Hydrogen Sulfide Removal via Sorption Process on Activated Carbon–Metal Oxide Composites Derived from Different Biomass Sources
Abstract
:1. Introduction
2. Results and Discussion
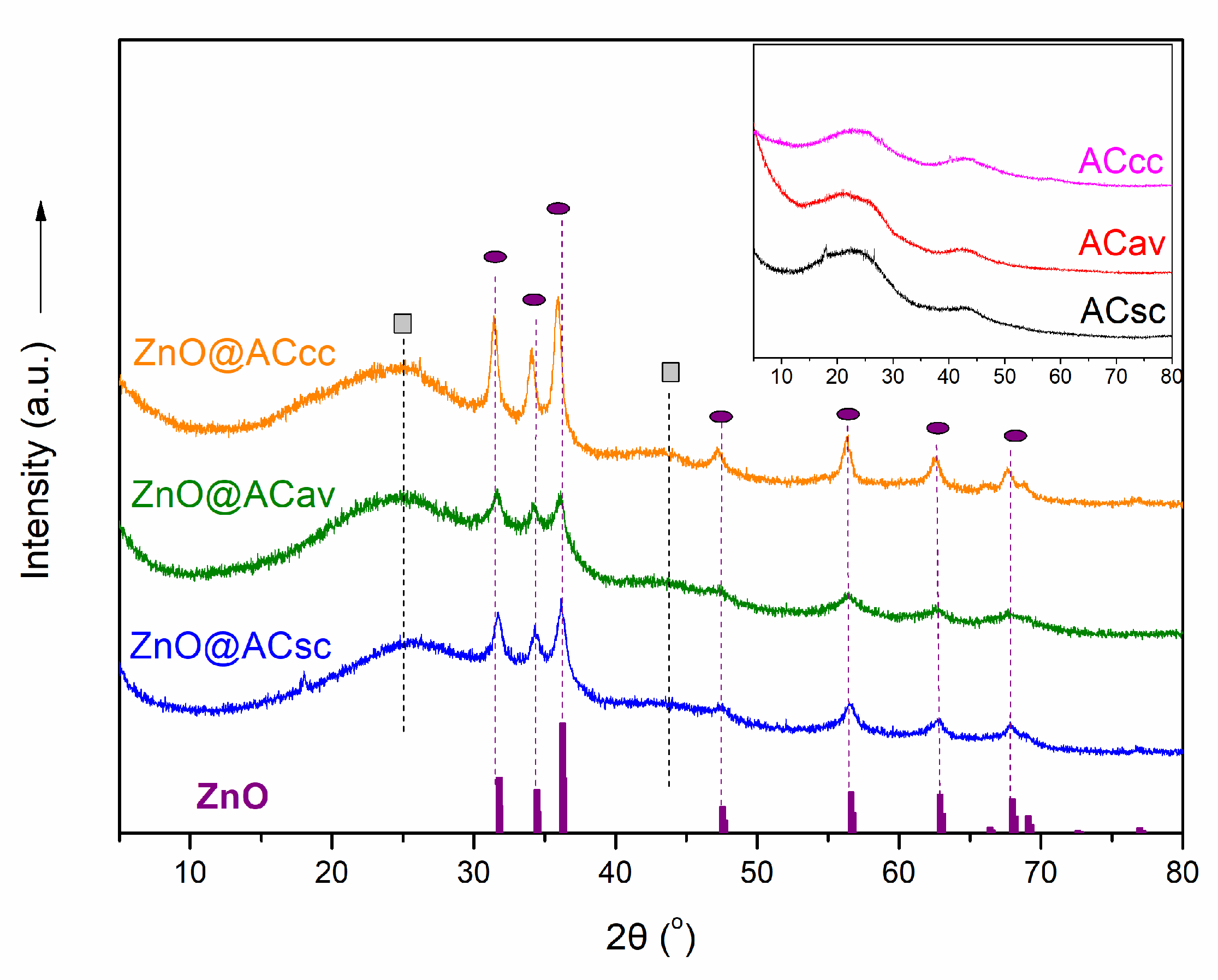
2.1. XRD Analysis of Pure AC and ZnO@AC Composites
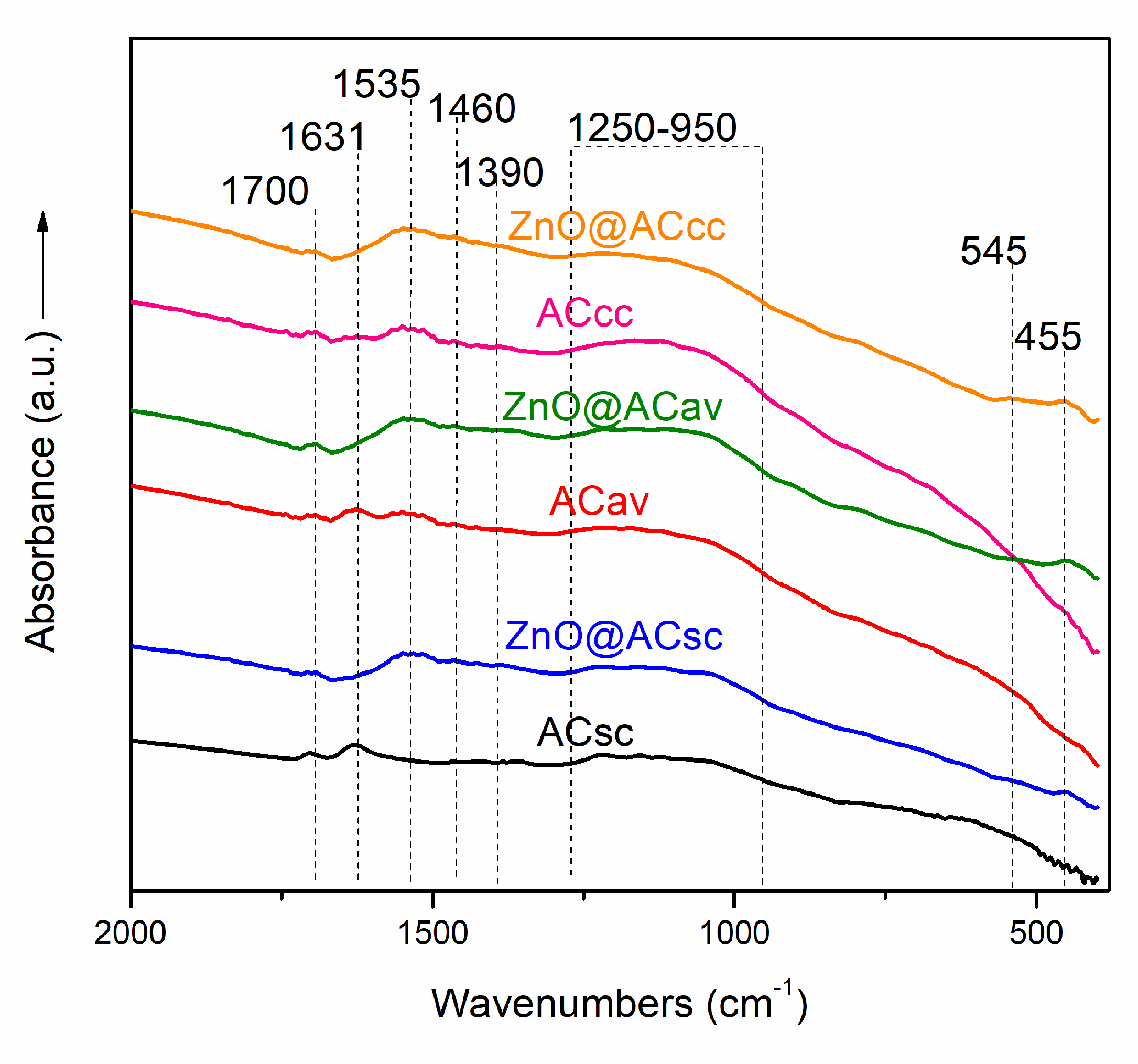
2.2. FTIR Spectroscopy of Pure AC and ZnO@AC Composites
2.3. Thermogravimetric TG% Analysis of Pure AC and ZnO@AC Composites
2.4. Nitrogen Porosimetry for Pore Structure Analysis of Pure AC and ZnO@AC Composites
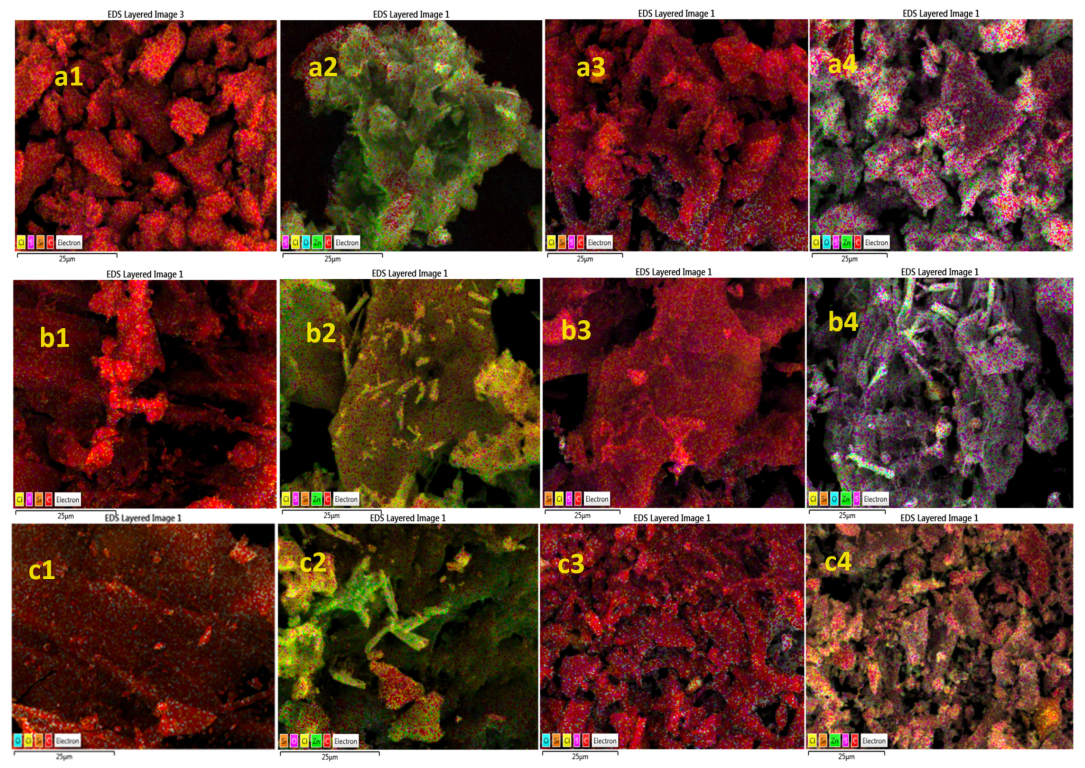
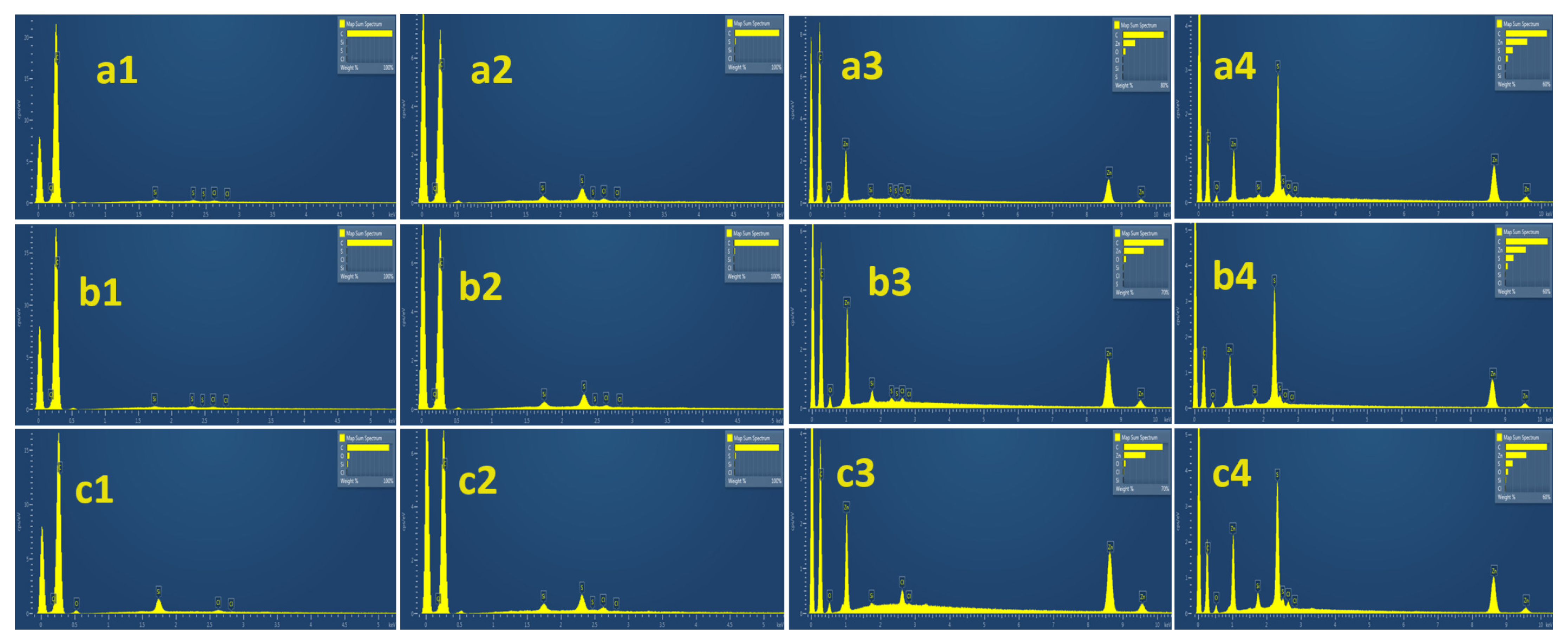
2.5. SEM-EDS Images Analysis of Pure AC and ZnO@AC Composites
2.6. Hydrogen Sulfide (H2S) Removal Experiments
3. Materials and Methods
3.1. Materials
3.2. Preparation of Activated Carbons
3.3. Preparation of ZnO@AC Composites
3.4. Characterization Techniques
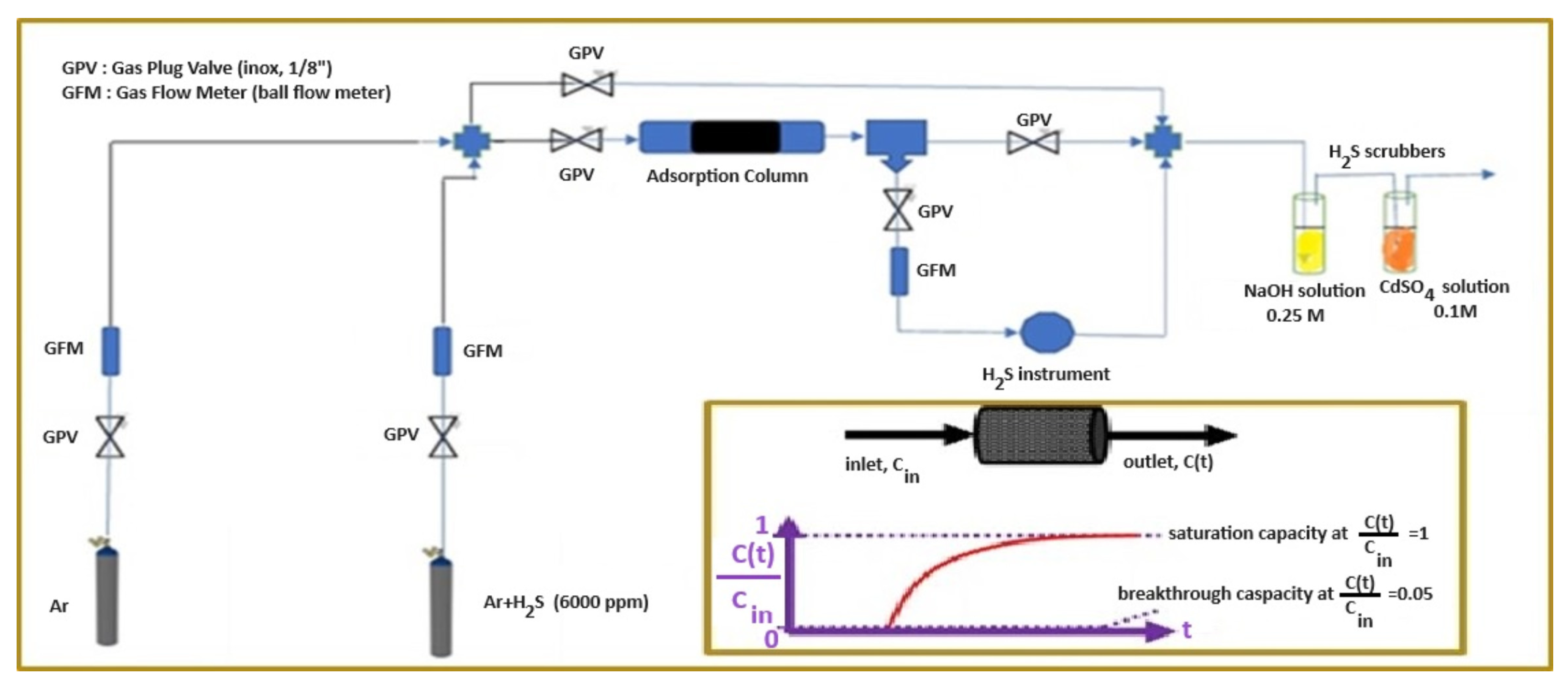
3.5. H2S Sorption Experimental Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomou, E.; Basina, G.; Spyrou, K.; Wahedi, Y.A.; Rudolf, P.; Gournis, D. H2S Removal by Copper Enriched Porous Carbon Cuboids. Carbon Trends 2022, 7, 100145. [Google Scholar] [CrossRef]
- Wiheeb, A.D.; Shamsudin, I.K.; Ahmad, M.A.; Murat, M.N.; Kim, J.; Othman, M.R. Present Technologies for Hydrogen Sulfide Removal from Gaseous Mixtures. Rev. Chem. Eng. 2013, 29, 449–470. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of Hydrogen Sulfide From Various Industrial Gases: A Review of The Most Promising Adsorbing Materials. Catalysts 2020, 10, 521. [Google Scholar] [CrossRef]
- Gary, J.H.; Handwerk, G.E. Petroleum Refining Technology and Economics, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1984; ISBN 0-8247-7150-8. [Google Scholar]
- Shoukat, U.; Pinto, D.D.D.; Knuutila, H.K. Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas. Processes 2019, 7, 160. [Google Scholar] [CrossRef]
- Vikrant, K.; Kailasa, S.K.; Tsang, D.C.W.; Lee, S.S.; Kumar, P.; Giri, B.S.; Singh, R.S.; Kim, K.-H. Biofiltration of Hydrogen Sulfide: Trends and Challenges. J. Clean. Prod. 2018, 187, 131–147. [Google Scholar] [CrossRef]
