The Mechanisms of Polysaccharides from Tonic Chinese Herbal Medicine on the Enhancement Immune Function: A Review
Abstract
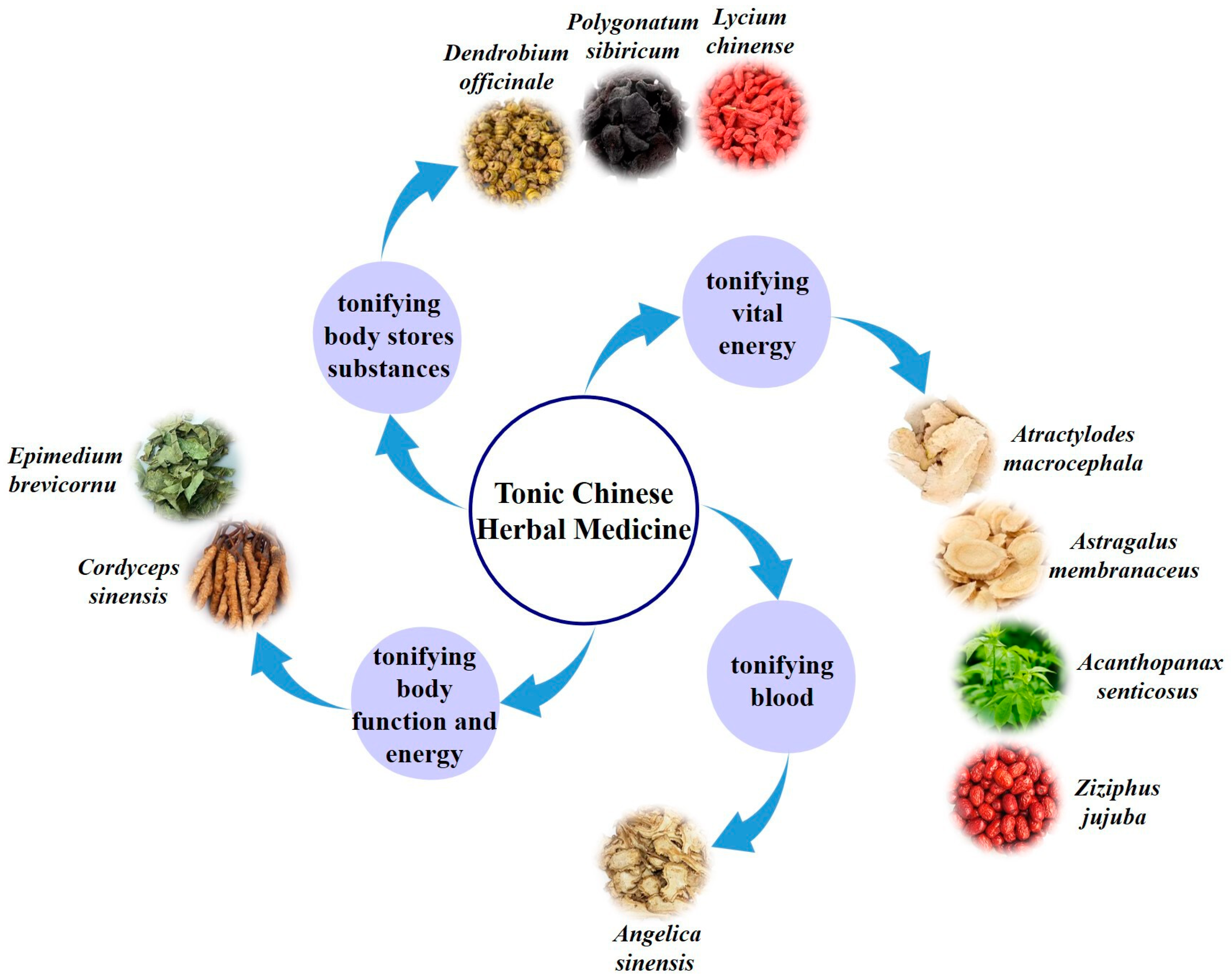
:1. Introduction
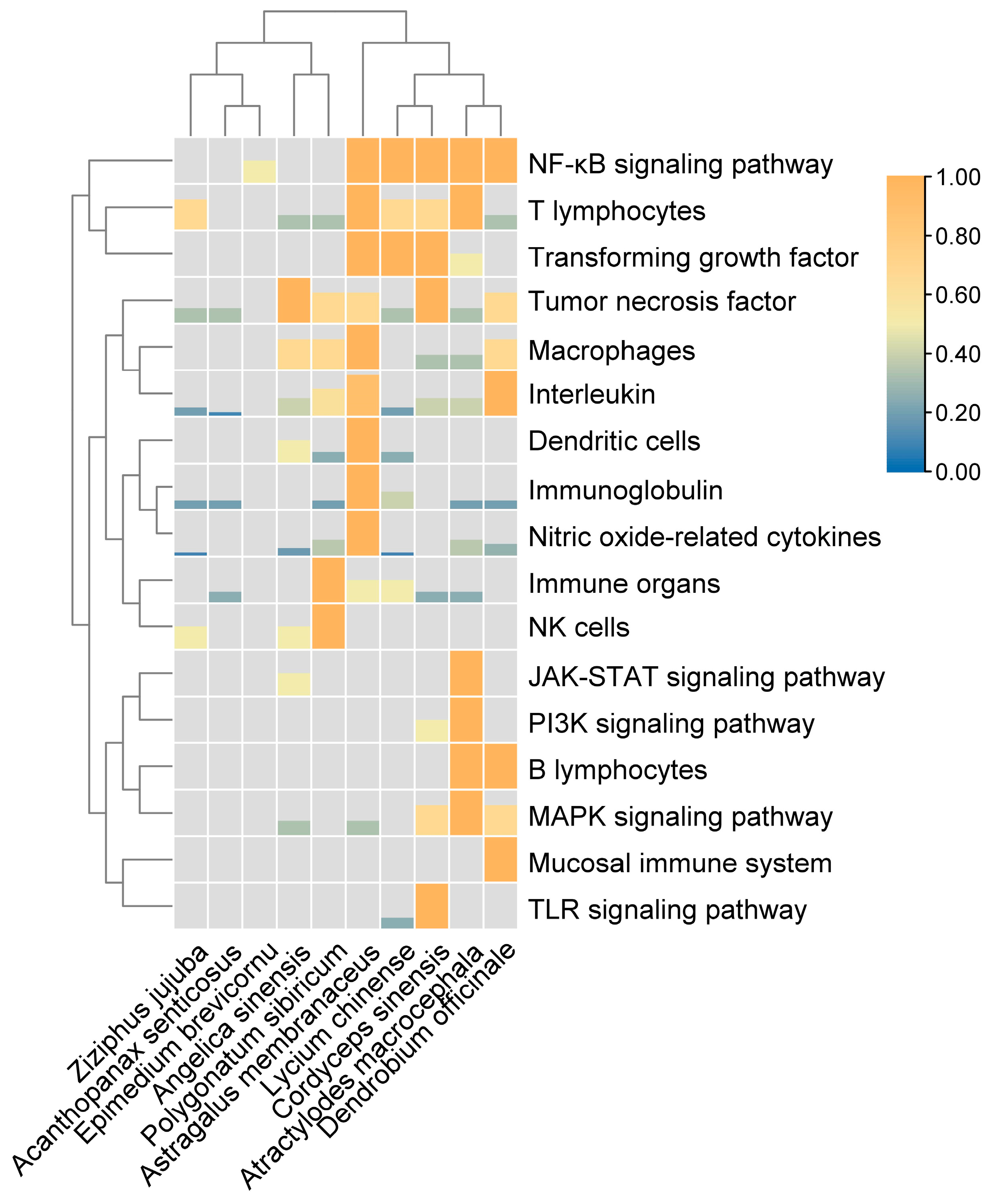
2. The Effects of Tonic Chinese Herbal Medicine Polysaccharides on Organs of the Immune System
2.1. The Effects of Tonic Chinese Herbal Medicine Polysaccharides on the Thymus, Spleen, and Bursa of Fabricius
2.2. The Effects of Tonic Chinese Herbal Medicine Polysaccharides on the Mucosal Immune System
3. The Effects of Tonic Chinese Herbal Medicine Polysaccharides on Immune Cells
3.1. The Effects of Tonic Chinese Herbal Medicine Polysaccharides on Macrophages
3.2. The Effects of Tonic Chinese Herbal Medicine Polysaccharides on Dendritic Cells
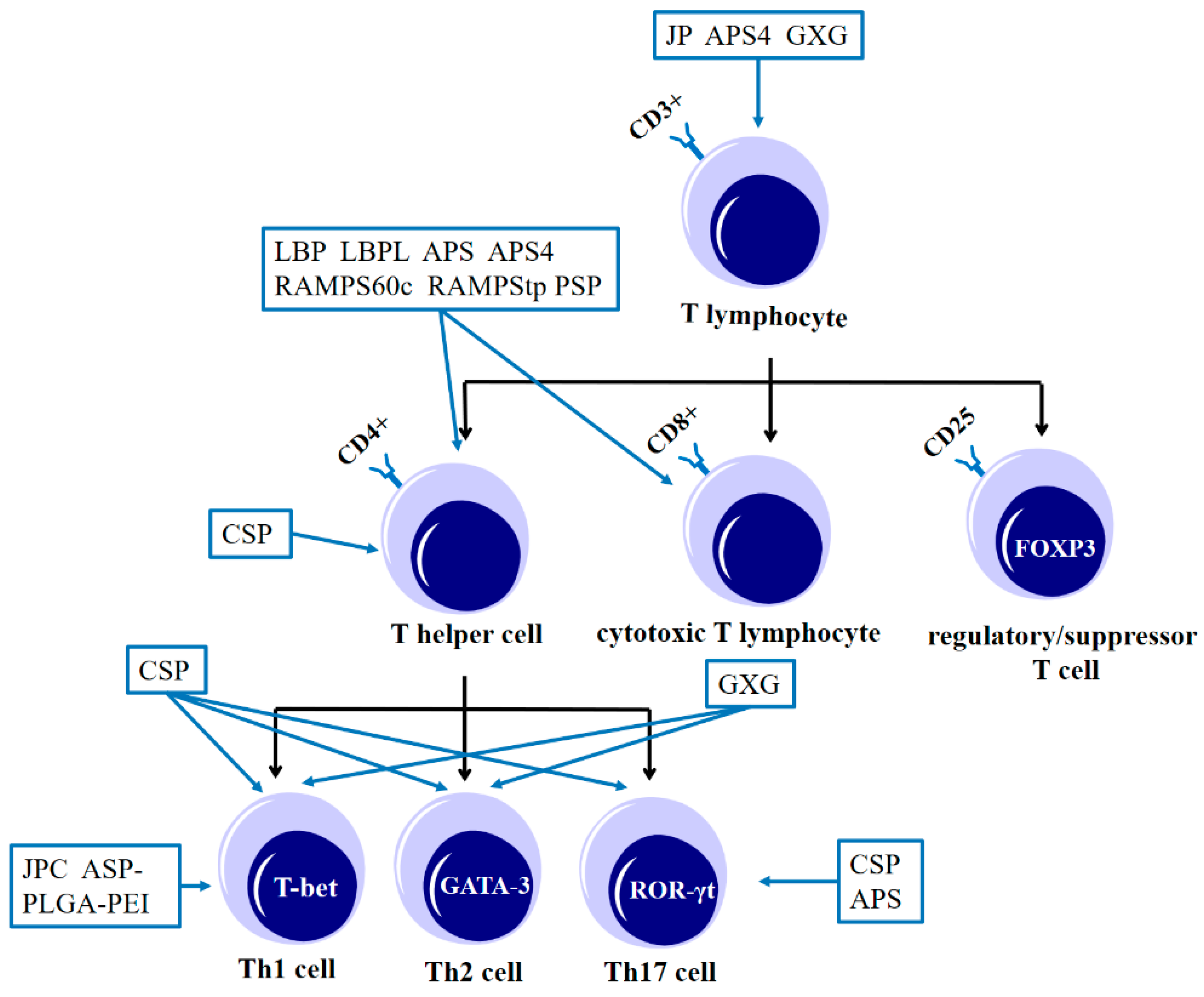
3.3. The Effect of Tonic Chinese Herbal Medicine Polysaccharide on T Lymphocytes
3.4. The Effect of Tonic Chinese Herbal Medicine Polysaccharide on B Lymphocytes
3.5. The Effects of Polysaccharides from Tonic Chinese Herbal Medicine on NK Cells
4. The Effect of Tonic Chinese Herbal Medicine Polysaccharides on Cytokines
4.1. The Effect of Tonic Chinese Herbal Medicine Polysaccharides on Immunoglobulin
4.2. The Effect of Tonic Chinese Herbal Medicine Polysaccharides on Interleukin
4.3. The Effects of Tonic Chinese Herbal Medicine Polysaccharides on Nitric Oxide-Related Cytokines
4.4. The Effect of Tonic Chinese Herbal Medicine Polysaccharides on Transforming Growth Factor
4.5. The Effect of Tonic Chinese Herbal Medicine Polysaccharides on Tumor Necrosis Factor
4.6. The Effects of Tonic Chinese Herbal Medicine Polysaccharides on Other Related Cytokines
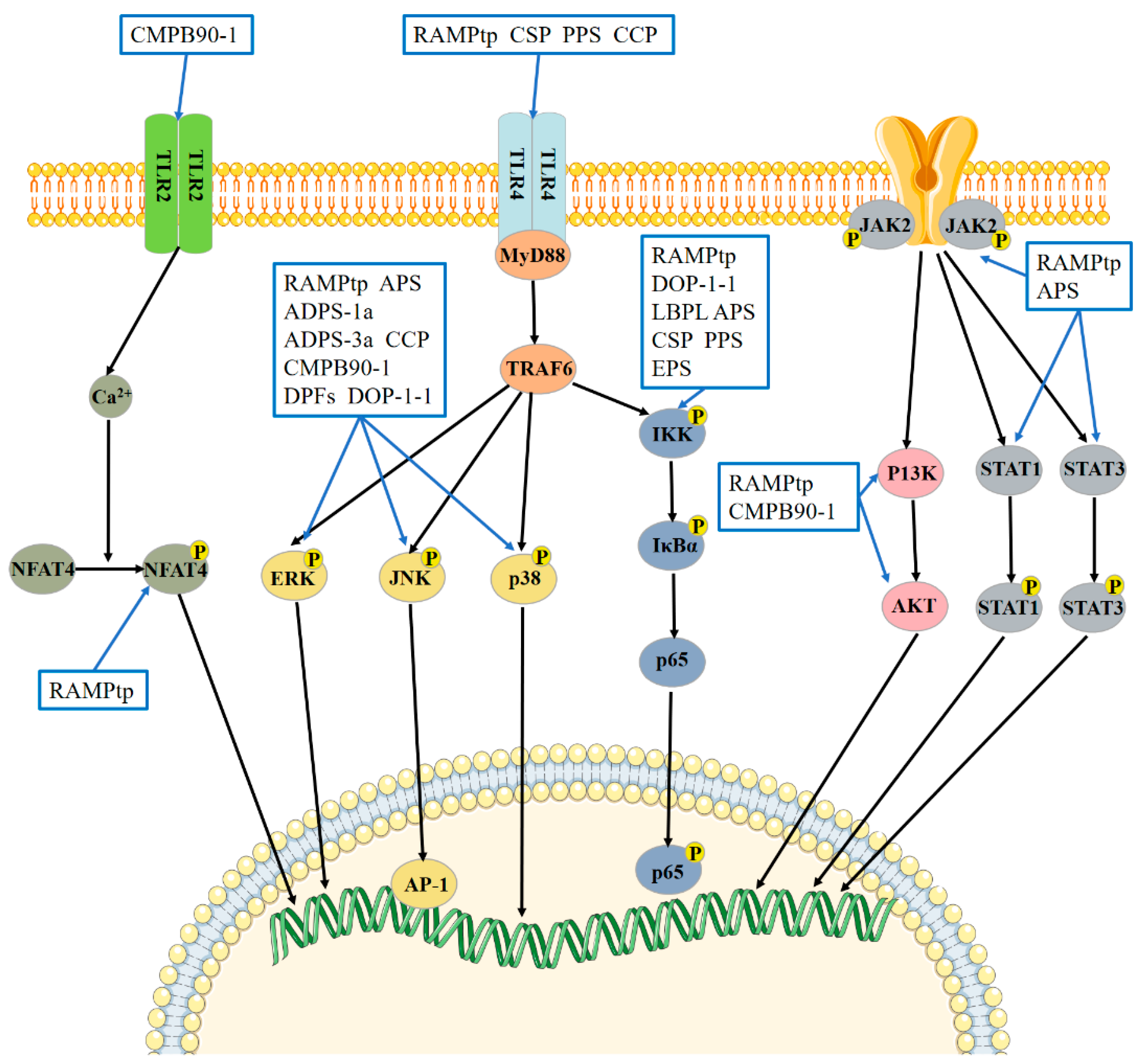
5. Study on the Mechanism of Tonic Chinese Herbal Medicine Polysaccharides to Enhancing Immunity
5.1. Polysaccharides of Tonic Chinese Herbal Medicine Activate the MAPK Signaling Pathway to Enhance Immunity
5.2. Tonic Chinese Herbal Medicine Polysaccharides Activates the NF-κB Signaling Pathway to Enhance Immunity
5.3. Polysaccharides of Tonic Chinese Herbal Medicine Activate the TLR Signaling Pathway to Enhance Immunity
5.4. Polysaccharides of Tonic Chinese Herbal Medicine Activate the JAK-STAT Signaling Pathway to Enhance Immunity
5.5. Tonic Chinese Herbal Medicine Polysaccharides Activate Other Signaling Pathways to Enhance Immunity
6. Safety Evaluation of Tonic Chinese Herbal Medicine Polysaccharides
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| LBP | A crude polysaccharide from Lycium chinense |
| APS | A polysaccharide from Astragalus membranaceus |
| ASPS | A crude polysaccharide from Acanthopanax senticosus |
| PPS | A purified polysaccharide from Cordyceps sinensis |
| PSP | A crude polysaccharide from Polygonatum sibiricum |
| PSP3 | A purified polysaccharide from PSP |
| RAMPS60c | A purified polysaccharide from RAMPS, a polysaccharide from Atractylodes macrocephala |
| RAMPStp | A purified polysaccharide from RAMPS, a polysaccharide from Atractylodes macrocephala |
| PP nodes | Pyle’s collecting lymph nodes |
| DOP-W3-b | A purified polysaccharide from Dendrobium officinale |
| MLNs | Mesenteric lymph nodes |
| NK cells | Natural killer cells |
| Dendrobium CPs | A crude polysaccharide from Dendrobium |
| ADPS-1a | A purified polysaccharide from Angelica sinensis |
| ADPS-3a | A purified polysaccharide from Angelica sinensis |
| PSPC | A purified polysaccharide from PSP |
| PSPW | A wine-processed PSP |
| APS | An alcohol-soluble polysaccharide was extracted from Astragalus membranaceus |
| DSP | A purified polysaccharide from Dendrobium officinale |
| IFN-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor α |
| IL-12 | Interleukin-12 |
| IL-1β | Interleukin-1β |
| CD80 | Cluster of differentiation 80 |
| CD86 | Cluster of differentiation 86 |
| MHC-II | Major histocompatibility complex-II |
| M2 | Macrophage 2 |
| IL-4 | Interleukin-4 |
| IL-10 | Interleukin-10 |
| CD206 | Cluster of differentiation 206 |
| CMPB90-1 | A purified polysaccharide from Cordyceps sinensis |
| ISAg | A purified polysaccharide from Angelica sinensis |
| RAMPtp | A purified polysaccharide from Atractylodes macrocephala Koidz. |
| PG2 | A purified polysaccharide from APS |
| PLGA | Polylactic acid/glycolic acid |
| M1 | Macrophage 1 |
| DC cells | Dendritic cells |
| MHC | Major histocompatibility complex |
| CTAB-modified PSP-Cubs/OVA | Cetyltrimethylammonium bromide-modified Polygonatum sibiricum polysaccharide cubosomes |
| ASP-PLGA-PEI | Polyethylenimine-coated PLGA nanoparticles containing ASP, a polysaccharide from Angelica sinensis, system |
| MDDCs | Monocyte-derived dendritic cells |
| CD25 | Cluster of differentiation 25 |
| JP | A crude polysaccharide from Ziziphus jujuba |
