Abstract
The N-functionalized indole is a privileged structural framework in a wide range of bioactive molecules. The nucleophilic addition between indoles with vinylene carbonate proceeded smoothly in the presence of K2CO3 as the catalyst to produce novel indolyl-containing skeletons and 4-indolyl-1,3-dioxolanones in satisfactory to excellent yields (up to >97% yield). Various synthetically useful functional groups, such as halogen atoms, cyano, nitro, and methoxycarbonyl groups, remained intact during the regioselective N-H addition reactions. The developed catalytic system also could accommodate 2-naphthalenol to achieve the target O-H additive product in good yield.
1. Introduction
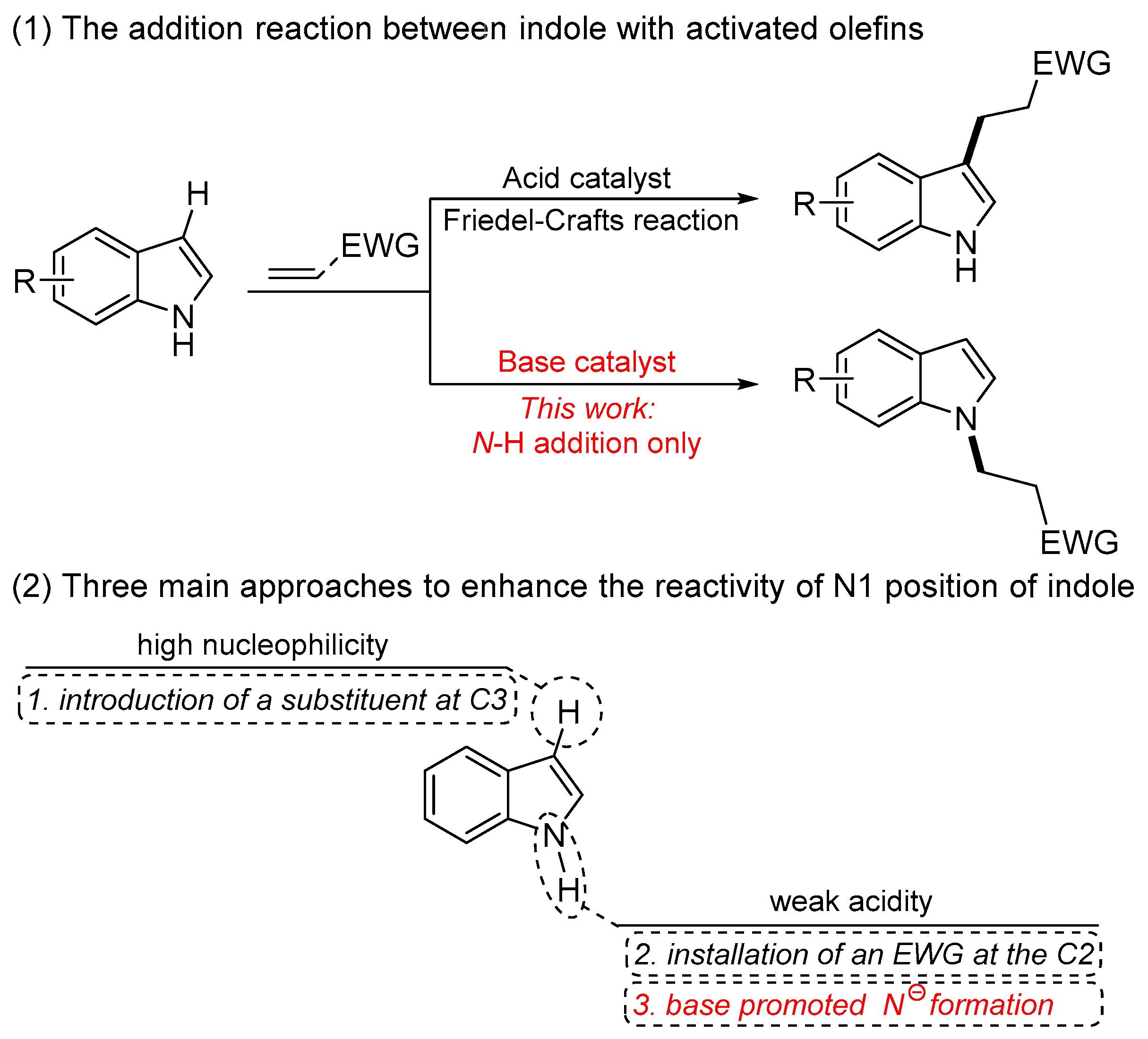
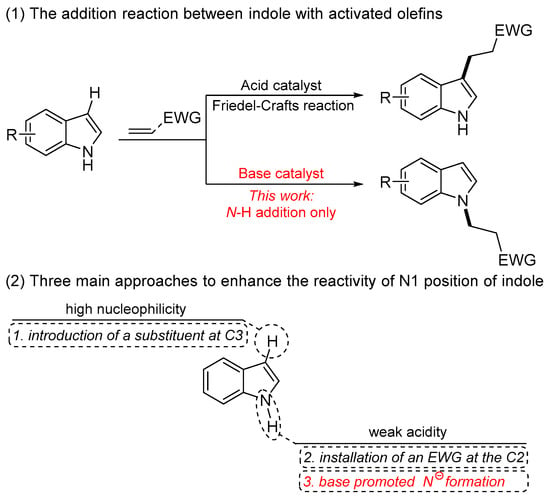
The indole moiety is a ubiquitous structural framework in natural products and widely recognized as privileged components in biologically, physiologically, and pharmacologically relevant compounds [1,2,3,4,5,6,7,8]. Therefore, an impressive number of practical techniques as well as methods have been developed for the synthesis of indole-containing compounds and related functionalization. Among them, N-functionalization of indoles is of particular importance for the synthesis of N-substituted indoles with bioactivity. The indoles normally serve as nucleophilic coupling partners to react with diverse electrophiles, such as activated olefins, ketones, imines, and alkynes [9,10,11,12,13]. Olefins, as a stable and atom-economic synthon, are one of the fundamental synthetic materials in organic chemistry and provide