Abstract
Consumers who are environmentally and health conscious are increasingly looking for plant-based alternatives to replace animal-based products in their daily diets. Among these alternatives, there is a growing demand for meat analogues that closely resemble the taste and texture of meat. As a result, significant efforts have been dedicated to developing meat analogues with a desirable meat-like structure. Currently, soy protein and wheat gluten are the main ingredients used for producing these meat analogues due to their availability and unique functionalities. This study observed that high moisture extrusion at moisture levels of 50–80% has become a common approach for creating fibrous structures, with soy protein and wheat gluten being considered incompatible proteins. After the structuring process, they form two-phase filled gels, with wheat gluten acting as the continuous phase and soy protein serving as a filler material. Moreover, the formation of soy protein and wheat gluten networks relies on a combination of covalent and non-covalent interaction bonds, including hydrogen bonds that stabilize the protein networks, hydrophobic interactions governing protein chain associations during thermo-mechanical processes, and disulfide bonds that potentially contribute to fibrous structure formation. This review provides case studies and examples that demonstrate how specific processing conditions can improve the overall structure, aiming to serve as a valuable reference for further research and the advancement of fibrous structures.
1. Introduction
Plant-based diets have gained global attention for their positive impact on both environmental sustainability and health benefits, resulting in a growing trend of replacing animal-based products with plant-based products [1,2]. A significant example of this shift can be seen in the market share of soy protein, which was previously dominated by its usage in animal feed. However, in 2022, the gap between its usage in animal feed and in food and beverages has nearly disappeared globally [3]. Notably, North America is the dominant player in the soy protein market, and the majority of its applications are found in food and beverages. Specifically, its market demand is led by meat and dairy alternatives, accounting for a volume share of 44% in 2022 [3].
The soy protein industry has undergone significant development, with substantial investments made throughout its value chain from farm to fork, ensuring a cost-effective price for soy protein ingredients [4]. Although anti-nutritional compounds, such as trypsin inhibitors, initially hinder the acceptance of soy by impeding pancreatic trypsin and chymotrypsin action in the gut, leading to various disorders, thermal treatment in food processing has been found to effectively reduce or eliminate the negative effects of these anti-nutritional factors in soy protein ingredients [5,6]. Moreover, soy protein has one of the highest scores for digestibility and has a well-balanced amino acid composition, making it a versatile ingredient with a neutral taste profile [7,8]. Furthermore, soy has nitrogen-fixing properties [9], and the production of soy protein for direct human consumption is associated with reduced deforestation and comparatively lower environmental impacts. This is because it requires significantly less land and emits far fewer greenhouse gases compared to its use as animal feed for livestock husbandry [7,10,11,12]. Overall, all these attributes position soy protein as the most valuable alternative to animal protein options, and soy protein continues to be the primary ingredient in most meat analogue products worldwide. In fact, the meat analogue sub-segment accounts for approximately 22% of the global soy protein market [3,13].
The fibrous structure is a crucial element in ensuring consumer acceptance of meat analogue products [14,15]. This is why wheat gluten, alongside soy protein, plays a vital role in developing the desired texture. Wheat gluten possesses a distinctive capability to form thin protein films upon elongation, making it ideal for creating fibrous protein-based materials [16]. Moreover, its functional properties make it suitable as both a binding agent and a structuring agent in the formulation [17,18,19]. An additional advantage is its cost-effectiveness for the industry, as wheat gluten is obtained as a by-product of wheat starch production [20]. The extraction process of gluten from wheat involves only water to wash out the soluble and dispersible components, leaving behind the insoluble proteins [21].
In this overview, we provide an initial introduction to soy protein and wheat gluten, which act as the main ingredients in plant-based meat analogues. Then, we delve into the discussion of advanced technologies used for creating the desired structure. Emphasis is placed on protein interactions and the potential underlying mechanisms behind fibrous structure formation. Furthermore, we elaborate on different formulations for the development of meat analogues and present case studies and examples that highlight how specific processing conditions can enhance the overall structure.
2. Protein Ingredients
2.1. Soy Protein
Soybeans contain both water-soluble and insoluble proteins, which can be separated into storage globulin and whey fractions by acidification to an approximate pH of 4.5 [22]. The whole extractable globular proteins present in aqueous solutions can be categorized into four main groups: 2S, 7S, 11S, and 15S. These categories have different sedimentation properties under centrifugal force, in which the 7S (β-conglycinin) and 11S (glycinin) fractions account for over 80% of the proteins [23]. The functionalities of soy protein ingredients are determined by several factors, including fractionation pathways, protein content, protein composition, and the presence of other compounds such as oil and carbohydrates [24,25,26].
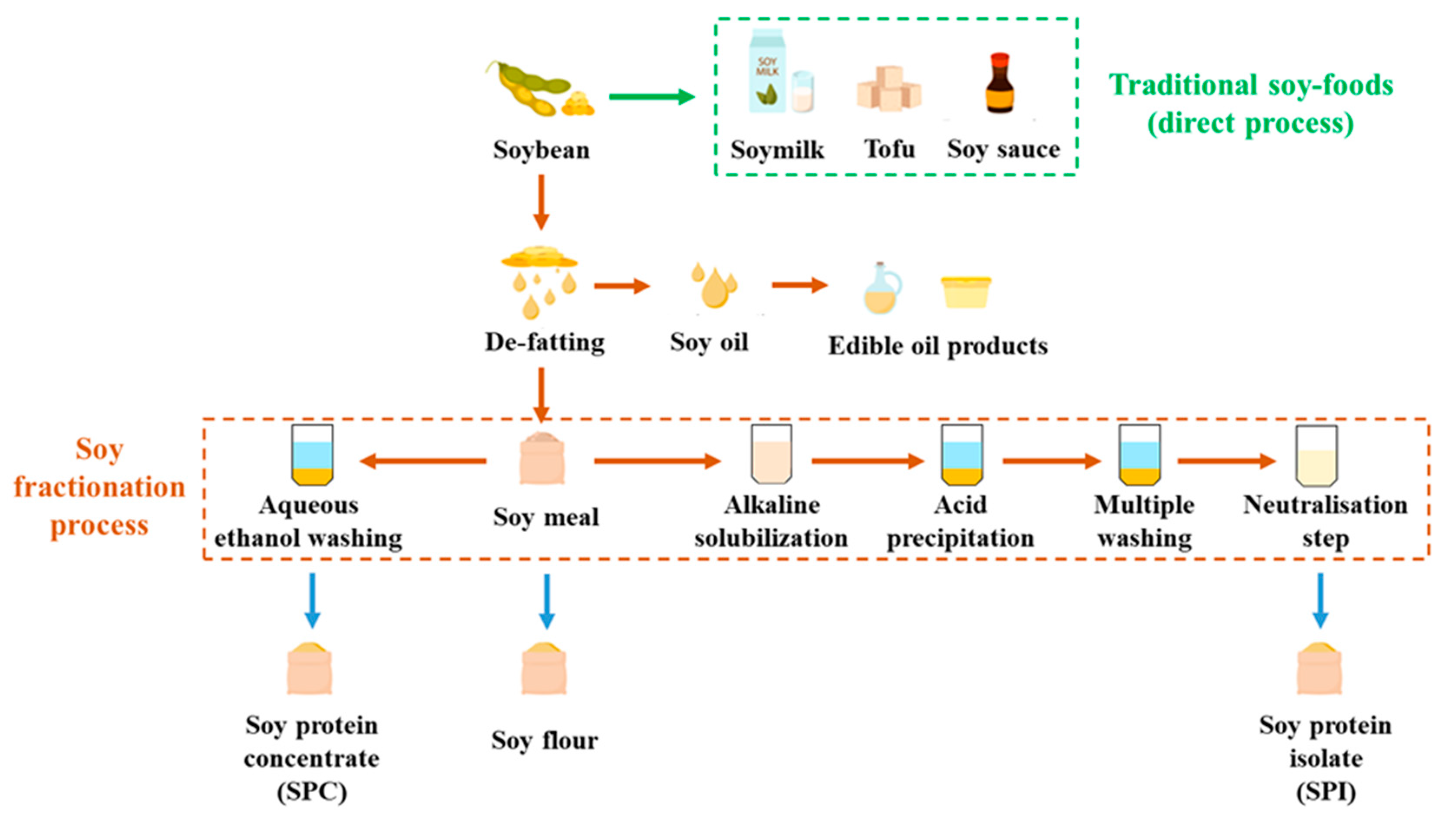
Soy protein ingredients, such as soy protein concentrate (SPC) and soy protein isolate (SPI), are broadly used in various industries. SPC typically contains around 65% protein, while SPI contains at least 85% protein. There are two common pathways for fractionating SPC and SPI (Figure 1). To produce SPC, de-fatted soy meal (DFSM) is washed with hot water, acidic solution, or aqueous ethanol, with the latter being the most industrially used approach [27]. During this washing process, the soy protein is retained in the solid phase along with the insoluble carbohydrates such as fiber, while the soluble carbohydrates are removed with the solvent. On the other hand, the fractionation of SPI involves an alkaline solubilization step. The protein in the DFSM is extracted in the liquid phase, while the insoluble carbohydrates are separated and discarded in the solid phase [28]. This step aims to achieve high protein solubility for maximum protein extraction and increased yield. The soluble carbohydrates are further eliminated during protein precipitation. Consequently, SPI is considered the most refined soy protein ingredient with the highest protein purity.
