Recent Advances in the Synthesis and Antioxidant Activity of Low Molecular Mass Organoselenium Molecules
Abstract
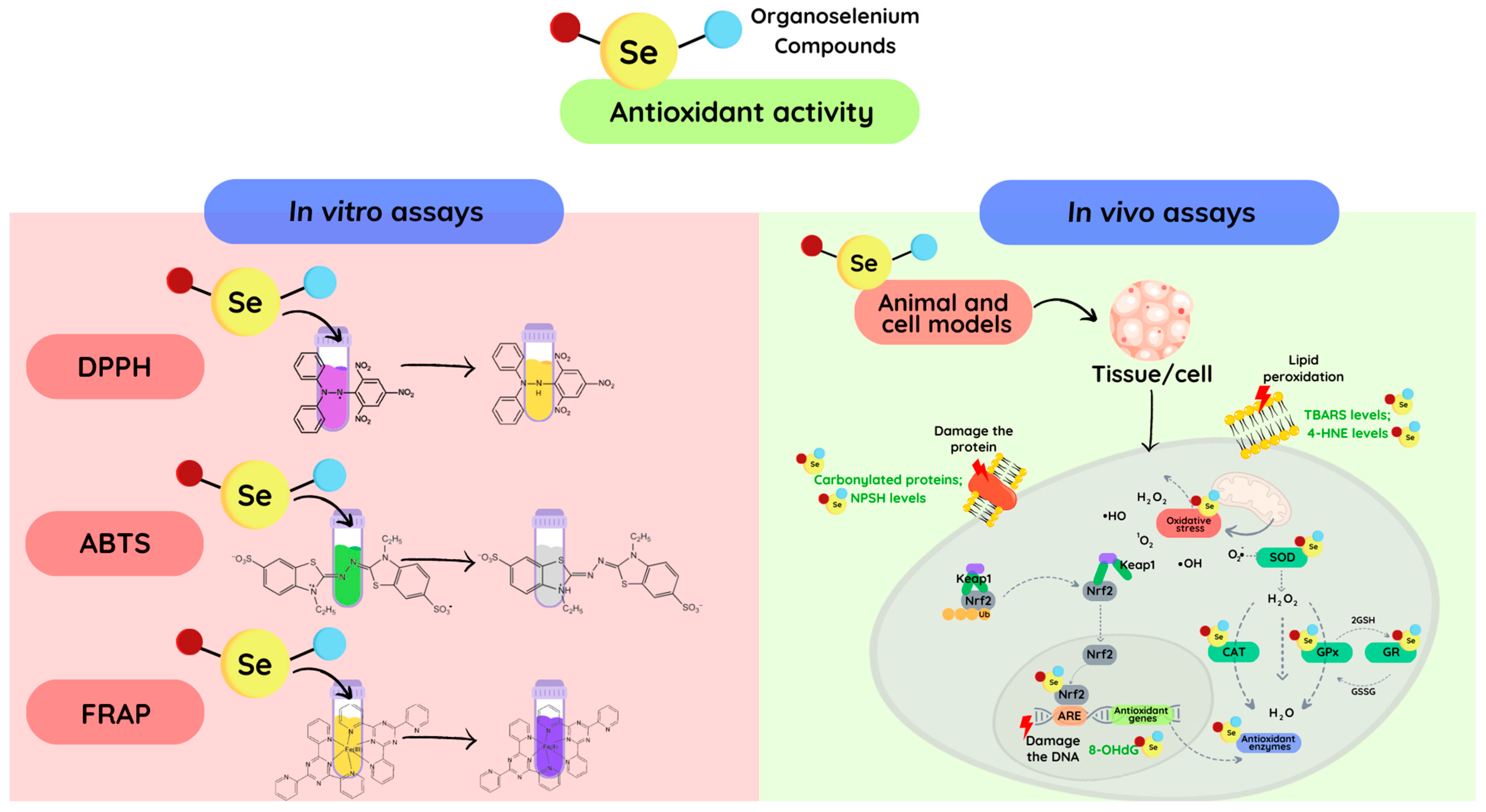
:1. Introduction
2. Oxidative Stress
3. Synthesis and Antioxidant Evaluation of Organoselenium Compounds
3.1. Diselenides
3.2. Ebselen Derivatives
3.3. Se-Functionalized N-Heterocycles
3.4. Se-Heterocycles
3.5. Selenides
3.6. Miscellaneous
4. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef]
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Mamgain, R.; Kostic, M.; Singh, F.V. Synthesis and Antioxidant Properties of Organoselenium Compounds. Curr. Med. Chem. 2023, 30, 2421–2448. [Google Scholar] [CrossRef] [PubMed]
- Obieziurska-Fabisiak, M.; Pacuła-Miszewska, A.J.; Laskowska, A.; Ścianowski, J. Organoselenium compounds as antioxidants. Arkivoc 2022, 5, 69–92. [Google Scholar] [CrossRef]
- Kumar, M.; Chhillar, B.; Verma, D.; Nain, S.; Singh, V.P. Introduction of Methyl Group in Substituted Isoselenazolones: Catalytic and Mechanistic Study. J. Org. Chem. 2023, 88, 4273–4285. [Google Scholar] [CrossRef]
- Deshmukh, Y.; Gandhi, V.V.; Singh, B.G.; Kumbhare, L.B.; Debnath, A.K.; Kunwar, A. 3,3′-Diselenodipropionic acid (DSePA) forms 1:1 complex with Hg(II) and prevents oxidative stress in cultured cells and mice model. J. Inorg. Biochem. 2022, 226, 111638. [Google Scholar] [CrossRef]
- Upadhyay, A.; Bhakuni, B.S.; Meena, R.; Kumar, S. Radical Chain Breaking Bis(ortho-organoselenium) Substituted Phenolic Antioxidants. Chem. Asian J. 2021, 16, 966–973. [Google Scholar] [CrossRef]
- Peglow, T.J.; Martins, C.C.; da Motta, K.P.; Luchese, C.; Wilhelm, E.A.; Stieler, R.; Schneider, P.H. Synthesis and biological evaluation of 5-chalcogenyl-benzo[h]quinolines via photocyclization of arylethynylpyridine derivatives. New J. Chem. 2022, 46, 23030–23038. [Google Scholar] [CrossRef]
- Soares, A.T.G.; Junior, L.B.L.R.; Salgueiro, W.G.; Forno, A.H.C.D.; Rodrigues, C.F.; Sacramento, M.; Franco, J.; Alves, D.; Oliveira, R.P.; Pinton, S.; et al. Organoselenotriazoles attenuate oxidative damage induced by mitochondrial dysfunction in mev-1 Caenorhabditis elegans mutants. J. Trace Elem. Med. Biol. 2019, 53, 34–40. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Moore, K.; Roberts, L.J. Measurement of Lipid Peroxidation. Free Radic. 1998, 28, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 106, 21–30. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Roy, Z.; Bansal, R.; Siddiqui, L.; Chaudhary, N. Understanding the Role of Free Radicals and Antioxidant Enzymes in Human Diseases. Curr. Pharm. Biotechnol. 2023, 24, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Mohideen, K.; Jeddy, N.; Krithika, C.; Faizee, S.H.; Dhungel, S.; Ghosh, S. Assessment of glutathione peroxidase enzyme response and total antioxidant status in oral cancer—Systematic review and meta-analysis. Cancer Rep. 2023, 6, e1842. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Barchielli, J.; Caperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Selenium and redox signaling. Arch. Biochem. Biophys. 2017, 617, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sudati, J.H.; Nogara, P.A.; Saraiva, R.A.; Wagner, C.; Alberto, E.E.; Braga, A.L.; Fachinetto, R.; Piquini, P.C.; Batista, J.; Rocha, T. Diselenoamino acid derivatives as GPx mimics and as substrates of TrxR: In vitro and in silico studies. Org. Biomol. Chem. 2018, 16, 3777–3787. [Google Scholar] [CrossRef]
- Hecka, S.O.; Zborowskia, V.A.; Quinesb, C.B.; Nogueira, C.W. 4,4′-Dichlorodiphenyl diselenide reverses a depressive-like phenotype, modulates prefrontal cortical oxidative stress and dysregulated glutamatergic neurotransmission induced by subchronic dexamethasone exposure to mice. J. Psychiatr. Res. 2019, 116, 61–68. [Google Scholar] [CrossRef]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis, 1st ed.; Pergamon Press: Oxford, UK, 1986; pp. 25–51. [Google Scholar]