a desired alternative for alkylating agents. As a result, extensive efforts have been devoted to realizing the Friedel–Crafts reaction of indoles with electron-deficient olefins for the preparation of C3-alkylated indoles [14,15,16,17,18]; in contrast, N-alkylation of indoles with olefins is much more challenging, due to the higher nucleophilicity toward the C3 position of indoles compared to the N1 site (Scheme 1, Equation (1)). Typically, three main approaches have been developed to enhance the reactivity of the N1 position of indoles (Scheme 1, Equation (2)). One approach involves the installation of a substituent at the C3 position to absolutely overcome the competitive transformations and thus create more opportunities for the N-functionalization [19,20]. Another approach involving the introduction of an electron-withdrawing group at the C2 position can increase the acidity of the N-H bond to dramatically avoid the competitive transformations from the nucleophilicity of the C3 position [21,22,23,24,25]. On the other hand, the N1-H bond possessing weak acidity [26] enables the generation of nitrogen anions in the presence of a base, thus enhancing the nucleophilicity of N1. Indeed, such an elegant strategy is the most straightforward, economical, and convenient method to realize the N-functionalization of N-H indoles without complex transition-metal catalysts and organocatalysts.
Scheme 1.
The addition reaction between indole with activated olefins toward the formation of alkylated indoles and three main approaches to enhance the reactivity of N1 position of indole.