| APS4 | A purified polysaccharide from APS |
| GXG | A purified polysaccharide from DOP |
| LBPL | LBP liposome |
| CSP | A crude polysaccharide from Cordyceps sinensis |
| GATA3 | GATA binding protein 3 |
| ROR-γt | Retinoic acid-related orphan receptor-γt |
| JPC | JP conjugates |
| ADP | A crude polysaccharide from Angelica dahurica |
| IgG | Immunoglobulin G |
| IgA | Immunoglobulin A |
| IgM | Immunoglobulin M |
| IL-1 | Interleukin-1 |
| IL-2 | Interleukin-2 |
| TNF-α | Tumor necrosis factor-α |
| TGF-β | Transforming growth factor-β |
| IgE | Immunoglobulin E |
| LMw-APS | A purified polysaccharide from APS |
| SIgA | Secretory immunoglobulin A |
| APH | Acetyl phenyl hydrazine |
| CTX | Cyclophosphamide |
| SPSP | A polysaccharide from steam-processed Polygonatum sibiricum |
| DDP | A polysaccharide from Dendrobium devonianum |
| UDP-1 | A purified polysaccharide from unfermented Dendrobium |
| FDP-1 | A polysaccharide from fermented Dendrobium |
| FLP-1 | A polysaccharide from fermented FDP-1 liquid |
| DOPA-1 | A purified polysaccharides from DOPA |
| DOPA-2 | A purified polysaccharides from DOPA |
| DOP-1-1 | A purified polysaccharide from DOP |
| THP-1 | Tohoku Hospital Pediatrics-1 |
| CCP | A purified polysaccharide from CSP |
| CFS | Chronic fatigue |
| iNOS | Nitric oxide synthase |
| NO | Nitric oxide |
| sCAP2 | A purified polysaccharides from ADP |
| RAMAP-1 | A purified polysaccharides from RAMP |
| RAMAP-2 | A purified polysaccharides from RAMP |
| RAMAP-3 | A purified polysaccharides from RAMP |
| GLP | A polysaccharide from Ganoderma lucidum |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-beta |
| TLR | Toll-like receptor |
| JAK-STAT | Janus kinase-signal transducer and activator of transcription |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| DPFs | A purified polysaccharide from DOP |
| EPS | A polysaccharide from Epimedium |
| MyD88 | Myeloiddifferentiationfactor88 |
| PI3K | Phosphatidylinositol 3-kinase |
| Akt | Protein kinase-B |
References
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, M.; Sun, R.; Pan, L. Extraction, characterization of a Ginseng fruits polysaccharide and its immune modulating activities in rats with Lewis lung carcinoma. Carbohydr. Polym. 2015, 127, 215–221. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J. Scutellaria polysaccharide inhibits the infectivity of Newcastle disease virus to chicken embryo fibroblast. J. Sci. Food Agric. 2014, 94, 779–784. [Google Scholar] [CrossRef]
- Chu, Q.; Jia, R.; Chen, M.; Li, Y.; Yu, X.; Wang, Y.; Chen, W.; Ye, X.; Liu, Y.; Jiang, Y.; et al. Tetrastigma hemsleyanum tubers polysaccharide ameliorates LPS-induced inflammation in macrophages and Caenorhabditis elegans. Int. J. Biol. Macromol. 2019, 141, 611–621. [Google Scholar] [CrossRef]
- Ayeka, P.A.; Bian, Y.; Githaiga, P.M.; Zhao, Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement. Altern. Med. 2017, 17, 536. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Xian, L.; Song, M.; Zeng, L.; Xiong, W.; Liu, J.; Sun, W.; Wang, D.; Hu, Y. Effects of Bush Sophora Root polysaccharide and its sulfate on immuno-enhancing of the therapeutic DVH. Int. J. Biol. Macromol. 2015, 80, 217–224. [Google Scholar] [CrossRef]
- Madej, J.P.; Stefaniak, T.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chickens. Poult. Sci. 2015, 94, 1209–1219. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.C.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020, 121, 109591. [Google Scholar] [CrossRef]
- Yu, J.; Dong, X.D.; Jiao, J.S.; Ji, H.Y.; Liu, A.J. Antitumor and immunoregulatory activities of a novel polysaccharide from Astragalus membranaceus on S180 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 189, 930–938. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef]
- Meng, Q.; Pan, J.; Liu, Y.; Chen, L.; Ren, Y. Anti-tumour effects of polysaccharide extracted from Acanthopanax senticosus and cell-mediated immunity. Exp. Ther. Med. 2018, 15, 1694–1701. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef]
- Meng, M.; Wang, H.; Li, Z.; Guo, M.; Hou, L. Protective effects of polysaccharides from Cordyceps gunnii mycelia against cyclophosphamide-induced immunosuppression to TLR4/TRAF6/NF-kappaB signalling in BALB/c mice. Food Funct. 2019, 10, 3262–3271. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Kong, X.; Li, H. Characterization and Immunological Activities of Polysaccharides from Polygonatum sibiricum. Biol. Pharm. Bull. 2020, 43, 959–967. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, N.; Xue, X.; Li, Q.; Sun, D.; Zhao, Z. Purification, structural characterization and in vivo immunoregulatory activity of a novel polysaccharide from Polygonatum sibiricum. Int. J. Biol. Macromol. 2020, 160, 688–694. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Zhao, J.; Yin, L.; Lin, J.; Fu, H.; Tang, H.; Fang, J.; Peng, X.; Zhao, X. Protective effect of Polygonatum sibiricum polysaccharide on cyclophosphamide-induced immunosuppression in chickens. Res. Vet. Sci. 2021, 135, 96–105. [Google Scholar] [CrossRef]