- Garcia, C.S.; Garcia, P.R.; Espíndola, C.N.S.; Nunes, G.A.; Jardim, N.S.; Müller, S.G.; Bortolatto, C.F.; Brüning, C.A. Effect of m-Trifluoromethyl-diphenyl diselenide on the Pain–Depression Dyad Induced by Reserpine: Insights on Oxidative Stress, Apoptotic, and Glucocorticoid Receptor Modulation. Mol. Neurobiol. 2021, 58, 5078–5089. [Google Scholar] [CrossRef]
- Dos Santos, M.M.; de Macedo, G.T.; Prestes, A.S.; Eckera, A.; Müller, T.E.; Leitempergera, J.; Fontana, B.D.; Daniel, M.P.; Araújo, A.; Rosemberga, D.B.; et al. Modulation of redox and insulin signaling underlie the anti-hyperglycemic and antioxidant effects of diphenyl diselenide in zebrafish. Free Radic. Biol. Med. 2020, 158, 20–31. [Google Scholar] [CrossRef]
- Wanga, X.; Huanb, Y.; Lib, C.; Caob, H.; Sunb, S.; Leib, L.; Liub, Q.; Liub, S.; Jib, W.; Liua, H.; et al. Diphenyl diselenide alleviates diabetic peripheral neuropathy in rats with streptozotocin-induced diabetes by modulating oxidative stress. Biochem. Pharmacol. 2020, 182, 114221. [Google Scholar] [CrossRef]
- Botteselle, G.V.; Elias, W.C.; Bettanin, L.; Canto, R.F.S.; Salin, D.N.O.; Barbosa, F.A.R.; Saba, S.; Gallardo, H.; Ciancaleoni, G.; Domingos, J.B.; et al. Catalytic Antioxidant Activity of Bis-Aniline-Derived Diselenides as GPx Mimics. Molecules 2021, 26, 4446. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Huan, Y.; Cao, H.; Sun, S.; Lei, L.; Liu, Q.; Liu, S.; Ji, W.; Huang, K.; et al. Diphenyl diselenide ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats via suppressing oxidative stress and inflammation. Chem. Biol. Interact. 2021, 338, 109427. [Google Scholar] [CrossRef] [PubMed]
- Capperucci, A.; Coronnello, M.; Salvini, F.; Tanini, D.; Dei, S.; Teodori, E.; Giovannelli, L. Synthesis of functionalised organochalcogenides and in vitro evaluation of their antioxidant activity. Bioorg. Chem. 2021, 110, 104812. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Yadav, M.; Chhillar, B.; Singh, V.P. Regenerable Radical-Trapping and Preventive Selenazolonamine Antioxidants. Asian J. Org. Chem. 2021, 10, 1492–1499. [Google Scholar] [CrossRef]
- Singh, V.P.; Poon, J.; Yan, J.; Lu, X.; Ott, M.K.; Butcher, R.J.; Gates, P.J.; Engman, L. Nitro-, Azo-, and Amino Derivatives of Ebselen: Synthesis, Structure, and Cytoprotective Effects. J. Org. Chem. 2017, 82, 313–321. [Google Scholar] [CrossRef]
- Carroll, L.; Gardiner, K.; Ignasiak, M.; Holmehave, J.; Shimodaira, S.; Breitenbach, T.; Iwaoka, M.; Ogilby, P.R.; Pattison, D.I.; Davies, M.J. Interaction kinetics of selenium-containing compounds with oxidants. Free Radic. Biol. Med. 2020, 155, 58–68. [Google Scholar] [CrossRef]
- Carroll, L.; Pattison, D.I.; Fu, S.; Schiesser, C.H.; Davies, M.J.; Hawkins, C.L. Reactivity of selenium-containing compounds with myeloperoxidase-derived chlorinating oxidants: Second-order rate constants and implications for biological damage. Free Radic. Biol. Med. 2015, 84, 279–288. [Google Scholar] [CrossRef]
- Carroll, L.; Davies, M.J.; Pattison, D.I. Reaction of low-molecular-mass organoselenium compounds (and their sulphur analogues) with inflammation-associated oxidants. Free Radic. Res. 2015, 49, 750–767. [Google Scholar] [CrossRef]
- Storkey, C.; Pattison, D.I.; Ignasiak, M.T.; Schiesser, C.H.; Davies, M.J. Kinetics of reaction of peroxynitrite with selenium- and sulfur-containing compounds: Absolute rate constants and assessment of biological significance. Free Radic. Biol. Med. 2015, 89, 1049–1056. [Google Scholar] [CrossRef]