- Pudi, A.; Rezaei, M.; Signorini, V.; Andersson, M.P.; Baschetti, M.G.; Mansouri, S.S. Hydrogen Sulfide Capture and Removal Technologies: A Comprehensive Review of Recent Developments and Emerging Trends. Sep. Purif. Technol. 2022, 298, 121448. [Google Scholar] [CrossRef]
- Khabazipour, M.; Anbia, M. Removal of Hydrogen Sulfide from Gas Streams Using Porous Materials: A Review. Ind. Eng. Chem. Res. 2019, 58, 22133–22164. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Noor Azam, A.M.; Masdar, M.S.; Isahak, W.N. Adsorption–Desorption Behavior of Hydrogen Sulfide Capture on a Modified Activated Carbon Surface. Materials 2023, 16, 462. [Google Scholar] [CrossRef]
- Bashkova, S.; Baker, F.S.; Wu, X.; Armstrong, T.R.; Schwartz, V. Activated Carbon Catalyst for Selective Oxidation of Hydrogen Sulphide: On the Influence of Pore Structure, Surface Characteristics, and Catalytically-Active Nitrogen. Carbon 2007, 45, 1354–1363. [Google Scholar] [CrossRef]
- Sawalha, H.; Maghalseh, M.; Qutaina, J.; Junaidi, K.; Rene, E.R. Removal of Hydrogen Sulfide from Biogas Using Activated Carbon Synthesized from Different Locally Available Biomass Wastes—A Case Study from Palestine. Bioengineered 2020, 11, 607–618. [Google Scholar] [CrossRef]
- Rambabu, N.; Azargohar, R.; Dalai, A.K.; Adjaye, J. Evaluation and Comparison of Enrichment Efficiency of Physical/Chemical Activations and Functionalized Activated Carbons Derived from Fluid Petroleum Coke for Environmental Applications. Fuel Process. Technol. 2013, 106, 501–510. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kubota, M.; Matsuda, H.; D’Elia Camacho, L.F. Adsorption Behaviors of Ammonia and Hydrogen Sulfide on Activated Carbon Prepared from Petroleum Coke by KOH Chemical Activation. Fuel Process. Technol. 2016, 144, 164–169. [Google Scholar] [CrossRef]
- Nam, H.; Wang, S.; Jeong, H.-R. TMA and H2S Gas Removals Using Metal Loaded on Rice Husk Activated Carbon for Indoor Air Purification. Fuel 2018, 213, 186–194. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Gralak-Podemska, B.; Nowicki, P.; Pietrzak, R. The Use of Microwave Radiation for Obtaining Activated Carbons from Sawdust and Their Potential Application in Removal of NO2 and H2S. Chem. Eng. J. 2015, 269, 352–358. [Google Scholar] [CrossRef]
