Selected Fungicides as Potential EDC Estrogenic Micropollutants in the Environment
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity
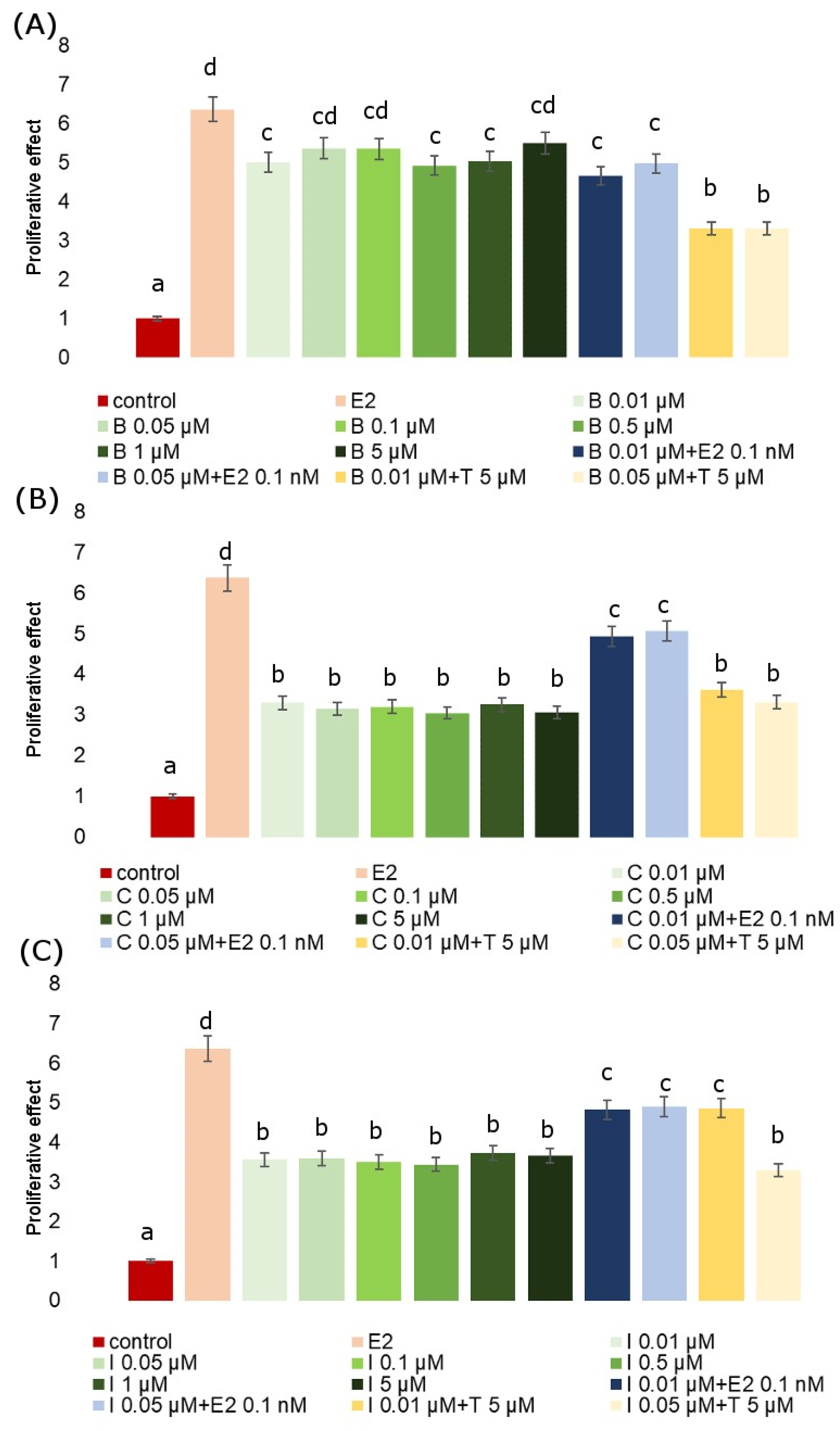
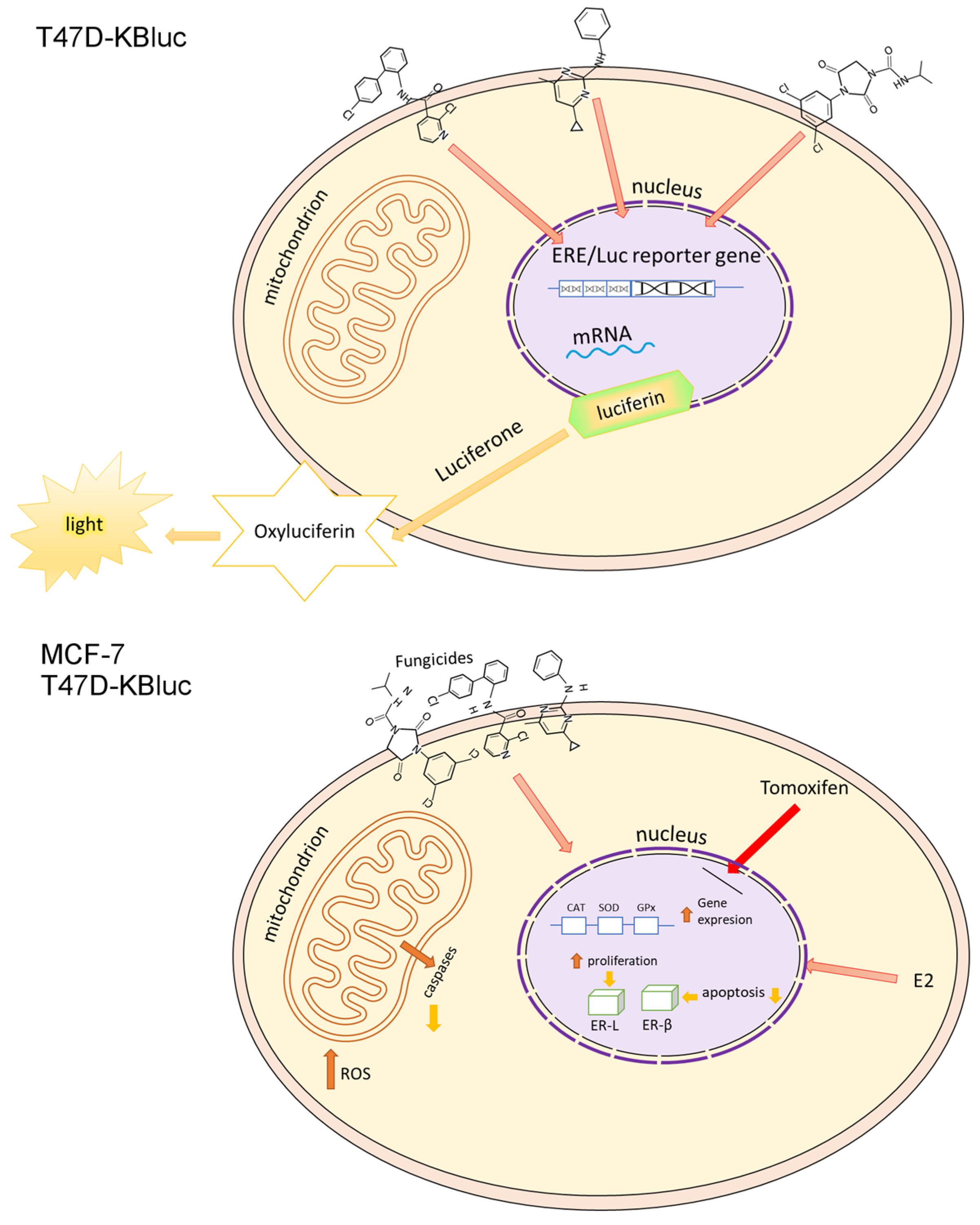
2.2. E-screen Assay and T47D-KBluc Gene-Reporter Assay
2.3. Oxidative Stress
2.4. Effects of the Fungicides on the Zeta Potential and Surface Charge of Cell Membranes
2.5. LC–ESI–MS/MS Analysis of Boscalid, Cyprodinil and Iprodione in MCF-7 Cells
2.6. Morphological Changes in Live Cells
3. Discussion
4. Materials and Methods
4.1. Chemical Treatment of Cells
4.2. Fungicide Cytotoxicity
4.3. E-screen Test
4.4. Bioassay for Measurement of Estrogenic Activity
4.5. Flow Cytometry Detection of Intracellular ROS
4.6. Extraction and Preparation of RNA
4.7. Reverse Transcription-Quantitative PCR (RT-qPCR)
4.8. Determination of Surface Charge and Zeta Potential
4.9. LC–ESI–MS/MS Analysis of Boscalid, Cyprodinil and Iprodione
4.10. Statistical Analysis
4.11. Morphological Analysis of MCF-7 and T47DKBluc Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global Trends in Pesticides: A Looming Threat and Viable Alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Ma, M.; Chen, C.; Yang, G.; Wang, Y.; Wang, T.; Li, Y.; Qian, Y. Combined anti-androgenic effects of mixtures of agricultural pesticides using in vitro and in silico methods. Ecotoxicol. Environ. Saf. 2019, 186, 109652. [Google Scholar] [CrossRef]
