Study on the Biomolecular Competitive Mechanism of Polybrominated Diphenyl Ethers and Their Derivatives on Thyroid Hormones
Abstract
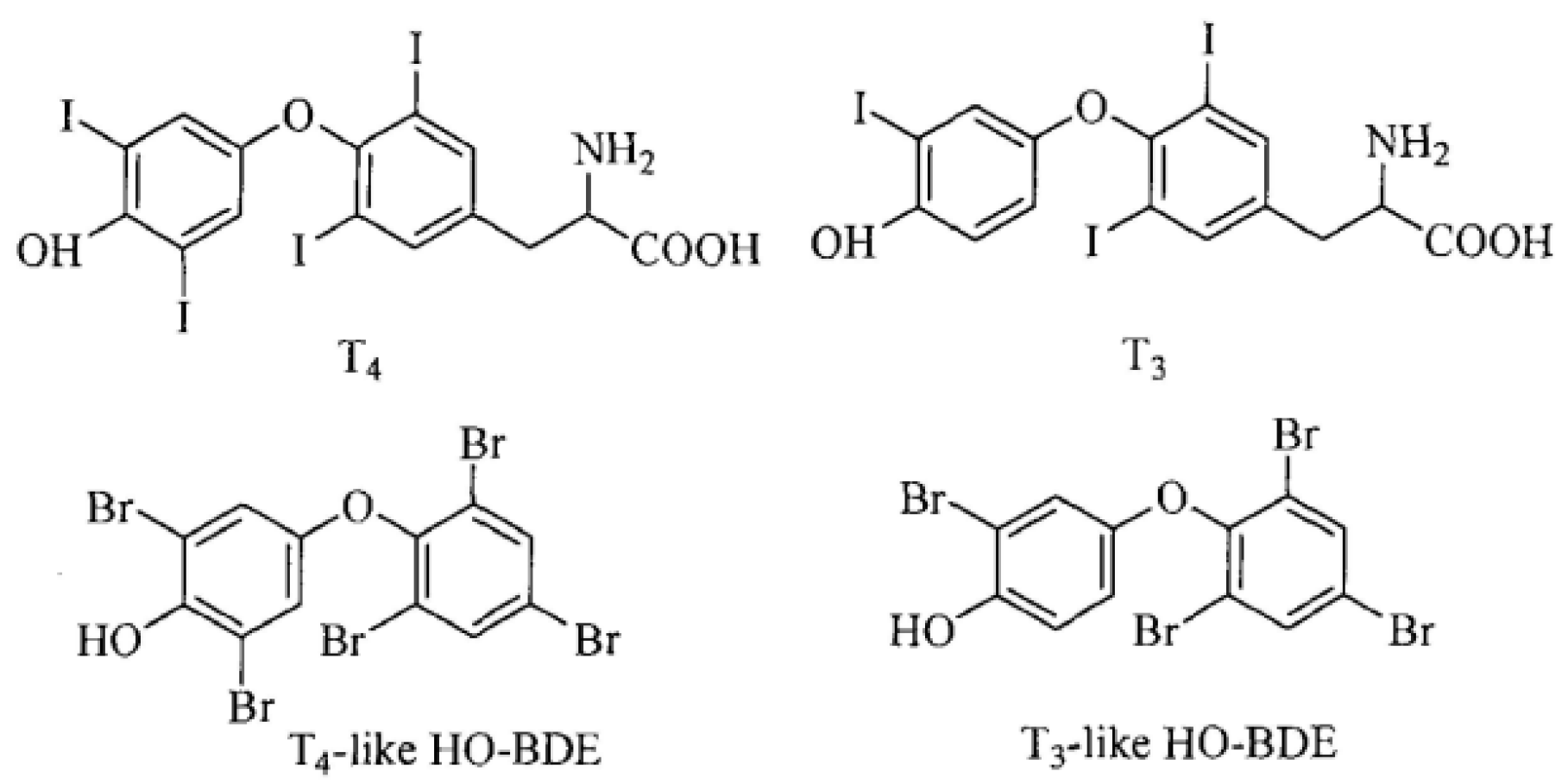
:1. Introduction
2. Results and Discussion