- De Oliveira, L.H.; Meneguin, J.G.; Pereira, M.V.; do Nascimento, J.F.; Arroyo, P.A. Adsorption of Hydrogen Sulfide, Carbon Dioxide, Methane, and Their Mixtures on Activated Carbon. Chem. Eng. Commun. 2019, 206, 1533–1553. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, C.; Wu, Y.; Ke, C.; Liu, X.; Wang, J.; Qiao, W.; Ling, L. Efficient Removal of H2S with Zinc Oxide/Nitrogen-Doped Ordered Mesoporous Carbons at Room Temperature. Microporous Mesoporous Mater. 2022, 333, 111712. [Google Scholar] [CrossRef]
- Elyassi, B.; Wahedi, Y.A.; Rajabbeigi, N.; Kumar, P.; Jeong, J.S.; Zhang, X.; Kumar, P.; Balasubramanian, V.V.; Katsiotis, M.S.; Andre Mkhoyan, K.; et al. A High-Performance Adsorbent for Hydrogen Sulfide Removal. Microporous Mesoporous Mater. 2014, 190, 152–155. [Google Scholar] [CrossRef]
- Wang, L.-J.; Fan, H.-L.; Shangguan, J.; Croiset, E.; Chen, Z.; Wang, H.; Mi, J. Design of a Sorbent to Enhance Reactive Adsorption of Hydrogen Sulfide. ACS Appl. Mater. Interfaces 2014, 6, 21167–21177. [Google Scholar] [CrossRef]
- Karvan, O.; Atakül, H. Investigation of CuO/Mesoporous SBA-15 Sorbents for Hot Gas Desulfurization. Fuel Process. Technol. 2008, 89, 908–915. [Google Scholar] [CrossRef]
- Yang, J.H. Hydrogen Sulfide Removal Technology: A Focused Review on Adsorption and Catalytic Oxidation. Korean J. Chem. Eng. 2021, 38, 674–691. [Google Scholar] [CrossRef]
- Portela, R.; Rubio-Marcos, F.; Leret, P.; Fernández, J.F.; Bañares, M.A.; Ávila, P. Nanostructured ZnO/Sepiolite Monolithic Sorbents for H2S Removal. J. Mater. Chem. A 2014, 3, 1306–1316. [Google Scholar] [CrossRef]
- Samokhvalov, A.; Tatarchuk, B.J. Characterization of Active Sites, Determination of Mechanisms of H2S, COS and CS2 Sorption and Regeneration of ZnO Low-Temperature Sorbents: Past, Current and Perspectives. Phys. Chem. Chem. Phys. 2011, 13, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Awume, B.; Tajallipour, M.; Nemati, M.; Predicala, B. Application of ZnO Nanoparticles in Control of H2S Emission from Low-Temperature Gases and Swine Manure Gas. Water. Air Soil Pollut. 2017, 228, 147. [Google Scholar] [CrossRef]