- Rizzati, V.; Briand, O.; Guillou, H.; Gamet-Payrastre, L. Effects of Pesticide Mixtures in Human and Animal Models: An Update of the Recent Literature. Chem. Biol. Interact. 2016, 254, 231–246. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Butarewicz, A. Toxicological Effects of Traumatic Acid and Selected Herbicides on Human Breast Cancer Cells: In Vitro Cytotoxicity Assessment of Analyzed Compounds. Molecules 2019, 24, 1710. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of Novel Pesticides in the 21st Century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Kamp, H.; Wahrheit, J.; Stinchcombe, S.; Walk, T.; Stauber, F.; Ravenzwaay, B.V. Succinate Dehydrogenase Inhibitors: In Silico Flux Analysis and in Vivo Metabolomics Investigations Show No Severe Metabolic Consequences for Rats and Humans. Food Chem. Toxicol. 2021, 150, 112085. [Google Scholar] [CrossRef]
- Bénit, P.; Kahn, A.; Chretien, D.; Bortoli, S.; Huc, L.; Schiff, M.; Gimenez-Roqueplo, A.-P.; Favier, J.; Gressens, P.; Rak, M.; et al. Evolutionarily Conserved Susceptibility of the Mitochondrial Respiratory Chain to SDHI Pesticides and Its Consequence on the Impact of SDHIs on Human Cultured Cells. PLoS ONE 2019, 14, e0224132. [Google Scholar] [CrossRef]
- Hu, S.M.; Zhang, J.; Zhang, Y.C.; He, S.; Zhu, F.X. Baseline sensitivity and toxic actions of boscalid against Sclerotinia sclerotiorum. Crop Prot. 2018, 110, 83–90. [Google Scholar]
- Qian, L.; Cui, F.; Yang, Y.; Liu, Y.; Qi, S.Z.; Wang, C.J. Mechanisms of developmental toxicity in zebrafish embryos (Danio rerio) induced by boscalid. Sci. Total Environ. 2018, 634, 478–487. [Google Scholar]