Vinylene carbonate has been regarded as a valuable and versatile C2 surrogate in organic synthesis [27,28,29,30,31]. In particular, vinylene carbonate has been extensively employed in the transition-metal-catalyzed C−H bond activation and annulation, resulting in the construction of value-added N-heterocyclic frameworks [32,33,34,35,36]. Vinylene carbonate is an inexpensive electron-deficient olefin; nevertheless, the nucleophilic additions between indoles with vinylene carbonate for the preparation of indolyl-containing ethylene carbonates have not been reported. Furthermore, indolyl-containing ethylene carbonates can undergo further transformation so as to obtain structurally diverse indolyl-containing skeletons [37,38,39,40,41].
In the course of our continuous research on the development of novel methods to achieve indole functionalization [42,43,44,45,46,47,48,49,50,51], we achieved the efficient synthesis of 4-indolyl-1,3-dioxolanones through the base-catalyzed nucleophilic addition reaction of N-H indoles with vinylene carbonate. The results are described in this paper.
2. Results
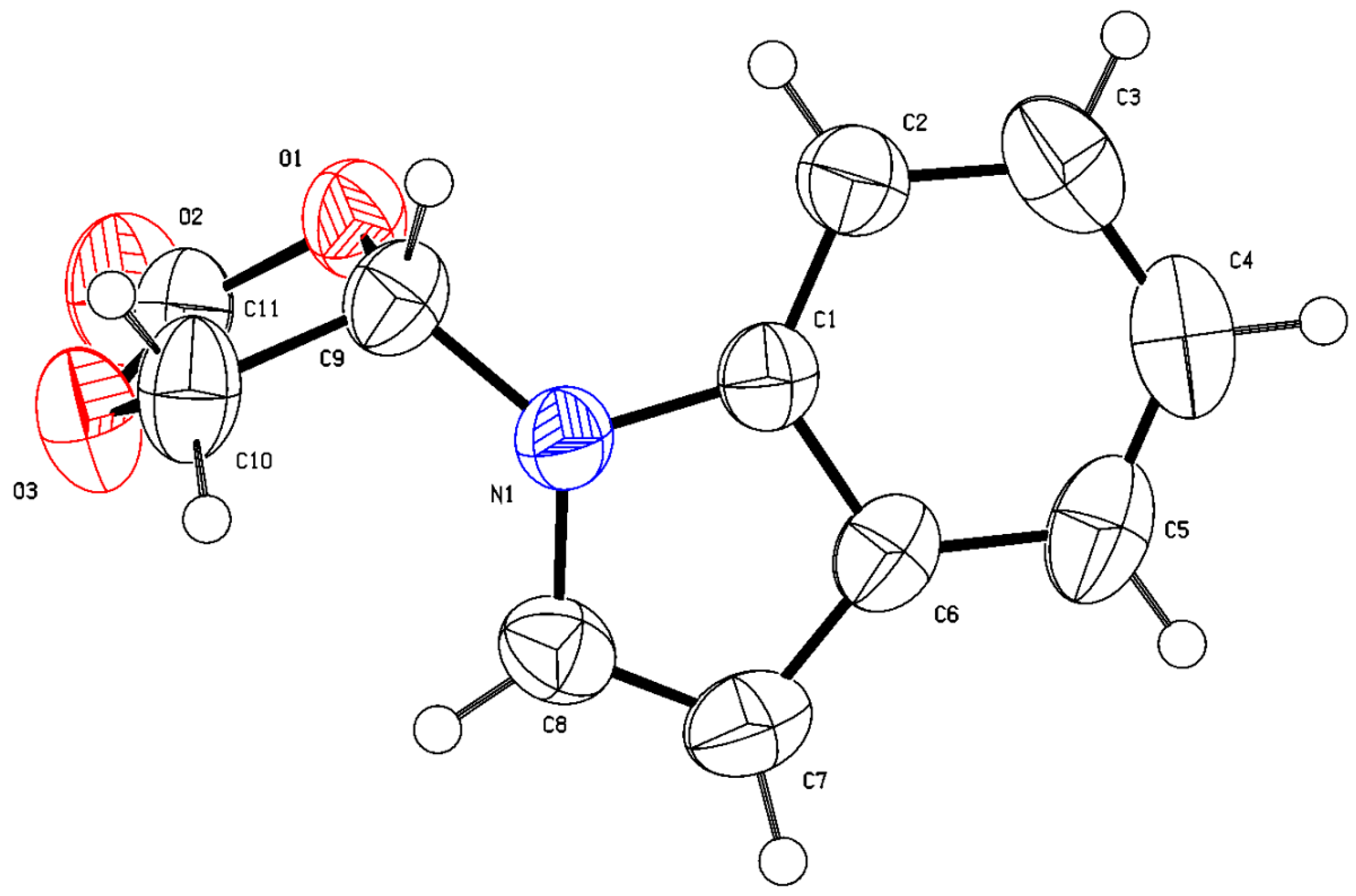
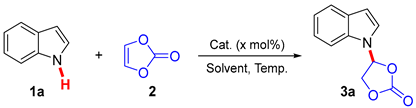
Initially, the nucleophilic addition reaction of indole (1a) with vinylene carbonate (2, 4 equiv.) was performed as a model to optimize the reaction parameters. The results are summarized in Table 1. The base catalyst was firstly screened using acetonitrile (CH3CN) as the solvent at 70 °C. Among the examined bases [triethylamine (NEt3), sodium bicarbonate (NaHCO3), sodium carbonate (Na2CO3), sodium acetate (NaOAc), potassium carbonate (K2CO3), sodium formate (HCOONa∙2H2O), DABCO (1,4-diaza[2.2.2]bicyclooctane), and