Treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Radović Vučić, M.; Baošić, R.; Mitrović, J.; Petrović, M.; Velinov, N.; Kostić, M.; Bojić, A. Comparison of the Advanced Oxidation Processes in the Degradation of Pharmaceuticals and Pesticides in Simulated Urban Wastewater: Principal Component Analysis and Energy Requirements. Process Saf. Environ. Prot. 2021, 149, 786–793. [Google Scholar] [CrossRef]
- Klančar, A.; Trontelj, J.; Kristl, A.; Meglič, A.; Rozina, T.; Justin, M.Z.; Roškar, R. An Advanced Oxidation Process for Wastewater Treatment to Reduce the Ecological Burden from Pharmacotherapy and the Agricultural Use of Pesticides. Ecol. Eng. 2016, 97, 186–195. [Google Scholar] [CrossRef]
- de Oliveira Guidolin, T.; Possolli, N.M.; Polla, M.B.; Wermuth, T.B.; Franco de Oliveira, T.; Eller, S.; Klegues Montedo, O.R.; Arcaro, S.; Cechinel, M.A.P. Photocatalytic Pathway on the Degradation of Methylene Blue from Aqueous Solutions Using Magnetite Nanoparticles. J. Clean. Prod. 2021, 318, 128556. [Google Scholar] [CrossRef]
- Deshannavar, U.B.; Kumar Singa, P.; Gaonkar, D.; Gayathri, A.; Patil, A.; Malade, L.V. Removal of Acid Violet 49 and Acid Red 88 Dyes from Aqueous Solutions Using Advanced Oxidation Process. Mater. Today Proc. 2020, 24, 1011–1019. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent Advances in Syntheses, Properties and Applications of TiO2 Nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- FUJISHIMA, A.; HONDA, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium Dioxide Nanoparticles in Food and Personal Care Products—What Do We Know about Their Safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef]