- Zang, X.; Ji, M.; Wang, K.; Li, X.; Zhang, Y.; Li, X.; Tian, H.; Zhu, H.; Du, F. Effects of boscalid on the antioxidant enzyme system of adult zebrafish (Danio rerio). Agric. Sci. Technol. 2017, 18, 287–293. [Google Scholar]
- Wang, H.; Meng, Z.; Liu, F.; Zhou, L.; Su, M.; Meng, Y.; Zhang, S.; Liao, X.; Cao, Z.; Lu, H. Characterization of Boscalid-Induced Oxidative Stress and Neurodevelopmental Toxicity in Zebrafish Embryos. Chemosphere 2020, 238, 124753. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, J.; Chen, X.; Qi, S.; Wu, P.; Wang, C.; Wang, C. Toxic Effects of Boscalid in Adult Zebrafish (Danio Rerio) on Carbohydrate and Lipid Metabolism. Environ. Pollut. 2019, 247, 775–782. [Google Scholar] [CrossRef]
- Çayır, A.; Coskun, M.; Coskun, M. Micronuclei, Nucleoplasmic Bridges, and Nuclear Buds Induced in Human Lymphocytes by the Fungicide Signum and Its Active Ingredients (Boscalid and Pyraclostrobin). Environ. Toxicol. 2014, 29, 723–732. [Google Scholar] [CrossRef]
- Graillot, V.; Tomasetig, F.; Cravedi, J.-P.; Audebert, M. Evidence of the in Vitro Genotoxicity of Methyl-Pyrazole Pesticides in Human Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 748, 8–16. [Google Scholar] [CrossRef]
- d’Hose, D.; Isenborghs, P.; Brusa, D.; Jordan, B.F.; Gallez, B. The Short-Term Exposure to SDHI Fungicides Boscalid and Bixafen Induces a Mitochondrial Dysfunction in Selective Human Cell Lines. Molecules 2021, 26, 5842. [Google Scholar] [CrossRef]
- Available online: https://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 16 October 2023).