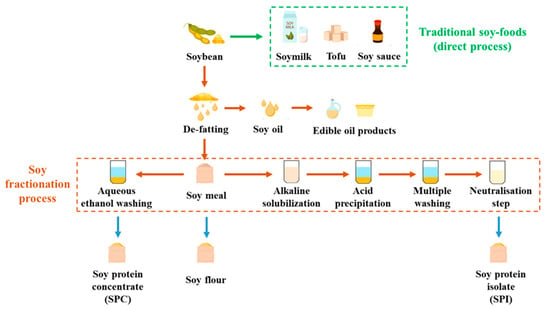
Figure 1.
Flow chart of soy protein fractionations.
Soy protein ingredients were developed to enhance the economic feasibility of soy refining [29]. They have been widely utilized as functional ingredients or cost-effective substitutes in various products. For instance, SPC is commonly added to processed meat, fish, and poultry items to enhance their color and flavor. SPI, with its high viscosity and solubility, finds application in soups, sauces, and beverages. Additionally, SPI with its forming and whipping characteristics can effectively replace egg whites, reducing ingredient costs [30]. In all of the mentioned food products, the required level of soy protein ingredients to achieve the desired effects is low, without introducing negative attributes such as off-flavors, odor problems, or unwelcome textures. Consequently, current soy fractionation processes are optimized to attain high protein purity and specific functionalities, including increased solubility, water absorption, and viscosity, in order to maximize functionality while minimizing adverse effects [31]. However, the growing popularity of plant-based products like meat analogues imposes new requirements for soy protein ingredient purity and functionality.
2.2. Wheat Gluten
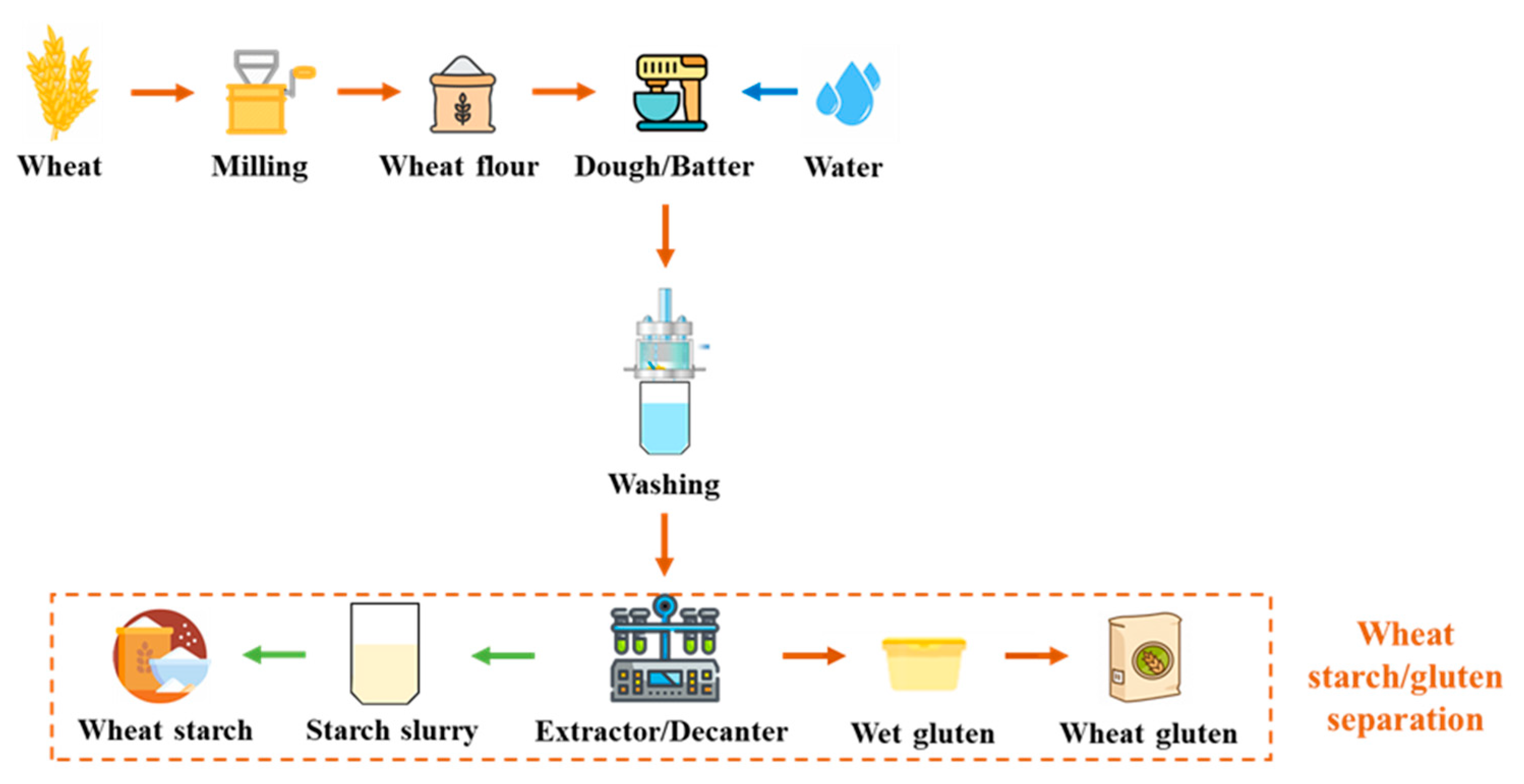
The approach of separating wheat flour into starch and gluten has been understood for nearly 300 years. However, it was only during the second half of the 20th century that wheat gluten began to be traded as a commodity, eventually gaining recognition as a valuable vegetable protein (Figure 2) [32]. Currently, it is widely employed as a food additive to enhance flour for bread production, and as an ingredient in various food and non-food systems. Its distinctive characteristics, including its capability to form an elastic mass when mixed with water, its ability to retain water, and its thermosetting properties, make it well-suited for a diverse range of applications [33].
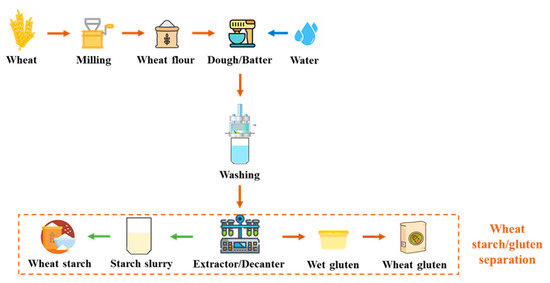
Figure 2.
Flow chart of wheat starch/gluten separation.
Wheat gluten is a cohesive and viscoelastic proteinaceous substance obtained as the by-product of wheat starch production. Gluten protein constitutes approximately 85% of all wheat proteins that are deposited in the wheat endosperm cells, and consists of a diverse mixture of proteins. About half of these proteins are monomeric gliadins, while the remaining portion forms the polymeric glutenin fraction through disulfide cross-linking [34]. Gliadins can be extracted using aqueous ethanol, and have a molecular weight ranging from 30,000 to 60,000. When cysteine residues are present, they are involved in intrachain disulfide bonds [35,36]. Glutenins, on the other hand, are large polymers with a molecular weight of up to twenty million, and are composed of GS units linked by disulfide bonds. Within the GS units, there are high molecular weight (HMW 70,000 to 90,000) and low molecular weight (LMW 30,000 to 45,000) variants [37]. Unlike gliadins, GS units are capable of forming interchain disulfide bonds even at room temperature. When heated, glutenin and gliadin undergo cross-linking, due to oxidation of free sulfhydryl groups, and interchange reactions involving disulfide bonds, resulting in the formation of a significant protein network. Additionally, the formation of disulfide bonds can be regulated by introducing redox agents [38].
Gluten has an extensible nature and can be stretched and pulled, which corresponds with its unique film-forming properties. The elastic properties of gluten can be attributed to glutenin, while gliadin contributes to the viscosity of the protein network [39]. Glutenin exhibits high strength but limited elongation, whereas gliadin enhances extensibility but compromises water stability in gluten films [33]. Previous research has demonstrated that wheat gluten, when subjected to high moisture extrusion or shear structuring as a single component, forms protein gels with anisotropic structures [18,40]. Additionally, wheat gluten facilitates the creation of thin protein films through deformation and elongation, transforming meat analogue dough into a three-dimensional fibrous structure [41]. The water absorption and swelling properties of gluten further contribute to reducing cooking losses during processing and enhancing slicing characteristics [15]. Hence, wheat gluten has been used as key matrix material in the formulation and production of meat analogue products with a fibrous structure.
Soy protein and wheat gluten are plant proteins with distinct fractionation approaches, protein profiles, properties, and functionalities. However, they fulfill the requirements for nutritional and functional characteristics, and act as bulk ingredients in the development of meat alternatives. In order to achieve the desired texture and appearance in current meat analogues, the selection of appropriate protein-rich ingredients is important; furthermore, the utilization of established texturization methods also play a crucial role in creating meat-like structures.
3. Structure Formation
Various technologies have been studied and developed for creating a meat-like fibrous structure using soy protein and wheat gluten, including low/high moisture extrusion (LME/HME), spinning, shear cell, and 3D printing. HME is the most commonly used technology in the industry, while the others are still in the developing stage at laboratories. Table 1 summarizes some of the formulations and processing conditions that are soy protein- and wheat gluten-based for the development of meat analogue products.

Table 1.
An overview of soy protein–wheat gluten formulations, techniques, and processing conditions used to produce meat-like fibrous structures.
3.1. Low/High Moisture Extrusion
Extrusion is a popular method used in the production of protein-based foods, and it was first introduced in the 1970s to create soy-based meat substitutes; the obtained products appear as a relatively flat, elongated, and longitudinally aligned fibrous mass that closely resembles meat in terms of compactness and chewiness [59]. Currently, these texturized proteins are commercially manufactured using two extrusion methods: low moisture extrusion (LME) and high moisture extrusion (HME) [60].