- Adeleye, A.T.; John, K.I.; Adeleye, P.G.; Akande, A.A.; Banjoko, O.O. One-Dimensional Titanate Nanotube Materials: Heterogeneous Solid Catalysts for Sustainable Synthesis of Biofuel Precursors/Value-Added Chemicals—A Review. J. Mater. Sci. 2021, 56, 18391–18416. [Google Scholar] [CrossRef] [PubMed]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene Blue Decomposition on TiO2/Reduced Graphene Oxide Hybrid Photocatalysts Obtained by a Two-Step Hydrothermal and Calcination Synthesis. Catal. Today 2020, 357, 630–637. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, Á.; Manassero, A.; Satuf, M.L.; Alfano, O.M.; Casas, J.A.; Bahamonde, A. Influence of TiO2-RGO Optical Properties on the Photocatalytic Activity and Efficiency to Photodegrade an Emerging Pollutant. Appl. Catal. B 2019, 246, 1–11. [Google Scholar] [CrossRef]
- Garrafa-Gálvez, H.E.; Alvarado-Beltrán, C.G.; Almaral-Sánchez, J.L.; Hurtado-Macías, A.; Garzon-Fontecha, A.M.; Luque, P.A.; Castro-Beltrán, A. Graphene Role in Improved Solar Photocatalytic Performance of TiO2-RGO Nanocomposite. Chem. Phys. 2019, 521, 35–43. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Kale, D.P.; Kar, S.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K.; Sonawane, S.H. Ultrasound Assisted Preparation of RGO/TiO2 Nanocomposite for Effective Photocatalytic Degradation of Methylene Blue under Sunlight. Nano-Struct. Nano-Objects 2020, 21, 100407. [Google Scholar] [CrossRef]
- Duan, Y.; Gou, M.-L.; Guo, Y.; Cai, J.; Song, W.; Liu, Z.; Zhou, E. In Situ Hydrothermal Synthesis of TiO2–RGO Nanocomposites for 4-Nitrophenol Degradation under Sunlight Irradiation. J. Mater. Res. 2021, 36, 906–915. [Google Scholar] [CrossRef]