- Hernández, S.P.; Chiappero, M.; Russo, N.; Fino, D. A Novel ZnO-Based Adsorbent for Biogas Purification in H2 Production Systems. Chem. Eng. J. 2011, 176–177, 272–279. [Google Scholar] [CrossRef]
- Yang, C.; Yang, S.; Fan, H.; Wang, Y.; Shangguan, J. Tuning the ZnO-Activated Carbon Interaction through Nitrogen Modification for Enhancing the H2S Removal Capacity. J. Colloid Interface Sci. 2019, 555, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Liang, D.T.; Tsen, L.; Tay, J.H. Kinetics and Mechanisms of H2S Adsorption by Alkaline Activated Carbon. Environ. Sci. Technol. 2002, 36, 4460–4466. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Wang, L.-J.; Yang, C.; Zhang, H.-Y.; Zhao, Y.-R.; Fan, H.-L.; Huo, C. Room-Temperature Hydrogen Sulfide Removal with Zinc Oxide Nanoparticle/Molecular Sieve Prepared by Melt Infiltration. Fuel Process. Technol. 2019, 185, 26–37. [Google Scholar] [CrossRef]
- Scherrer, P. Nachrichten von Der Gesellschaft Der Wissenschaften Zu Göttingen. Gött. Nachr. Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Daffalla, S.; Mukhtar, H.; Shaharun, M. Properties of Activated Carbon Prepared from Rice Husk with Chemical Activation. Int. J. Glob. Environ. Issues 2012, 12, 107–129. [Google Scholar] [CrossRef]
- Baikousi, M.; Georgiou, Y.; Daikopoulos, C.; Bourlinos, A.B.; Filip, J.; Zbořil, R.; Deligiannakis, Y.; Karakassides, M.A. Synthesis and Characterization of Robust Zero Valent Iron/Mesoporous Carbon Composites and Their Applications in Arsenic Removal. Carbon 2015, 93, 636–647. [Google Scholar] [CrossRef]
- Altıntıg, E.; Yenigun, M.; Sarı, A.; Altundag, H.; Tuzen, M.; Saleh, T.A. Facile Synthesis of Zinc Oxide Nanoparticles Loaded Activated Carbon as an Eco-Friendly Adsorbent for Ultra-Removal of Malachite Green from Water. Environ. Technol. Innov. 2021, 21, 101305. [Google Scholar] [CrossRef]