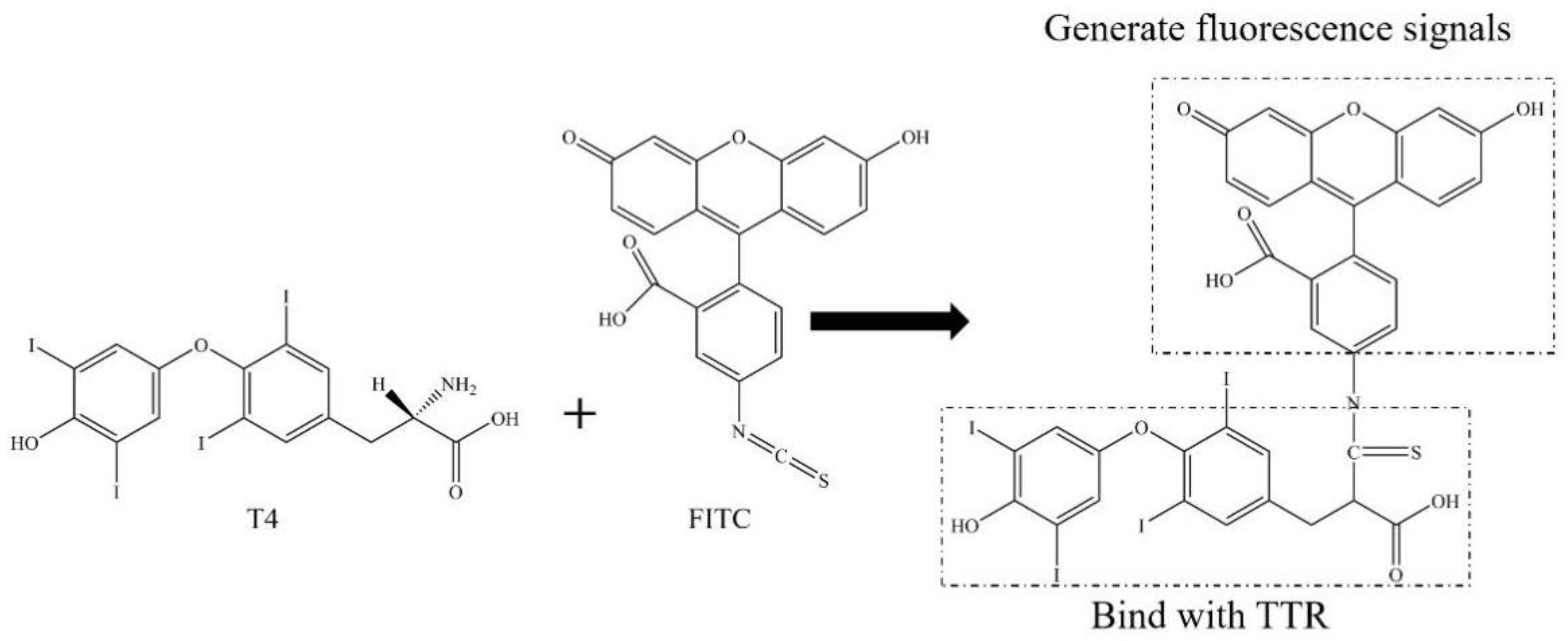
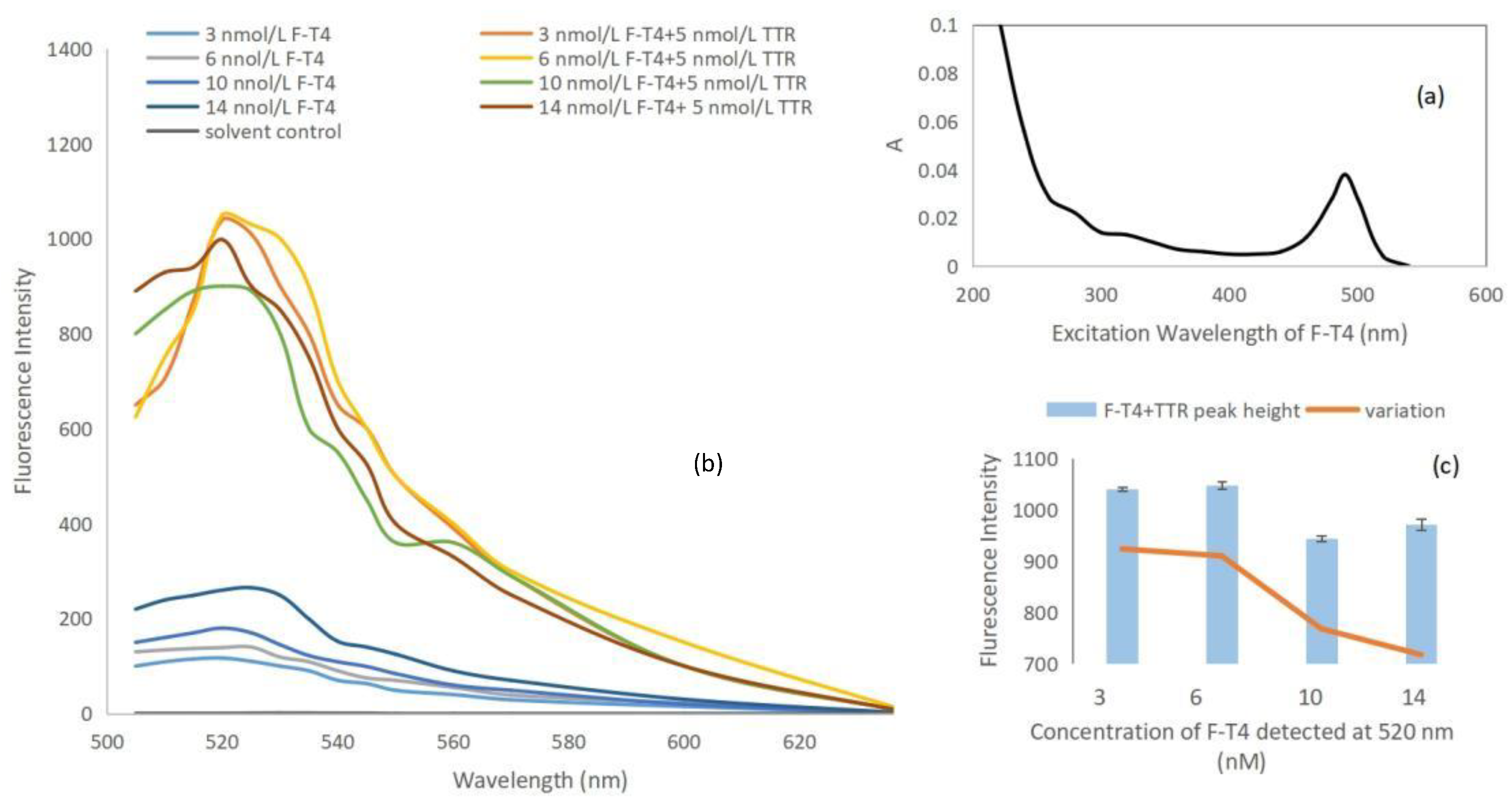
2.1. Synthesis, Purification, and Optimization of F-T4 Fluorescent Probe
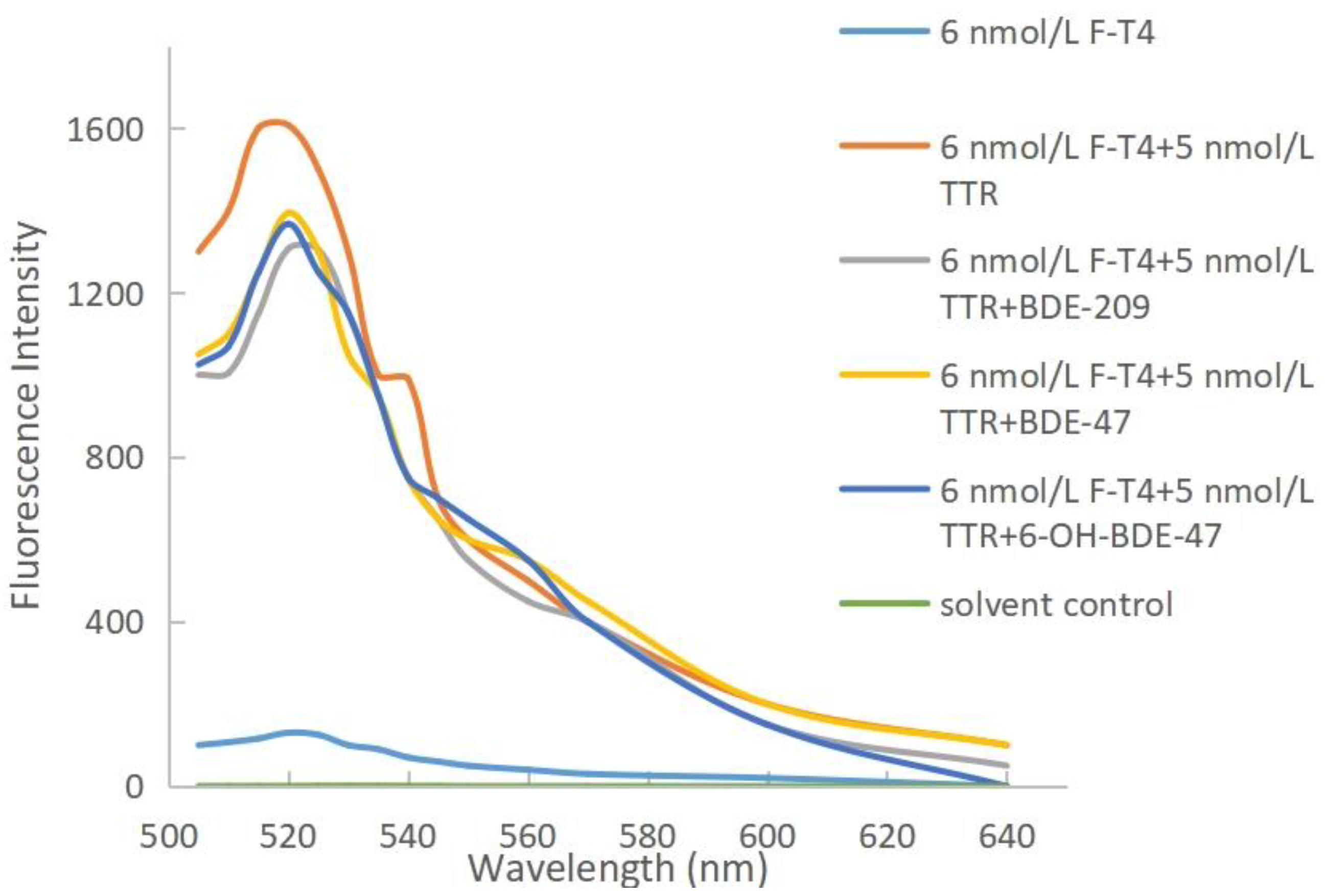
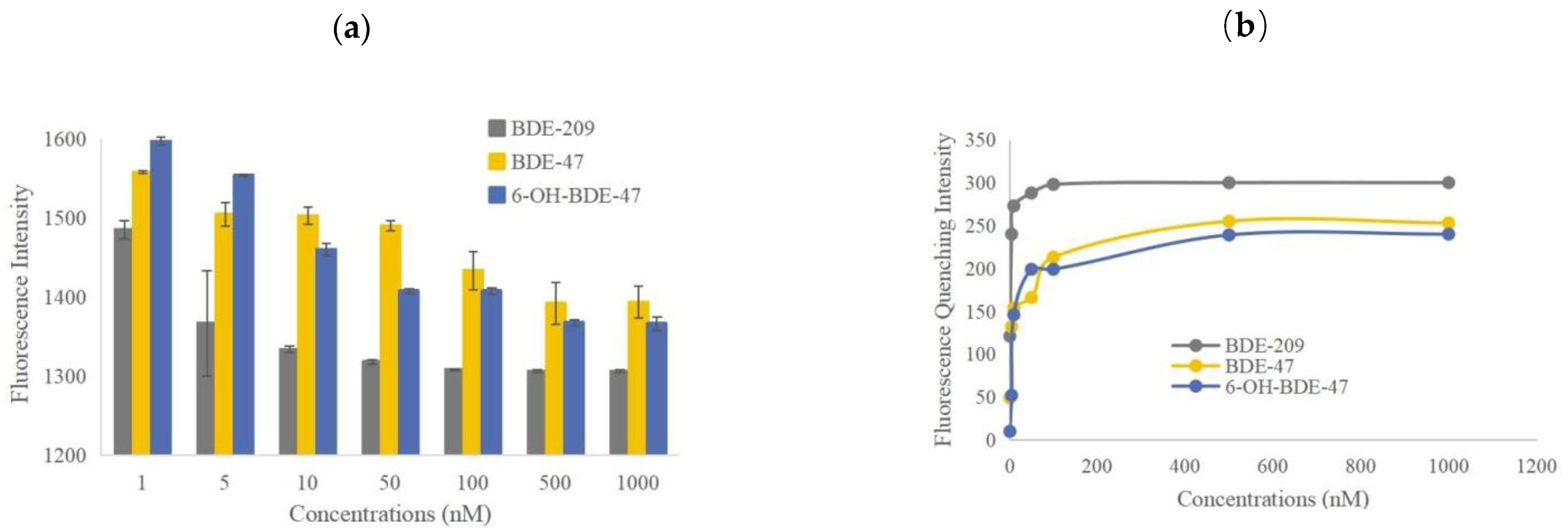