- Wu, S.; Jia, Q.; Dai, W. Synthesis of RGO/TiO2 Hybrid as a High Performance Photocatalyst. Ceram. Int. 2017, 43, 1530–1535. [Google Scholar] [CrossRef]
- Amir Faiz, M.S.; Che Azurahanim, C.A.; Raba’ah, S.A.; Ruzniza, M.Z. Low Cost and Green Approach in the Reduction of Graphene Oxide (GO) Using Palm Oil Leaves Extract for Potential in Industrial Applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Huang, N.M.; Lim, H.N.; Zakaria, R. One-Pot Sonochemical Synthesis of Reduced Graphene Oxide Uniformly Decorated with Ultrafine Silver Nanoparticles for Non-Enzymatic Detection of H2O2 and Optical Detection of Mercury Ions. RSC Adv. 2014, 4, 36401–36411. [Google Scholar] [CrossRef]
- Mikhaylov, P.A.; Vinogradov, M.I.; Levin, I.S.; Shandryuk, G.A.; Lubenchenko, A.V.; Kulichikhin, V.G. Synthesis and Characterization of Polyethylene Terephthalate-Reduced Graphene Oxide Composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 693, 012036. [Google Scholar] [CrossRef]
- Jiao, X.; Qiu, Y.; Zhang, L.; Zhang, X. Comparison of the Characteristic Properties of Reduced Graphene Oxides Synthesized from Natural Graphites with Different Graphitization Degrees. RSC Adv. 2017, 7, 52337–52344. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?-Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Ricci, P.C.; Carbonaro, C.M.; Stagi, L.; Salis, M.; Casu, A.; Enzo, S.; Delogu, F. Anatase-to-Rutile Phase Transition in TiO2 Nanoparticles Irradiated by Visible Light. J. Phys. Chem. C 2013, 117, 7850–7857. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Ljubas, D.; Mužina, K.; Bačić, I.; Radošević, T.; Podlogar, M.