Evaluation of the Biological Activity of Hydrogel with Cornus mas L. Extract and Its Potential Use in Dermatology and Cosmetology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Analysis of Extracts from Fruit of C. mas L.
2.2. Assessment of Antioxidant Activity
2.2.1. DPPH and ABTS Radical Scavenging
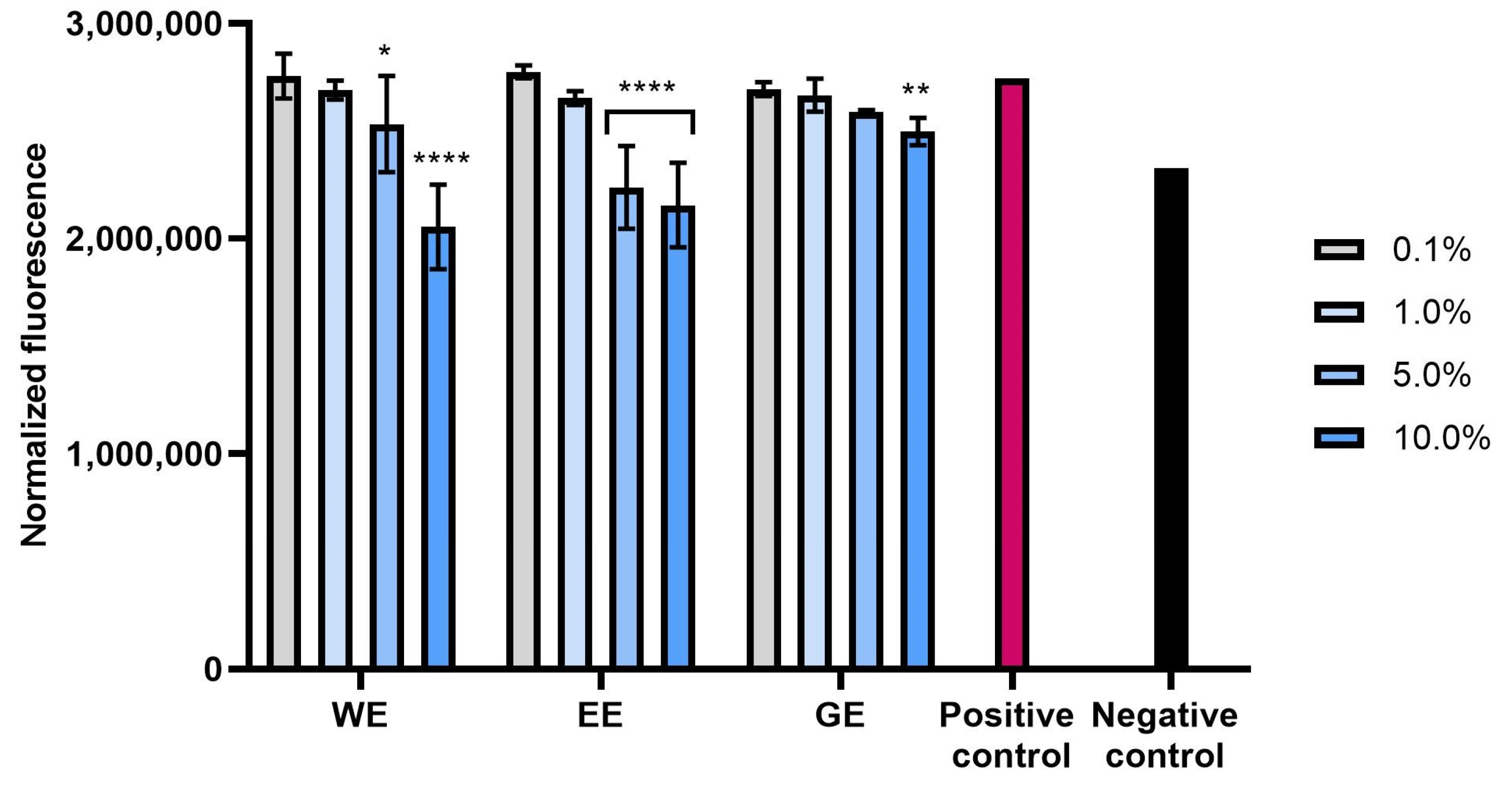
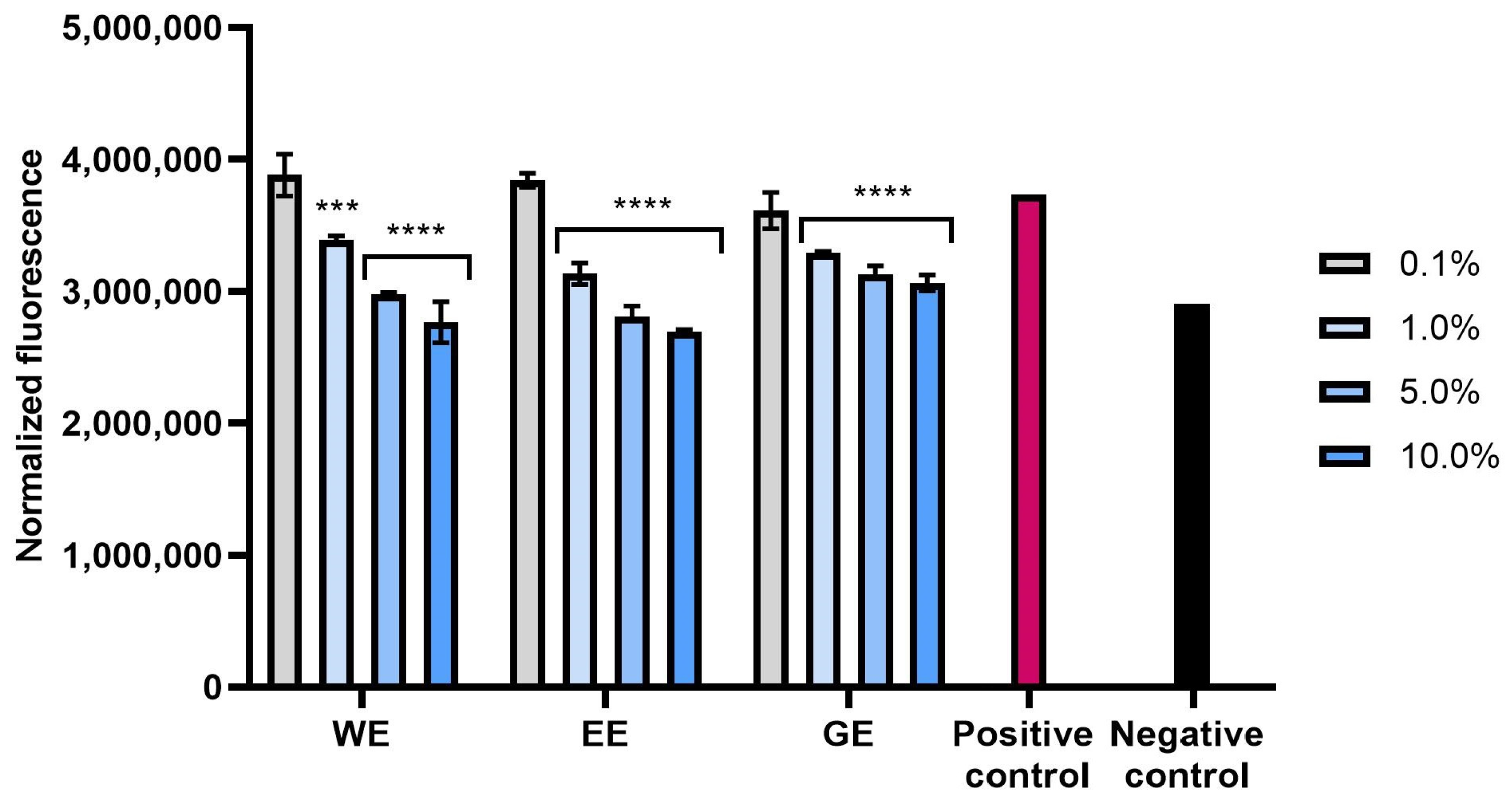
2.2.2. Intracellular ROS Levels in Skin Cells
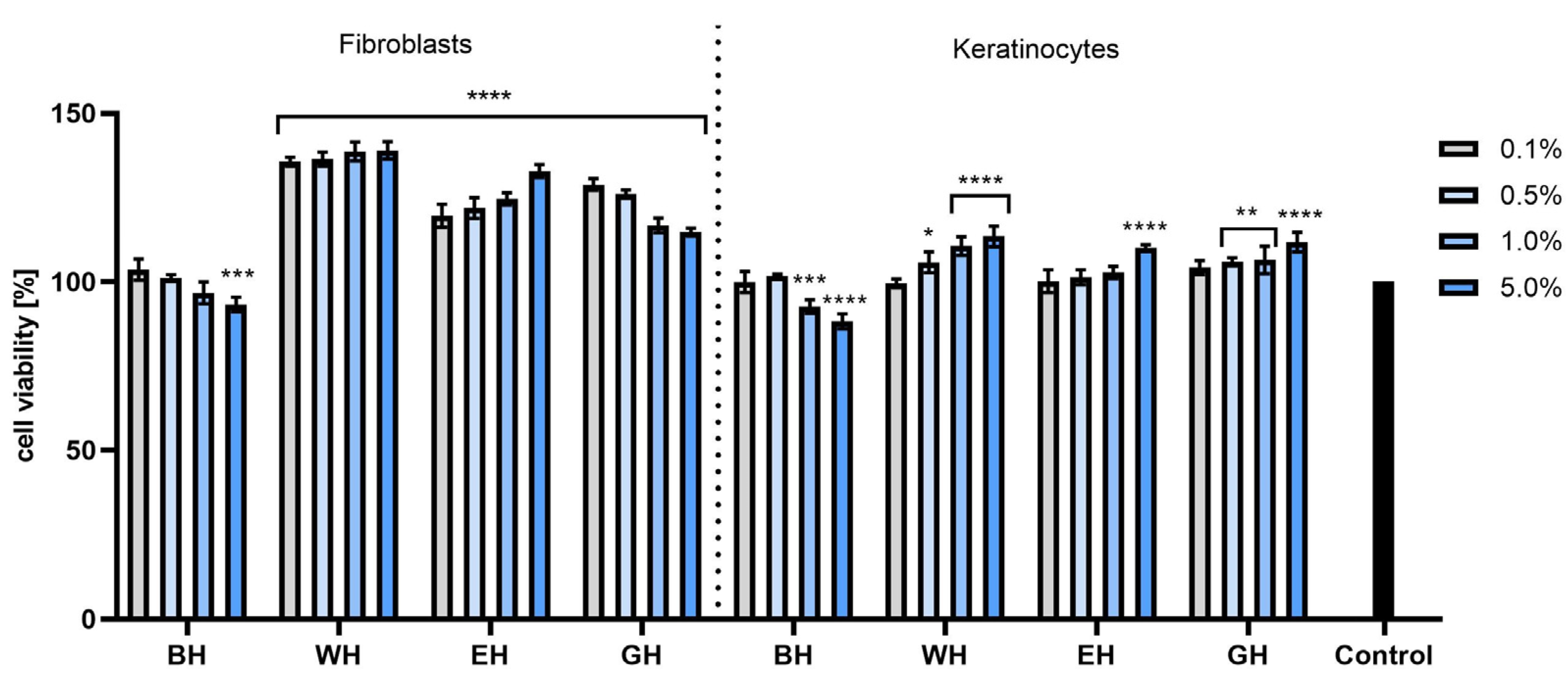
2.3. Cytotoxicity Assessment
2.4. Scratch Wound Assay
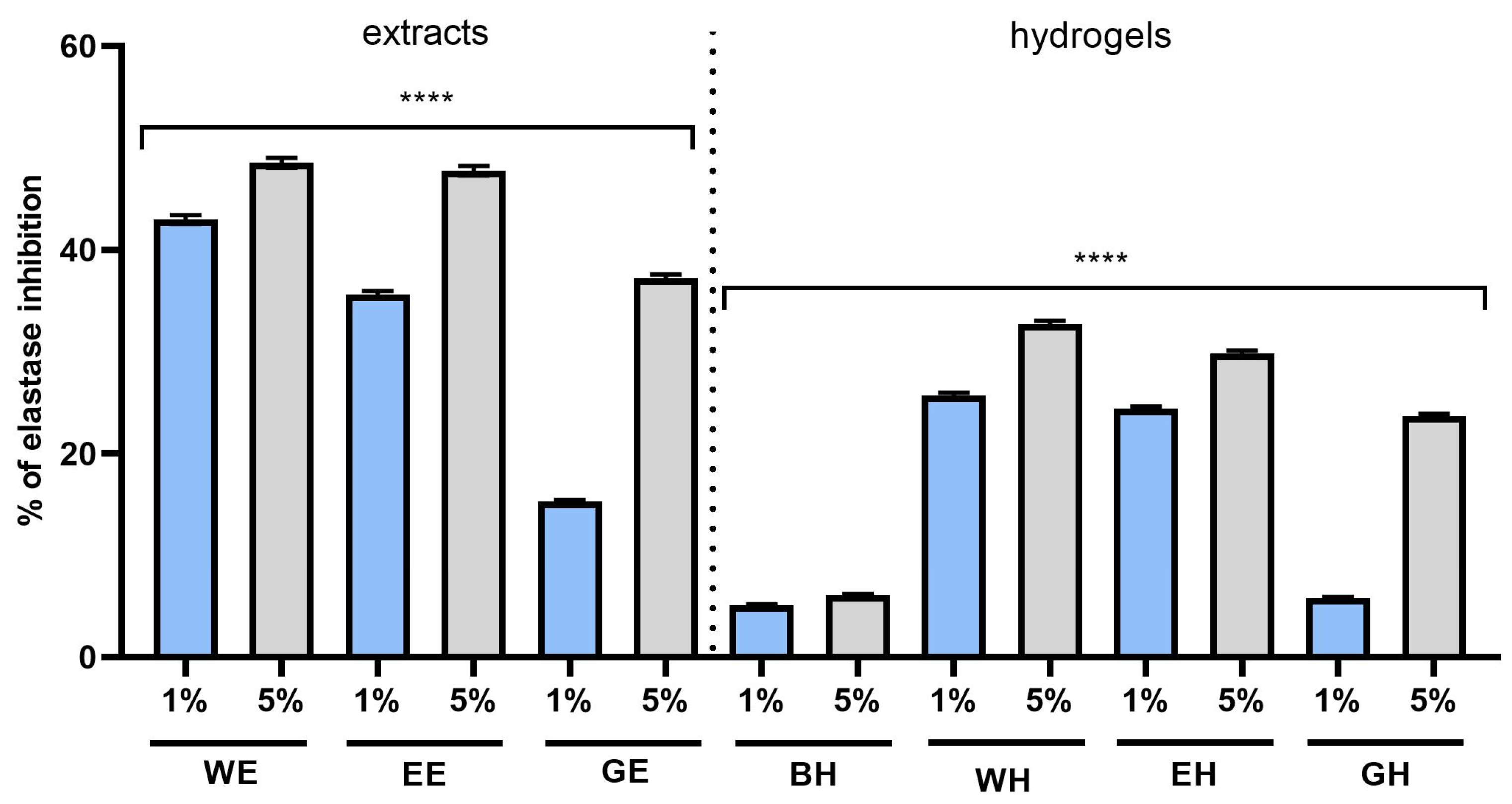