- Wang, X.; Huan, Y.; Liu, S.; Li, C.; Cao, H.; Lei, L.; Liu, Q.; Ji, W.; Sun, S.; Huang, K.; et al. Diphenyl Diselenide Alleviates Tert-Butyl Hydrogen Peroxide-Induced Oxidative Stress and Lipopolysaccharide-Induced Inflammation in Rat Glomerular Mesangial Cells. Int. J. Mol. Sci. 2022, 23, 11215. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdallah, B.; Al-Faiyz, Y.S.; Shaaban, S. Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates. Biomolecules 2022, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Obieziurska-Fabisiak, M.; Pacuła, A.J.; Capoccia, L.; Drogosz-Stachowicz, J.; Janecka, A.; Santi, C.; Scianowski, J. Phenylselanyl Group Incorporation for “Glutathione Peroxidase-like” Activity Modulation. Molecules 2020, 25, 3354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chhillar, B.; Yadav, M.; Sagar, P.; Singhal, N.K.; Gates, P.J.; Butcher, R.J.; Singh, V.P. Catalytic and highly regenerable aminic organoselenium antioxidants with cytoprotective effects. Org. Biomol. Chem. 2021, 19, 2015–2022. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, V.P. Synthesis and antioxidant activities of N-thiophenyl ebselenamines: A 77Se{1H} NMR mechanistic study. New J. Chem. 2022, 46, 12010–12022. [Google Scholar] [CrossRef]
- Vogt, A.G.; Voss, G.T.; de Oliveira, R.L.; Paltian, J.J.; Duarte, L.F.B.; Alves, D.; Jessec, C.R.; Roman, S.S.; Roehrs, J.A.; Wilhelm, E.A.; et al. Organoselenium group is critical for antioxidant activity of 7-chloro-4-phenylselenyl-quinoline. Chem. Biol. Interact. 2018, 282, 7–12. [Google Scholar] [CrossRef]
- Couto, S.F.; Araujo, S.M.; Bortolotto, V.C.; Poetinia, M.R.; Pinheiro, F.C.; Musachio, E.A.S.; Meichtrya, L.B.; do Sacramento, M.; Alves, D.; Novo, D.R.; et al. 7-chloro-4-(phenylselanyl) quinoline prevents dopamine depletion in a Drosophila melanogaster model of Parkinson’s-like disease. J. Trace Elem. Med. Biol. 2019, 54, 232–243. [Google Scholar] [CrossRef]
- Voss, G.T.; de Oliveira, R.L.; do Sacramento, M.; Roehrs, J.A.; Alves, D.; Luchese, C.; Wilhelm, E.A. Contribution of antioxidant action of 7-chloro-4-(phenylselanyl)quinoline to treat streptozotocin-induced diabetic neuropathy in mice. New J. Chem. 2022, 46, 19773–19784. [Google Scholar] [CrossRef]
- Domingues, M.; Casaril, A.M.; Birmann, P.T.; Lourenço, D.A.; Vieira, B.; Begnini, K.; Lenardão, E.J.; Collares, T.; Seixas, F.K.; Savegnago, L. Selanylimidazopyridine Prevents Lipopolysaccharide-Induced Depressive-Like Behavior in Mice by Targeting Neurotrophins and Inflammatory/Oxidative Mediators. Front. Neurosci. 2018, 12, 486. [Google Scholar] [CrossRef]
- Domingues, M.; Casaril, A.M.; Smaniotto, T.A.; Birmann, P.T.; Lourenço, D.A.; Bampi, S.R.; Vieira, B.; Lenardão, E.J.; Savegnago, L. Selanylimidazopyridine abolishes inflammation- and stress-induced depressive-like behaviors by modulating the oxido-nitrosative system. Eur. J. Pharmacol. 2022, 914, 174570. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; Rafique, J.; Saba, S.; Grinevicius, V.M.A.S.; Filho, D.W.; Zamoner, A.; Braga, A.L.; Pedrosa, R.C.; Ourique, F. IP-Se-06, a Selenylated Imidazo[1,2-a]pyridine, Modulates Intracellular Redox State and Causes Akt/mTOR/HIF-1α and MAPK Signaling Inhibition, Promoting Antiproliferative Effect and Apoptosis in Glioblastoma Cells. Oxid. Med. Cell. Longev. 2022, 2022, 3710449. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.T.; Seus, N.; de Souza, D.; Rodrigues, O.E.D.; Paixão, M.W.; Jacob, R.G.; Lenardão, E.J.; Perin, G.; Alves, D. Synthesis of [(Arylselanyl)alkyl]-1,2,3-triazoles by Copper-Catalyzed 1,3-Dipolar Cycloaddition of (Arylselanyl)alkynes with Benzyl Azides. Synthesis 2012, 44, 1997–2004. [Google Scholar] [CrossRef]
- Vieira, B.M.; Thurow, S.; Brito, J.S.; Perin, G.; Alves, D.; Jacob, R.G.; Santi, C.; Lenardão, E.J. Sonochemistry: An efficient alternative to the synthesis of 3-selanylindoles using CuI as catalyst. Ultrason. Sonochem. 2015, 27, 192–199. [Google Scholar] [CrossRef]