2.2. Binding Competition of PBDEs and OH-PBDEs with F-T4 and TTR
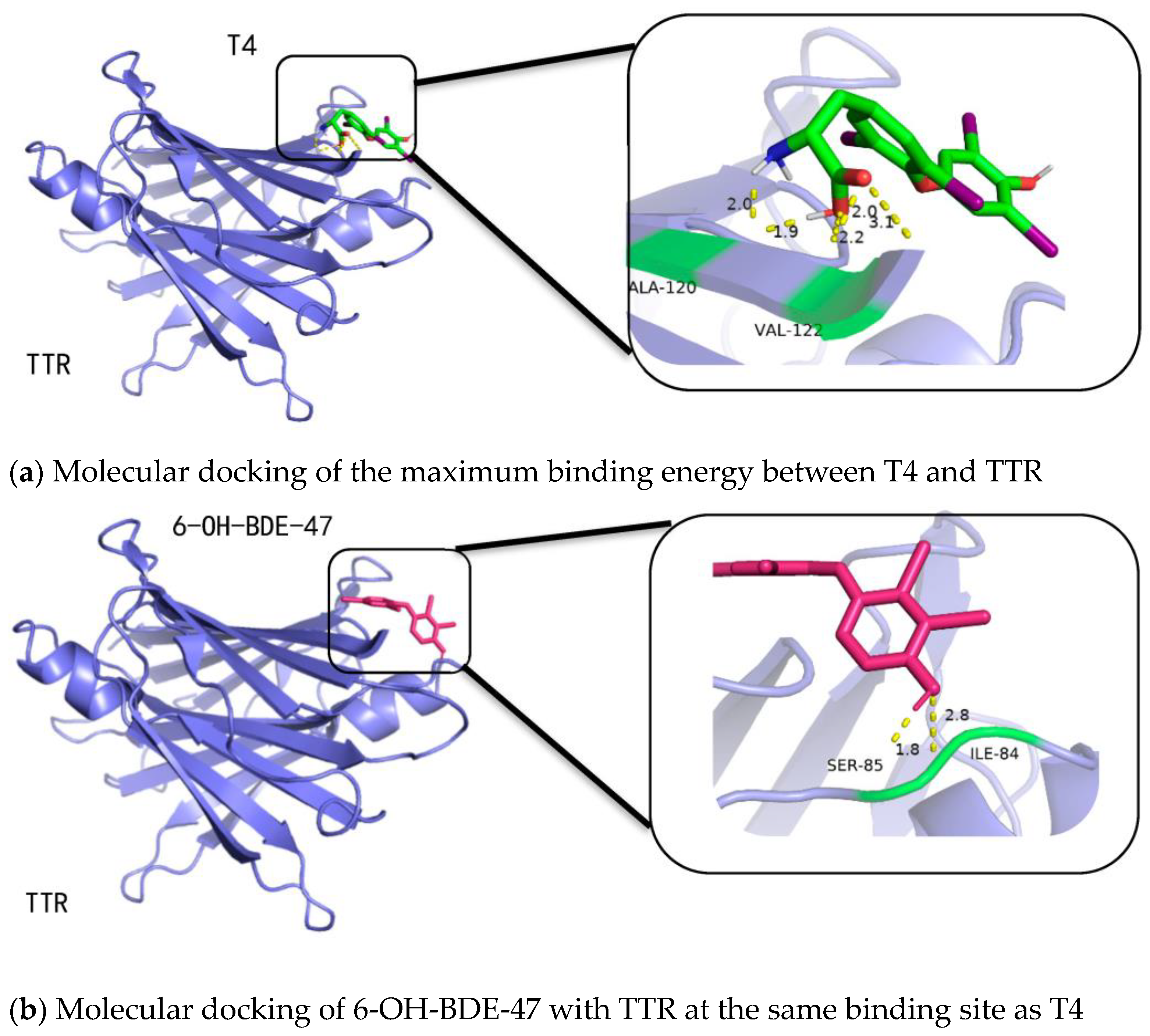
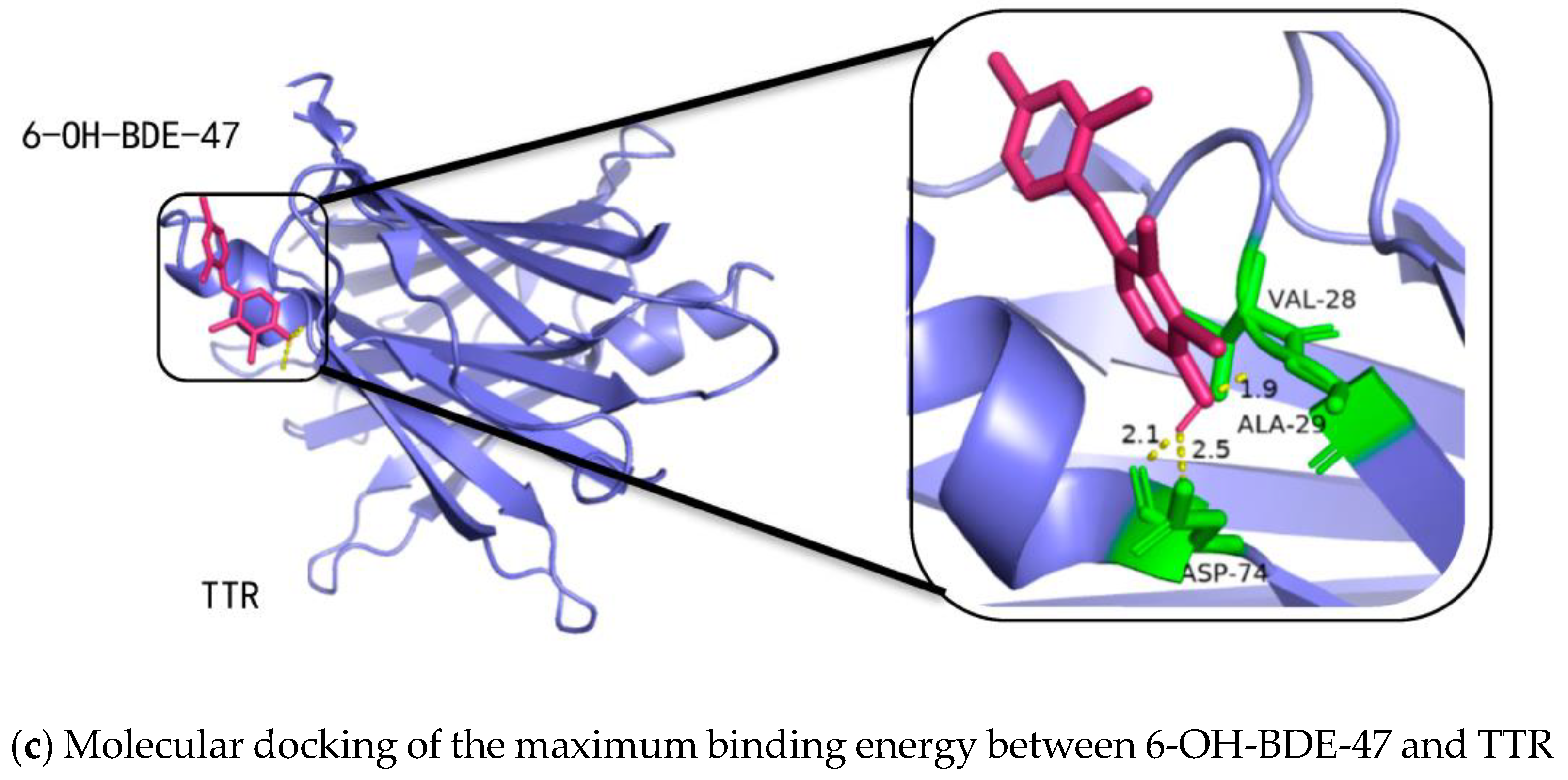
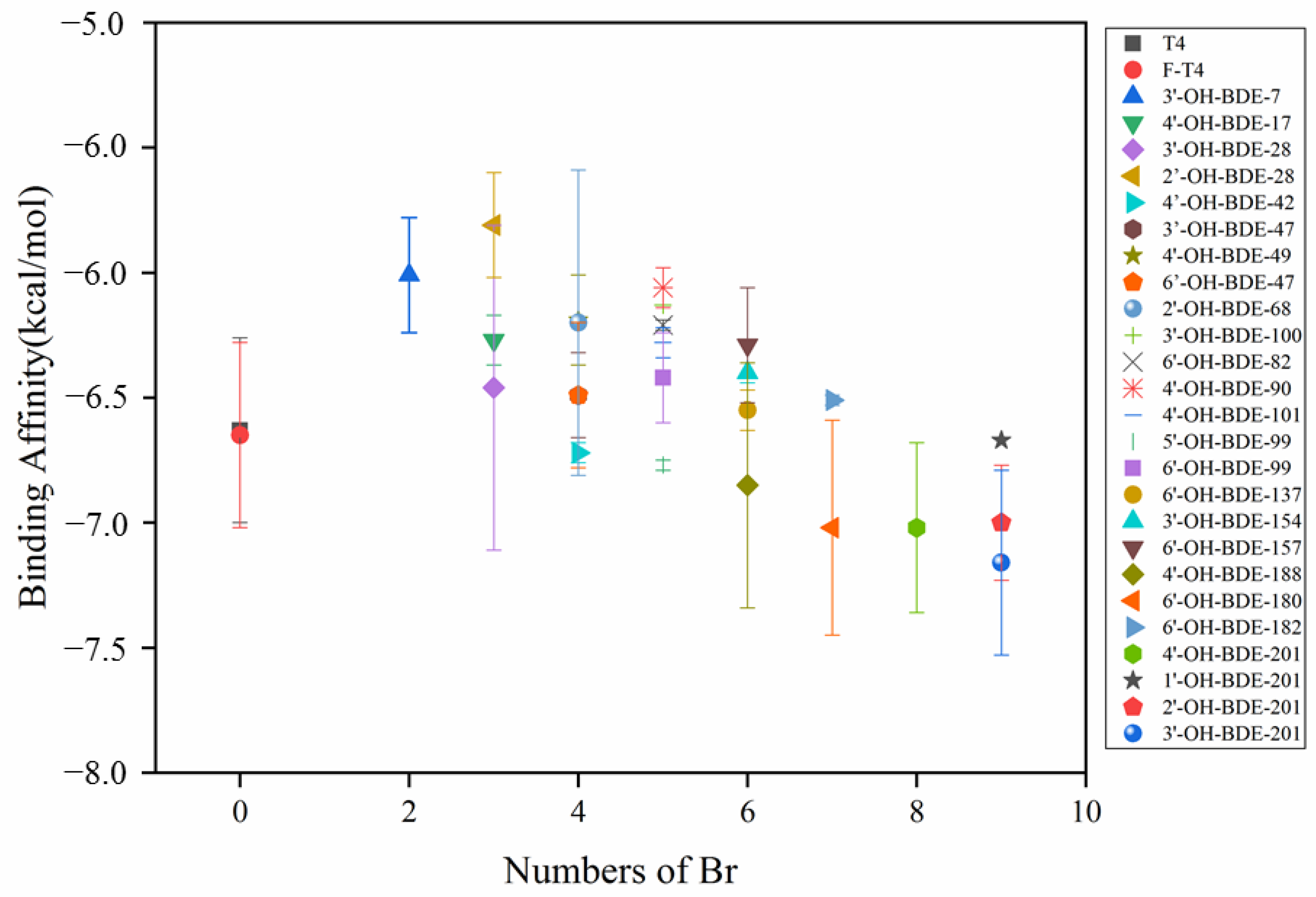
2.3. Binding Affinities of OH-PBDEs and T4 to TTR
3. Materials and Methods
3.1. Materials
3.2. Preparation and Synthesis of F-T4 Fluorescent Probe
3.3. Purification and Storage of F-T4 Fluorescent Probe
3.4. Binding of F-T4 Fluorescent Probe with TTR
3.5. Competition between F-T4 and BDE-209, BDE-47, and 6-OH-BDE-47
3.6. Determination of Binding Affinity of 6-OH-PBDEs
4. Conclusions
- (1)
- PBDEs and OH-PBDEs have certain thyroid interference effects. Different types of interferents having different effects. The binding capacity of PBDEs and OH-PBDEs to TTR is correlated with the number of bromine atoms in the PBDEs. The more bromine atoms in the molecule, the stronger the binding affinity with TTR.