DBU (1,8-diazabicyclo[5,4,0]-7-undecene)], K2CO3 proved to be the best base, providing the product, 4-(1H-indol-1-yl)-1,3-dioxolan-2-one (3a), with 71% yield (entries 1–8). The structure of product 3a was confirmed through X-ray crystallographic analysis (Figure 1, CCDC 2299714). A relatively high yield was observed when the loading of catalyst K2CO3 was increased to 30 mol% and 40 mol%, respectively (entries 9 and 10 vs. entry 5). However, the yield was found to be decreased when the base loading was further increased (entry 11 vs. entries 9 and 10). The reaction temperature was subsequently screened using 40 mol% of K2CO3 as the catalyst. The results obtained indicated that 60 °C was the best reaction temperature (entry 10 vs. entries 12–14). Then, the solvent was investigated under 60 °C conditions. Non-polar solvents [benzene and toluene] and polar solvents [CH3CN, tert-butyl methyl ether (MTBE), 1,2-ethanediol dimethyl ether (DME), tetrahydrofuran (THF), and 1,4-dioxane] were examined, respectively (entry 13, 15–20). Except for CH3CN, the use of other solvents showed almost no reaction, and most of the starting materials were recovered. This reaction had a strong dependence on solvent, whereas the exact factors involved remain unclear. Relatively low yields were obtained when either decreasing or increasing the amounts of vinylene carbonate (entry 13 vs. entries 21 and 22). Therefore, the subsequent nucleophilic addition reaction of various N-H indoles with vinylene carbonate (4.0 equiv.) was performed in the presence of K2CO3 (40 mol%) in CH3CN (3 mL) at 60 °C.

Table 1.
Optimization of reaction conditions a.
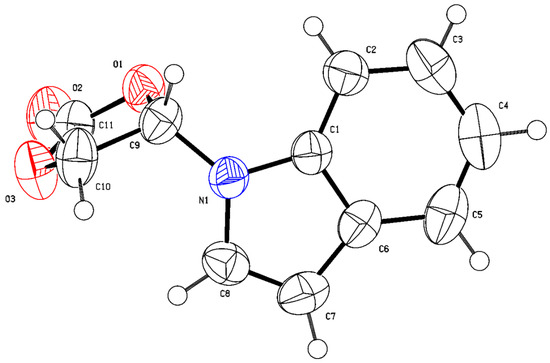
Figure 1.