- Fan, B.; Guo, H.; Shi, J.; Shi, C.; Jia, Y.; Wang, H.; Chen, D.; Yang, Y.; Lu, H.; Xu, H.; et al. Facile One-Pot Preparation of Silver/Reduced Graphene Oxide Nanocomposite for Cancer Photodynamic and Photothermal Therapy. J. Nanosci. Nanotechnol. 2016, 16, 7049–7054. [Google Scholar] [CrossRef]
- Rochman, R.; Wahyuningsih, S.; Ramelan, A.; Hanif, Q. Preparation of Nitrogen and Sulphur Co-Doped Reduced Graphene Oxide (rGO-NS) Using N and S Heteroatom of Thiourea. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012119. [Google Scholar] [CrossRef]
- Chunlan, L.; Shaoping, X.; Yixiong, G.; Shuqin, L.; Changhou, L. Effect of Pre-Carbonization of Petroleum Cokes on Chemical Activation Process with KOH. Carbon 2005, 43, 2295–2301. [Google Scholar] [CrossRef]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel Green Route of Synthesis of ZnO Nanoparticles by Using Natural Biodegradable Polymer and Its Application as a Catalyst for Oxidation of Aldehydes. J. Macromol. Sci. Part Pure Appl. Chem. 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, G.; Zheng, H.; Jiang, X.; Shen, F. Combustion Performance of Pulverized Coal and Corresponding Kinetics Study after Adding the Additives of Fe2O3 and CaO. Int. J. Miner. Metall. Mater. 2023, 30, 314–323. [Google Scholar] [CrossRef]
- Gong, X.; Guo, Z.; Wang, Z. Reactivity of Pulverized Coals during Combustion Catalyzed by CeO2 and Fe2O3. Combust. Flame 2010, 157, 351–356. [Google Scholar] [CrossRef]
- Saroyan, H.; Arampatzidou, A.; Voutsa, D.; Lazaridis, N.; Deliyanni, E. Activated Carbon Supported MnO2 for Catalytic Degradation of Reactive Black 5. Colloids Surf. Physicochem. Eng. Asp. 2019, 566, 166–175. [Google Scholar] [CrossRef]
- Tanji, K.; El Mrabet, I.; Fahoul, Y.; Jellal, I.; Benjelloun, M.; Belghiti, M.; El Hajam, M.; Naciri, Y.; El Gaidoumi, A.; El Bali, B.; et al. Epigrammatic Progress on the Photocatalytic Properties of ZnO and TiO2 Based Hydroxyapatite@photocatlyst toward Organic Molecules Photodegradation: A Review. J. Water Process Eng. 2023, 53, 103682. [Google Scholar] [CrossRef]
- Tanji, K.; El Mrabet, I.; Fahoul, Y.; Soussi, A.; Belghiti, M.; Jellal, I.; Naciri, Y.; El Gaidoumi, A.; Kherbeche, A. Experimental and Theoretical Investigation of Enhancing the Photocatalytic Activity of Mg Doped ZnO for Nitrophenol Degradation. React. Kinet. Mech. Catal. 2023, 136, 1125–1142. [Google Scholar] [CrossRef]