- Ma, Z.H.; Ye, Z.Y. The New Target Fungicide-Pyrimidine Amines. World Pestic. 1997, 3, 12–13. [Google Scholar]
- Shen, C.; Tang, C.; Zhu, K.; He, C.; Yang, C.; Zuo, Z. Toxicity and ecological risk assessment for two AhR agonistic pesticides mepanipyrim and cyprodinil and their metabolites. Environ. Sci. Pollut. Res. Int. 2023, 30, 58944–58955. [Google Scholar] [CrossRef]
- Go, R.E.; Kim, C.W.; Choi, K.C. Effect of fenhexamid and cyprodinil on the expression of cell cycle- and metastasis-related genes via an estrogen receptor-dependent pathway in cellular and xenografted ovarian cancer models. Toxicol. Appl. Pharmacol. 2015, 289, 48–57. [Google Scholar]
- Huang, X.; Jin, Y.; Zhou, D.; Xu, G.; Huang, J.; Shen, L. IQGAP1 modulates the proliferation and migration of vascular smooth muscle cells in response to estrogen. Int. J. Mol. Med. 2015, 35, 1460–1466. [Google Scholar]
- Fang, C.C.; Chen, F.Y.; Chen, C.R.; Liu, C.C.; Wong, L.C.; Liu, Y.W.; Su, J.G. Cyprodinil as an activator of aryl hydrocarbon receptor. Toxicology 2013, 304, 32–40. [Google Scholar]
- Masner, P.; Muster, P.; Schmid, J. Possible Methionine Biosynthesis Inhibition by Pyrim-Idineamine Fungicides. Pestic. Sci. 1997, 42, 163–166. [Google Scholar]
- Kanetis, L.; Förster, H.; Jones, C.A.; Borkovich, K.A.; Adaskaveg, J.E. Characterization of Genetic and Biochemical Mechanisms of Fludioxonil and Pyrimethanil Resistance in Field Isolates of Penicillium Digitatum. Phytopathology 2008, 98, 205–214. [Google Scholar] [CrossRef]
- Coleman, M.D.; O’Neil, J.D.; Woehrling, E.K.; Ndunge, O.B.A.; Hill, E.J.; Menache, A.; Reiss, C.J. A Preliminary Investigation into the Impact of a Pesticide Combination on Human Neuronal and Glial Cell Lines in Vitro. PLoS ONE 2012, 7, e42768. [Google Scholar] [CrossRef]
- Özdoğan, N.; Kapukıran, F.; Mutluoğlu, G.; Chormey, D.S.; Bakırdere, S. Simultaneous Determination of Iprodione, Procymidone, and Chlorflurenol in Lake Water and Wastewater Matrices by GC-MS after Multivariate Optimization of Binary Dispersive Liquid-Liquid Microextraction. Environ. Monit. Assess. 2018, 190, 607. [Google Scholar] [CrossRef]
- U.S. EPA: Environmental Protection Agency. Reregistration Eligibility Decision (RED), Iprodione; U.S. EPA: Environmental Protection Agency: Washington, DC, USA, 1998.
- Radice, S.; Marabini, L.; Gervasoni, M.; Ferraris, M.; Chiesara, E. Adaptation to oxidative stress: Effects of vinclozolin and iprodione on the HepG2 cell line. Toxicology 1998, 129, 183–191. [Google Scholar]
- Radice, S.; Ferraris, M.; Marabini, L.; Grande, S.; Chiesara, E. Effect of iprodione, a dicarboximide fungicide, on primary cultured rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 2001, 54, 51–58. [Google Scholar]
- Commission Implementing Regulation (EU) 2017/2091 of 14 November 2017 Concerning the Non-Renewal of Approval of the Active Substance Iprodione, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending Commission Implementing Regulation (EU) No 540/2011. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/2091/oj?locale=en (accessed on 16 October 2023).