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; et al. Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-like Radiation. Appl. Sci. 2021, 11, 3966. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, S.; Ma, F.; Xu, Y. Synergistic Effects and Kinetics of RGO-Modified TiO2 Nanocomposite on Adsorption and Photocatalytic Degradation of Humic Acid. J. Environ. Manag. 2019, 235, 293–302. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled Synthesis, Characterization and Reduction of Graphene Oxide: A Convenient Method for Large Scale Production. Egypt. J. Basic. Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, S.A.; Aladresi, A.A.M.; Alharbi, S.A.; Chinnathambi, A. Ovalbumin-Mediated Synthesis and Simultaneous Functionalization of Graphene with Increased Protein Stability. Green. Chem. Lett. Rev. 2020, 13, 60–67. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Preparation, Characterization and Photocatalytic Activity of TiO2/Reduced Graphene Oxide Nanocomposite. In Proceedings of the Annals of DAAAM and Proceedings of the International DAAAM Symposium, DAAAM International Vienna, Vienna, Austria, 23–26 November 2020; Volume 31, pp. 668–676. [Google Scholar]
- Avar, B.; Panigrahi, M. Synthesis and Characterization of Binary Reduced Graphene Oxide/Metal Oxide Nanocomposites. Phys. Chem. Solid State 2022, 23, 101–112. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Wang, X.; Zhang, J.; Long, T. A Critical Review on Challenges and Trend of Ultrapure Water Production Process. Sci. Total Environ. 2021, 785, 147254. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Liu, Z.; Yang, W.; Liu, J. A Highly Conductive Porous Graphene Electrode Prepared via in Situ Reduction of Graphene Oxide Using Cu Nanoparticles for the Fabrication of High Performance Supercapacitors. RSC Adv. 2015, 5, 54275–54282. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.-G. Hydrothermally Reduced Graphene Oxide as a Supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, L.; Li, D.-W.; Karpuzov, D.; Long, Y.-T. Electrocatalytic Oxidation of NADH on Graphene Oxide and Reduced Graphene Oxide Modified Screen-Printed Electrode. Int. J. Electrochem. Sci. 2011, 6, 819–829. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Khan, S.A.; Arshad, Z.; Shahid, S.; Arshad, I.; Rizwan, K.; Sher, M.; Fatima, U. Synthesis of TiO2/Graphene Oxide Nanocomposites for Their Enhanced Photocatalytic Activity against Methylene Blue Dye and Ciprofloxacin. Compos. B Eng. 2019, 175, 107120. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Yu, C.; Zhang, H. Influence of TiO2 Morphology on Adsorption-Photocatalytic Efficiency of TiO2-Graphene Composites for Methylene Blue Degradation. J. Environ. Chem. Eng. 2018, 6, 4899–4907. [Google Scholar] [CrossRef]
- Wu, K.; Shi, M.; Pan, X.; Zhang, J.; Zhang, X.; Shen, T.; Tian, Y. Decolourization and Biodegradation of Methylene Blue Dye by a Ligninolytic Enzyme-Producing Bacillus Thuringiensis: Degradation Products and Pathway. Enzym. Microb. Technol. 2022, 156, 109999. [Google Scholar] [CrossRef] [PubMed]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Deling; Zhang, Y.; Qi, J.; Rao, Y.; Lu, G.; Li, B.; Wang, K.; Yin, K. Enhanced Photocatalytic Performance of BiVO4 for Degradation of Methylene Blue under LED Visible Light Irradiation Assisted by Peroxymonosulfate. Int. J. Electrochem. Sci. 2020, 15, 2470–2480. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lin, Z.; Yang, J.; Li, C.; Gu, R. Combination of Plasma Oxidation Process with Microbial Fuel Cell for Mineralizing Methylene Blue with High Energy Efficiency. J. Hazard. Mater. 2020, 384, 121307. [Google Scholar] [CrossRef]
- Islam, M.A.; Siddiquey, I.A.; Rahman Khan, M.A.; Alam, M.M.; Islam, S.S.M.; Hasnat, M.A. Adsorption and UV-Visible Light Induced Degradation of Methylene Blue over ZnO Nano-Particles. Int. J. Chem. React. Eng. 2011, 9. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Khaleel, A.; Ahmed, A. Photocatalytic Degradation of Methylene Blue Using a Mixed Catalyst and Product Analysis by LC/MS. Chem. Eng. J. 2010, 157, 373–378. [Google Scholar] [CrossRef]
- Li, X.; Ye, J. Photocatalytic Degradation of Rhodamine B over Pb3Nb4O13/Fumed SiO2 Composite under Visible Light Irradiation. J. Phys. Chem. C 2007, 111, 13109–13116. [Google Scholar] [CrossRef]
- Asiltürk, M.; Sayılkan, F.; Arpaç, E. Effect of Fe3+ Ion Doping to TiO2 on the Photocatalytic Degradation of Malachite Green Dye under UV and Vis-Irradiation. J. Photochem. Photobiol. A Chem. 2009, 203, 64–71. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Tang, H.; Huang, T.; Xing, B. Revisit the Adsorption of Aromatic Compounds on Graphene Oxide: Roles of Oxidized Debris. Chem. Eng. J. 2022, 450, 137996. [Google Scholar] [CrossRef]
- Sangareswari, M.; Meenakshi Sundaram, M. Development of Efficiency Improved Polymer-Modified TiO2 for the Photocatalytic Degradation of an Organic Dye from Wastewater Environment. Appl. Water Sci. 2017, 7, 1781–1790. [Google Scholar] [CrossRef]