The crystal structure of 3a.
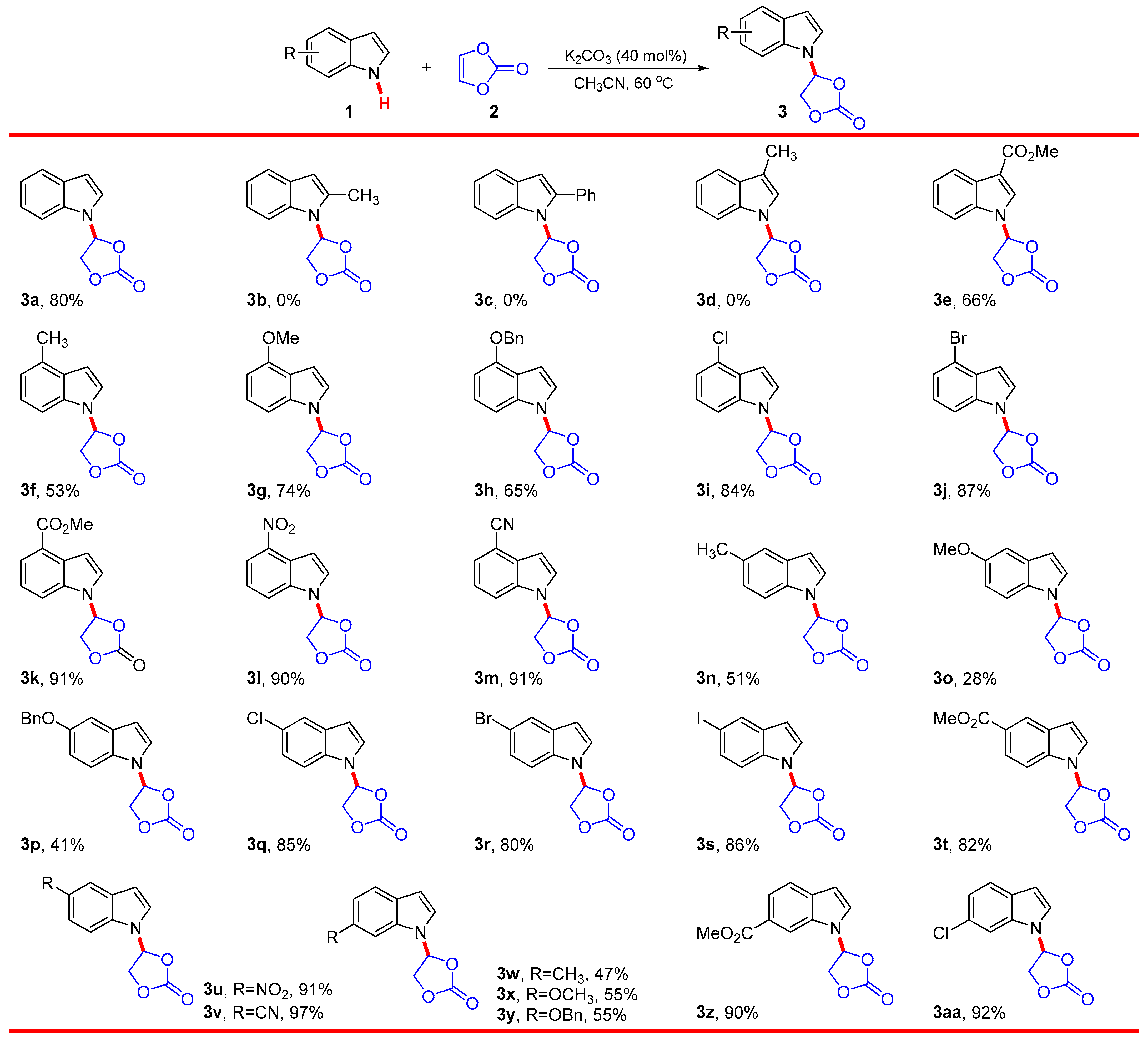
Given the optimized reaction conditions, the scope and limitation of this reaction were explored, and the results are summarized in Scheme 2. The electron-donating group, such as methyl (Me) and phenyl (Ph) linked on the pyrrole ring in substrate 1, would severely hamper the reaction, and no target products were detected. Nevertheless, the electron-withdrawing group, such as methoxycarbonyl (COOMe) linked on the pyrrole ring, was tolerated well, and the corresponding addition product 3e was obtained with 66% yield. The results suggested that the electron-donating group linked on the pyrrole ring could greatly weaken the acidity of the N–H bond, which was consistent with the formation of the indole nitrogen anion. In contrast, reactions of indole substrates 1 bearing the Me group linked on different positions of benzene ring proceeded smoothly under standard conditions; the corresponding 4-indolyl-1,3-dioxolanone products 3f, 3n, and 3w were obtained in moderate yields. Other electron-donating groups, such as methoxyl (OMe) and benzyloxyl (OBn), were also tolerated well in this nucleophilic addition reaction, providing the desired products 3g–3h, 3o–3p, and 3x–3y in 28–76% yields, respectively. Reactions of N-H indole substrates 1 bearing the electron-withdrawing group, such as COOMe, nitro (NO2), and cyano (CN), linked on different positions of the benzene ring were subsequently investigated under the optimized reaction