- Fitó Friedrichs, G.; Berenstein, G.; Nasello, S.; Dutra Alcoba, Y.Y.; Hughes, E.A.; Basack, S.; Montserrat, J.M. Human Exposure and Mass Balance Distribution during Procymidone Application in Horticultural Greenhouses. Heliyon 2020, 6, e03093. [Google Scholar] [CrossRef]
- Lai, Q.; Sun, X.; Li, L.; Li, D.; Wang, M.; Shi, H. Toxicity effects of procymidone, iprodione and their metabolite of 3,5-dichloroaniline to zebrafish. Chemosphere 2021, 272, 129577. [Google Scholar] [CrossRef]
- Maltby, L.; Brock, T.C.M.; van den Brink, P.J. Fungicide Risk Assessment for Aquatic Ecosystems: Importance of Interspecific Variation, Toxic Mode of Action, and Exposure Regime. Environ. Sci. Technol. 2009, 43, 7556–7563. [Google Scholar] [CrossRef]
- Rifai, A.; Souissi, Y.; Genty, C.; Clavaguera, C.; Bourcier, S.; Jaber, F.; Bouchonnet, S. Ultraviolet Degradation of Procymidone—Structural Characterization by Gas Chromatography Coupled with Mass Spectrometry and Potential Toxicity of Photoproducts Using in Silico Tests: UV Degradation of Procymidone. Rapid Commun. Mass Spectrom. 2013, 27, 1505–1516. [Google Scholar] [CrossRef]
- Blystone, C.; Lambright, C.; Furr, J.; Wilson, V.; Grayjr, L. Iprodione Delays Male Rat Pubertal Development, Reduces Serum Testosterone Levels, and Decreases Ex Vivo Testicular Testosterone Production. Toxicol. Lett. 2007, 174, 74–81. [Google Scholar] [CrossRef]
- Pisani, C.; Voisin, S.; Arafah, K.; Durand, P.; Perrard, M.H.; Guichaoua, M.R.; Bulet, P.; Prat, O. Ex vivo assessment of testicular toxicity induced by carbendazim and iprodione, alone or in a mixture. ALTEX-Altern. Anim. Exp. 2016, 33, 393–413. [Google Scholar]
- Bletsou, A.A.; Hanafi, A.H.; Dasenaki, M.E.; Thomaidis, N.S. Development of Specific LC-ESI-MS/MS Methods to Determine Bifenthrin, Lufenuron, and Iprodione Residue Levels in Green Beans, Peas, and Chili Peppers under Egyptian Field Conditions. Food Anal. Methods 2013, 6, 1099–1112. [Google Scholar] [CrossRef]
- Loutfy, N.; Malhat, F.; Kamel, E.; Saber, A. Residual Pattern and Dietary Intake of Iprodione on Grapes under Egyptian Field Conditions: A Prelude to Risk Assessment Profile. Hum. Ecol. Risk Assess. 2015, 21, 265–279. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Li, S.; Liu, C. Evaluation of Nine Pesticide Residues in Three Minor Tropical Fruits from Southern China. Food Control 2016, 60, 677–682. [Google Scholar] [CrossRef]
- Bernardes, P.M.; Andrade-Vieira, L.F.; Aragao, F.B.; Ferreira, A.; Ferreira, M.F.D. Toxicological effects of comercial formulations of fungicides based on procy-midone and iprodione in seedlings and root tip cells of Allium cepa. Environ. Sci. Pollut. Res. 2019, 26, e21013–e21021. [Google Scholar]
- Wang, Z.; Cang, T.; Qi, P.; Zhao, X.; Xu, H.; Wang, X.; Zhang, H.; Wang, X. Dissipation of Four Fungicides on Greenhouse Strawberries and an Assessment of Their Risks. Food Control 2015, 55, 215–220. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of Methods for Assessing Bacterial Cell Surface Charge Properties Based on Zeta Potential Measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- van Meeuwen, J.A.; Ter Burg, W.; Piersma, A.H.; van den Berg, M.; Sanderson, J.T. Mixture Effects of Estrogenic Compounds on Proliferation and PS2 Expression of MCF-7 Human Breast Cancer Cells. Food Chem. Toxicol. 2007, 45, 2319–2330. [Google Scholar] [CrossRef]