2.5. Assessment of Matrix Metallopeptidase Inhibition Using ELISA Method
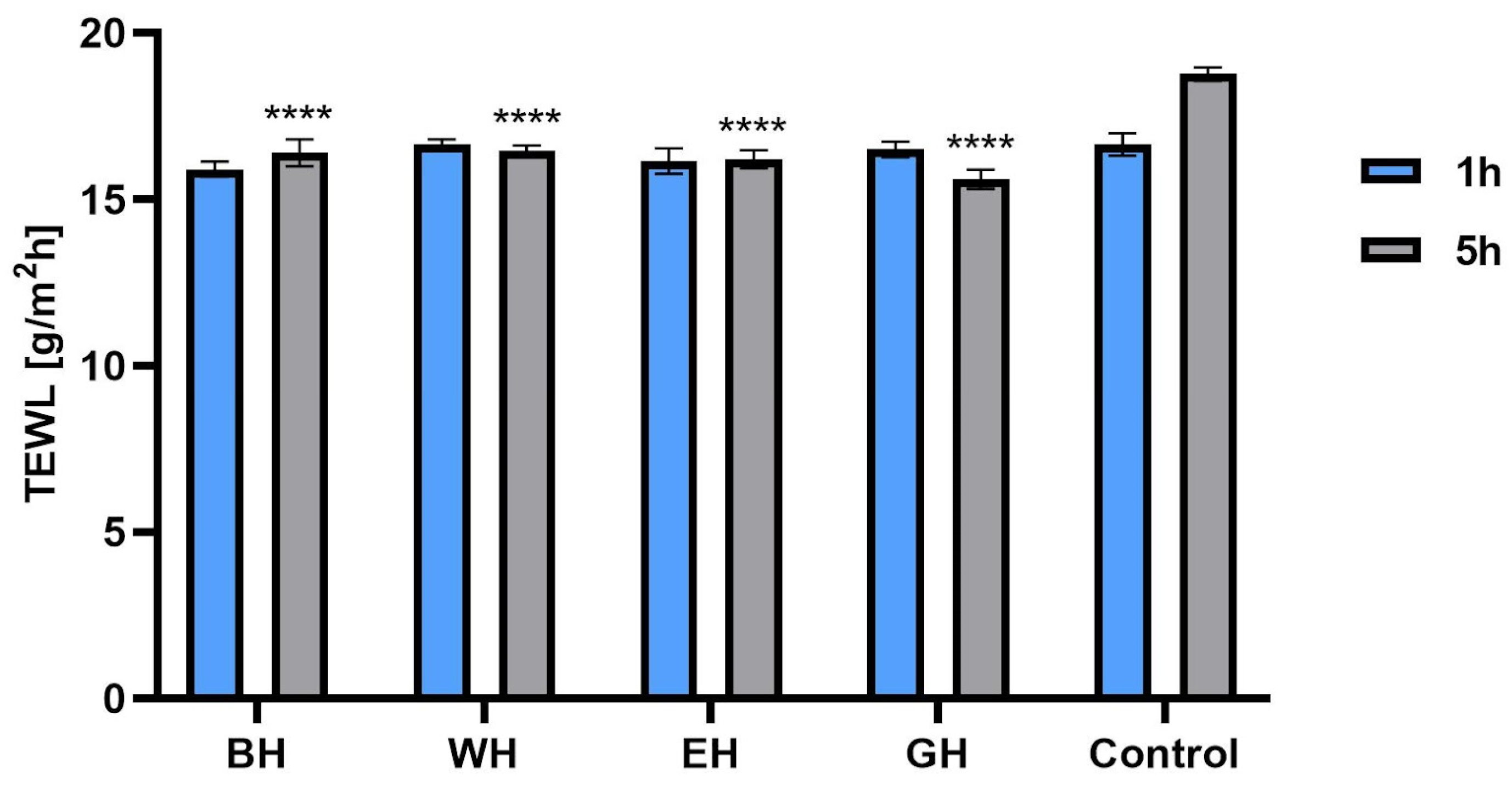
2.6. Transepidermal Water Loss (TEWL) and Skin Hydration Measurements
3. Materials and Methods
3.1. Materials
3.2. Plant Material and Extraction Procedure
3.3. Hydrogel Preparation
3.4. Determination of Biologically Active Compounds
3.5. Determination of Antioxidant Properties
3.5.1. DPPH (1,1-Diphenyl-2-picrylhydrazyl) Radical Scavenging Assay
3.5.2. ABTS Scavenging Assay
3.5.3. Determination of Intracellular Levels of Reactive Oxygen Species (ROS)
3.6. Cytotoxicity Analysis
3.6.1. Cell Culture
3.6.2. Alamar Blue Assay
3.6.3. Neutral Red Uptake Assay
3.7. Scratch Wound Assay
3.8. Evaluation of Inhibition of Collagenase and Elastase Activity
3.9. Transepidermal Water Loss (TEWL) and Skin Hydration Measurements
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. Prevalence of Most Common Skin Diseases in Europe: A Population-Based Study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1088–1096. [Google Scholar] [CrossRef]
- Faccio, G. Plant Complexity and Cosmetic Innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef]
- Stallings, A.F.; Lupo, M.P. Practical Uses of Botanicals in Skin Care. J. Clin. Aesthet. Dermatol. 2009, 2, 36. [Google Scholar]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional Properties of Cornelian Cherry (Cornus mas L.): A Comprehensive Review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Determination of Physical and Chemical Properties of Cornelian Cherry (Cornus mas L.) Fruits Depending on Degree of Ripening and Ecotypes—Acta Scientiarum Polonorum. Hortorum Cultus—Tom 18, Numer 2 (2019)—AGRO—Yadda. Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-3c1358e0-2c3f-47ee-a4cc-c13e6eab01af (accessed on 20 October 2023).