conditions. The 4-indolyl-1,3-dioxolanone products 3k–3m, 3t–3v, and 3z were obtained in good to excellent yields (82–97%). These results mentioned above indicated that the electron properties (electron donating or electron withdrawing) of substituents linked on either pyrrole or benzene ring significantly affect the reactivity of 1. The reason for these results may be that the electron-withdrawing group, compared to electron-donating group, is more conducive to the formation and stabilization of indole nitrogen anion. The suitability of indole substrates 1i–1j, 1q–1s, and 1aa having halogen atoms (Cl, Br, and I) in the current nucleophilic addition reaction was investigated. The desired products 3i–3j, 3q–3s, and 3aa were also obtained in good to excellent yields (80–92%). The survival of synthetically useful functional groups, such as halogen atoms (Cl, Br, and I), CN, and CO2Me, under the reaction conditions will further increase structural diversification. In general, this nucleophilic addition exhibits broad scope and proceeds efficiently with electron-poor and -rich indoles, especially electron-poor indoles. However, the presence of groups at the C2-position of indole, with significant steric hindrance, would severely hamper the reaction.
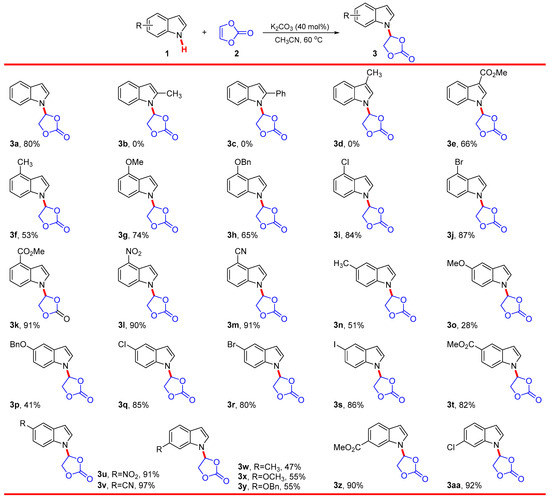
Scheme 2.
Substrate scope. Reaction conditions: 1 (0.50 mmol), 2 (172 mg, 2.0 mmol, 2.0 equiv.), and K2CO3 (27.6 mg, 0.20 mmol, 40 mol%) in CH3CN (3 mL) at 60 °C under an air atmosphere for 24 h; isolated yield.