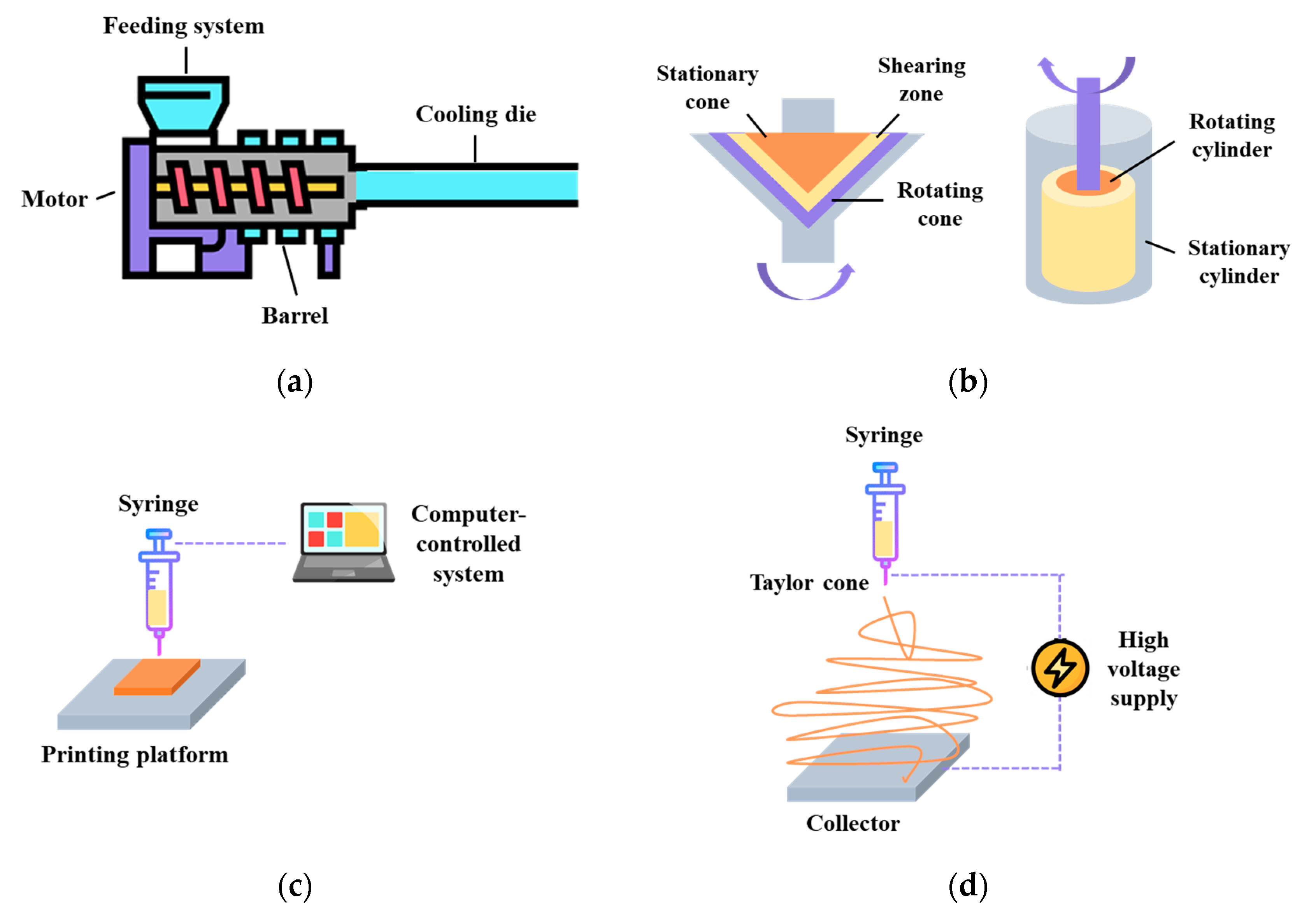
The process of creating meat analogues through extrusion Involves three main steps: preconditioning, mixing/cooking, and cooling. This process typically requires a setup that includes a preconditioning system, a feeding system, a screw, a heated barrel equipped with a single screw or twin screw, and a cooling die (Figure 3a) [61]. In the case of LME, protein ingredients and other additives are mixed under low moisture conditions. The shaping of the mixture is achieved through thermo-mechanical means, making the preconditioning and cooling steps less crucial in this process [62]. The end products produced through LME have a sponge-like structure, and require rehydration before cooking or frying. Since they have excellent water absorption properties, they are commonly used as meat extenders in the industry to improve the water-holding capacities of processed meat products like burger patties and sausages [63]. The HME technology, developed in the late 1980s, is based on the traditional food extrusion process. Unlike the process of LME, HME is primarily used for food mixtures with moisture contents exceeding 50% [64]. As a result, the end products obtained through HME have a relatively higher water content. During the production of meat analogues, fibrous structures are achieved by extrusion cooking of soy protein and wheat gluten at moisture levels ranging from 50 to 80%. This process takes place at barrel temperatures above 140 °C, with the use of long cooling dies [65]. The moisture levels play a crucial role in the HME process, as they reduce or prevent the dissipation of energy and product expansion. However, they also facilitate necessary operations such as protein gelation, fat emulsification, and the restructuring and shaping of protein constituents [66].
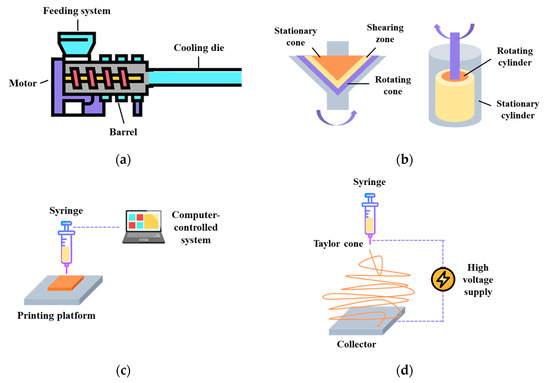
Figure 3.
Current structuring technologies for meat-like fibrous structure formation. (a) Extrusion, (b) shear cell and Couette cell, (c) 3D printing, and (d) protein spinning.
The successful production of meat analogue products with a fibrous structure is influenced by various processing conditions, such as the screw diameter, rotor speed, barrel temperatures, and the specific plant proteins and formulations used (Table 1). Several studies have highlighted the significance of processing conditions in extrusion processes, with particular emphasis on the importance of the processing temperatures. It has been reported that the temperature during processing is a critical parameter for achieving fibrillation of soy protein and wheat gluten, as it affects protein–protein interactions [67,68,69,70]. Therefore, there is a need for dedicated efforts to optimize extrusion parameters to meet the specific requirements of each meat analogue product.
3.2. Shear Cell
In the early 2000s, shear cell devices were developed for studying the effects of simple shear on the overall properties of biopolymers like starch or proteins [71,72]. Two types of shearing devices were developed over the years: a conical device based on a cone-plate rheometer (shear cell) and a cylindrical device for scaling up (Couette cell). The shear cell device has a stationary top cone and a rotating bottom, while the Couette cell has a stationary outer cylinder and a rotating inner cylinder [63] (Figure 3b). Both the shear cell and HME processes utilize thermo-mechanical forces to generate fibrous structure formations, and they follow similar key stages, which include preconditioning, mixing/cooking, and cooling [73]. Preconditioning can be done manually (lab-scale) or using a Z-blade mixer (pilot-scale), while mixing/cooking can be achieved through circulating heated oil (shear cell) or a steam jacket (Couette cell). The cooling step is achieved by circulating cooled oil with the shear flow stopped [73].
The implementation of the shearing process to induce fibrous hierarchical structures in dense calcium caseinate has led to the recognition of shear cell technology as a potential tool for creating fibrous anisotropic structures for meat analogue development [74]. Evaluations of the impacts of the shearing process on the structure formation of plant-based biopolymers have been conducted. Fibrous-structured samples have been processed using soy protein and wheat gluten mixtures. These mixtures were processed under different conditions and formulations using both the shear cell and Couette cell (Table 1). Typically, a dough is prepared by mixing soy protein and wheat gluten with water and allowing it to hydrate for 30 min at room temperature. The moisture contents used in the shear cell process are also comparable to HME, and range from approximately 50 to 70%. The hydrated dough is then transferred to a pre-heated shearing device to initiate the shearing process [73]. Thermo-mechanical treatment is carried out at a constant shear rate for 15 min while heating, with distinct fibrous structures predominantly forming at a processing temperature of 140 °C for soy protein–wheat gluten blends [75]. The blends are subsequently cooled and taken out once the temperature is lower than 50 °C, a temperature level that effectively solidifies the material and prevents expansion caused by water evaporation during device opening.
Apart from soy protein and wheat gluten systems, shear cell technology has successfully created a meat-like fibrous structure using a greater variety of plant-based materials [52,76,77,78]. Additionally, research has indicated that the mechanical energy input required for the structuring process can be significantly lower compared to forced assembly processes like extrusion [79]. While shear cell technology is predominantly conducted in small-scale laboratory setups and typically involves prolonged exposure to shear forces for the desired structural changes, the design of the shear cell allows for increased productivity of meat analogues [63]. This can be achieved by increasing the capacity of the device through adjustments to cylinder size, length, or product thickness. However, further studies are necessary to comprehend the underlying principles and complexities of shear flow, and its impact on material structuring. Overall, shear cell technology holds significant potential for scaling up the industrial production of meat analogues.