- Hosseinpour-Jaghdani, F.; Shomali, T.; Gholipour-Shahraki, S.; Rahimi-Madiseh, M.; Rafieian-Kopaei, M. Cornus mas: A Review on Traditional Uses and Pharmacological Properties. J. Complement. Integr. Med. 2017, 14, 1–16. [Google Scholar] [CrossRef]
- Bayram, H.M.; Arda Ozturkcan, S. Bioactive Components and Biological Properties of Cornelian Cherry (Cornus mas L.): A Comprehensive Review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Klymenko, S.; Kucharska, A.Z.; Sokół-łętowska, A.; Piórecki, N.; Przybylska, D.; Grygorieva, O. Iridoids, Flavonoids, and Antioxidant Capacity of Cornus mas, C. officinalis, and C. mas × C. officinalis Fruits. Biomolecules 2021, 11, 776. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for Hydrogels in Cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 6. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The Application of Hydrogels Based on Natural Polymers for Tissue Engineering. Curr. Med. Chem. 2019, 27, 2658–2680. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Minhas, M.U.; Ahmad, M.; Sohail, M.; Khalid, Q.; Abdullah, O. Synthesis and Evaluation of Topical Hydrogel Membranes; a Novel Approach to Treat Skin Disorders. J. Mater. Sci. Mater. Med. 2018, 29, 191. [Google Scholar] [CrossRef]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the Delivery of Plant-Derived (Poly)Phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef]
- Martinović, A.; Cavoski, I. The Exploitation of Cornelian Cherry (Cornus mas L.) Cultivars and Genotypes from Montenegro as a Source of Natural Bioactive Compounds. Food Chem. 2020, 318, 126549. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Szumny, A.; Sokól-Letowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and Anthocyanins in Cornelian Cherry (Cornus mas L.) Cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Camangi, F.; Braca, A. Quali-Quantitative Analysis of Flavonoids of Cornus mas L. (Cornaceae) Fruits. Food Chem. 2010, 119, 1257–1261. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z.; Piórecki, N. Characteristics of Biologically Active Compounds in Cornelian Cherry Meads. Molecules 2018, 23, 2024. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Dziok, M.; Ziemlewska, A.; Mokrzyńska, A.; Nizioł-Łukaszewska, Z.; Sowa, I.; Szczepanek, D.; Wójciak, M. Comparative Study of Cytotoxicity and Antioxidant, Anti-Aging and Antibacterial Properties of Unfermented and Fermented Extract of Cornus mas L. Int. J. Mol. Sci. 2023, 24, 13232. [Google Scholar] [CrossRef] [PubMed]
- Chavan, U.D.; Shahidi, F.; Naczk, M. Extraction of Condensed Tannins from Beach Pea (Lathyrus maritimus L.) as Affected by Different Solvents. Food Chem. 2001, 75, 509–512. [Google Scholar] [CrossRef]
- Atik, F.; Mohammedi, Z. Impact of Solvent Extraction Type on Total Polyphenols Content and Biological Activity from Tamarix aphylla (L.) Karst. Int. J. Pharma Bio Sci. 2011, 2, 609–615. [Google Scholar]
- Sun, T.; Ho, C.T. Antioxidant Activities of Buckwheat Extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Aurori, M.; Andrei, S.; Dreanca, A.I.; Morohoschi, A.G.; Cotul, M.; Niculae, M.; Nan, M.I.; Codea, A.R.; Gal, A.F. The Nephroprotective Effect of Cornelian Cherry (Cornus mas L.) and Rowanberry (Sorbus aucuparia L.) in Gentamicin-Induced Nephrotoxicity on Wistar Rats with Emphasis on the Evaluation of Novel Renal Biomarkers and the Antioxidant Capacity in Correlation with Nitro-Oxidative Stress. Nutrients 2023, 15, 4392. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Barreca, D. Mechanisms of Plant Antioxidants Action. Plants 2020, 10, 35. [Google Scholar] [CrossRef]