- (2)
- The binding affinity of PBDEs and their derivatives to TTR is related to the presence of hydroxyl functional groups. When the number of bromine atoms is the same, OH-PBDEs exhibit stronger binding affinity to TTR than PBDEs.
- (3)
- The binding affinity of PBDEs and OH-PBDEs to TTR is influenced by the concentration of the disruptors. Higher concentrations result in stronger competition between the disruptors and F-T4 for binding to TTR. When the concentrations of BDE-209, BDE-47, and 6-OH-BDE-47 increased from 1 nmol/L to 1 μmol/L, the changes in fluorescence quenching of F-T4 upon competition with TTR increase from 121 to 300, 49 to 213, and 10 to 240, respectively.
- (4)
- When OH-PBDEs have four or more bromine atoms and exhibit the most structural similarity to T4, their binding affinity to TTR is stronger than that of T4.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Sun, L.; Wu, S.; Wang, P.; Wang, Z.; Zhai, M.; Xu, J.; Zhang, D.; Yu, D.; Li, C. Toxicity and risk priority ranking of polybrominated diphenyl ethers (PBDEs): A relative receptor-bound concentration approach. Sci. Total Environ. 2023, 892, 164714. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Breivik, K. Global historical stocks and emissions of PBDEs. Environ. Sci. Technol. 2019, 53, 6330–6340. [Google Scholar]
- Sarkar, D.; Singh, V.K.; Singh, S.K. Maternal BDE-209 exposure during lactation perturbs steroidogenesis, germ cell kinetics and THR alpha 1 expression in testes of prepubertal mice offspring. Food Chem. Toxicol. 2018, 122, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, J.; Sun, J. Research status of photodegradation of polybromin-ated diphenyl ethers (PBDES). Environ. Chem. 2015, 34, 270–278. [Google Scholar]
- Yao, B.; Luo, Z.; Zhi, D.; Hou, D.; Luo, L.; Du, S.; Zhou, Y. Current progress in degradation and removal methods of polybrominated diphenyl ethers from water and soil: A review. J. Hazard. Mater. 2021, 403, 123674. [Google Scholar] [CrossRef] [PubMed]
- Linares, V.; Belles, M.; Domingo, J.L. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 2015, 89, 335–356. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Zhao, Y.; Ma, F.; Lin, Z.; Liu, Y.; Dong, Z.; Chen, G.; Liu, D. PBDEs disrupt homeostasis maintenance and regeneration of planarians due to DNA damage, proliferation and apoptosis anomaly. Ecotoxicol. Environ. Safe 2022, 248, 114287. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, C.; Guo, L.; Ren, X.; Zhang, J. Binding and activity of polybrominated diphenyl ether sulfates to thyroid hormone transport proteins and nuclear receptors. Environ. Sci.-Proc. Impacts 2019, 21, 950–956. [Google Scholar] [CrossRef]
- Lindqvist, D.; Wincent, E. Kinetics and toxicity of an environmentally relevant mixture of halogenated organic compounds in zebrafish embryo. Aquat. Toxicol. 2022, 252, 106311. [Google Scholar] [CrossRef]
- Gu, C.; Cai, J.; Fan, X.; Bian, Y.; Yang, X.; Xia, Q.; Sun, C.; Jiang, X. Theoretical investigation of AhR binding property with relevant structural requirements for AhR-mediated toxicity of polybrominated diphenyl ethers. Chemosphere 2020, 249, 126554. [Google Scholar] [CrossRef]
- Klaren, P.H.M.; Geven, E.J.W.; Nagelkerke, A.; Flik, G. Kinetics and thiol requirements of iodothyronine 5′-deiodination are tissue-specific in common carp (Cyprinus carpi L.). Comp. Biochem. Phys. B 2012, 161, 275–282. [Google Scholar] [CrossRef]
- Vuong, A.M.; Webster, G.M.; Romano, M.E.; Braun, J.M.; Zoeller, R.T.; Hoofnagle, A.N.; Sjoedin, A.; Yolton, K.; Lanphear, B.P.; Chen, A. Maternal polybrominated diphenyl ether (PBDE) exposure and thyroid hormones in maternal and cord sera: The home study, Cincinnati, USA. Environ. Health Perspect. 2015, 123, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Geller, R.J.; Romano, L.E.; Coleman-Phox, K.; Adler, N.E.; Parry, E.; Wang, M.; Park, J.-S.; Elmi, A.F.; Laraia, B.A.; et al. Association between persistent endocrine-disrupting chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and biomarkers of inflammation and cellular aging during pregnancy and postpartum. Environ. Int. 2018, 115, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.; Legradi, J.; Lamoree, M.H.; Asplund, L.; Leonards, P.E.G. Metabolite alterations in zebrafish embryos exposed to hydroxylated polybrominated diphenyl ethers. Sci. Total Environ. 2023, 857, 159269. [Google Scholar] [CrossRef] [PubMed]