3.3. 3D Printing
3D printing, initially developed in the 1980s for material science applications, is a revolutionary digital process that allows for the creation of intricate solid forms [80]. The process begins with the design of a digital template that defines the desired 3D shape. Then, this template guides a digitally-controlled XYZ robotics system, which constructs the item, layer by layer, starting from the bottom and moving upwards [81] (Figure 3c). The layers can be connected during the construction process or through a separate post-construction step. The supplied materials can be classified into liquids, powders, and cultures of cells, while the printing technologies can be grouped into extrusion printing, inkjet printing, binder jetting, and selective sintering printing [57,82]. In recent years, the food industry has shown a keen interest in 3D printing technology due to its multiple advantages, such as the ability to customize food designs, personalize nutrition, simplify the supply chain, and broaden the availability of food materials [83]. In the case of meat analogue development, the fibrous structure formation is being investigated using 3D printing mainly because it can control the distribution of different components easily to achieve a more similar appearance and appealing shapes [84].
Depositing liquid-based materials can be achieved through extrusion and inkjet printing, with extrusion printing being the most commonly used method [85]. Coaxial extrusion is particularly beneficial as it allows for the cross-linking of two materials, resulting in an encapsulated core and shell configuration. This enables the production of complex and re-defined appealing products with desired shapes [84]. Coaxial extrusion 3D printing is ideal for depositing components independently and simultaneously, making it suitable for creating fibrous structures like meat analogues [84]. Initially, SPI-based mixtures with carrageenan, xanthan gum, sodium alginate, and gelatin were developed as 3D printing materials [86,87]. Subsequently, soy-based fibrous structures were created using a coaxial nozzle incorporating hydrocolloids [88]. High-protein edible printing materials were further developed by mixing soy protein and wheat gluten powders. To create meat analogue products, composite gels were 3D-printed with the facilitation of thermos-sensitive cocoa butter and rice protein [56,57,89].
3D printing technology offers the advantage of creating intricate and visually appealing structures in edible products, thus enhancing consumer interest and appetite [83]. In theory, this technology can be utilized to produce fibrous and anisotropic structures that closely resemble meat muscular tissues. Furthermore, the composition of meat analogue products, including the ratios of soy protein/wheat gluten, moisture content, and types of fat, can be easily adjusted to customize the texture, taste, and flavor according to individual preferences [88]. However, research on 3D-printed plant-based meat analogues is currently limited. To achieve precise and accurate printing of meat-like fibrous structures, several factors must be considered, such as the functionalities of plant protein ingredients, process parameters, and post-processing methods. Although some attempts have been made in producing meat analogues using 3D printing technology, their widespread application remains limited. Therefore, more efforts should be dedicated to the precise and efficient printing of fibrous structures, with the aim of maximizing the technical and commercial potential of 3D printing technology in creating exceptional meat analogue products.
3.4. Protein Spinning
Spinning, a well-established technique, was first observed in the early 1900s and patented in the 1930s [90,91]. Wet spinning and electrospinning have been extensively studied for the formation of fibrous structures, with a key distinction in the alignment of biopolymers. During the wet spinning process, a viscous polymeric solution is forced through a spinneret, leading to stretched and aligned fibers. These fibers are subsequently solidified in a coagulation bath containing salts, acids, or alkalis, requiring a vital washing step and generating large waste streams. Consequently, a more appealing alternative method that has gained attention is electrospinning [63,92]. Electrospinning is a technique that employs electrical forces to create polymer fibers, ranging in diameter from 2 nm to several micrometers. The basic components required for an electrospinning apparatus include a high-power voltage supply, a capillary tube with a needle or pipette, and a collector or target (Figure 3d). This process allows for the production of unique natural nanofibers and fabrics with a controllable pore structure, offering great potential for various applications [93,94].
The wet spinning process of proteins presents a challenge as most proteins cannot be spun under food-grade conditions. In the case of the electrospinning process, it has been investigated to structure proteins on a nanometer-length scale, and it is able to act as a means to produce thin fibrils that can serve as textural building blocks for meat analogue products [95]. Due to the complex secondary and tertiary structures, plant proteins pose a considerable challenge when it comes to electrospinning. To successfully electrospin proteins or protein blends with other polymers, the solution must meet certain requirements, for example, high solubility, conductivity, viscosity, and surface tension [92]. Accordingly, SPI that possesses high solubility and viscosity could potentially be used as an electrospinning material. Moreover, solvents with good solvent quality can disrupt protein interactions, leading to the solubilization of the resulting unstructured protein. By employing this approach, plant proteins can possibly become suitable for electrospinning [95]. Although the utilization of wheat gluten and soy protein–wheat gluten blends for fibrous structure formation by electrospinning process has not yet been reported, zein has been successfully spun after solubilization in 70% ethanol [96]. Consequently, further research is necessary to investigate electrospinnable plant proteins and align the fibers for the creation of plant-based meat analogues with a defined structure.
Despite significant advancements in the technology used to structure protein-rich ingredients, the impact of structuring processes on the physical and covalent interactions between proteins remains a topic of extensive research. This complexity arises from the intricate chemical bonding responsible for protein structure. By investigating the interactions between proteins during the structuring process, we can gain valuable insights into the nature of protein–protein bonding.
4. Protein–Protein Interactions
Gaining a comprehensive understanding of the interactions between soy protein and wheat gluten, as well as their interactions with other components in the system, could greatly contribute to the comprehension of the underlying mechanisms involved in the formation of fibrous structures during the development of meat analogue products. This knowledge has the potential to resolve discrepancies found in existing literature, enhance production techniques, and ultimately elevate the quality of the end products.
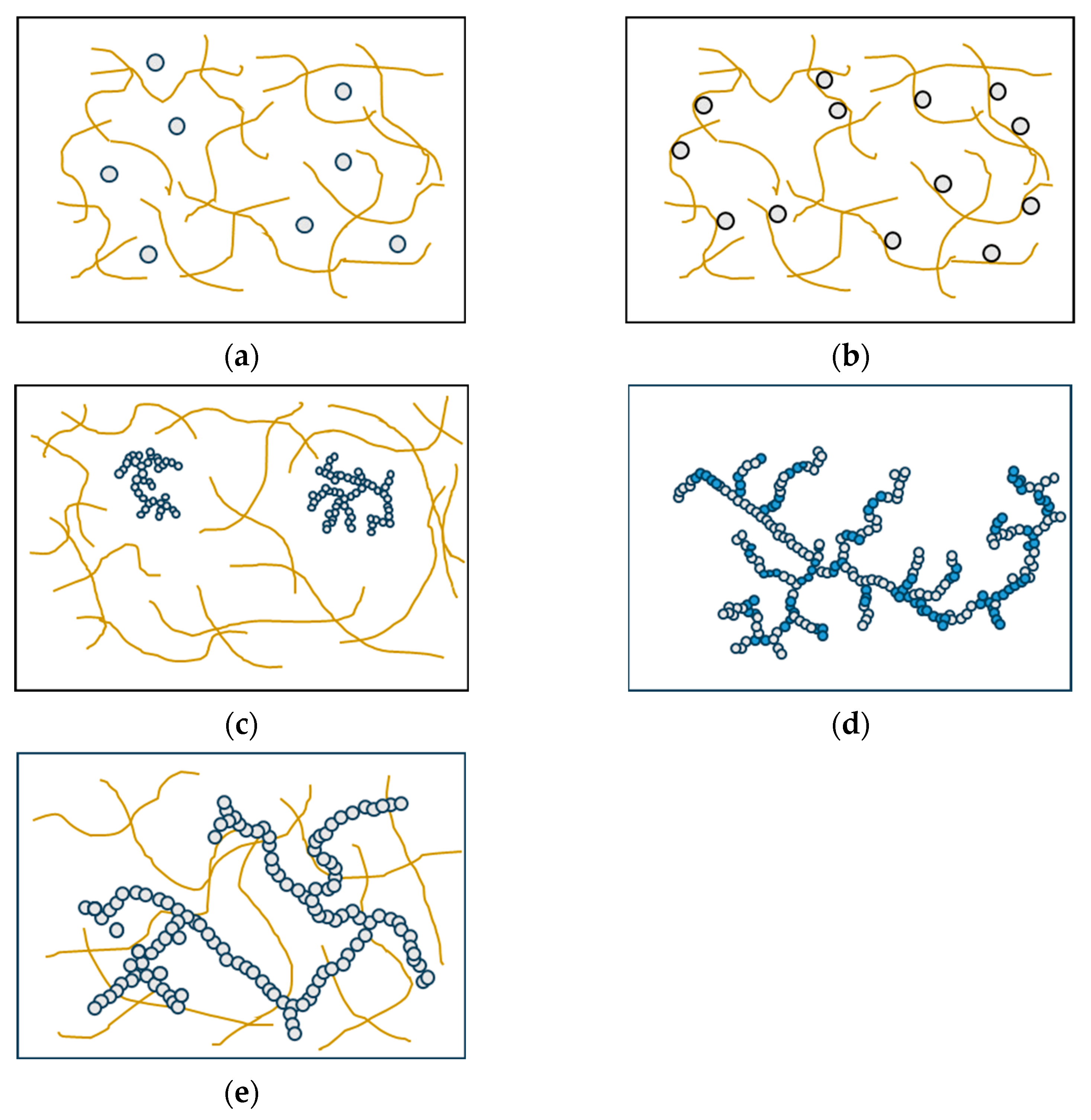
Various factors influence the structural formation of multi-component gels through the interaction of two or more proteins. These factors include the proteins’ thermodynamic compatibility, their potential for interaction, and their individual mechanisms of gelation. When different types of protein are mixed, they can be classified as incompatible, semi-compatible, or compatible, depending on whether they form two or more immiscible phases, partially mix at the molecular level, or form a single thermodynamically stable phase [97]. The classification of mixed gels into five distinct types is determined based on the specific functionalities exhibited by individual proteins and the interactions that occur among proteins within the gel system. Filled gels can be formed by incorporating additional protein components into the primary protein gel network. Depending on the phase state of the system, two types of filled gels can be distinguished: single-phase gels, where the filler remains soluble (Figure 4a), and two-phase gels, where phase separation occurs due to thermodynamic incompatibility (Figure 4b) [98,99]. Complex gels are formed when the proteins interact and physically associate with each other. This association can happen randomly through non-specific interactions, where a non-gelling protein reduces the flexibility of the primary network chains and increases the rigidity of the gel (Figure 4c). In this case, the non-gelling protein can be the protein with gelation properties, but the concentration in the mixture is below its least gelling concentration [100]. Another way complex gels form is through the copolymerization of two or more proteins, resulting in a single, heterogeneous network (Figure 4d) [101]. A unique type of multi-component gel is the interpenetrating protein network, in which both protein networks are continuous throughout the gel system (Figure 4e) [97].