- Birmann, P.T.; Sousa, F.S.S.; Domingues, M.; Brüning, C.A.; Vieira, B.M.; Lenardão, E.J.; Savegnago, L. 3-(4-Chlorophenylselanyl)-1-methyl-1H-indole promotes recovery of neuropathic pain and depressive-like behavior induced by partial constriction of the sciatic nerve in mice. J. Trace Elem. Med. Biol. 2019, 54, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; Bampi, S.R.; Lourenço, D.A.; Padilha, N.B.; Lenardão, E.J.; Sonego, M.; Seixas, F.K.; Collares, T.; Nogueira, C.W.; et al. The selenium-containing compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole reverses depressive-like behavior induced by acute restraint stress in mice: Modulation of oxido-nitrosative stress and inflammatory pathway. Psychopharmacology 2019, 236, 2867–2880. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; Bampi, S.R.; Lourenço, D.A.; Smaniotto, T.Â.; Segatto, N.; Vieira, B.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. The antioxidant and immunomodulatory compound 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole attenuates depression-like behavior and cognitive impairment developed in a mouse model of breast tumor. Brain Behav. Immun. 2020, 84, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; Lourenço, D.A.; Vieira, B.; Begnini, K.; Corcini, C.D.; França, R.T.; Junior, A.S.V.; Seixas, F.K.; Collares, T.; et al. 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole ameliorates long-lasting depression- and anxiogenic-like behaviors and cognitive impairment in post-septic mice: Involvement of neuroimmune and oxidative hallmarks. Chem. Biol. Interact. 2020, 331, 109278. [Google Scholar] [CrossRef]
- Casaril, A.M.; Lourenço, D.A.; Domingues, M.; Smaniotto, T.A.; Birmann, P.T.; Vieira, B.; Sonego, M.S.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. Anhedonic- and anxiogenic-like behaviors and neurochemical alterations are abolished by a single administration of a selenium-containing compound in chronically stressed mice. Compr. Psychoneuroendocrinol. 2021, 6, 100054. [Google Scholar] [CrossRef]
- Casaril, A.M.; Segatto, N.; Simões, L.; Paschoal, J.; Domingues, M.; Vieira, B.; Sousa, F.S.S.; Lenardão, E.J.; Seixas, F.K.; Collares, T.; et al. Neuroprotective Effect of 3-[(4-Chlorophenyl)selanyl]-1-methyl-1H-indole on Hydrogen Peroxide-Induced Oxidative Stress in SH-SY5Y Cells. Compr. Psychoneuroendocrinol. 2021, 46, 535–549. [Google Scholar] [CrossRef]
- Bampi, S.R.; Casaril, A.M.; Sousa, F.S.S.; Pesarico, A.P.; Vieira, B.; Lenardão, E.J.; Savegnago, L. Repeated administration of a selenium-containing indolyl compound attenuates behavioural alterations by streptozotocin through modulation of oxidative stress in mice. Pharmacol. Biochem. Behav. 2019, 183, 46–55. [Google Scholar] [CrossRef]
- Bampi, S.R.; Casaril, A.M.; Fronza, M.G.; Domingues, M.; Vieira, B.; Begninic, K.R.; Seixas, F.K.; Collares, T.C.; Lenardão, E.J.; Savegnago, L. The selenocompound 1-methyl-3-(phenylselanyl)-1H-indole attenuates depression-like behavior, oxidative stress, and neuroinflammation in streptozotocin-treated mice. Brain Res. Bull. 2020, 161, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Bampi, S.R.; Casaril, A.M.; Domingues, M.; Lourenço, D.A.; Pesarico, A.P.; Vieira, B.; Begnini, K.R.; Seixas, F.K.; Collares, T.V.; Lenardão, E.J.; et al. Depression-like behavior, hyperglycemia, oxidative stress, and neuroinflammation presented in diabetic mice are reversed by the administration of 1-methyl-3-(phenylselanyl)-1H-indole. J. Psychiatr. Res. 2020, 120, 91–102. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.H.; Sousa, F.S.S.; Birmann, P.T.; Pesarico, A.P.; Alves, D.; Jacob, R.G.; Savegnago, L. Evaluation of antioxidant activity and toxicity of sulfur- or selenium-containing 4-(arylchalcogenyl)-1H-pyrazoles. Can. J. Physiol. Pharmacol. 2020, 98, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.H.; Aquino, T.B.; Nascimento, J.E.R.; Perin, G.; Jacob, R.G.; Alves, D. Direct Synthesis of 4-Organylselanylpyrazoles by Copper-Catalyzed One-Pot Cyclocondensation and C-H Bond Selenylation Reactions. Adv. Synth. Catal. 2015, 357, 4041–4049. [Google Scholar] [CrossRef]