- E Silva, S.A.M.; Leonardi, G.R.; Michniak-Kohn, B. An Overview about Oxidation in Clinical Practice of Skin Aging. An. Bras. Dermatol. 2017, 92, 367–374. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, Y.-W.; Liu, Q.; Liu, L.-P.; Luo, F.-L.; Zhou, H.-C.; Isoda, H.; Ohkohchi, N.; Li, Y.-M.; Xu, H.; et al. Reactive Oxygen Species in Skin Repair, Regeneration, Aging, and Inflammation. React. Oxyg. Species (ROS) Living Cells 2017, 8, 69–88. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.C.; Simmonds, M.S.J.; Sampson, J.; Houghton, P.J.; Grice, P. Wound Healing Activity of Acylated Iridoid Glycosides from Scrophularia nodosa. Phytother. Res. 2002, 16, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Nazir, M.; Green, I.R.; Saleem, M.; Raza, M.L. Therapeutic Potential of Iridoid Derivatives: Patent Review. Inventions 2019, 4, 29. [Google Scholar] [CrossRef]
- Delicato, A.; Masi, M.; De Lara, F.; Rubiales, D.; Paolillo, I.; Lucci, V.; Falco, G.; Calabrò, V.; Evidente, A. In Vitro Characterization of Iridoid and Phenylethanoid Glycosides from Cistanche Phelypaea for Nutraceutical and Pharmacological Applications. Phytother. Res. 2022, 36, 4155. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid–loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef]
- Perde-Schrepler, M.; David, L.; Olenic, L.; Potara, M.; Fischer-Fodor, E.; Virag, P.; Imre-Lucaci, F.; Brie, I.; Florea, A. Gold Nanoparticles Synthesized with a Polyphenols-Rich Extract from Cornelian Cherry (Cornus mas) Fruits: Effects on Human Skin Cells. J. Nanomater. 2016, 2016, 6986370. [Google Scholar] [CrossRef]
- Vang Mouritzen, M.; Jenssen, H. Optimized Scratch Assay for In Vitro Testing of Cell Migration with an Automated Optical Camera. J. Vis. Exp. 2018, 2018, e57691. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Lu, W.; Xu, M.; Yuan, Y.; Zhang, X.; Tan, J.; He, J.; Tian, Y. Effect of Raspberry Extract on Wound Healing. Food Qual. Saf. 2021, 5, fyab013. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Z.; Wang, Z.; Wang, X. Topical Application of Quercetin Improves Wound Healing in Pressure Ulcer Lesions. Exp. Dermatol. 2018, 27, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kaptaner İğci, B.; Aytaç, Z. An Investigation on the in Vitro Wound Healing Activity and Phytochemical Composition of Hypericum Pseudolaeve N. Robson Growing in Turkey. Turk. J. Pharm. Sci. 2020, 17, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.E.; Ebrahimi, S.N.; Salehi, P.; Farimani, M.M.; Hamburger, M.; Jabbarzadeh, E. Wound Healing Potential of Chlorogenic Acid and Myricetin-3-O-β-Rhamnoside Isolated from Parrotia Persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Curr. Aging Sci. 2019, 13, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wahab, N.A.; Rahman, R.A.; Ismail, A.; Mustafa, S.; Hashim, P. Assessment of Antioxidant Capacity, Anti-Collagenase and Anti-Elastase Assays of Malaysian Unfermented Cocoa Bean for Cosmetic Application. Nat. Prod. Chem. Res. 2014, 2, 132. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, Anti-Collagenase and Anti-Elastase Activities of Phyllanthus Emblica, Manilkara Zapota and Silymarin: An in Vitro Comparative Study for Anti-Aging Applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-Collagenase, Anti-Elastase and Anti-Oxidant Activities of Extracts from 21 Plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Eun, C.H.; Kang, M.S.; Kim, I.J. Elastase/Collagenase Inhibition Compositions of Citrus Unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity. Appl. Sci. 2020, 10, 4838. [Google Scholar] [CrossRef]
- Wittenauer, J.; MäcKle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory Effects of Polyphenols from Grape Pomace Extract on Collagenase and Elastase Activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Teramachi, F.; Koyano, T.; Kowithayakorn, T.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Collagenase Inhibitory Quinic Acid Esters from Ipomoea Pes-Caprae. J. Nat. Prod. 2005, 68, 794–796. [Google Scholar] [CrossRef]