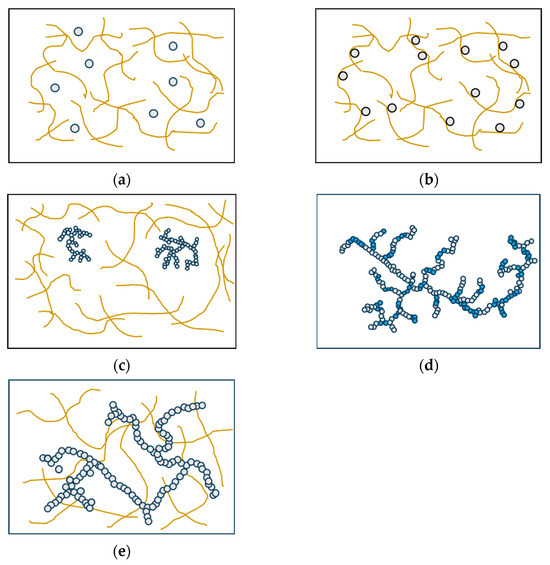
Figure 4.
Types of mixed gels. (a) Single-phase gels, (b) two-phase gels, (c) complex gels formed by a non-gelling protein, (d) complex gels formed by the copolymerization of two or more proteins, and (e) interpenetrating protein gels [96].
Wheat gluten has the ability to form fibrous structures independently after undergoing thermo-mechanical processes such as extrusion and shear cell, and fibrous formations were still observed with the addition of up to 50% soy protein [19,75]. Hence, it can be inferred that soy protein and wheat gluten are incompatible, and the combination of soy protein and wheat gluten falls under the classification of two-phase filled gels, with wheat gluten acting as the continuous phase while soy protein functions as a filler material. The analysis of protein gels developed in a shear cell, using small-angle neutron and X-ray scattering, yielded valuable insights into the micro- and nano-scale structure of flowing two-phase systems [102]. The formation of two phases can align in the shear flow direction during the structuring process [55,73]. Furthermore, in the SPI–WG system, SPI competes with gluten for hydration and the distribution of water is unevenly spread. A noticeably higher water content was observed in the SPI phase compared to the WG phase; as a result, the volumetric fraction of the SPI phase is larger than its mass fraction, and vice versa for the WG phase. Consequently, the higher water absorption by the SPI phase results in a lower concentration of SPI within that phase, leading it to exist either as dispersed liquid particles or as a secondary gel network. It is worth mentioning that the heating and/or structuring processes did not affect the distribution of water within the SPI–WG blend [55].
The heat-induced gelation of soy protein occurs in four steps: dissociation of small aggregates, association into dense spherical particles, formation of self-similar aggregates, and ultimately, network formation [103]. Hydrophobic interactions are predominant for the formation of SPI gels, while disulfide bonds generally play a role at temperatures over 100 °C (Figure 5) [17,34,104]. Wheat gluten forms a cross-linked network upon hydration, while the formation of disulfide bonds mainly contributes to the heat-induced gluten aggregation and the formation of a three-dimensional network [105]. Furthermore, disulfide bond formations also dominated the wheat gluten polymerization during extrusion, with non-disulfide covalent bonds playing a minor role [19].
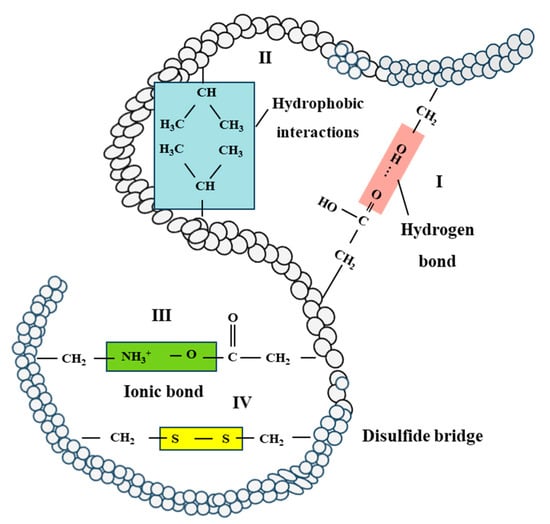
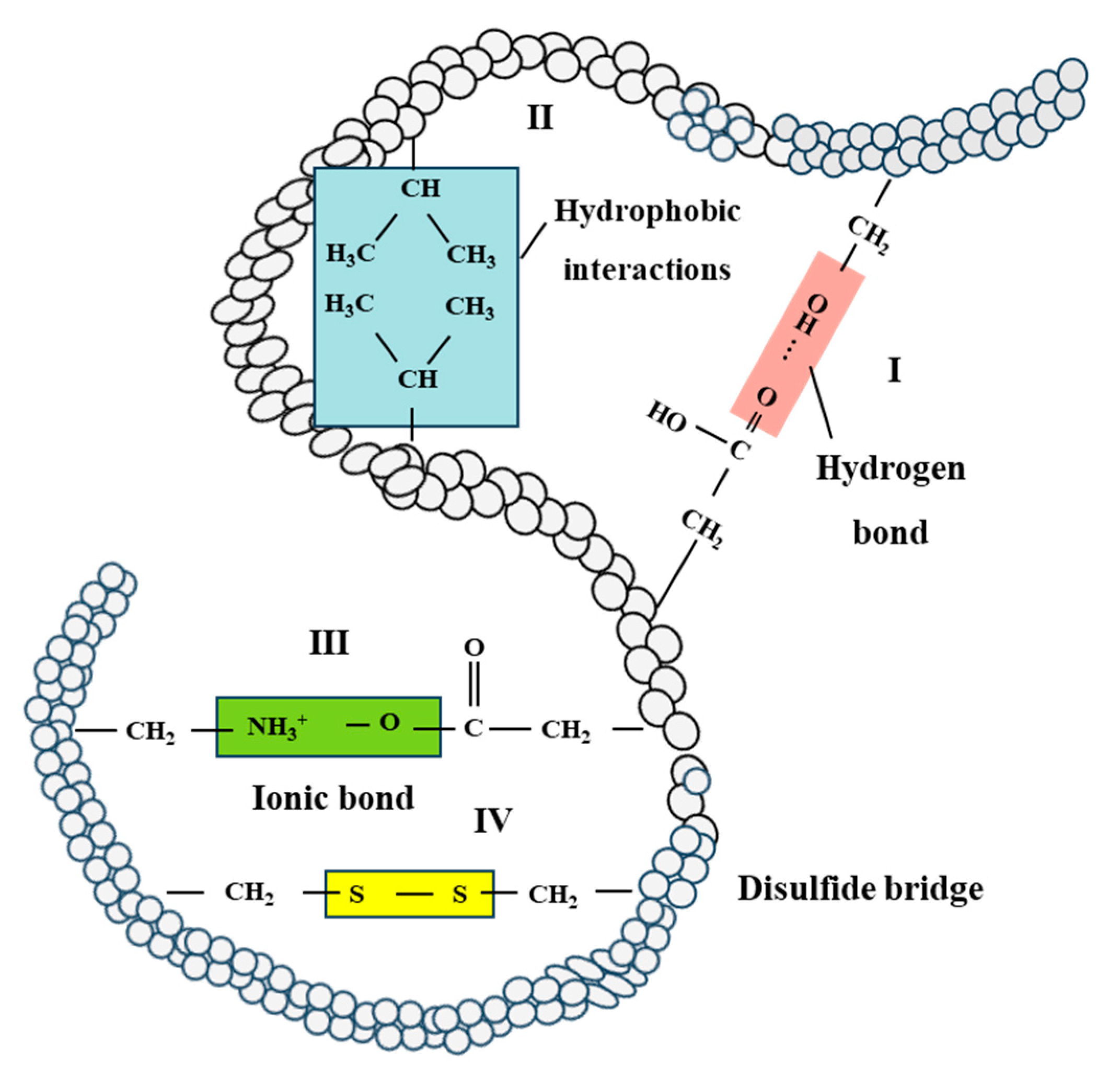
Figure 5.
Flow chart of protein–protein interactions in the tertiary structure of protein networks. I: hydrogen bond, II: hydrophobic interactions, III: ionic bond and IV: disulfide bridge [17,34].