- Birmann, P.T.; Casaril, A.M.; Hartwig, D.; Jacob, R.G.; Seixas, F.K.; Collares, T.; Savegnago, L. A novel pyrazole-containing selenium compound modulates the oxidative and nitrergic pathways to reverse the depression-pain syndrome in mice. Brain Res. 2020, 1741, 146880. [Google Scholar] [CrossRef]
- Birmann, P.T.; Domingues, M.; Casaril, A.M.; Smaniotto, T.A.; Hartwig, D.; Jacob, R.G.; Savegnago, L. A pyrazole-containing selenium compound modulates neuroendocrine, oxidative stress, and behavioral responses to acute restraint stress in mice. Behav. Brain Res. 2021, 396, 112874. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Busatto, F.F.; Schaefer, B.T.; Tomasini, P.P.; Nunes, I.J.; Machado, T.S.; Cargnelutti, R.; de Aquino, T.F.B.; Ferreira, K.Q.; Casaril, A.M.; et al. Synthesis, characterization, antioxidant potential, and cytotoxicity screening of new Cu(II) complexes with 4-(arylchalcogenyl)-1H-pyrazoles ligands. J. Inorg. Biochem. 2022, 237, 112013. [Google Scholar] [CrossRef]
- Jacob, R.G.; Hartwig, D.; Nascimento, J.E.R.; Abib, P.B.; Ebersol, C.P.; Nunes, P.P.P.; Birmann, P.T.; Casaril, A.M.; Savegnago, L.; Schumacher, R.F. Sequential one-pot synthesis and antioxidant evaluation of 5-amino-4-(arylselanyl)-1H-pyrazoles. Tetrahedron Lett. 2022, 103, 153992. [Google Scholar] [CrossRef]
- Sheikhi-Mohammareh, S.; Shiri, A.; Beyzaei, H.; Yarmohammadi, E. New efficient design and synthesis of novel antioxidant and antifungal 7-imino[1,3]selenazolo[4,5-d]pyrimidine-5(4H)-thiones utilizing a base-promoted cascade addition/cyclization sequence. Monatsh. Chem. 2020, 151, 963–969. [Google Scholar] [CrossRef]
- Mániková, D.; Šestáková, Z.; Rendeková, J.; Vlasáková, D.; Lukácová, P.; Paegle, E.; Arsenyan, P.; Chovanec, M. Resveratrol-Inspired Benzo[b]selenophenes Act as Anti-Oxidants in Yeast. Molecules 2018, 23, 507. [Google Scholar] [CrossRef]
- Paegle, E.; Domracheva, I.; Turovska, B.; Petrova, M.; Kanepe-Lapsa, I.; Gulbe, A.; Liepinsh, E.; Arsenyan, P. Natural-Antioxidant-Inspired Benzo[b]selenophenes: Synthesis, Redox Properties, and Antiproliferative Activity. Chem. Asian J. 2016, 11, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, M.; Hamama, W.S.; Zooroba, H.H. An Easy Synthetic Approach to Construct Some Ebselen Analogues and Benzo[b]selenophene Derivatives: Their Antioxidant and Cytotoxic Assessment. J. Heterocycl. Chem. 2018, 55, 1645–1650. [Google Scholar] [CrossRef]
- Peglow, T.J.; Bartz, R.H.; Martins, C.C.; Belladona, A.L.; Luchese, C.; Wilhelm, E.A.; Schumacher, R.F.; Perin, G. Synthesis of 2-Organylchalcogenopheno[2,3-b]pyridines from Elemental Chalcogen and NaBH4/PEG-400 as a Reducing System: Antioxidant and Antinociceptive Properties. ChemMedChem 2020, 15, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, A.C.; Plano, D.; Encío, I.; Aydillo, C.; Sharma, A.K.; Carmen Sanmartín, C. Novel selenadiazole derivatives as selective antitumor and radical scavenging agents. Eur. J. Med. Chem. 2018, 157, 14–27. [Google Scholar] [CrossRef]
- Plano, D.; Moreno, E.; Font, M.; Encio, I.; Palop, J.A.; Sanmartin, C. Synthesis and in vitro Anticancer Activities of some Selenadiazole Derivatives. Arch. Pharm. Chem. Life Sci. 2010, 343, 680–691. [Google Scholar] [CrossRef]
- Banerjee, K.; Bhattacherjee, D.; Mahato, S.K.; Sufian, A.; Bhabak, K.P. Benzimidazole- and Imidazole-Fused Selenazolium and Selenazinium Selenocyanates: Ionic Organoselenium Compounds with Efficient Peroxide Scavenging Activities. Inorg. Chem. 2021, 60, 12984–12999. [Google Scholar] [CrossRef]
- Singh, B.G.; Kumar, P.; Phadnis, P.; Iwaoka, M.; Priyadarsini, K.I. Free radical induced selenoxide formation in isomeric organoselenium compounds: The effect of chemical structures on antioxidant activity. New J. Chem. 2019, 43, 13357–13362. [Google Scholar] [CrossRef]