- Ermilova, I.; Stenberg, S.; Lyubartsev, A.P. Quantum chemical and molecular dynamics modelling of hydroxylated polybrominated diphenyl ethers. Phys. Chem. Chem. Phys. 2017, 19, 28263–28274. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Liu, H.; He, J.; Yang, X. Development of chicken and fish muscle protein-Water partition coefficients predictive models for ionogenic and neutral organic chemicals. Ecotoxicol. Environ. Safe 2018, 157, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, Q.; Li, X.; Li, N.; Chi, P.; Chen, J.; Wang, Z.; Hao, C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-beta: In vitro and in silico investigations. Environ. Health Perspect. 2010, 118, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.M. Molecular Toxicology of Hydroxylated Polybrominated Diphenyl Ethers and Perfluoroalkyl Acid on the Thyroid Hormone System; University of Chinese Academy of Sciences: Beijing, China, 2013. [Google Scholar]
- Ren, X.M.; Guo, L.H. Assessment of the binding of hydroxylated polybrominated diphenyl ethers to thyroid hormone transport proteins using a site-specific fluorescence probe. Environ. Sci. Technol. 2012, 46, 4633–4640. [Google Scholar] [CrossRef] [PubMed]
- He, X. Study on Interaction Properties of Polybrominated Diphenyl Ethers and Their Derivatives with Biomolecules; Fuzhou University: Fuzhou, China, 2015. [Google Scholar]
- Su, Q.; Jiang, C.; Gou, D.; Long, Y. Surface plasmon-assisted fluorescence enhancing and quenching: From theory to application. ACS Appl. Bio Mater. 2021, 4, 4684–4705. [Google Scholar] [CrossRef]
- Wang, L.; Liang, N.; Li, H.; Yang, Y.; Zhang, D.; Liao, S.; Pan, B. Quantifying the dynamic fluorescence quenching of phenanthrene and ofloxacin by dissolved humic acids. Environ. Pollut. 2015, 196, 379–385. [Google Scholar] [CrossRef]
- Paterson, K.A.; Arlt, J.; Jones, A.C. Dynamic and static quenching of 2-aminopurine fluorescence by the natural DNA nucleotides in solution. Methods Appl. Fluoresc. 2020, 8, 025002. [Google Scholar] [CrossRef]
- Youssef, L.; Patra, D. Interaction of carbon nanotubes with curcumin: Effect of temperature and pH on simultaneous static and dynamic fluorescence quenching of curcumin using carbon nanotubes. Luminescence 2020, 35, 659–666. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Qi, P. Effects of BDE-209 and its mixtures with BDE-47 and BDE-99 on multiple biomarkers in Carassius auratus. Environ. Toxicol. Pharmacol. 2014, 38, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sun, Y.; Wang, L.; Zhao, C.; Fua, J.; Zhang, A. Understanding the microscopic binding mechanism of hydroxylated and sulfated polybrominated diphenyl ethers with transthyretin by molecular docking, molecular dynamics simulations and binding free energy calculations. Mol. Biosyst. 2017, 13, 736–749. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, X.; Xu, T.; Yin, D. Structure-dependent activities of polybrominated diphenyl ethers and hydroxylated metabolites on zebrafish retinoic acid receptor. Environ. Sci. Pollut. Res. 2015, 22, 1723–1730. [Google Scholar] [CrossRef]
- Cao, L.; Zheng, Z.; Ren, X.; Andersson, P.L.; Guo, L. Structure-dependent activity of polybrominated diphenyl ethers and their hydroxylated metabolites on estrogen related receptor gamma: In vitro and in silico study. Environ. Sci. Technol. 2018, 52, 8894–8902. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Hu, R.; Zhan, H.; You, W.; Tao, J.; Jiang, L. Study on the Biomolecular Competitive Mechanism of Polybrominated Diphenyl Ethers and Their Derivatives on Thyroid Hormones. Molecules 2023, 28, 7374. https://doi.org/10.3390/molecules28217374
Liu S, Hu R, Zhan H, You W, Tao J, Jiang L. Study on the Biomolecular Competitive Mechanism of Polybrominated Diphenyl Ethers and Their Derivatives on Thyroid Hormones. Molecules. 2023; 28(21):7374. https://doi.org/10.3390/molecules28217374
Chicago/Turabian StyleLiu, Shaoheng, Rong Hu, Hao Zhan, Wanli You, Jianjun Tao, and Luhua Jiang. 2023. "Study on the Biomolecular Competitive Mechanism of Polybrominated Diphenyl Ethers and Their Derivatives on Thyroid Hormones" Molecules 28, no. 21: 7374. https://doi.org/10.3390/molecules28217374
APA StyleLiu, S., Hu, R., Zhan, H., You, W., Tao, J., & Jiang, L. (2023). Study on the Biomolecular Competitive Mechanism of Polybrominated Diphenyl Ethers and Their Derivatives on Thyroid Hormones. Molecules, 28(21), 7374. https://doi.org/10.3390/molecules28217374