The heating and structuring process of soy protein and wheat gluten involves the application of heat and shear, impacting various bonds that stabilize protein structure. As temperature increases, hydrogen bonds are weakened, and hydrophobic interactions can increase [106,107]. Reactive free thiol groups and labile disulfide bonds contribute to thiol-disulfide interchange reactions, forming a transient or reversible network [108,109]. Moreover, shear stress can promote these thiol-disulfide interchange reactions as well [110,111]. Overall, hydrogen bonds, hydrophobic interactions, and disulfide bonds are vital for the formation, stabilization, and retention of fibrous structures in the development of meat analogues. Hydrogen bonds primarily stabilize soy protein and wheat gluten networks, while hydrophobic interactions govern protein chain associations during the thermo-mechanical process, and disulfide bonds may contribute to fiber formation.
5. Conclusions
Environmentally and health conscious consumers are increasingly seeking plant-based alternatives to replace animal-based products in their diets, particularly in relation to dairy. The market is seeing a growing demand for meat analogue products that mimic the taste and texture of meat. Currently, the most commonly used plant protein resources for developing these products are soy protein and wheat gluten, mainly due to their availability and specific functionalities. Extensive research has focused on understanding how these proteins interact and form a fibrous structure through various structuring processes. Our findings indicate that both HME and shear cell processes share similar basic processing steps, while 3D-printing and spinning processes exhibit some similarities to a certain extent. Among all of the techniques, extrusion has become the industrially used technique for fibrous structure formation, while other advanced technologies are actively being developed and tested at both the lab-scale and pilot-scale levels. In addition to processing techniques, our research also summarizes formulations and processing conditions and introduces hypotheses regarding the protein–protein interactions during the process. According to our hypothesis, soy protein and wheat gluten generally form two-phase filled gels, and a combination of covalent and non-covalent interaction bonds plays a crucial role in forming soy protein and wheat gluten networks. These bonds include hydrogen bonds, which help to stabilize the networks of proteins, hydrophobic interactions that govern protein chain associations during thermo-mechanical processes, and disulfide bonds that potentially contribute to the formation of fibrous structures.
6. Future Directions
In light of the increasing demand for plant-based meat analogue products and the advancements made in understanding protein interactions and structuring processes, future research should focus on several key areas. Firstly, it is necessary to explore different fractionation pathways to produce a wider range of soy protein and wheat gluten ingredients. Currently, their existing fractionation processes are not fully optimized for meat analogue development. By doing so, the applicability and sustainability of plant protein ingredients can be further enhanced. Moreover, more in-depth studies are required to understand the network-forming behavior of soy protein and wheat gluten. The mechanisms behind their formation have not been fully elucidated yet. Gaining a better understanding of these mechanisms would enable precise control over the texture and structure of meat analogue products, ultimately improving consumer acceptance. Furthermore, additional research is needed to optimize the production processes of meat analogues, such as 3D-printing and spinning techniques. Taking a comprehensive approach that incorporates multiple developed technologies will ensure that future developments in meat analogues meet the evolving needs of environmentally and health conscious consumers.
Author Contributions
Conceptualization, Y.P.; validation, X.W. and Y.N.; formal analysis, Y.P.; investigation, Y.P.; resources, Y.P. and D.Z.; data curation, D.Z. and M.L.; writing—original draft preparation, Y.P.; writing—review and editing, D.Z., M.L. and Y.N.; visualization, Y.P.; supervision, Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a science and technology plan project in the Ali area of Tibet, “Research and demonstration on key technologies of highland barley food processing and high-value products in the Ali area of Tibet”, project number: QYXTZX-AL2023-02.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alfaro-Diaz, A.; Escobedo, A.; Luna-Vital, D.A.; Castillo-Herrera, G.; Mojica, L. Common Beans as a Source of Food Ingredients: Techno-Functional and Biological Potential. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2910–2944. [Google Scholar] [CrossRef] [PubMed]
- Allotey, D.K.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. Life Cycle Sustainability Assessment Outlook of Plant-Based Protein Processing and Product Formulations. Sustain. Prod. Consum. 2023, 36, 108–125. [Google Scholar] [CrossRef]
- Mordor Intelligence Global Soy Protein Market (2017–2029). 2023. Available online: https://www-mordorintelligence-com.webpkgcache.com/doc/-/s/www.mordorintelligence.com/industry-reports/global-soy-protein-market (accessed on 17 October 2023).
- Bashi, Z.; McCullough, R.; Ong, L.; Ramirez, M. Alternative Proteins: The Race for Market Share Is On; McKinsey & Company: New York, NY, USA, 2019; pp. 1–11. [Google Scholar]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R.; Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R.; Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use in Food Products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Rocha-Pizaña, M.D.R.; García-Lara, S.; López-Castillo, L.M.; Serna-Saldívar, S.O. Effect of Thermal Processing and Reducing Agents on Trypsin Inhibitor Activity and Functional Properties of Soybean and Chickpea Protein Concentrates. LWT 2018, 98, 629–634. [Google Scholar] [CrossRef]
- Messina, M.J. Legumes and Soybeans: Overview of Their Nutritional Profiles and Health Effects. Am. J. Clin. Nutr. 2018, 70, 439s–450s. [Google Scholar] [CrossRef]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy Protein: Impacts, Production, and Applications. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2017; pp. 23–45. [Google Scholar]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Harvey, F.; Phillips, D. A Fifth of Brazilian Soy in Europe Is Result of Deforestation. Environ. Guard. 2020, 16–17. [Google Scholar]
- Amaral, D.F.; de Souza Ferreira Filho, J.B.; Chagas, A.L.S.; Adami, M. Expansion of Soybean Farming into Deforested Areas in the Amazon Biome: The Role and Impact of the Soy Moratorium. Sustain. Sci. 2021, 16, 1295–1312. [Google Scholar] [CrossRef]
- Haidar, C.N.; Coscueta, E.; Cordisco, E.; Nerli, B.B.; Malpiedi, L.P. Aqueous Micellar Two-Phase System as an Alternative Method to Selectively Remove Soy Antinutritional Factors. LWT 2018, 93, 665–672. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat Analogues: Health Promising Sustainable Meat Substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Meat Analog as Future Food: A Review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Malav, O.P.; Talukder, S.; Gokulakrishnan, P.; Chand, S. Meat Analog: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Don, C.; Lichtendonk, W.; Plijter, J.J.; Hamer, R.J. Glutenin Macropolymer: A Gel Formed by Glutenin Particles. J. Cereal Sci. 2003, 37, 1–7. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of Ingredients and Additives in Plant-Based Meat Analogues. Foods 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, V.L.; Emin, M.A.; Schuchmann, H.P. Process Conditions Influencing Wheat Gluten Polymerization during High Moisture Extrusion of Meat Analog Products. J. Food Eng. 2017, 198, 28–35. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Karbstein, H.P.; Emin, M.A. Kinetics of Wheat Gluten Polymerization at Extrusion-like Conditions Relevant for the Production of Meat Analog Products. Food Hydrocoll. 2018, 85, 102–109. [Google Scholar] [CrossRef]
- Rausch, K.D.; Hummel, D.; Johnson, L.A.; May, J.B. Wet Milling: The Basis for Corn Biorefineries. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 501–535. [Google Scholar]
- Ooms, N.; Jansens, K.J.A.; Pareyt, B.; Reyniers, S.; Brijs, K.; Delcour, J.A. The Impact of Disulfide Bond Dynamics in Wheat Gluten Protein on the Development of Fermented Pastry Crumb. Food Chem. 2018, 242, 68–74. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Nagano, T.; Guo, S.; Wang, R. Soy as a Food Ingredient, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081007297. [Google Scholar]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy Proteins: A Review on Composition, Aggregation and Emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Tarone, A.G.; Fasolin, L.H.; de Assis Perrechil, F.; Hubinger, M.D.; da Cunha, R.L. Influence of Drying Conditions on the Gelling Properties of the 7S and 11S Soy Protein Fractions. Food Bioprod. Process. 2013, 91, 111–120. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, P.; Xie, T.; Liu, X.; Yang, L.; Wang, S.; Li, J.; Liu, H. Interaction between Soyasaponin and Soy β-Conglycinin or Glycinin: Air-Water Interfacial Behavior and Foaming Property of Their Mixtures. Colloids Surf. B Biointerfaces 2020, 186, 110707. [Google Scholar] [CrossRef]