- Arai, K.; Kumakura, F.; Takahira, M.; Sekiyama, N.; Kuroda, N.; Suzuki, T.; Iwaoka, M. Effects of Ring Size and Polar Functional Groups on the Glutathione Peroxidase-Like Antioxidant Activity of Water-Soluble Cyclic Selenides. J. Org. Chem. 2015, 80, 5633–5642. [Google Scholar] [CrossRef]
- Milton, M.D.; Khan, S.; Singh, J.D.; Mishra, V.; Khandelwal, B.L. A facile access to chalcogen and dichalcogen bearing dialkylamines and diols. Tetrahedron Lett. 2005, 46, 755–758. [Google Scholar] [CrossRef]
- Karimi, M.; Ignasiak, M.T.; Chan, B.; Croft, A.K.; Radom, L.; Schiesser, C.H.; Pattison, D.I.; Davies, M.J. Reactivity of disulfide bonds is markedly affected by structure and environment: Implications for protein modification and stability. Sci. Rep. 2016, 6, 38572. [Google Scholar] [CrossRef]
- Gao, Q.; Grzyb, K.; Gamon, L.F.; Ogilby, P.R.; Pędziński, T.; Davies, M.J. The structure of model and peptide disulfides markedly affects their reactivity and products formed with singlet oxygen. Free Radic. Biol. Med. 2023, 207, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Schiesser, C.H. 1,4-Anhydro-4-seleno-d-talitol (SeTal): A remarkable selenium-containing therapeutic molecule. New J. Chem. 2019, 43, 9759–9765. [Google Scholar] [CrossRef]
- Storkey, C.; Davies, M.J.; White, J.M.; Schiesser, C.H. Synthesis and antioxidant capacity of 5-selenopyranose derivatives. Chem. Commun. 2011, 47, 9693–9695. [Google Scholar] [CrossRef] [PubMed]
- Storkey, C.; Pattison, D.I.; White, J.M.; Schiesser, C.H.; Davies, M.J. Preventing Protein Oxidation with Sugars: Scavenging of Hypohalous Acids by 5-Selenopyranose and 4-Selenofuranose Derivatives. Chem. Res. Toxicol. 2012, 25, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Pattison, D.I.; Fu, S.; Schiesser, C.H.; Davies, M.J.; Hawkins, C.L. Catalytic oxidant scavenging by selenium-containing compounds: Reduction of selenoxides and N-chloramines by thiols and redox enzymes. Redox Biol. 2017, 12, 872–882. [Google Scholar] [CrossRef]
- Zacharias, T.; Flouda, K.; Jepps, T.A.; Gammelgaard, B.; Schiesser, C.H.; Davies, M.J. Effects of a novel selenium substituted-sugar (1,4-anhydro-4-seleno-d-talitol, SeTal) on human coronary artery cell lines and mouse aortic rings. Biochem. Pharmacol. 2020, 173, 113631. [Google Scholar] [CrossRef]
- Voss, G.T.; Davies, M.J.; Schiesser, C.H.; de Oliveira, R.L.; Nornberg, A.B.; Soares, V.R.; Barcellos, A.M.; Luchese, C.; Fajardo, A.R.; Wilhelm, E.A. Treating atopic-dermatitis-like skin lesions in mice with gelatin-alginate films containing 1,4-anhydro-4-seleno-d-talitol (SeTal). Int. J. Pharm. 2023, 642, 123174. [Google Scholar] [CrossRef]
- Voss, G.T.; de Oliveira, R.L.; Davies, M.J.; Domingues, W.B.; Campos, V.F.; Soares, M.P.; Luchese, C.; Schiesser, C.H.; Wilhelm, E.A. Suppressive effect of 1,4-anhydro-4-seleno-D-talitol (SeTal) on atopic dermatitis-like skin lesions in mice through regulation of inflammatory mediators. J. Trace Elem. Med. Biol. 2021, 67, 126795. [Google Scholar] [CrossRef]
- Ng, H.H.; Leo, C.H.; O’Sullivan, K.; Alexander, S.-A.; Davies, M.J.; Schiesser, C.H.; Parry, L.J. 1,4-Anhydro-4-seleno-d-talitol (SeTal) protects endothelial function in the mouse aorta by scavenging superoxide radicals under conditions of acute oxidative stress. Biochem. Pharmacol. 2017, 128, 34–45. [Google Scholar] [CrossRef]
- Farzamnezhad, I.; Sheikhi-Mohammareh, S.; Beyzaei, H.; Yarmohammadi, E.; Shiri, A. Synthesis of Novel DPPH-Free Radical Scavenger Se-Containing Fused Chalcogenophenes: 2-Alkyl-7-Cyano-4-Imino-3-Phenyl-6-(pyrrolidin-1-yl)-3,4-Dihydroselenopheno[3,2-d]Pyrimidines. Polycycl. Aromat. Compd. 2023, 1–11. [Google Scholar] [CrossRef]
- Sousa, F.S.S.; Birmann, P.T.; Balaguez, R.; Alves, D.; Brüning, C.A.; Savegnago, L. α-(phenylselanyl) acetophenone abolishes acute restraint stress induced-comorbid pain, depression and anxiety-related behaviors in mice. Neurochem. Int. 2018, 120, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Victoria, F.N.; Radatz, C.S.; Sachini, M.; Jacob, R.G.; Perin, G.; da Silva, W.P.; Lenardão, E.J. KF/Al2O3 and PEG-400 as a recyclable medium for the selective α-selenation of aldehydes and ketones. Preparation of potential antimicrobial agents. Tetrahedron Lett. 2009, 50, 6761–6763. [Google Scholar] [CrossRef]