- Shaw, I.; McCully, S. A Review of the Potential Impact of Dietary Endocrine Disrupters on the Consumer. Int. J. Food Sci. Technol. 2002, 37, 471–476. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wydro, U.; Serra-Majem, L.; Wołejko, E.; Butarewicz, A. The Analysis of Bifenox and Dichlobenil Toxicity in Selected Microorganisms and Human Cancer Cells. Int. J. Environ. Res. Public Health 2019, 16, 4137. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Krętowski, R.; Świderski, G.; Cechowska-Pasko, M.; Lewandowski, W. Cichoric Acid Attenuates the Toxicity of Mesotrione. Effect on in Vitro Skin Cell Model. Environ. Toxicol. Pharmacol. 2020, 77, 103375. [Google Scholar] [CrossRef]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate Induces Human Breast Cancer Cells Growth via Estrogen Receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- Huovinen, M.; Loikkanen, J.; Naarala, J.; Vähäkangas, K. Toxicity of Diuron in Human Cancer Cells. Toxicol. In Vitro 2015, 29, 1577–1586. [Google Scholar] [CrossRef]
- Schilirò, T.; Porfido, A.; Longo, A.; Coluccia, S.; Gilli, G. The E-Screen Test and the MELN Gene-Reporter Assay Used for Determination of Estrogenic Activity in Fruits and Vegetables in Relation to Pesticide Residues. Food Chem. Toxicol. 2013, 62, 82–90. [Google Scholar] [CrossRef]
- Soto, A.M.; Sonnenschein, C.; Chung, K.L.; Fernandez, M.F.; Olea, N.; Serrano, F.O. The E-SCREEN Assay as a Tool to Identify Estrogens: An Update on Estrogenic Environmental Pollutants. Environ. Health Perspect. 1995, 103, 113–122. [Google Scholar] [CrossRef]
- Chou, H.-M.; Chao, H.-R.; Lin, C.; Chiang, P.-C.; Wang, G.-S.; Tsou, T.-C. An Improved Estrogenic Activity Reporter Gene Assay (T47D-KBluc) for Detecting Estrogenic Activity in Wastewater and Drinking Water. Toxicol. Environ. Chem. 2016, 98, 376–384. [Google Scholar] [CrossRef]
- Wehmas, L.C.; Cavallin, J.E.; Durhan, E.J.; Kahl, M.D.; Martinovic, D.; Mayasich, J.; Tuominen, T.; Villeneuve, D.L.; Ankley, G.T. Screening Complex Effluents for Estrogenic Activity with the T47D-KBluc Cell Bioassay: Assay Optimization and Comparison with in Vivo Responses in Fish. Environ. Toxicol. Chem 2011, 30, 439–445. [Google Scholar]
- Beischlag, T.V.; Perdew, G.H. ERα-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J. Biol. Chem. 2005, 280, 21607–21611. [Google Scholar]
- Bemanian, V.; Male, R.; Goksoyr, A. The aryl hydrocarbon receptor-mediated disruption of vitellogenin synthesis in the fish liver: Cross-talk between AHR- and ERα-signalling pathways. Comp. Hepatol. 2004, 3, 2–16. [Google Scholar]
- Medjakovic, S.; Zoechling, A.; Gerster, P.; Ivanova, M.M.; Teng, Y.; Klinge, C.M.; Schildberger, B.; Gartner, M.; Jungbauer, A. Effect of nonpersistent pesticides on estrogen receptor, androgen receptor, and aryl hydrocarbon receptor. Environ. Toxicol. 2014, 29, 1201–1216. [Google Scholar] [CrossRef]
- Lee, H.M.; Hwang, K.A.; Choi, K.C. Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process. Mol. Cell. Endocrinol. 2017, 457, 103–113. [Google Scholar]
- Kim, B.-G.; Kim, J.-W.; Kim, S.-M.; Go, R.-E.; Hwang, K.-A.; Choi, K.-C. 3,3′-Diindolylmethane Suppressed Cyprodinil-Induced Epithelial-Mesenchymal Transition and Metastatic-Related Behaviors of Human Endometrial Ishikawa Cells via an Estrogen Receptor-Dependent Pathway. Int. J. Mol. Sci. 2018, 19, 189. [Google Scholar] [CrossRef]
- Aksakal, F.I. Evaluation of boscalid toxicity on Daphnia magna by using antioxidant enzyme activities, the expression of genes related to antioxidant and detoxification systems, and life-history parameters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 237, 108830. [Google Scholar] [CrossRef]
- Naumowicz, M.; Kusaczuk, M.; Zając, M.; Gál, M.; Kotyńska, J. Monitoring of the Surface Charge Density Changes of Human Glioblastoma Cell Membranes upon Cinnamic and Ferulic Acids Treatment. Int. J. Mol. Sci. 2020, 21, 6972. [Google Scholar] [CrossRef]
- Zając, M.; Kotyńska, J.; Worobiczuk, M.; Breczko, J.; Naumowicz, M. The Effect of Submicron Polystyrene on the Electrokinetic Potential of Cell Membranes of Red Blood Cells and Platelets. Membranes 2022, 12, 366. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Mekapati, S.B.; Kurup, A. QSAR and ADME. Bioorg. Med. Chem. 2004, 12, 3391–3400. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 18 October 2023).