- Peng, Y.; Kyriakopoulou, K.; Ndiaye, M.; Bianeis, M.; Keppler, J.K.; van der Goot, A.J. Characteristics of Soy Protein Prepared Using an Aqueous Ethanol Washing Process. Foods 2021, 10, 2222. [Google Scholar] [CrossRef]
- Alibhai, Z.; Mondor, M.; Moresoli, C.; Ippersiel, D.; Lamarche, F. Production of Soy Protein Concentrates/Isolates: Traditional and Membrane Technologies. Desalination 2006, 191, 351–358. [Google Scholar] [CrossRef]
- Deak, N.A.; Murphy, P.A.; Johnson, L.A. Characterization of Fractionated Soy Proteins Produced by a New Simplified Procedure. J. Am. Oil Chem. Soc. 2007, 84, 137–149. [Google Scholar] [CrossRef]
- Senti, F.R. Soy Protein Foods in U.S. Assistance Programs. J. Am. Oil Chem. Soc. 1974, 51, 138–140. [Google Scholar] [CrossRef]
- Egbert, W.R. Isolated Soy Protein: Technology, Properties, and Applications. In Soybeans as Functional Foods and Ingredients; AOCS Press: Champaign, IL, USA, 2004; pp. 134–162. [Google Scholar]
- Goldsmith, P.D. Economics of Soybean Production, Marketing, and Utilization. In Soybeans Chemistry, Production, Processing, and Utilization; AOCS Press: Champaign, IL, USA, 2008; pp. 117–150. [Google Scholar]
- Day, L. Wheat Gluten: Production, Properties and Application; Woodhead Publishing Limited: Sawston, UK, 2011. [Google Scholar]
- Menon, V.; Kaur, M.; Gupta, S.; Nadda, A.K.; Singh, G.B.; Sharma, S. Fabrication, Properties and Applications of Gluten Protein; Woodhead Publishing Limited: Sawston, UK, 2022; ISBN 9780323905459. [Google Scholar]
- Veraverbeke, W.S.; Roels, S.P.; Delcour, J.A. Heat-Induced Changes in Sodium Dodecyl Sulphate-Sedimentation Volume and Functionality of Vital Wheat Gluten. J. Cereal Sci. 1997, 26, 177–181. [Google Scholar] [CrossRef]
- Veraverbeke, W.S.; Delcour, J.A.; Bekes, F. Wheat Protein Composition and Properties of Wheat Glutenin in Relation to Breadmaking. Crit. Rev. Food Sci. Nutr. 2002, 42, 179–208. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat Gluten Functionality as a Quality Determinant in Cereal-Based Food Products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef]
- Gianibelli, M.C.; Larroque, O.R.; MacRitchie, F.; Wrigley, C.W. Biochemical, Genetic, and Molecular Characterization of Wheat Glutenin and Its Component Subunits. Cereal Chem. 2001, 78, 635–646. [Google Scholar] [CrossRef]
- Ng, T.S.K.; McKinley, G.H. Power Law Gels at Finite Strains: The Nonlinear Rheology of Gluten Gels. J. Rheol. 2008, 52, 417–449. [Google Scholar] [CrossRef]
- Sissons, M.J.; Egan, N.E.; Gianibelli, M.C. New Insights into the Role of Gluten on Durum Pasta Quality Using Reconstitution Method. Cereal Chem. 2005, 82, 601–608. [Google Scholar] [CrossRef]
- Grabowska, K.J.; Tekidou, S.; Boom, R.M.; van der Goot, A.J. Shear Structuring as a New Method to Make Anisotropic Structures from Soy–Gluten Blends. Food Res. Int. 2014, 64, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Krintiras, G.A.; Göbel, J.; Van Der Goot, A.J.; Stefanidis, G.D. Production of Structured Soy-Based Meat Analogues Using Simple Shear and Heat in a Couette Cell. J. Food Eng. 2015, 160, 34–41. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, T.; Zhang, Y.; Jiang, L.; Sui, X. Potential of Hydrolyzed Wheat Protein in Soy-Based Meat Analogues: Rheological, Textural and Functional Properties. Food Chem. X 2023, 20, 100921. [Google Scholar] [CrossRef]
- Hou, Y.; Xia, S.; Ma, C.; Xue, C.; Jiang, X. Effects of the Soy Protein to Wheat Gluten Ratio on the Physicochemical and Structural Properties of Alaska Pollock Surimi-Based Meat Analogs by High Moisture Extrusion. Food Res. Int. 2023, 173, 113469. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of Soy Protein to Wheat Gluten Ratio on the Physicochemical Properties of Extruded Meat Analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Xie, S.H.; Wang, Z.J.; He, Z.Y.; Zeng, M.M.; Qin, F.; Adhikari, B.; Chen, J. The Effects of Maltodextrin/Starch in Soy Protein Isolate–Wheat Gluten on the Thermal Stability of High-Moisture Extrudates. J. Integr. Agric. 2023, 22, 1590–1602. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Zhang, M.; Zheng, H.; Li, L. Preservation of Soy Protein-Based Meat Analogues by Using PLA/PBAT Antimicrobial Packaging Film. Food Chem. 2022, 380, 132022. [Google Scholar] [CrossRef]
- Mateen, A.; Mathpati, M.; Singh, G. A Study on High Moisture Extrusion for Making Whole Cut Meat Analogue: Characterization of System, Process and Product Parameters. Innov. Food Sci. Emerg. Technol. 2023, 85, 103315. [Google Scholar] [CrossRef]
- Samard, S.; Gu, B.Y.; Ryu, G.H. Effects of Extrusion Types, Screw Speed and Addition of Wheat Gluten on Physicochemical Characteristics and Cooking Stability of Meat Analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef]
- Liu, K.S.; Hsieh, F.H. Protein-Protein Interactions during High-Moisture Extrusion for Fibrous Meat Analogues and Comparison of Protein Solubility Methods Using Different Solvent Systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, T.; Zhang, Y.; Jiang, L.; Sui, X. High Moisture Extrusion of Soy Protein and Wheat Gluten Blend: An Underlying Mechanism for the Formation of Fibrous Structures. LWT 2022, 163, 113561. [Google Scholar] [CrossRef]
- Krintiras, G.A.; Göbel, J.; Bouwman, W.G.; Jan Van Der Goot, A.; Stefanidis, G.D. On Characterization of Anisotropic Plant Protein Structures. Food Funct. 2014, 5, 3233–3240. [Google Scholar] [CrossRef] [PubMed]
- Schreuders, F.K.G.; Dekkers, B.L.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Comparing Structuring Potential of Pea and Soy Protein with Gluten for Meat Analogue Preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Cornet, S.H.V.; Snel, S.J.E.; Lesschen, J.; van der Goot, A.J.; van der Sman, R.G.M. Enhancing the Water Holding Capacity of Model Meat Analogues through Marinade Composition. J. Food Eng. 2021, 290, 110283. [Google Scholar] [CrossRef]
- Estrada, P.D.; Berton-Carabin, C.C.; Schlangen, M.; Haagsma, A.; Pierucci, A.P.T.R.; Van Der Goot, A.J. Protein Oxidation in Plant Protein-Based Fibrous Products: Effects of Encapsulated Iron and Process Conditions. J. Agric. Food Chem. 2018, 66, 11105–11112. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.L.; Emin, M.A.; Boom, R.M.; van der Goot, A.J. The Phase Properties of Soy Protein and Wheat Gluten in a Blend for Fibrous Structure Formation. Food Hydrocoll. 2018, 79, 273–281. [Google Scholar] [CrossRef]
- Qiu, Y.; McClements, D.J.; Chen, J.; Li, C.; Liu, C.; Dai, T. Construction of 3D Printed Meat Analogs from Plant-Based Proteins: Improving the Printing Performance of Soy Protein- and Gluten-Based Pastes Facilitated by Rice Protein. Food Res. Int. 2023, 167, 112635. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S. 3D Printing of Soy Protein- and Gluten-Based Gels Facilitated by Thermosensitive Cocoa Butter in a Model Study. ACS Food Sci. Technol. 2021, 1, 1990–1996. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Bhandari, B. 3D Printing of Steak-Like Foods Based on Textured Soybean Protein. Foods 2021, 10, 2011. [Google Scholar] [CrossRef]
- Puski, G.; Konwinski, A.H. Process of Making a Soy-Based Meat Substitute. U.S. Patent 3950564A, 13 April 1976. [Google Scholar]
- Verbeek, C.J.R.; Van Den Berg, L.E. Extrusion Processing and Properties of Protein-Based Thermoplastics. Macromol. Mater. Eng. 2010, 295, 10–21. [Google Scholar] [CrossRef]
- Maurya, A.K.; Said, P.P. Extrusion Processing on Physical and Chemical Properties of Protein Rich Products-An Overview. J. Bioresour. Eng. Technol. 2014, 2, 61–67. [Google Scholar]
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as Agricultural Polymers for Packaging Production. Cereal Chem. 1998, 75, 1–9. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Plant-Based Meat Analogues; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128148747. [Google Scholar]
- Cheftel, J.C.; Kitagawa, M.; Queguiner, C. New Protein Texturization Processes by Extrusion Cooking at High Moisture Levels. Food Rev. Int. 1992, 8, 235–275. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, B.; Fu, H.; Li, J.; Ji, L.; Gong, H.; Zhang, R.; Liu, J.; Yu, H. The Development Process of Plant-Based Meat Alternatives: Raw Material Formulations and Processing Strategies. Food Res. Int. 2023, 167, 112689. [Google Scholar] [CrossRef] [PubMed]
- Navale, S.A.; Swami, S.B.; Thakor, N.J. Extrusion Cooking Technology for Foods: A Review. J. Ready Eat Food 2015, 2, 66–80. [Google Scholar]