- Xue, L.; Wang, D.; Zhang, D.; Ju, A.; Duan, A.; Xing, J.; Qin, Y.; Yang, S.; Luan, W. The immune adjuvant effect of Astragalus polysaccharide on in ovo injection of Newcastle disease vaccine. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1719–1726. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, W.; Zhang, S.; Meng, G.; Qi, C.; Fan, W.; Wang, Y.; Liu, J. The immune adjuvant response of polysaccharides from Atractylodis macrocephalae Koidz in chickens vaccinated against Newcastle disease (ND). Carbohydr. Polym. 2016, 141, 190–196. [Google Scholar] [CrossRef]
- Xie, S.Z.; Liu, B.; Zhang, D.D.; Zha, X.Q.; Pan, L.H.; Luo, J.P. Intestinal immunomodulating activity and structural characterization of a new polysaccharide from stems of Dendrobium officinale. Food Funct. 2016, 7, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zuo, H.; Mahony, T.J.; Zhang, B.; Rolfe, B.; Xu, Z.P. Efficient induction of comprehensive immune responses to control pathogenic E. coli by clay nano-adjuvant with the moderate size and surface charge. Sci. Rep. 2017, 7, 13367. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.Z.; Lv, G.P.; Hu, D.J.; Cheong, K.L.; Xie, J.; Zhao, J.; Li, S.P. Effects of polysaccharides from different species of Dendrobium (Shihu) on macrophage function. Molecules 2013, 18, 5779–5791. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Zhang, H.; Liu, Z.; Ma, C.; Kang, W. Immunomodulation of ADPs-1a and ADPs-3a on RAW264.7 cells through NF-κB/MAPK signaling pathway. Int. J. Biol. Macromol. 2019, 132, 1024–1030. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, H.; Li, Y.; Liu, Y.; Dai, W.; Fang, J.; Cao, C.; Die, Y.; Liu, Q.; Wang, C.; et al. Physicochemical properties and immunological activities of polysaccharides from both crude and wine-processed Polygonatum sibiricum. Int. J. Biol. Macromol. 2020, 143, 255–264. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.Y.; Liu, A.J. Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 2018, 118, 2057–2064. [Google Scholar] [CrossRef]
- Liu, W.; Yan, R.; Zhang, L. Dendrobium sonia polysaccharide regulates immunity and restores the dysbiosis of the gut microbiota of the cyclophosphamide-induced immunosuppressed mice. Chin. J. Nat. Med. 2019, 17, 600–607. [Google Scholar] [CrossRef]
- Gong, M.; Zhuo, X.; Ma, A. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med. Sci. Monit. Basic. Res. 2017, 23, 240–249. [Google Scholar] [CrossRef]
- Savai, R.; Schermuly, R.T.; Pullamsetti, S.S.; Schneider, M.; Greschus, S.; Ghofrani, H.A.; Traupe, H.; Grimminger, F.; Banat, G.A. A combination hybrid-based vaccination/adoptive cellular therapy to prevent tumor growth by involvement of T cells. Cancer Res. 2007, 67, 5443–5453. [Google Scholar] [CrossRef]
- Bi, S.; Huang, W.; Chen, S.; Huang, C.; Li, C.; Guo, Z.; Yang, J.; Zhu, J.; Song, L.; Yu, R. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int. J. Biol. Macromol. 2020, 150, 261–280. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Park, H.J.; Lee, S.H.; Im, W.K.; Kim, Y.D.; Kim, K.H.; Park, S.J.; Hong, S.; Jeon, S.H. Anti-cancer activity of Angelica gigas by increasing immune response and stimulating natural killer and natural killer T cells. BMC Complement. Altern. Med. 2018, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fang, S.; Wang, Y.; Zhang, T.; Hu, S. Molecular mechanisms associated with macrophage activation by Rhizoma Atractylodis macrocephalae polysaccharides. Int. J. Biol. Macromol. 2020, 147, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Bamodu, O.A.; Kuo, K.T.; Wang, C.H.; Huang, W.C.; Wu, A.T.H.; Tsai, J.T.; Lee, K.Y.; Yeh, C.T.; Wang, L.S. Astragalus polysaccharides (PG2) Enhances the M1 Polarization of Macrophages, Functional Maturation of Dendritic Cells, and T Cell-Mediated Anticancer Immune Responses in Patients with Lung Cancer. Nutrients 2019, 11, 2264. [Google Scholar] [CrossRef]
- Xu, S.; Feng, Z.; Zhang, Y.; Ni, H.; Liu, Z.; Wang, D. pH-responsive Astragalus polysaccharide-loaded PLGA nanoparticles as an adjuvant system to improve immune responses. Int. J. Biol. Macromol. 2022, 222, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.W.; Park, C.G.; et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ni, H.; Yu, L.; Xu, S.; Bo, R.; Qiu, T.; Gu, P.; Zhu, T.; He, J.; Wusiman, A.; et al. Adjuvant activities of CTAB-modified Polygonatum sibiricum polysaccharide cubosomes on immune responses to ovalbumin in mice. Int. J. Biol. Macromol. 2020, 148, 793–801. [Google Scholar] [CrossRef]