- Belghiti, M.; Tanji, K.; El Mersly, L.; Lamsayety, I.; Ouzaouit, K.; Faqir, H.; Benzakour, I.; Rafqah, S.; Outzourhit, A. Fast and Non-Selective Photodegradation of Basic Yellow 28, Malachite Green, Tetracycline, and Sulfamethazine Using a Nanosized ZnO Synthesized from Zinc Ore. React. Kinet. Mech. Catal. 2022, 135, 2265–2278. [Google Scholar] [CrossRef]
- Salmas, C.E. DOWNLOADS, CPSM_Nitrogen. Available online: http://users.uoi.gr/ksalmas/ (accessed on 1 August 2023).
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Salmas, C.E.; Androutsopoulos, G.P. Rigid Sphere Molecular Model Enables an Assessment of the Pore Curvature Effect upon Realistic Evaluations of Surface Areas of Mesoporous and Microporous Materials. Langmuir 2005, 21, 11146–11160. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the Bet Equation Applicable to Microporous Adsorbents? In Studies in Surface Science and Catalysis; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Characterization of Porous Solids VII; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 49–56. [Google Scholar]
- Surya, K.; Michael, M.S. Hierarchical Porous Activated Carbon Prepared from Biowaste of Lemon Peel for Electrochemical Double Layer Capacitors. Biomass Bioenergy 2021, 152, 106175. [Google Scholar] [CrossRef]
- Ding, Y.; Qi, J.; Hou, R.; Liu, B.; Yao, S.; Lang, J.; Chen, J.; Yang, B. Preparation of High-Performance Hierarchical Porous Activated Carbon via a Multistep Physical Activation Method for Supercapacitors. Energy Fuels 2022, 36, 5456–5464. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Li, A.; Li, H.; Cui, D.; Dong, M.; Lin, J.; Fan, J.; Zhang, J.; Hou, H.; et al. Advanced Porous Hierarchical Activated Carbon Derived from Agricultural Wastes toward High Performance Supercapacitors. J. Alloys Compd. 2020, 820, 153111. [Google Scholar] [CrossRef]