- Silva, A.M.; Martins-Gomes, C.; Silva, T.L.; Coutinho, T.E.; Souto, E.B.; Andreani, T. In Vitro Assessment of Pesticides Toxicity and Data Correlation with Pesticides Physicochemical Properties for Prediction of Toxicity in Gastrointestinal and Skin Contact Exposure. Toxics 2022, 10, 378. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Jackson, T.A.; Richer, J.K.; Bain, D.L.; Takimoto, G.S.; Tung, L.; Horwitz, K.B. The Partial Agonist Activity of Antagonist-Occupied Steroid Receptors Is Controlled by a Novel Hinge Domain-Binding Coactivator L7/SPA and the Corepressors N-CoR or SMRT. Mol. Endocrinol. 1997, 11, 693–705. [Google Scholar] [CrossRef]
- O’Leary, D.P.; Bhatt, L.; Woolley, J.F.; Gough, D.R.; Wang, J.H.; Cotter, T.G. Redmond HP. TLR-4 signalling accelerates colon cancer cell adhesion via NF-κB mediated transcriptional up-regulation of Nox-1. PLoS ONE 2012, 7, e44176. [Google Scholar] [CrossRef]
- Piana, C.; Wirth, M.; Gerbes, S.; Viernstein, H.; Gabor, F.; Toegel, S. Validation of Reference Genes for QPCR Studies on Caco-2 Cell Differentiation. Eur. J. Pharm. Biopharm. 2008, 69, 1187–1192. [Google Scholar] [CrossRef]
- Alexander, A.E. Some Applications of Surface Chemistry to Problems in Colloid Science. J. Chem. Soc. 1947, 1422–1425. [Google Scholar] [CrossRef]
- Barrow, G.M. Physical Chemistry; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- da Costa Morais, E.H.; Collins, C.H.; Jardim, I.C.S.F. Pesticide determination in sweet peppers using QuEChERS and LC–MS/MS. Food Chem. 2018, 249, 77–83. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef]





| Physicochemical/Toxicological Properties | Iprodione | Cyprodinil | Boscalid |
|---|---|---|---|
| Chemical formula | C₁₃H₁₃Cl₂N₃O₃ | C₁₄H₁₅N₃ | C₁₈H₁₂Cl₂N₂O |
| 2D structure diagram |  |  |  |
| Molecular mass | 330.17 | 225.29 | 343.21 |
| CAS name | 3-(3,5-dichlorophenyl)-N-(1-methylethyl)-2,4-dioxo-1-imidazolidinecarboxamide | 4-cyclopropyl-6-methyl-N-phenyl-2-pyrimidinamine | 2-chloro-N-(4′-chloro[1,1′-biphenyl]-2-yl)-3-pyridinecarboxamide |
| Solubility—in water at 20 °C (mg/L) | 6.8 (Low) | 13 (Low) | 4.6 (Low) |
| Known metabolites | N-(3,5-dichlorophenylcarbamoyl)- N-isopropylcarbamoyl-glycine 3,5-dichloroaniline 3-(3,5-dichlorophenyl)-2,4- dioxoimidazolidine 1-[(3,5-dichlorophenyl) carbamoymethyl]-3-isopropylurea | 4-cyclopropyl-6-methyl- pyrimidin-2-ol 4-(4-cyclopropyl-6-methyl- pyrimidin-2-yl-amino)-phenol (2-amino-6-cyclopropyl- pyrimidin-4-yl)-methanol 4-(4-cyclopropyl-6-methyl- pyrimidin-2-yl-amino)-phenol | 4-chlorobenzoic acid 2-chloronicotinic acid 2-chloro-N-(4′-chloro-5-hydroxybiphenyl-2-yl)nicotinamide N-(4-chlorobiphenyl-2-yl)-2-hydroxynicotinamide |