- Sun, N.; Ma, J.; Wang, C.; Xue, J.; Qiang, L.; Tang, J. A Facile and Efficient Method to Directly Synthesize TiO2/rGO with Enhanced Photocatalytic Performance. Superlattices Microstruct. 2018, 121, 1–8. [Google Scholar] [CrossRef]
- Gonçalves, G.; Cruz, S.M.A.; Ramalho, A.; Grácio, J.; Marques, P.A.A.P. Graphene Oxide versus Functionalized Carbon Nanotubes as a Reinforcing Agent in a PMMA/HA Bone Cement. Nanoscale 2012, 4, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Trevizo, A.; Pizá-Ruiz, P.; Chávez-Flores, D.; Ogaz-Parada, J.; Amézaga-Madrid, P.; Vega-Ríos, A.; Miki-Yoshida, M. On the Discoloration of Methylene Blue by Visible Light. J. Fluoresc. 2019, 29, 15–25. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of Water Matrix on the Removal of Micropollutants by Advanced Oxidation Technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Wanag, A.; Kusiak-Nejman, E.; Czyżewski, A.; Moszyński, D.; Morawski, A.W. Influence of Rgo and Preparation Method on the Physicochemical and Photocatalytic Properties of TiO2/Reduced Graphene Oxide Photocatalysts. Catalysts 2021, 11, 1333. [Google Scholar] [CrossRef]
- Ćurković, L.; Ljubas, D.; Šegota, S.; Bačić, I. Photocatalytic Degradation of Lissamine Green B Dye by Using Nanostructured Sol–Gel TiO2 Films. J. Alloys Compd. 2014, 604, 309–316. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Enhanced Photocatalytic Activity of Hybrid RGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation. Catalysts 2021, 11, 1023. [Google Scholar] [CrossRef]