- Gu, P.; Cai, G.; Yang, Y.; Hu, Y.; Liu, J.; Wang, D. Polyethylenimine-coated PLGA nanoparticles containing Angelica sinensis polysaccharide promote dendritic cells activation and associated molecular mechanisms. Int. J. Biol. Macromol. 2022, 207, 559–569. [Google Scholar] [CrossRef]
- Xiong, J.; Jiang, B.; Luo, Y.; Zou, J.; Gao, X.; Xu, D.; Du, Y.; Hao, L. Multifunctional Nanoparticles Encapsulating Astragalus Polysaccharide and Gold Nanorods in Combination with Focused Ultrasound for the Treatment of Breast Cancer. Int. J. Nanomed. 2020, 15, 4151–4169. [Google Scholar] [CrossRef]
- Du, X.; Zhao, B.; Li, J.; Cao, X.; Diao, M.; Feng, H.; Chen, X.; Chen, Z.; Zeng, X. Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice. Int. Immunopharmacol. 2012, 14, 463–470. [Google Scholar] [CrossRef]
- Bo, R.; Liu, Z.; Zhang, J.; Gu, P.; Ou, N.; Sun, Y.; Hu, Y.; Liu, J.; Wang, D. Mechanism of Lycium barbarum polysaccharides liposomes on activating murine dendritic cells. Carbohydr. Polym. 2019, 205, 540–549. [Google Scholar] [CrossRef]
- An, E.K.; Zhang, W.; Kwak, M.; Lee, P.C.; Jin, J.O. Polysaccharides from Astragalus membranaceus elicit T cell immunity by activation of human peripheral blood dendritic cells. Int. J. Biol. Macromol. 2022, 223, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Zhang, W.; Dhananjay, Y.; An, E.K.; Kwak, M.; You, S.; Lee, P.C.; Jin, J.O. Astragalus membranaceus polysaccharides potentiate the growth-inhibitory activity of immune checkpoint inhibitors against pulmonary metastatic melanoma in mice. Int. J. Biol. Macromol. 2021, 182, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, D.; Sher, A.; Yap, G. Th1/Th2 effector choice in parasitic infection: Decision making by committee. Curr. Opin. Immunol. 2001, 13, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Knocke, S.; Fleischmann-Mundt, B.; Saborowski, M.; Manns, M.P.; Kuhnel, F.; Wirth, T.C.; Woller, N. Tailored Tumor Immunogenicity Reveals Regulation of CD4 and CD8 T Cell Responses against Cancer. Cell Rep. 2016, 17, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: Molecular biology. Int. J. Clin. Oncol. 2010, 15, 544–551. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chang, M.C.; Chen, C.A.; Lin, H.W.; Cheng, W.F.; Chien, C.L. Depletion of regulatory T lymphocytes reverses the imbalance between pro- and anti-tumor immunities via enhancing antigen-specific T cell immune responses. PLoS ONE 2012, 7, e47190. [Google Scholar] [CrossRef]
- Xu, W.; Fang, S.; Cui, X.; Guan, R.; Wang, Y.; Shi, F.; Hu, S. Signaling pathway underlying splenocytes activation by polysaccharides from Atractylodis macrocephalae Koidz. Mol. Immunol. 2019, 111, 19–26. [Google Scholar] [CrossRef]
- Sun, W.; Meng, K.; Qi, C.; Yang, X.; Wang, Y.; Fan, W.; Yan, Z.; Zhao, X.; Liu, J. Immune-enhancing activity of polysaccharides isolated from Atractylodis macrocephalae Koidz. Carbohydr. Polym. 2015, 126, 91–96. [Google Scholar] [CrossRef]
- Han, X.; Bai, B.; Zhou, Q.; Niu, J.; Yuan, J.; Zhang, H.; Jia, J.; Zhao, W.; Chen, H. Dietary supplementation with polysaccharides from Ziziphus Jujuba cv. Pozao intervenes in immune response via regulating peripheral immunity and intestinal barrier function in cyclophosphamide-induced mice. Food Funct. 2020, 11, 5992–6006. [Google Scholar] [CrossRef]
- Xie, S.Z.; Liu, B.; Ye, H.Y.; Li, Q.M.; Pan, L.H.; Zha, X.Q.; Liu, J.; Duan, J.; Luo, J.P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef]
- Bo, R.; Sun, Y.; Zhou, S.; Ou, N.; Gu, P.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Simple nanoliposomes encapsulating Lycium barbarum polysaccharides as adjuvants improve humoral and cellular immunity in mice. Int. J. Nanomed. 2017, 12, 6289–6301. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Fang, Q.; Dong, N.; Fang, Q.; Cui, S.W.; Nie, S. A polysaccharide from natural Cordyceps sinensis regulates the intestinal immunity and gut microbiota in mice with cyclophosphamide-induced intestinal injury. Food Funct. 2021, 12, 6271–6282. [Google Scholar] [CrossRef]
- Murphy, K.M.; Reiner, S.L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002, 2, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Kuchroo, V.K. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 2015, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Chi, A.; Kang, C.; Zhang, Y.; Tang, L.; Guo, H.; Li, H.; Zhang, K. Immunomodulating and antioxidant effects of polysaccharide conjugates from the fruits of Ziziphus Jujube on Chronic Fatigue Syndrome rats. Carbohydr. Polym. 2015, 122, 189–196. [Google Scholar] [CrossRef]
- Gu, P.; Wusiman, A.; Wang, S.; Zhang, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant to enhance immune responses. Carbohydr. Polym. 2019, 223, 115128. [Google Scholar] [CrossRef]