- Hussain, M.; Abbas, N.; Fino, D.; Russo, N. Novel Mesoporous Silica Supported ZnO Adsorbents for the Desulphurization of Biogas at Low Temperatures. Chem. Eng. J. 2012, 188, 222–232. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Androutsopoulos, G.P.; Salmas, C.E. A New Model for Capillary Condensation−Evaporation Hysteresis Based on a Random Corrugated Pore Structure Concept: Prediction of Intrinsic Pore Size Distributions. 1. Model Formulation. Ind. Eng. Chem. Res. 2000, 39, 3747–3763. [Google Scholar] [CrossRef]
- Androutsopoulos, G.P.; Salmas, C.E. A New Model for Capillary Condensation−Evaporation Hysteresis Based on a Random Corrugated Pore Structure Concept: Prediction of Intrinsic Pore Size Distribution. 2. Model Application. Ind. Eng. Chem. Res. 2000, 39, 3764–3777. [Google Scholar] [CrossRef]
- Dombrowski, R.J.; Hyduke, D.R.; Lastoskie, C.M. Pore Size Analysis of Activated Carbons from Argon and Nitrogen Porosimetry Using Density Functional Theory. Langmuir 2000, 16, 5041–5050. [Google Scholar] [CrossRef]
- Salmas, C.E.; Androutsopoulos, G.P. Preparation and Characterization of Anodic Aluminum Oxide Films Exhibiting Microporosity. Chem. Eng. Commun. 2008, 196, 407–442. [Google Scholar] [CrossRef]
- Marsh, H.; Rand, B. The Characterization of Microporous Carbons by Means of the Dubinin-Radushkevich Equation. J. Colloid Interface Sci. 1970, 33, 101–116. [Google Scholar] [CrossRef]

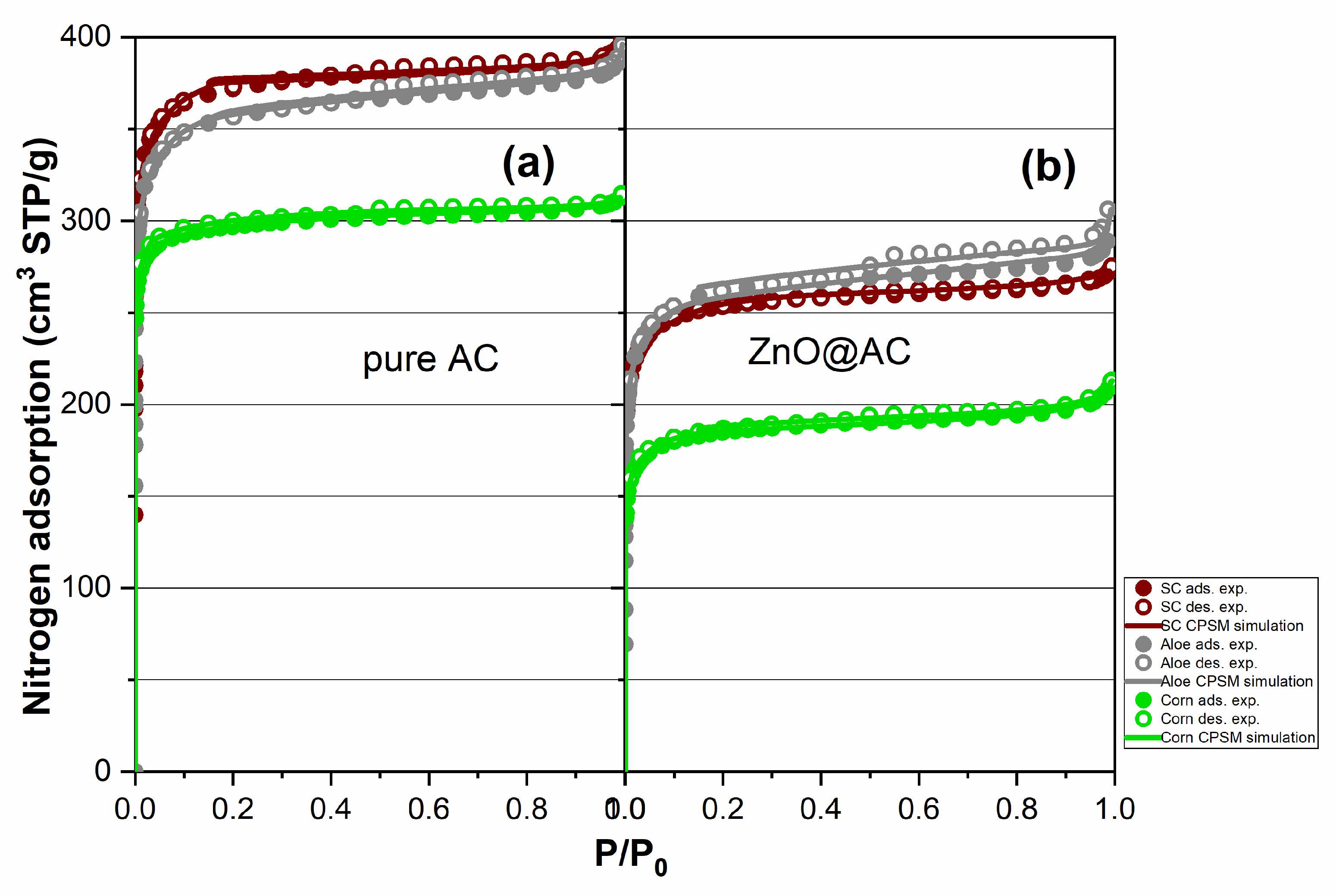
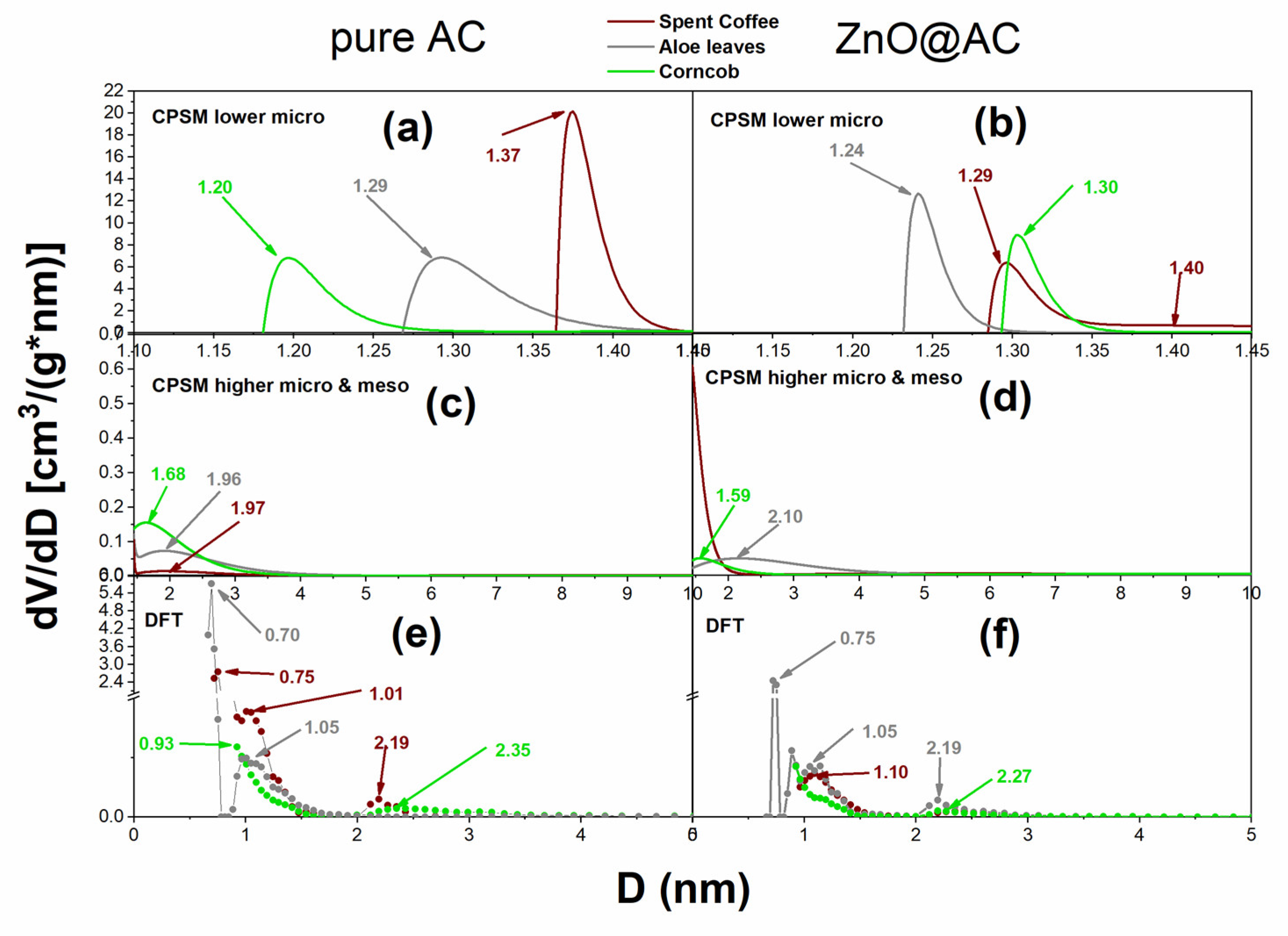
| Material Code | Sg(m2/g) (BET) | Sg(m2/g) (BETRouq.) | Sg(m2/g) (Lang.) | Sg(m2/g) (CPSM) | Decreasing Ratio |
|---|---|---|---|---|---|
| ACsc | 1195 | 1452 | 1643 | 1653 | 1.5 |
| ZnO@ACsc | 818 | 989 | 1121 | 1132 | |
| ACav | 1148 | 1498 | 1577 | 1594 | 1.3 |
| ZnO@ACav | 846 | 990 | 1156 | 1188 | |
| ACcc | 953 | 1210 | 1300 | 1341 | 1.6 |
| ZnO@ACcc | 597 | 726 | 816 | 832 |
| Material Code | Total Pore Volume (cm3/g) | Dmean (nm) CPSM Low Micro | %Microp. (CPSM) | %Microp. (Dubinin) |
|---|---|---|---|---|
| ACsc | 0.615 | 1.37 | 91 | 93 |
| ZnO@ACsc | 0.422 | 1.29 | 91 | 93 |
| ACav | 0.613 | 1.29 | 80 | 90 |
| ZnO@ACav | 0.474 | 1.24 | 72 | 84 |
| ACcc | 0.484 | 1.20 | 82 | 95 |
| ZnO@ACcc | 0.329 | 1.30 | 81 | 87 |