- Tanini, D.; Lupori, B.; Nostro, P.; Capperucci, A. Synthesis and catalytic antioxidant activity of functionalized chalcogen-containing GPx mimics. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 746–749. [Google Scholar] [CrossRef]
- He, X.; Nie, Y.; Zhong, M.; Li, S.; Li, X.; Guo, Y.; Liu, Z.; Gao, Y.; Ding, F.; Wen, D.; et al. New organoselenides (NSAIDs-Se derivatives) as potential anticancer agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2021, 218, 113384. [Google Scholar] [CrossRef] [PubMed]
- Marwa Sak, M.; Al-Faiyz, Y.S.; Elsawy, H.; Shaaban, S. Novel Organoselenium Redox Modulators with Potential Anticancer, Antimicrobial, and Antioxidant Activities. Antioxidants 2022, 11, 1231. [Google Scholar] [CrossRef]
- Leal, J.G.; Piccoli, B.C.; Oliveira, C.S.; da Silva, F.A.; Omage, F.B.; da Rocha, J.B.T.; Sonego, M.S.; Segatto, N.V.; Seixas, F.K.; Collares, T.V.; et al. Synthesis, antioxidant and antitumoral activity of new 50-arylchalcogenyl-30-N-(E)-feruloyl-30,50-dideoxy-amino-thymidine (AFAT) derivatives. New J. Chem. 2022, 46, 22306–22313. [Google Scholar] [CrossRef]
- Mailahn, D.H.; Araujo, D.R.; Nobre, P.C.; Fonseca, C.A.R.; Penteado, F.; Lenardão, E.J.; Luchese, C.; Wilhelm, E.A.; Perin, G. Benzeneseleninic Acid Promoting the Selenofunctionalization of 2-naphthol Derivatives: Synthesis and Antioxidant Activity of 1-organoselanyl-naphthalen-2-ols. Curr. Chem. Biol. 2023, 17, 56–66. [Google Scholar] [CrossRef]
- Shaaban, S.; Vervandier-Fasseur, D.; Andreoletti, P.; Zarrouk, A.; Richard, P.; Negm, A.; Jacob, G.M.C.; Cherkaoui-Malki, M. Cytoprotective and antioxidant properties of organic selenides for the myelin-forming cells, oligodendrocytes. Bioorg. Chem. 2018, 80, 43–56. [Google Scholar] [CrossRef]
- McMillan, J.D.R.; Sands, K.N.; Cooney, G.S.; Gelfand, B.S.; Back, T.G. Unexpected Formation and Potent Antioxidant Activity of Macrocyclic Dimers Containing Disulfide and Selenide Groups. Angew. Chem. Int. Ed. 2022, 61, e202213744. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, M.; Chahal, A.; Sodhi, N.; Chhillar, B.; Alajangi, H.K.; Barnwal, R.P.; Singh, V.P. Synthesis, Reactions, and Antioxidant Properties of Bis(3-amino-1-hydroxybenzyl)diselenide. J. Org. Chem. 2023, 88, 3509–3522. [Google Scholar] [CrossRef]
- Nobre, P.C.; Vargas, H.A.; Jacoby, C.G.; Schneider, P.H.; Casaril, A.M.; Savegnago, L.; Schumacher, R.F.; Lenardão, E.J.; Ávila, D.S.; Junior, L.B.L.R.; et al. Synthesis of enantiomerically pure glycerol derivatives containing an organochalcogen unit: In vitro and in vivo antioxidant activity. Arab. J. Chem. 2020, 13, 883–899. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Ramos-Inza, S.; Aydillo, C.; Talavera, I.; Encío, I.; Plano, D.; Sanmartín, C. Novel N,N′-Disubstituted Acylselenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2020, 9, 55. [Google Scholar] [CrossRef]
- Calvo-Martín, G.; Plano, D.; Encío, I.; Sanmartín, C. Novel N,N′-Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2021, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Ahmed, N.; Pezzuto, J.M.; Kondratyuk, T.P.; Park, E.-J.; Hussain, I. Synthesis, characterization and biological applications of selenoureas having ferrocene and substituted benzoyl functionalities. Polyhedron 2019, 170, 12–24. [Google Scholar] [CrossRef]
- Tanini, D.; Bonardi, C.; Viglianisi, C.; Capperucci, A.; Menichetti, S. Towards New Catalytic Antioxidants: A Simple and Mild Synthesis of Selenenylsulfides. Catalysts 2019, 9, 333. [Google Scholar] [CrossRef]