- Song, B.; Li, P.; Yan, S.; Liu, Y.; Gao, M.; Lv, H.; Lv, Z.; Guo, Y. Effects of Dietary Astragalus Polysaccharide Supplementation on the Th17/Treg Balance and the Gut Microbiota of Broiler Chickens Challenged with Necrotic Enteritis. Front. Immunol. 2022, 13, 781934. [Google Scholar] [CrossRef]
- LeBien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Li, W.; Guo, S.; Xu, D.; Li, B.; Cao, N.; Tian, Y.; Jiang, Q. Polysaccharide of Atractylodes macrocephala Koidz (PAMK) Relieves Immunosuppression in Cyclophosphamide-Treated Geese by Maintaining a Humoral and Cellular Immune Balance. Molecules 2018, 23, 932. [Google Scholar] [CrossRef]
- Dong, X.D.; Liu, Y.N.; Zhao, Y.; Liu, A.J.; Ji, H.Y.; Yu, J. Structural characterization of a water-soluble polysaccharide from Angelica dahurica and its antitumor activity in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 193, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Coffman, R.L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 1989, 46, 111–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018, 97, 3489–3493. [Google Scholar] [CrossRef] [PubMed]
- Long, L.N.; Zhang, H.H.; Wang, F.; Yin, Y.X.; Yang, L.Y.; Chen, J.S. Research Note: Effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult. Sci. 2021, 100, 101028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, D.; Li, X.; Guo, Z.; Liu, Y.; Ma, X.; Zheng, S. PEI-modified macrophage cell membrane-coated PLGA nanoparticles encapsulating Dendrobium polysaccharides as a vaccine delivery system for ovalbumin to improve immune responses. Int. J. Biol. Macromol. 2020, 165, 239–248. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, L.; Zhang, W.; Cui, X.; Zhen, Y.; Suolangzhaxi; Song, X. Liposome can improve the adjuvanticity of astragalus polysaccharide on the immune response against ovalbumin. Int. J. Biol. Macromol. 2013, 60, 206–212. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, C.; Qu, J.; Wang, G. Structural characterization of low molecular weight polysaccharide from Astragalus membranaceus and its immunologic enhancement in recombinant protein vaccine against systemic candidiasis. Carbohydr. Polym. 2016, 145, 48–55. [Google Scholar] [CrossRef]
- Schmidt, L.D.; Xie, Y.; Lyte, M.; Vulchanova, L.; Brown, D.R. Autonomic neurotransmitters modulate immunoglobulin A secretion in porcine colonic mucosa. J. Neuroimmunol. 2007, 185, 20–28. [Google Scholar] [CrossRef]
- Corthesy, B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun. Rev. 2013, 12, 661–665. [Google Scholar] [CrossRef]
- Xie, F.; Sakwiwatkul, K.; Zhang, C.; Wang, Y.; Zhai, L.; Hu, S. Atractylodis macrocephalae Koidz. polysaccharides enhance both serum IgG response and gut mucosal immunity. Carbohydr. Polym. 2013, 91, 68–73. [Google Scholar] [CrossRef]
- Shan, C.; Sun, B.; Dalloul, R.A.; Zhai, Z.; Sun, P.; Li, M.; Yang, S.; Luan, W. Effect of the oral administration of astragalus polysaccharides on jejunum mucosal immunity in chickens vaccinated against Newcastle disease. Microb. Pathog. 2019, 135, 103621. [Google Scholar] [CrossRef]
- Kong, Y.; Gao, C.; Du, X.; Zhao, J.; Li, M.; Shan, X.; Wang, G. Effects of single or conjoint administration of lactic acid bacteria as potential probiotics on growth, immune response and disease resistance of snakehead fish (Channa argus). Fish. Shellfish. Immunol. 2020, 102, 412–421. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, J.L.; Ren, H.T.; Zhou, B.H.; Wu, Q.J.; Sun, P. Effect of tributyltin on antioxidant ability and immune responses of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Xu, W.; Fang, S.; Wang, Y.; Chi, X.; Ma, X.; Zhang, T.; Hu, S. Receptor and signaling pathway involved in bovine lymphocyte activation by Atractylodis macrocephalae polysaccharides. Carbohydr. Polym. 2020, 234, 115906. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Cao, N.; Chen, F.; Qian, L.; Wang, Y.; Huang, Y.; Tian, Y.; Xu, D.; Li, W. Polysaccharide of Atractylodes macrocephala Koidz (PAMK) Alleviates Cyclophosphamide-induced Immunosuppression in Mice by Upregulating CD28/IP3R/PLCgamma-1/AP-1/NFAT Signal Pathway. Front. Pharmacol. 2020, 11, 529657. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ding, H.; Liu, L.; Peng, C.; Huang, Y.; Zhong, F.; Li, W.; Meng, T.; Li, J.; Wang, X.; et al. Astragalus polysaccharide enhances the immune function of RAW264.7 macrophages via the NF-κB p65/MAPK signaling pathway. Exp. Ther. Med. 2021, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Wang, B.; Lu, Y.; Li, L.; Li, Y.; Liu, S. Evaluation of the effects of Astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 253, 109249. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Yuan, L.; Ruan, H.; Zhu, Z.; Fan, X.; Zhu, L.; Peng, X. Blood-Enriching Effects and Immune-Regulation Mechanism of Steam-Processed Polygonatum sibiricum Polysaccharide in Blood Deficiency Syndrome Mice. Front. Immunol. 2022, 13, 813676. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, Q.; Wang, G.; Tan, J.; Liu, S.; Pu, S.; Chen, W.; Xie, P.; Zhang, Y.; Zhang, J.; et al. Effects of non-thermal plasma treatment on the polysaccharide from Dendrobium nobile Lindl. And its immune activities in vitro. Int. J. Biol. Macromol. 2020, 153, 942–950. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Chen, L.X.; Chen, X.Q.; Lu, J.H.; Zhao, J.; Li, S.P. Chemical characterization and immunomodulatory activity of acetylated polysaccharides from Dendrobium devonianum. Carbohydr. Polym. 2018, 180, 238–245. [Google Scholar] [CrossRef]
- Tian, W.; Dai, L.; Lu, S.; Luo, Z.; Qiu, Z.; Li, J.; Li, P.; Du, B. Effect of Bacillus sp. DU-106 fermentation on Dendrobium officinale polysaccharide: Structure and immunoregulatory activities. Int. J. Biol. Macromol. 2019, 135, 1034–1042. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef]
- He, T.B.; Huang, Y.P.; Yang, L.; Liu, T.T.; Gong, W.Y.; Wang, X.J.; Sheng, J.; Hu, J.M. Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 83, 34–41. [Google Scholar] [CrossRef]
- Chen, Z.; Kwong Huat Tan, B.; Chan, S.H. Activation of T lymphocytes by polysaccharide-protein complex from Lycium barbarum L. Int. Immunopharmacol. 2008, 8, 1663–1671. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, M.; Li, L.; Chen, M.; Puno, P.T.; Bao, W.; Zheng, H.; Wen, X.; Cheng, H.; Fung, H.; et al. Cordyceps polysaccharide marker CCP modulates immune responses via highly selective TLR4/MyD88/p38 axis. Carbohydr. Polym. 2021, 271, 118443. [Google Scholar] [CrossRef]
- Chen, Z.E.; Wufuer, R.; Ji, J.H.; Li, J.F.; Cheng, Y.F.; Dong, C.X.; Taoerdahong, H. Structural Characterization and Immunostimulatory Activity of Polysaccharides from Brassica rapa L. J. Agric. Food Chem. 2017, 65, 9685–9692. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, K.; Tian, W.; Wang, H.; Liu, Z.; Li, Y.; Li, E.; Liu, C.; Li, X.; Hou, R.; et al. Effects of selenizing angelica polysaccharide and selenizing garlic polysaccharide on immune function of murine peritoneal macrophage. Int. Immunopharmacol. 2015, 27, 104–109. [Google Scholar] [CrossRef]
- Cui, Y.S.; Li, Y.X.; Jiang, S.L.; Song, A.N.; Fu, Z.; Dong, C.X.; Yao, Z.; Qiao, W. Isolation, purification, and structural characterization of polysaccharides from Atractylodis macrocephalae Rhizoma and their immunostimulatory activity in RAW264.7 cells. Int. J. Biol. Macromol. 2020, 163, 270–278. [Google Scholar] [CrossRef]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Mumberg, D.; Monach, P.A.; Wanderling, S.; Philip, M.; Toledano, A.Y.; Schreiber, R.D.; Schreiber, H. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc. Natl. Acad. Sci. USA 1999, 96, 8633–8638. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, Z.; Cai, G.; Zhang, Y.; Peng, S.; Jiao, L.; Liu, Z.; Yang, Y.; Wang, D. Astragalus polysaccharides combined with simvastatin as an immunostimulant enhances the immune adjuvanticity of oil-in-water emulsion and immune responses in mice. Vaccine 2023, 41, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Gong, Q.; Ma, J.; Liu, X.; Wang, Y.; Cheng, X. Immunosuppressive activity is attenuated by Astragalus polysaccharides through remodeling the gut microenvironment in melanoma mice. Cancer Sci. 2021, 112, 4050–4063. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y.; Lai, X.; Zhou, L. Polysaccharides from Chinese Herbal Lycium barbarum Induced Systemic and Local Immune Responses in H22 Tumor-Bearing Mice. J. Immunol. Res. 2018, 2018, 3431782. [Google Scholar] [CrossRef]
- Chung, S.S.; Wu, Y.; Okobi, Q.; Adekoya, D.; Atefi, M.; Clarke, O.; Dutta, P.; Vadgama, J.V. Proinflammatory Cytokines IL-6 and TNF-α Increased Telomerase Activity through NF-κB/STAT1/STAT3 Activation, and Withaferin A Inhibited the Signaling in Colorectal Cancer Cells. Mediators Inflamm. 2017, 2017, 5958429. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Zhong, C.; Pu, Y.; Yang, Z.; Bao, Y. Structure characteristics and immunomodulatory activities of a polysaccharide RGRP-1b from radix ginseng Rubra. Int. J. Biol. Macromol. 2021, 189, 980–992. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Lin, G.; Da, F.; Wan, X.; Huang, Y.; Yang, S.; Jian, J.; Cai, S. Immune-enhancing effects of Astragalus polysaccharides and Ganoderma lucidum polysaccharides on Vibrio harveyi flgJ DNA vaccine in grouper. J. Fish. Dis. 2023, 46, 147–156. [Google Scholar] [CrossRef]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases—Regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef]