- Alam, M.S.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and Extruded Products: Changes in Quality Attributes as Affected by Extrusion Process Parameters: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef]
- Crowe, T.W.; Johnson, L.A. Twin-Screw Extrusion Texturization of Extruded-Expelled Soybean Flour. J. Am. Oil Chem. Soc. 2001, 78, 781–786. [Google Scholar] [CrossRef]
- Basediya, A.L.; Pandey, S.; Shrivastava, S.P.; Khan, K.A.; Nema, A. Effect of Process and Machine Parameters on Physical Properties of Extrudate during Extrusion Cooking of Sorghum, Horse Gram and Defatted Soy Flour Blends. J. Food Sci. Technol. 2013, 50, 44–52. [Google Scholar] [CrossRef][Green Version]
- Guerrero, P.; Beatty, E.; Kerry, J.P.; De La Caba, K. Extrusion of Soy Protein with Gelatin and Sugars at Low Moisture Content. J. Food Eng. 2012, 110, 53–59. [Google Scholar] [CrossRef]
- Van Den Einde, R.M.; Van Der Goot, A.J.; Boom, R.M. Understanding Molecular Weight Reduction of Starch during Heating-Shearing Processes. J. Food Sci. 2003, 68, 2396–2404. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Van Der Goot, A.J.; Hamer, R.J.; Boom, R.M. A New Method to Study Simple Shear Processing of Wheat Gluten-Starch Mixtures. Cereal Chem. 2004, 81, 714–721. [Google Scholar] [CrossRef]
- Cornet, S.H.V.; Snel, S.J.E.; Schreuders, F.K.G.; van der Sman, R.G.M.; Beyrer, M.; van der Goot, A.J. Thermo-Mechanical Processing of Plant Proteins Using Shear Cell and High-Moisture Extrusion Cooking. Crit. Rev. Food Sci. Nutr. 2022, 62, 3264–3280. [Google Scholar] [CrossRef] [PubMed]
- Manski, J.M.; van der Goot, A.J.; Boom, R.M. Formation of Fibrous Materials from Dense Calcium Caseinate Dispersions. Biomacromolecules 2007, 8, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, K.J.; Zhu, S.; Dekkers, B.L.; De Ruijter, N.C.A.; Gieteling, J.; van der Goot, A.J. Shear-Induced Structuring as a Tool to Make Anisotropic Materials Using Soy Protein Concentrate. J. Food Eng. 2016, 188, 77–86. [Google Scholar] [CrossRef]
- Bühler, J.M.; Dekkers, B.L.; Bruins, M.E.; van der Goot, A.J. Modifying Faba Bean Protein Concentrate Using Dry Heat to Increase Water Holding Capacity. Foods 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Kyriakopoulou, K.; Roelofs, B.; Ndiaye, M.; Vincken, J.-P.; Keppler, J.K.; van der Goot, A.J. Removal of Phenolic Compounds from De-Oiled Sunflower Kernels by Aqueous Ethanol Washing. Food Chem. 2021, 362, 130204. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Rodriguez-Alonso, E.; Bianeis, M.; Keppler, J.K.; van der Goot, A.J. Assessing Functional Properties of Rapeseed Protein Concentrate versus Isolate for Food Applications. Innov. Food Sci. Emerg. Technol. 2021, 68, 102636. [Google Scholar] [CrossRef]
- Krintiras, G.A.; Diaz, J.G.; Van Der Goot, A.J.; Stankiewicz, A.I.; Stefanidis, G.D. On the Use of the Couette Cell Technology for Large Scale Production of Textured Soy-Based Meat Replacers. J. Food Eng. 2016, 169, 205–213. [Google Scholar] [CrossRef]
- Wegrzyn, T.F.; Golding, M.; Archer, R.H. Food Layered Manufacture: A New Process for Constructing Solid Foods. Trends Food Sci. Technol. 2012, 27, 66–72. [Google Scholar] [CrossRef]
- Uz Zaman, U.K.; Rivette, M.; Siadat, A.; Mousavi, S.M. Integrated Product-Process Design: Material and Manufacturing Process Selection for Additive Manufacturing Using Multi-Criteria Decision Making. Robot. Comput. Integr. Manuf. 2018, 51, 169–180. [Google Scholar] [CrossRef]
- Holland, S.; Foster, T.; MacNaughtan, W.; Tuck, C. Design and Characterisation of Food Grade Powders and Inks for Microstructure Control Using 3D Printing. J. Food Eng. 2018, 220, 12–19. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D Printing: Printing Precision and Application in Food Sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
- Wen, Y.; Chao, C.; Che, Q.T.; Kim, H.W.; Park, H.J. Development of Plant-Based Meat Analogs Using 3D Printing: Status and Opportunities. Trends Food Sci. Technol. 2023, 132, 76–92. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B. Recent Development in 3D Food Printing. Crit. Rev. Food Sci. Nutr. 2017, 57, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mu, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubruge, E. Application of Soy Protein Isolate and Hydrocolloids Based Mixtures as Promising Food Material in 3D Food Printing. J. Food Eng. 2019, 261, 76–86. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Devahastin, S. Investigation on 3D Printing Ability of Soybean Protein Isolate Gels and Correlations with Their Rheological and Textural Properties via LF-NMR Spectroscopic Characteristics. LWT 2020, 122, 109019. [Google Scholar] [CrossRef]
- Ko, H.J.; Wen, Y.; Choi, J.H.; Park, B.R.; Kim, H.W.; Park, H.J. Meat Analog Production through Artificial Muscle Fiber Insertion Using Coaxial Nozzle-Assisted Three-Dimensional Food Printing. Food Hydrocoll. 2021, 120, 106898. [Google Scholar] [CrossRef]
- Israeli, D.; Prigat Goldfriend, Y.; Dikovsky, D.; Benjamin, O. Novel Plant Proteins Used in 3D Printed Meat Analogues: Relationship between Protein Physicochemical and Functional Characteristics. Eur. Food Res. Technol. 2023, 249, 2335–2347. [Google Scholar] [CrossRef]
- Zeleny, J. The Electrical Discharge from Liquid Points, and a Hydrostatic Method of Measuring the Electric Intensity at Their Surfaces. Phys. Rev. 1914, 3, 69–91. [Google Scholar] [CrossRef]
- Formhals, A. Process and Apparatus for Artificial Threads. U.S. Patent 1975504A, 2 October 1934. [Google Scholar]
- Chiffman, J.D.; Schauer, C.L. A Review: Electrospinning of Biopolymer Nanofibers and Their Applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hsieh, A.J.; Rutledge, G.C. Electrospinning of Poly(MMA-Co-MAA) Copolymers and Their Layered Silicate Nanocomposites for Improved Thermal Properties. Polymer 2005, 46, 3407–3418. [Google Scholar] [CrossRef]
- Nieuwland, M.; Geerdink, P.; Brier, P.; Van Den Eijnden, P.; Henket, J.T.M.M.; Langelaan, M.L.P.; Stroeks, N.; Van Deventer, H.C.; Martin, A.H. Reprint of “Food-Grade Electrospinning of Proteins”. Innov. Food Sci. Emerg. Technol. 2014, 24, 138–144. [Google Scholar] [CrossRef]
- Kanjanapongkul, K.; Wongsasulak, S.; Yoovidhya, T. Investigation and Prevention of Clogging during Electrospinning of Zein Solution. J. Appl. Polym. Sci. 2010, 116, 1821–1829. [Google Scholar] [CrossRef]
- Ziegler, G.R.; Foegeding, E.A. The Gelation of Proteins. Adv. Food Nutr. Res. 1990, 34, 203–298. [Google Scholar] [CrossRef]
- Braudo, E.E.; Gotlieb, A.M.; Plashina, I.G.; Tolstoguzov, V.B. Protein-containing Multicomponent Gels. Food/Nahrung 1986, 30, 355–364. [Google Scholar] [CrossRef]
- Tolstoguzov, V. Some Thermodynamic Considerations in Food Formulation. Food Hydrocoll. 2003, 17, 1–23. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Some Physico-Chemical Aspects of Protein Processing in Foods. Multicomponent Gels. Top. Catal. 1995, 9, 317–332. [Google Scholar] [CrossRef]
- Polyakov, V.I.; Popello, I.A.; Grinberg, V.Y.; Tolstoguzov, V.B. Thermodynamic Compatibility of Proteins in Aqueous Medium. Food/Nahrung 1986, 30, 365–368. [Google Scholar] [CrossRef]
- Velichko, E.; Tian, B.; Nikolaeva, T.; Koning, J.; van Duynhoven, J.; Bouwman, W.G. A Versatile Shear Cell for Investigation of Structure of Food Materials under Shear. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 566, 21–28. [Google Scholar] [CrossRef]
- Nicolai, T.; Chassenieux, C. Heat-Induced Gelation of Plant Globulins. Curr. Opin. Food Sci. 2019, 27, 18–22. [Google Scholar] [CrossRef]
- Utsumi, S.; Kinsella, J.E. Forces Involved in Soy Protein Gelation: Effects of Various Reagents on the Formation, Hardness and Solubility of Heat-Induced Gels Made from 7S, 11S, and Soy Isolate. J. Food Sci. 1985, 50, 1278–1282. [Google Scholar] [CrossRef]
- Liu, K.S.; Hsieh, F.H. Protein-Protein Interactions in High Moisture-Extruded Meat Analogs and Heat-Induced Soy Protein Gels. J. Am. Oil Chem. Soc. 2007, 84, 741–748. [Google Scholar] [CrossRef]
- Cordier, F.; Grzesiek, S. Temperature-Dependence of Protein Hydrogen Bond Properties as Studied by High-Resolution NMR. J. Mol. Biol. 2002, 317, 739–752. [Google Scholar] [CrossRef]
- van Dijk, E.; Hoogeveen, A.; Abeln, S. The Hydrophobic Temperature Dependence of Amino Acids Directly Calculated from Protein Structures. PLoS Comput. Biol. 2015, 11, e1004277. [Google Scholar] [CrossRef]
- Volkin, D.B.; Klibanov, A.M. Thermal Destruction Processes in Proteins Involving Cystine Residues. J. Biol. Chem. 1987, 262, 2945–2950. [Google Scholar] [CrossRef]
- Schofield, J.D.; Bottomley, R.C.; Timms, M.F.; Booth, M.R. The Effect of Heat on Wheat Gluten and the Involvement of Sulphydryl-Disulphide Interchange Reactions. J. Cereal Sci. 1983, 1, 241–253. [Google Scholar] [CrossRef]
- Evans, E. Probing the Relation between Force-Lifetime-and Chemistry. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 105–128. [Google Scholar] [CrossRef]
- Nagy, P. Kinetics and Mechanisms of Thiol-Disulfide Exchange Covering Direct Substitution and Thiol Oxidation-Mediated Pathways. Antioxid. Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).