| (SEM-EDS) S % wt. | |||
|---|---|---|---|
| Material Code | Fresh | Used | Increasing Ratio |
| ACsc | 0.32 | 2.39 | 4.84 |
| ZnO@ACsc | 0.12 | 10.13 | |
| ACav | 0.38 | 2.31 | 5.65 |
| ZnO@ACav | 0.23 | 11.13 | |
| ACcc | 0 | 2.46 | 4.05 |
| ZnO@ACcc | 0 | 9.97 | |
| Material Code | % Yield | H2S Flow (mL/min) | GHSV (min−1) | Ads. Cap. (mgH2S/gads.) | Ads. Cap. (mmolH2S/gads.) | Times Increase |
|---|---|---|---|---|---|---|
| ACsc | 18.9 | 35.7 | 183 | 10.21 | 0.299 | 6.5 |
| ZnO@ACsc | 37.4 | 204 | 66.34 | 1.945 | ||
| ACav | 17.6 | 35.7 | 128 | 17.84 | 0.523 | 5.9 |
| ZnO@ACav | 35.7 | 136 | 106.03 | 3.109 | ||
| ACcc | 23.0 | 35.7 | 286 | 12.42 | 0.364 | 3.8 |
| ZnO@ACcc | 35.7 | 217 | 46.90 | 1.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baikousi, M.; Gantzoudi, A.; Gioti, C.; Moschovas, D.; Giannakas, A.E.; Avgeropoulos, A.; Salmas, C.E.; Karakassides, M.A. Hydrogen Sulfide Removal via Sorption Process on Activated Carbon–Metal Oxide Composites Derived from Different Biomass Sources. Molecules 2023, 28, 7418. https://doi.org/10.3390/molecules28217418
Baikousi M, Gantzoudi A, Gioti C, Moschovas D, Giannakas AE, Avgeropoulos A, Salmas CE, Karakassides MA. Hydrogen Sulfide Removal via Sorption Process on Activated Carbon–Metal Oxide Composites Derived from Different Biomass Sources. Molecules. 2023; 28(21):7418. https://doi.org/10.3390/molecules28217418
Chicago/Turabian StyleBaikousi, Maria, Anna Gantzoudi, Christina Gioti, Dimitrios Moschovas, Aris E. Giannakas, Apostolos Avgeropoulos, Constantinos E. Salmas, and Michael A. Karakassides. 2023. "Hydrogen Sulfide Removal via Sorption Process on Activated Carbon–Metal Oxide Composites Derived from Different Biomass Sources" Molecules 28, no. 21: 7418. https://doi.org/10.3390/molecules28217418
APA StyleBaikousi, M., Gantzoudi, A., Gioti, C., Moschovas, D., Giannakas, A. E., Avgeropoulos, A., Salmas, C. E., & Karakassides, M. A. (2023). Hydrogen Sulfide Removal via Sorption Process on Activated Carbon–Metal Oxide Composites Derived from Different Biomass Sources. Molecules, 28(21), 7418. https://doi.org/10.3390/molecules28217418