- Cho, J.W.; Lee, K.S.; Kim, C.W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int. J. Mol. Med. 2007, 19, 469–474. [Google Scholar] [PubMed]
- Ahmed-Hassan, H.; Abdul-Cader, M.S.; Sabry, M.A.; Hamza, E.; Abdul-Careem, M.F. Toll-like receptor (TLR)4 signalling induces myeloid differentiation primary response gene (MYD) 88 independent pathway in avian species leading to type I interferon production and antiviral response. Virus Res. 2018, 256, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cheng, L.; Zhu, Y.; Zhao, X.; Zhang, W.; Gao, X.; Xiong, T.; Guo, L. Immune-related effects of compound astragalus polysaccharide and sulfated epimedium polysaccharide on newborn piglets. Anim. Biotechnol. 2023, 34, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xie, Z.; Liu, F.; Han, C.; Zhang, D.; Wang, D.; Bao, X.; Sun, J.; Wen, C.; Fan, Y. Dihydroartemisinin inhibits activation of the Toll-like receptor 4 signaling pathway and production of type I interferon in spleen cells from lupus-prone MRL/lpr mice. Int. Immunopharmacol. 2014, 22, 266–272. [Google Scholar] [CrossRef]
- Weinlich, R.; Bortoluci, K.R.; Chehab, C.F.; Serezani, C.H.; Ulbrich, A.G.; Peters-Golden, M.; Russo, M.; Amarante-Mendes, G.P. TLR4/MYD88-dependent, LPS-induced synthesis of PGE2 by macrophages or dendritic cells prevents anti-CD3-mediated CD95L upregulation in T cells. Cell Death Differ. 2008, 15, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Li, M.; Niu, S.; Wang, J.; Shao, H.; Li, T.; Wang, H. Salvia miltiorrhiza polysaccharide activates T Lymphocytes of cancer patients through activation of TLRs mediated -MAPK and -NF-κB signaling pathways. J. Ethnopharmacol. 2017, 200, 165–173. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Rao, K.S.; Suryaprakash, V.; Senthilkumar, R.; Preethy, S.; Katoh, S.; Ikewaki, N.; Abraham, S.J.K. Role of Immune Dysregulation in Increased Mortality Among a Specific Subset of COVID-19 Patients and Immune-Enhancement Strategies for Combatting Through Nutritional Supplements. Front. Immunol. 2020, 11, 1548. [Google Scholar] [CrossRef]
- Lee, C.Y.; Nguyen, A.T.; Doan, L.H.; Chu, L.W.; Chang, C.H.; Liu, H.K.; Lee, I.L.; Wang, T.H.; Lai, J.M.; Tsao, S.M.; et al. Repurposing Astragalus Polysaccharide PG2 for Inhibiting ACE2 and SARS-CoV-2 Spike Syncytial Formation and Anti-Inflammatory Effects. Viruses 2023, 15, 641. [Google Scholar] [CrossRef] [PubMed]
| Plant Source | Compound | Administration Mothed | Administration Dosage | Model | Immune Organ | Immune Cell | Cytokine | Ref. |
|---|---|---|---|---|---|---|---|---|
| Atractylodes macrocephala Koidz. | RAMPtp | N/A | 12.5 mg/L | Mouse lymphocytes | N/A | N/A | IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, IFN-γ, TNF-α | [47] |
| RAMPtp | N/A | 25, 50, 100 μg/mL. | Bovine lymphocytes | N/A | N/A | IL-1α, IL-21, IFN-γ, TGF-β1 | [74] | |
| RAMPtp | N/A | 25, 50, 100 μg/mL. | Mouse macrophage | N/A | N/A | IL-6, IL-10, TNF-α, iNOS, NO | [32] | |
| RAMPS | ig | 0.25 mL of RAMPS (0.05 g) solution | FMVD O mice | N/A | N/A | IL-6, TNF-α, TGF-β, sIgA | [70] | |
| RAMPS60c, RAMPStp | ip | 0.5 mL 6 mg/mL | ND chicken | thymus, spleen, bursa of Fabricius | CD4+, CD8+ | N/A | [20] | |
| Acanthopanax senticosus | ASPS | added into forage | 1, 2, 4 g/kg | Chicken | N/A | N/A | IgA, IgM | [64] |
| ASPS | ig | 50, 100, 200 mg/kg | S180, H22, U14 tumor-bearing mice | thymus, spleen | N/A | IL-2, IL-12 | [12] | |
| Ziziphus jujuba | JP | ig | 150, 300, 600 mg/kg | Cyclophosphamide-injected mouse | N/A | CD3+, CD4+, CD8+ | IL-2, IL-4, IL-10, IFN-γ, TNF-α, sIgA | [49] |
| JPC | ig | 100, 200, 400 mg/kg | CSF rat | N/A | CD4+, CD8+, NK cell | IL-2, IL-10 | [56] | |
| Angelica sinensis | CAP, sCAP2 | N/A | 500μL 3.125, 1.563, 0.781 μg/mL | Mouse macrophage | N/A | N/A | NO | [88] |
| CAP, sCAP2 | ip | 0.4 mL 0.5, 1, 1.5 mg/mL | Mouse | N/A | N/A | IL-6, IL-10, TNF-α | [88] | |
| ASP-PLGA-PEI | N/A | 31.25 μg/mL | Bone marrow-derived dendritic cell | N/A | N/A | IL-12, TNF-α | [57] | |
| ISAg | ig | 4 mg/mice | B16 melanoma mice | N/A | N/A | IL-12, TNF-α | [31] | |
| ADPs-1a, ADPs-3a | N/A | ADPs-1a: 100, 200, 400, 800, 1600 μg/mLADPs-3a: 37.5, 75, 150, 300, 600 μg/mL | RAW264.7 cells | N/A | N/A | IL-6, TNF-α, NO | [24] | |
| Lycium chinense | LBP | ig | 50, 100, 200 mg/kg | Cyclophosphamide-injected mouse | thymus, spleen | N/A | IL-1β, IL-2, IL-6, TNF-α, IFN-γ | [13] |
| LBP | ig | 0.1 mL/10 g | Mouse | thymus, spleen | N/A | IL-2, IL-6, IFN-γ, TGF-β, IgA, sIgA | [9] | |
| LBP, LBPF1-5 | N/A | 1, 3, 10, 30, 100, 300 μg/mL | Mouse lymphocytes | N/A | CD3+, CD19+, CD25 | IL-2, IL-4, TNF-α, IFN-γ | [85] | |
| LBP1-5 | ig | 250 mg/kg | H22 tumor-bearing mice | N/A | CD4+, CD8+, CD25 | TGF-β, IL-10 | [94] | |