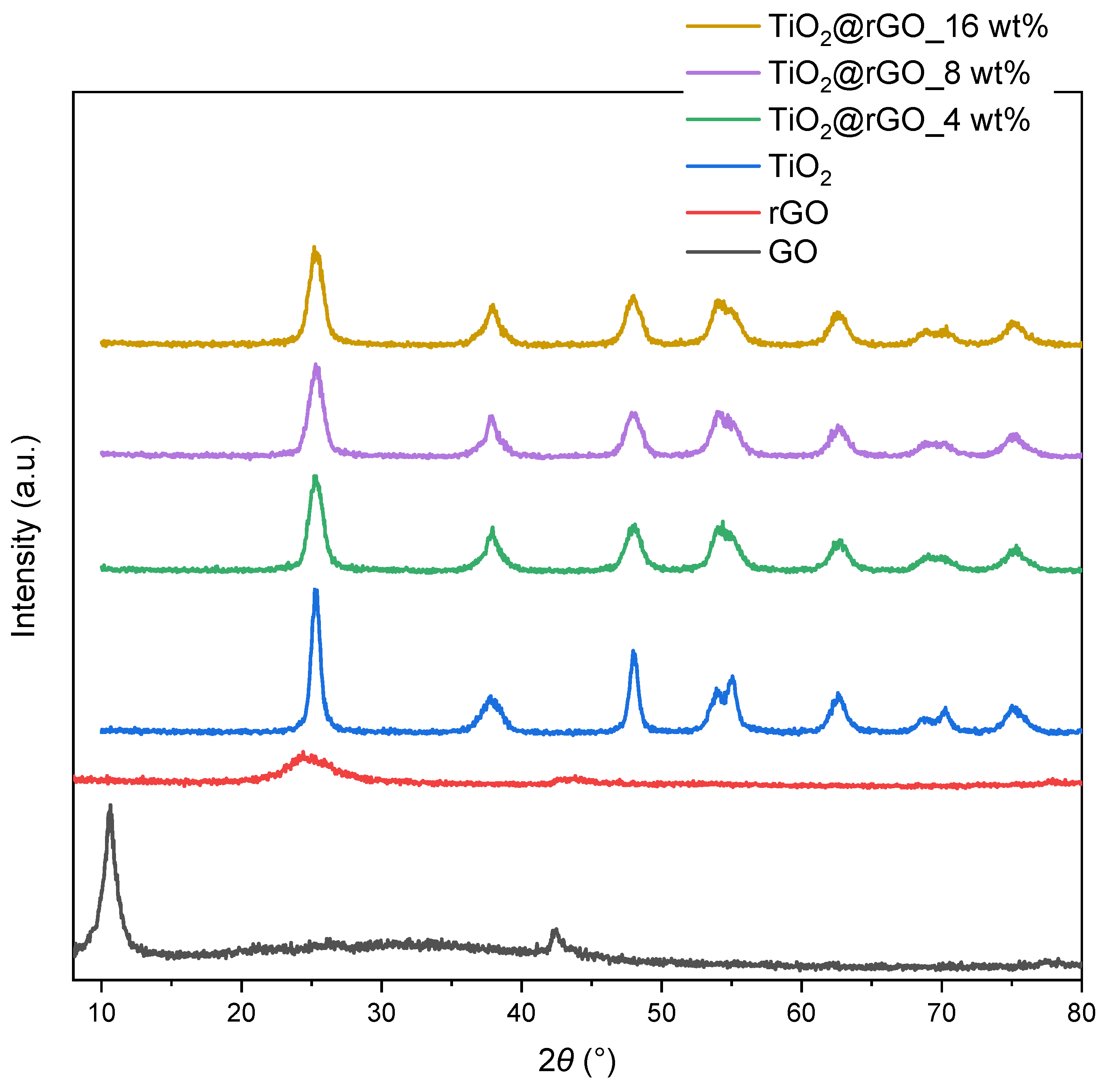
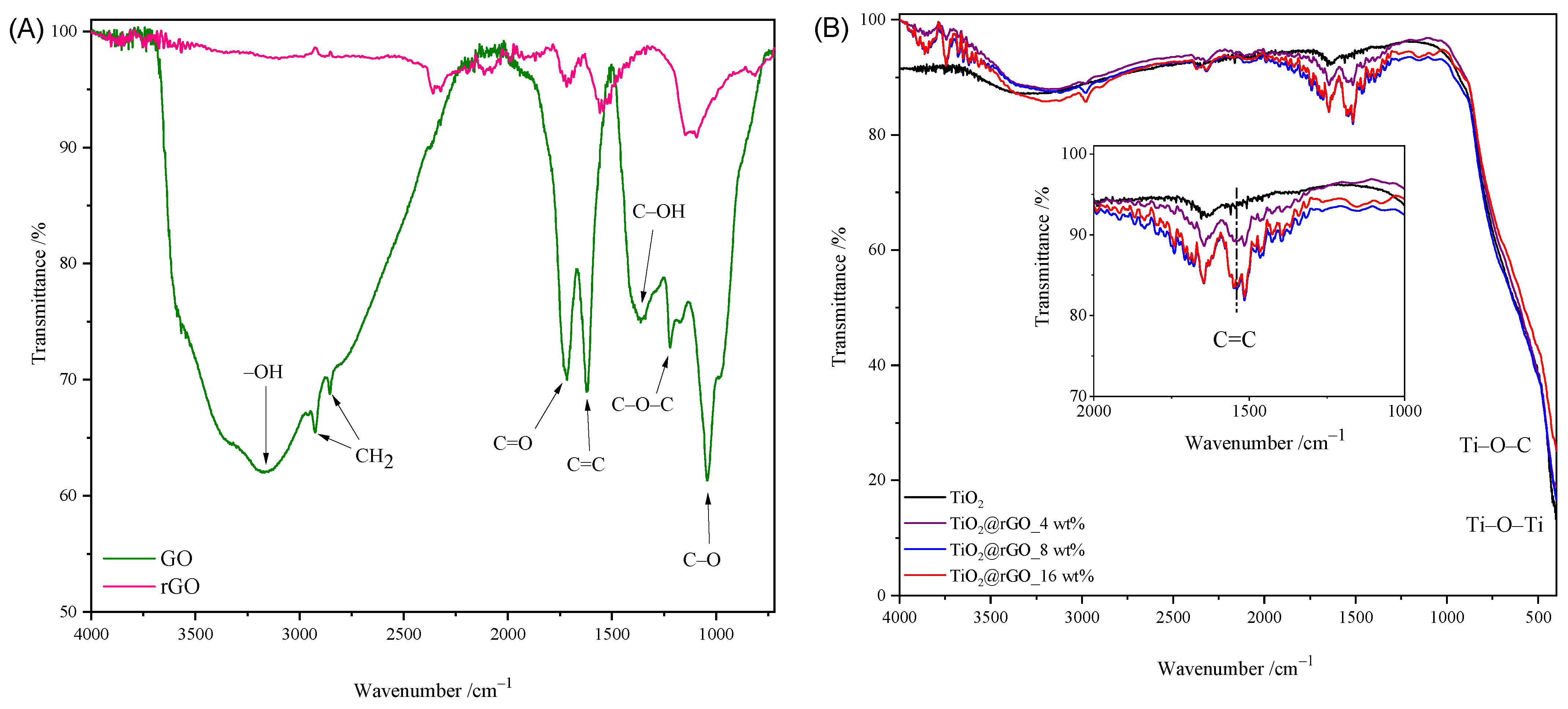
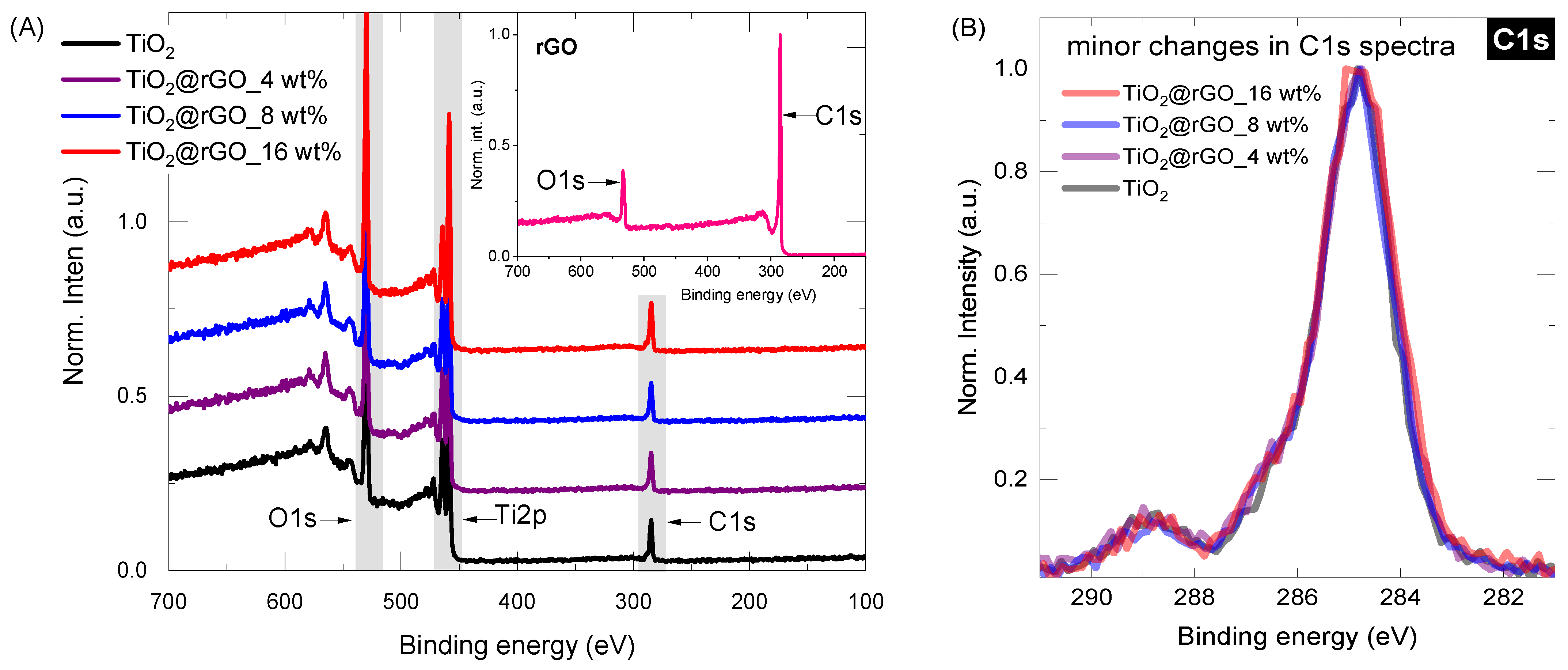
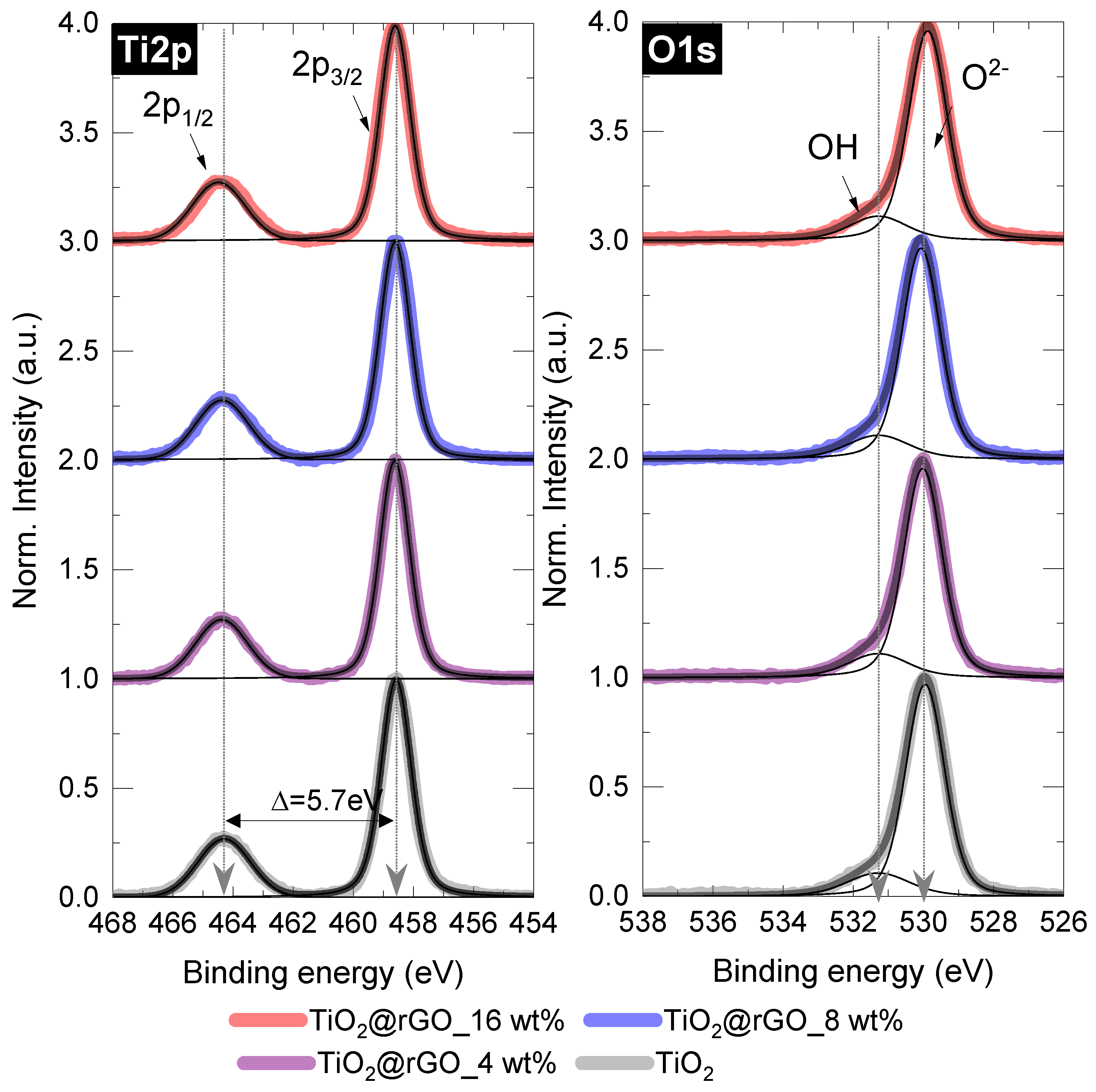
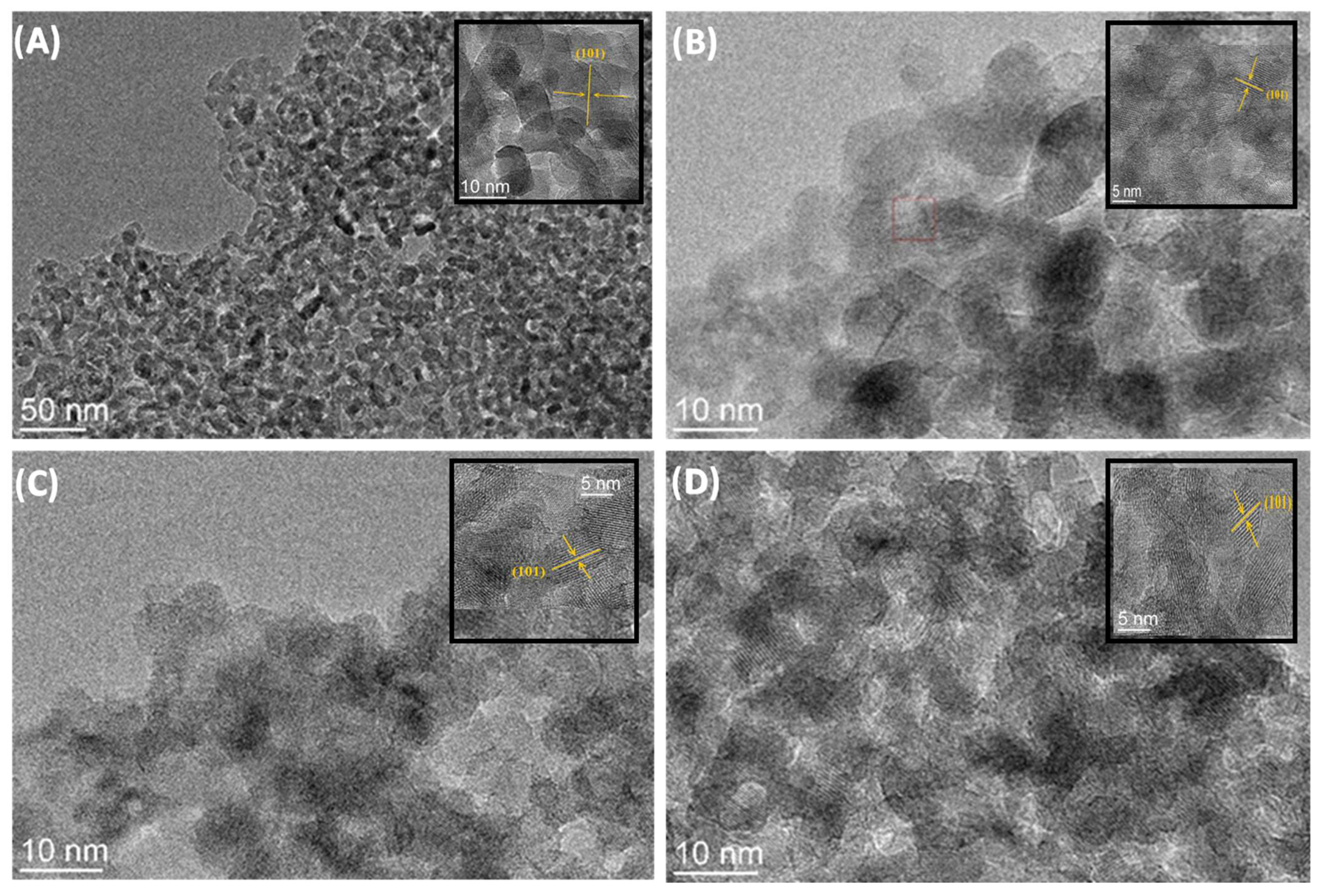


| Sample | C (at%) ± 0.3 | O (at%) ± 0.3 | Ti (at%) ± 0.3 | O/Ti Ratio |
|---|---|---|---|---|
| rGO | 85.5 | 14.5 | / | / |
| TiO2 | 19.9 | 56.8 | 23.3 | 2.43 |
| TiO2@rGO_4 wt% | 20.9 | 55.3 | 23.8 | 2.32 |
| TiO2@rGO_8 wt% | 21.2 | 55.8 | 23.0 | 2.42 |
| TiO2@rGO_16 wt% | 24.3 | 53.7 | 22.0 | 2.44 |
| Illumination Source | Power, W | λ, nm | Distance from Reactor, cm | UV-A and UV-B Intensity, mW·cm−2 |
|---|---|---|---|---|
| Simulated solar radiation | 300 | 280–780 | 20 | 5.3 ± 0.30 |
| Natural sunlight irradiation | 3.86 × 1026 | 100–1,000,000 | / | 6.5 ± 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocijan, M.; Ćurković, L.; Vengust, D.; Radošević, T.; Shvalya, V.; Gonçalves, G.; Podlogar, M. Synergistic Remediation of Organic Dye by Titanium Dioxide/Reduced Graphene Oxide Nanocomposite. Molecules 2023, 28, 7326. https://doi.org/10.3390/molecules28217326
Kocijan M, Ćurković L, Vengust D, Radošević T, Shvalya V, Gonçalves G, Podlogar M. Synergistic Remediation of Organic Dye by Titanium Dioxide/Reduced Graphene Oxide Nanocomposite. Molecules. 2023; 28(21):7326. https://doi.org/10.3390/molecules28217326
Chicago/Turabian StyleKocijan, Martina, Lidija Ćurković, Damjan Vengust, Tina Radošević, Vasyl Shvalya, Gil Gonçalves, and Matejka Podlogar. 2023. "Synergistic Remediation of Organic Dye by Titanium Dioxide/Reduced Graphene Oxide Nanocomposite" Molecules 28, no. 21: 7326. https://doi.org/10.3390/molecules28217326