- Vogt, A.G.; Perin, G.; Luchese, C.; da Silva, P.C.; Wilhelm, E.A.; Silva, M.S. Organylselanyl α-Amino Phosphonates: Synthesis, NMR Spectroscopic Study, and Antioxidant and Antinociceptive Activities. Eur. J. Org. Chem. 2018, 2018, 627–639. [Google Scholar] [CrossRef]
- Mailahn, D.H.; Iarocz, L.E.B.; Nobre, P.C.; Perin, G.; Sinott, A.; Pesarico, A.P.; Birmann, P.T.; Savegnago, L.; Silva, M.S. A greener protocol for the synthesis of phosphorochalcogenoates: Antioxidant and free radical scavenging activities. Eur. J. Med. Chem. 2021, 213, 113052. [Google Scholar] [CrossRef]
- Newton, T.D.; Bolton, S.G.; Garcia, A.C.; Chouinard, J.E.; Golledge, S.L.; Zakharov, L.N.; Pluth, M.D. Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donors for Intracellular H2Se Delivery. J. Am. Chem. Soc. 2021, 143, 19542–19550. [Google Scholar] [CrossRef]
- Frizon, T.E.A.; Cararo, J.H.; Saba, S.; Dal-Pont, G.C.; Michels, M.; Braga, H.C.; Pimentel, T.; Dal-Pizzol, F.; Valvassori, S.S.; Rafique, J. Synthesis of Novel Selenocyanates and Evaluation of Their Effect in Cultured Mouse Neurons Submitted to Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5417024. [Google Scholar] [CrossRef]
- Al-Abdallah, B.; Al-Faiyz, Y.S.; Shaaban, S. Organoselenocyanates Tethered Methyl Anthranilate Hybrids with Promising Anticancer, Antimicrobial, and Antioxidant Activities. Inorganics 2022, 10, 246. [Google Scholar] [CrossRef]
- Nornberg, A.B.; de Aquino, T.F.B.; Martins, C.C.; Luchese, C.; Wilhelm, E.A.; Jacob, R.G.; Hartwig, D.; Fajardo, A.R. Organoselenium-chitosan derivative: Synthesis via “click” reaction, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Elshaflu, H.; Todorovic, T.R.; Nikolic, M.; Lolic, A.; Višnjevac, A.; Hagenow, S.; Padrón, J.M.; García-Sosa, A.T.; Djordjevic, I.S.; Grubišic, S.; et al. Selenazolyl-hydrazones as Novel Selective MAO Inhibitors with Antiproliferative and Antioxidant Activities: Experimental and In-silico Studies. Front. Chem. 2018, 6, 247. [Google Scholar] [CrossRef]
- Gall, J.I.; Alves, A.G.; Júnior, L.R.C.; Rech, T.S.T.; Neto, J.S.S.; Alves, D.; Soares, M.S.P.; Spohr, L.; Spanevello, R.M.; Brüning, C.A.; et al. Insights into serotonergic and antioxidant mechanisms involved in antidepressant-like action of 2-phenyl-3-(phenylselanyl)benzofuran in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109956. [Google Scholar] [CrossRef] [PubMed]
- Gay, R.M.; Manarin, F.; Schneider, C.C.; Barancelli, D.A.; Costa, M.D.; Zeni, G. FeCl3-Diorganyl Dichalcogenides Promoted Cyclization of 2-Alkynylanisoles to 3-Chalcogen Benzo[b]furans. J. Org. Chem. 2010, 75, 5701–5706. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]





















































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghinoni, J.M.; Birmann, P.T.; da Rocha, M.J.; Gomes, C.S.; Davies, M.J.; Brüning, C.A.; Savegnago, L.; Lenardão, E.J. Recent Advances in the Synthesis and Antioxidant Activity of Low Molecular Mass Organoselenium Molecules. Molecules 2023, 28, 7349. https://doi.org/10.3390/molecules28217349
Anghinoni JM, Birmann PT, da Rocha MJ, Gomes CS, Davies MJ, Brüning CA, Savegnago L, Lenardão EJ. Recent Advances in the Synthesis and Antioxidant Activity of Low Molecular Mass Organoselenium Molecules. Molecules. 2023; 28(21):7349. https://doi.org/10.3390/molecules28217349
Chicago/Turabian StyleAnghinoni, João M., Paloma T. Birmann, Marcia J. da Rocha, Caroline S. Gomes, Michael J. Davies, César A. Brüning, Lucielli Savegnago, and Eder J. Lenardão. 2023. "Recent Advances in the Synthesis and Antioxidant Activity of Low Molecular Mass Organoselenium Molecules" Molecules 28, no. 21: 7349. https://doi.org/10.3390/molecules28217349
APA StyleAnghinoni, J. M., Birmann, P. T., da Rocha, M. J., Gomes, C. S., Davies, M. J., Brüning, C. A., Savegnago, L., & Lenardão, E. J. (2023). Recent Advances in the Synthesis and Antioxidant Activity of Low Molecular Mass Organoselenium Molecules. Molecules, 28(21), 7349. https://doi.org/10.3390/molecules28217349