| Polygonatum sibiricum | PSPC, PSPW | ig | 200, 400, 800 mg/kg | Spleen deficient mouse | N/A | N/A | IL-2, IL-6, TNF-α, IFN-γ, NO | [25] |
| PSP | added into forage | 800 mg/kg | Cyclophosphamide-injected chicken | thymus, spleen, bursa of Fabricius | N/A | IL-2, IL-6, IFN-γ, IgG, IgM | [18] | |
| PSP | ig | 100, 200, 400 mg/kg | Cyclophosphamide-injected mouse | thymus, spleen | NK cell, CD4+, CD8+ | IL-2, TNF-α | [15] | |
| PSP, PSP3 | ip | PSP: 400 mg/kg PSP3: 100, 200, 400 mg/kg | Cyclophosphamide-injected mouse | thymus, spleen | NK cell | IL-2, IL-4, IL-10, TNF-α | [16] | |
| PSP | ip | 100, 200, or 400 mg/kg | Cyclophosphamide-injected mouse | thymus, spleen | N/A | IL-2, IL-8, IL-10, TNF-α | [17] | |
| Astragalus membranaceus | APS | N/A | 25, 50, 100, 200 μg/mL | Mouse macrophage | N/A | N/A | IL-1β, IL-6, TNF-α, NO, iNOS | [76] |
| APS | N/A | 1, 2, 3, 4, 5 mg/mL | Mouse dendritic cell | N/A | N/A | IL-13, IFN-γ | [38] | |
| APS | ip | 0.2 mL 5 μg/mL | ND chicken | spleen, bursa of Fabricius | CD4+, CD8+ | IL-2, IL-4, IL-6, IFN-γ | [19] | |
| APS, APSL | ip | 0.5 mL1, 2, 4 mg/mL | OVA mouse | N/A | N/A | IL-6, IFN-γ, IgG, IgG1, IgG2a | [66] | |
| APS | ip | 500 μg | HBV mouse | N/A | CD4+, CTL, DC, Treg | IL-2, IL-4, IFN-γ | [39] | |
| LMw-APS | ip | 100 μg/mice | HSP90C mouse | N/A | N/A | IL-2, IL-4, IL-10, IL-12, IgG1, IgG2b | [67] | |
| APS | ig | 100, 200 and 300 mg/kg | H22 tumor-bearing mice | thymus, spleen | macrophages, NK cell | IL-2, TNF-α, IFN-γ | [26] | |
| APS | ip | 100, 200 mg/kg | 4T1 tumor-bearing mice | thymus, spleen | macrophages, lymphocytes, NK cell | IL-2, TNF-α, IFN-γ | [11] | |
| APS4 | ig | 150 and 300 mg/kg | S180 tumor-bearing mice | N/A | CD19+ B cell, CD4+, CD8+ | N/A | [10] | |
| APS | ip | 10 mg/mL | FUS treated tumor-bearing mice | N/A | N/A | IL-4, IL-10, TNF-α, IFN-γ, IgG1 | [38] | |
| APS | added into forage | 0-200 ppm/diet | Necrotizing enteritis chicken | thymus, spleen andbursa of Fabricius | N/A | IL-17 | [58] | |
| APS | added into forage | 1 g/kg/diet | SVCV-infected crucian carp | N/A | N/A | IL-1β, IL-8, IL-10, TNF-α, IFN-α, IFN-γ, IgM | [78] | |
| APS | sc | 1.25, 2.5,5 mg/mL | Mouse | N/A | CD4+, CD8+ | IL-6, IFN-γ, IgG | [92] | |
| Dendrobium officinale | Dendrobium CPs | N/A | 10, 30, 100, 300, 1000 μg/mL | Mouse macrophage | N/A | N/A | IL-1α, IL-6, IL-10, TNF-α, NO | [23] |
| DOP | N/A | 50, 150, 300 μg/mL | Mouse macrophage | N/A | N/A | IL-1, IL-6, TNF-α | [80] | |
| DOP-1-1 | N/A | 25, 50, 100 μg/mL | THP-1 cell | N/A | N/A | IL-1β, TNF-α | [84] | |
| DSP | ig | 100, 200, 300 mg·kg | Cyclophosphamide-injected mouse | N/A | N/A | IL-6, TNF-α, IFN-γ | [27] | |
| GXG | ig | 50, 200 mg/kg | Mouse | N/A | CD4+, CD8+, B cell, DC cell | IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, IL-17, TNF-α, IFN-γ, sIgA | [50] | |
| DOP-W3-b | ig | 500 mg/kg, 2 g/kg | Mouse | thymus, spleen | N/A | IL-4, IFN-γ | [21] | |
| Cordyceps sinensis | CMPB90-1 | N/A | 15.6, 31.3, 62.5, 125, 250 μg/mL | Mouse lymphocytes | N/A | N/A | IL-2 | [30] |
| CCP | N/A | 2, 20, 100 μg/mL | Mouse macrophage, BMDMs | N/A | N/A | IL-6, TNF-α, NO | [86] | |
| CSP | ig | 25, 50, 100 mg/kg | Cyclophosphamide-injected mouse | N/A | T lymphocytes | IL-17, IL-21, TGF-β3 | [52] | |
| CSP | ig | 25, 50, 100 mg/kg | Cyclophosphamide-injected mouse | N/A | N/A | IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, IL-17, IL-21, TNF-α, IFN-γ, TGF-β3 | [55] | |
| PPS | ig | 125, 250, 500 mg/kg | Cyclophosphamide-injected mouse | thymus, spleen | Macrophage, CTL, NK cell | IL-2, IL-12, IFN -γ, IgG | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Jiang, N.; Lin, M.; He, X.; Li, B.; Dong, Y.; Chen, S.; Lv, G. The Mechanisms of Polysaccharides from Tonic Chinese Herbal Medicine on the Enhancement Immune Function: A Review. Molecules 2023, 28, 7355. https://doi.org/10.3390/molecules28217355
Xie Z, Jiang N, Lin M, He X, Li B, Dong Y, Chen S, Lv G. The Mechanisms of Polysaccharides from Tonic Chinese Herbal Medicine on the Enhancement Immune Function: A Review. Molecules. 2023; 28(21):7355. https://doi.org/10.3390/molecules28217355
Chicago/Turabian StyleXie, Zhiyi, Ninghua Jiang, Minqiu Lin, Xinglishang He, Bo Li, Yingjie Dong, Suhong Chen, and Guiyuan Lv. 2023. "The Mechanisms of Polysaccharides from Tonic Chinese Herbal Medicine on the Enhancement Immune Function: A Review" Molecules 28, no. 21: 7355. https://doi.org/10.3390/molecules28217355
APA StyleXie, Z., Jiang, N., Lin, M., He, X., Li, B., Dong, Y., Chen, S., & Lv, G. (2023). The Mechanisms of Polysaccharides from Tonic Chinese Herbal Medicine on the Enhancement Immune Function: A Review. Molecules, 28(21), 7355. https://doi.org/10.3390/molecules28217355