| Ecotoxicology Mammals—acute oral LD₅₀ (mg/ kg) | >2000 | >2000 | >5000 |
| Human health and protection threshold of toxicological concern (Cramer class) | High (class III) | High (class III) | High (class III) |
| Specific human health issues | Endocrine disrupter; reproduction/development effects | Mammals’ chronic toxicity: moderate; reproduction/development effects | Possible carcinogen; reproduction/development effects |
| ADI—Acceptable Daily Intake (mg/kg bw/day) | 0.02 | 0.03 | 0.04 |
| WHO classification | III (Slightly hazardous) | III (c) (Slightly hazardous/Company classification) | U (Unlikely to present an acute hazard) |
| Fungicides Treatments | E-Screen RPE% | T47DKBluc Luciferase Assay TRANS% |
|---|---|---|
| B 0.01 µM | 37.212 | 34.179 |
| B 0.05 µM | 42.191 | 38.028 |
| B 0.1 µM | 42.090 | 41.645 |
| B 0.5 µM | 35.885 | 36.844 |
| B 1 µM | 37.544 | 33.734 |
| B 5 µM | 44.161 | 37.795 |
| B 10 µM | - | 95.388 |
| C 0.01 µM | 12.590 | 34.327 |
| C 0.05 µM | 10.437 | 35.744 |
| C 0.1 µM | 11.265 | 43.020 |
| C 0.5 µM | 9.026 | 41.433 |
| C 1 µM | 11.923 | 39.657 |
| C 5 µM | 9.259 | 39.128 |
| C 10 µM | - | 95.156 |
| I 0.01 µM | 16.358 | 49.492 |
| I 0.05 µM | 16.793 | 53.172 |
| I 0.1 µM | 15.490 | 72.377 |
| I 0.5 µM | 14.575 | 69.796 |
| I 1 µM | 18.919 | 74.492 |
| I 5 µM | 17.889 | 64.276 |
| I 10 µM | - | 83.438 |
| Fungicide | Applied Dose (µg/L) | Amount within the MCF-7 Cells (µg/L) | % Content |
|---|---|---|---|
| Boscalid (0.01 µM) | 0.34 | 0.24 | 70.5 |
| Boscalid (0.025 µM) | 0.85 | 0.36 | 42.35 |
| Cyprodinil (0.01 µM) | 0.22 | 0.17 | 77.27 |
| Cyprodinil (0.025 µM) | 0.56 | 0.22 | 39.28 |
| Iprodione (0.01 µM) | 0.33 | 0.21 | 63.63 |
| Iprodione (0.025 µM) | 0.825 | 0.26 | 31.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Makuła, M.; Krętowski, R.; Naumowicz, M.; Sokołowska, G.; Serra-Majem, L.; Cechowska-Pasko, M.; Łozowicka, B.; et al. Selected Fungicides as Potential EDC Estrogenic Micropollutants in the Environment. Molecules 2023, 28, 7437. https://doi.org/10.3390/molecules28217437
Jabłońska-Trypuć A, Wydro U, Wołejko E, Makuła M, Krętowski R, Naumowicz M, Sokołowska G, Serra-Majem L, Cechowska-Pasko M, Łozowicka B, et al. Selected Fungicides as Potential EDC Estrogenic Micropollutants in the Environment. Molecules. 2023; 28(21):7437. https://doi.org/10.3390/molecules28217437
Chicago/Turabian StyleJabłońska-Trypuć, Agata, Urszula Wydro, Elżbieta Wołejko, Marcin Makuła, Rafał Krętowski, Monika Naumowicz, Gabriela Sokołowska, Lluis Serra-Majem, Marzanna Cechowska-Pasko, Bożena Łozowicka, and et al. 2023. "Selected Fungicides as Potential EDC Estrogenic Micropollutants in the Environment" Molecules 28, no. 21: 7437. https://doi.org/10.3390/molecules28217437