- Mandrone, M.; Coqueiro, A.; Poli, F.; Antognoni, F.; Choi, Y.H. Identification of a Collagenase-Inhibiting Flavonoid from Alchemilla Vulgaris Using NMR-Based Metabolomics. Planta Med. 2018, 84, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, V.; Viel, A.; Vincenzi, S. Caftaric Acid Isolation from Unripe Grape: A “Green” Alternative for Hydroxycinnamic Acids Recovery. Molecules 2021, 26, 1148. [Google Scholar] [CrossRef] [PubMed]
- Mac-Mary, S.; Creidi, P.; Marsaut, D.; Courderot-Masuyer, C.; Cochet, V.; Gharbi, T.; Guidicelli-Arranz, D.; Tondu, F.; Humbert, P. Assessment of Effects of an Additional Dietary Natural Mineral Water Uptake on Skin Hydration in Healthy Subjects by Dynamic Barrier Function Measurements and Clinic Scoring. Ski. Res. Technol. 2006, 12, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Short, R.W.; Chan, J.L.; Choi, J.M.; Egbert, B.M.; Rehmus, W.E.; Kimball, A.B. Effects of Moisturization on Epidermal Homeostasis and Differentiation. Clin. Exp. Dermatol. 2007, 32, 88–90. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernández-nogueras, M.V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Nowak, I. Lipid Nanoparticles Loaded with Selected Iridoid Glycosides as Effective Components of Hydrogel Formulations. Materials 2021, 14, 4090. [Google Scholar] [CrossRef]
- Farboud, E.S.; Nasrollahi, S.A.; Tabbakhi, Z. Novel Formulation and Evaluation of a Q10-Loaded Solid Lipid Nanoparticle Cream: In Vitro and in Vivo Studies. Int. J. Nanomed. 2011, 6, 611–617. [Google Scholar] [CrossRef]
- Albèr, C.; Buraczewska-Norin, I.; Kocherbitov, V.; Saleem, S.; Lodén, M.; Engblom, J. Effects of Water Activity and Low Molecular Weight Humectants on Skin Permeability and Hydration Dynamics—A Double-Blind, Randomized and Controlled Study. Int. J. Cosmet. Sci. 2014, 36, 412–418. [Google Scholar] [CrossRef]
- Chen, H.; Chang, X.; Du, D.; Li, J.; Xu, H.; Yang, X. Microemulsion-Based Hydrogel Formulation of Ibuprofen for Topical Delivery. Int. J. Pharm. 2006, 315, 52–58. [Google Scholar] [CrossRef]
- Chen, H.; Chang, X.; Weng, T.; Zhao, X.; Gao, Z.; Yang, Y.; Xu, H.; Yang, X. A Study of Microemulsion Systems for Transdermal Delivery of Triptolide. J. Control. Release 2004, 98, 427–436. [Google Scholar] [CrossRef]
- Bolzinger, M.A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of Drugs through Skin, a Complex Rate-Controlling Membrane. Curr. Opin. Colloid. Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P. Methods for Evaluating Penetration of Drug into the Skin: A Review. Ski. Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, X.; Gao, Y. Extraction Optimization of Bioactive Compounds (Crocin, Geniposide and Total Phenolic Compounds) from Gardenia (Gardenia jasminoides Ellis) Fruits with Response Surface Methodology. Innov. Food Sci. Emerg. Technol. 2009, 10, 610–615. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors Influencing the Antioxidant Activity Determined by the ABTS.+ Radical Cation Assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago Officinalis Extracts on Skin Cells. Molecules 2023, 28, 868. [Google Scholar] [CrossRef] [PubMed]
- Page, B.; Page, M.; Noel, C. A New Fluorometric Assay for Cytotoxicity Measurements in Vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity Determined in Vitro by Morphological Alterations and Neutral Red Absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Zagórska- Dziok, M.; Ziemlewska, A.; Bujak, T. Comparison of the Antiaging and Protective Properties of Plants from the Apiaceae Family. Oxid. Med. Cell. Longev. 2020, 2020, 5307614. [Google Scholar] [CrossRef]
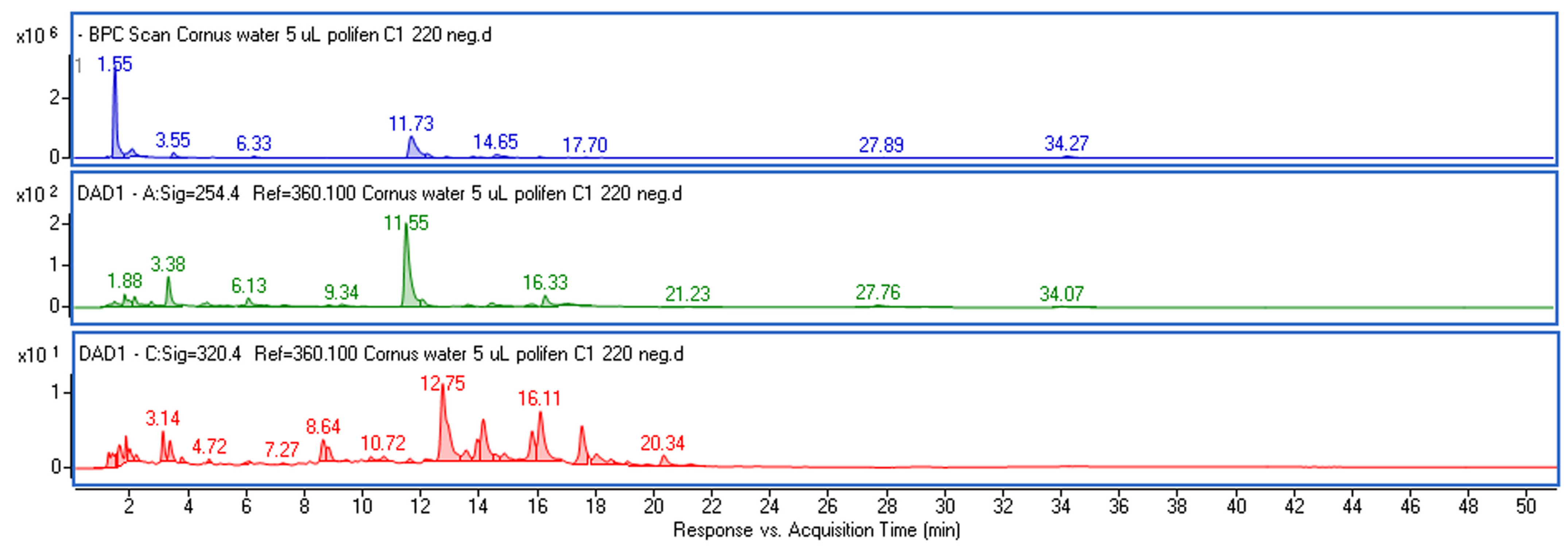








| Rt (min) | Observed Ion Mass [M-H]-/(Fragments) | Δ ppm | Formula | Identified | WE (µg/mL) | EE (µg/mL) | GE (µg/mL) |
|---|---|---|---|---|---|---|---|
| 1.55 | 191.05677 | 3.43 | C7H12O6 | Quinic acid * | 45.10 ± 2.55 a | 49.83 ± 2.24 a | 32.12 ± 1.54 b |
| 3.55 | 169.01363 (125) | −3.63 | C7H6O5 | Gallic acid * | 36.41 ± 1.91 a | 16.51 ± 0.99 b | 9.73 ± 0.41 c |
| 4.12 | 361.07686 (125, 169) | −2.14 | C14H18O11 | Galloyl-d-sedoheptulose | + | + | + |
| 6.33 | 153.01905 | −1.83 | C7H6O4 | Protocatechuic acid * | 7.21 ± 0.33 a | 3.12 ± 0.16 b | 2.54 ± 0.15 b |
| 6.80 | 243.05031 (125, 169) | −2.93 | C10H12O7 | Galloylglycerol 1 | 4.60 ± 0.28 a | 1.82 ± 0.09 b | 1.71 ± 0.08 b |
| 11.73 | 375.13013 | 1.22 | C16H24O10 | Loganic acid * | 45.51 ± 2.34 a | 34.11 ± 1.96 b | 17.90 ± 0.98 c |
| 12.93 | 311.04099 (179, 135) | 0.43 | C13H12O9 | Caftaric acid * | 10.32 ± 0.57 a | 3.53 ± 0.25 b | 1.73 ± 0.09 c |
| 13.83 | 549.18248 (375, 213) | −0.03 | C23H34O15 | Loganic acid derivative 2 | 0.98 ± 0.05 a | 1.10 ± 0.06 a | 0.99 ± 0.04 a |
| 14.15 | 353.08843 (191, 179) | 1.76 | C16H18O9 | Chlorogenic acid * | 2.61 ± 0.13 a | 0.31 ± 0.05 b | n.d. |
| 14.65 | 491.14011 (375) | −1.05 | C20H28O14 | Loganic acid derivative 2 | 2.12 ± 0.10 a | 2.51 ± 0.09 a | 2.33 ± 0.12 a |
| 16.13 | 491.14082 (375) | 0.39 | C20H28O14 | Loganic acid derivative 2 | 1.20 ± 0.06 a | 0.65 ± 0.02 b | 0.66 ± 0.03 b |
| 17.70 | 337.09214 (191, 173) | −2.22 | C16H18O8 | p-coumaroylquinic acid 3 | 1.93 ± 0.08 a | 1.21 ± 0.09 b | 0.33 ± 0.01 c |
| 17.79 | 447.09402 (285) | 1.64 | C21H20O11 | Cyanidin 3-O-galactoside * | 0.41 ± 0.06 a | 1.10 ± 0.08 b | n.d. |
| 18.79 | 431.09891 (269) | 1.25 | C21H20O10 | Pelargonidin 3-O-glucoside | 0.38 ± 0.04 a | 0.93 ± 0.07 b | n.d. |
| 19.04 | 403.12484 | 0.63 | C17H24O11 | Secoxyloganin | + | + | + |
| 27.89 | 300.99924 | 0.83 | C14H6O8 | Ellagic acid * | 2.81 ± 0.17 a | 4.61 ± 0.30 b | 1.51 ± 0.06 c |
| 28.67 | 477.06655 | −1.91 | C21H18O13 | Quercetin 3-glucuronide * | 1.92 ± 0.10 a | 2.92 ± 0.14 b | 0.61 ± 0.03 c |
| 34.27 | 541.15619 | −0.16 | C24H30O14 | Cornuside * | 1.12 ± 0.08 a | 0.44 ± 0.03 b | 1.21 ± 0.06 a |
| IC50 (%(v/v)) | Water Extract (WE) | Water–Ethanol Extract (EE) | Water–Glycerin Extract (GE) |
| DPPH assay | |||
| 5.62 ± 0.12 **** | 6.03 ± 0.09 **** | 7.16 ± 0.09 **** | |
| ABTS assay | |||
| 5.72 ± 0.08 **** (**) | 6.69 ± 0.11 **** (**) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagórska-Dziok, M.; Ziemlewska, A.; Mokrzyńska, A.; Nizioł-Łukaszewska, Z.; Wójciak, M.; Sowa, I. Evaluation of the Biological Activity of Hydrogel with Cornus mas L. Extract and Its Potential Use in Dermatology and Cosmetology. Molecules 2023, 28, 7384. https://doi.org/10.3390/molecules28217384
Zagórska-Dziok M, Ziemlewska A, Mokrzyńska A, Nizioł-Łukaszewska Z, Wójciak M, Sowa I. Evaluation of the Biological Activity of Hydrogel with Cornus mas L. Extract and Its Potential Use in Dermatology and Cosmetology. Molecules. 2023; 28(21):7384. https://doi.org/10.3390/molecules28217384
Chicago/Turabian StyleZagórska-Dziok, Martyna, Aleksandra Ziemlewska, Agnieszka Mokrzyńska, Zofia Nizioł-Łukaszewska, Magdalena Wójciak, and Ireneusz Sowa. 2023. "Evaluation of the Biological Activity of Hydrogel with Cornus mas L. Extract and Its Potential Use in Dermatology and Cosmetology" Molecules 28, no. 21: 7384. https://doi.org/10.3390/molecules28217384
APA StyleZagórska-Dziok, M., Ziemlewska, A., Mokrzyńska, A., Nizioł-Łukaszewska, Z., Wójciak, M., & Sowa, I. (2023). Evaluation of the Biological Activity of Hydrogel with Cornus mas L. Extract and Its Potential Use in Dermatology and Cosmetology. Molecules, 28(21), 7384. https://doi.org/10.3390/molecules28217384