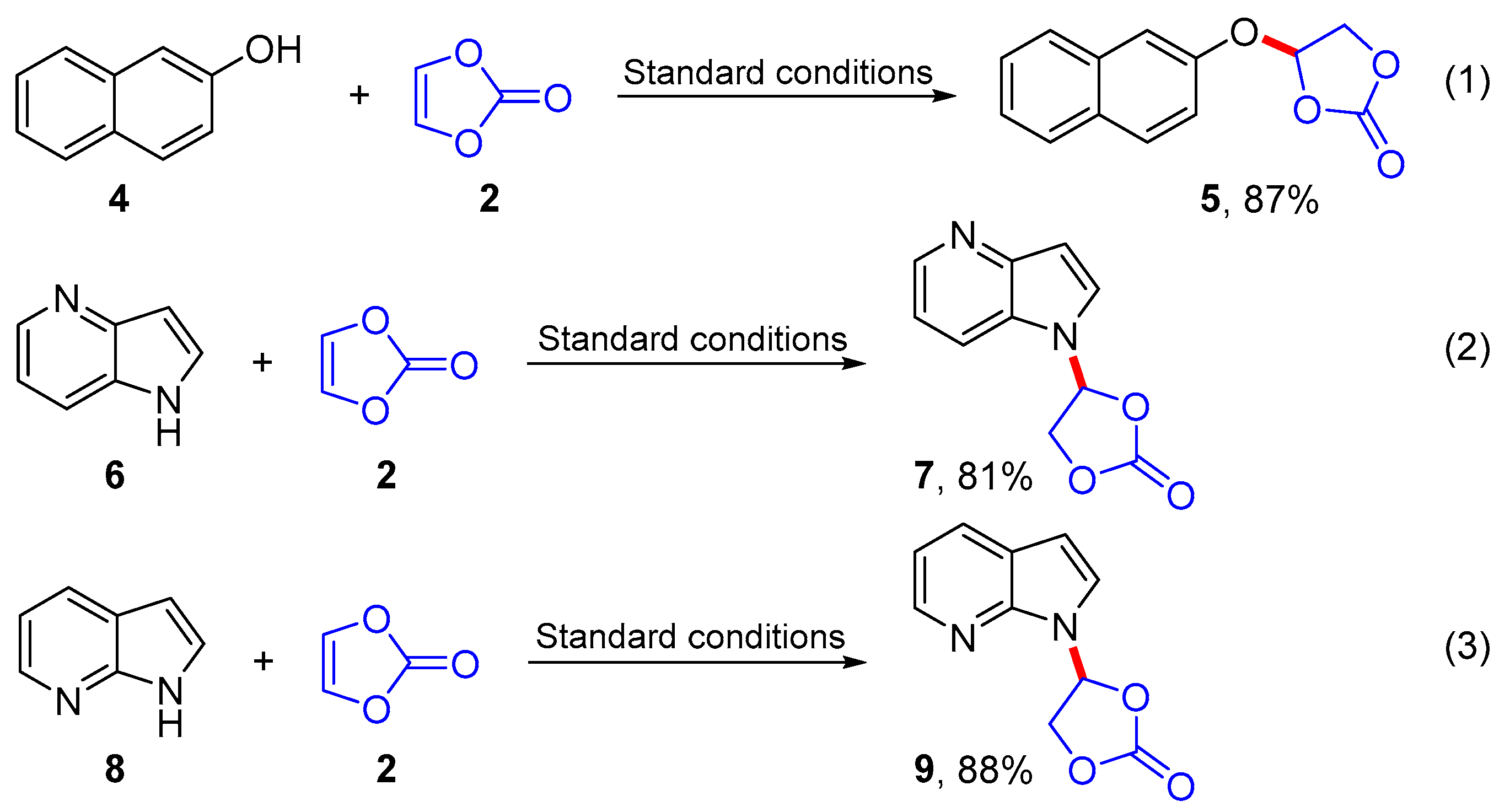
Continued substrate extension studies showed that the 2-naphthalenol (4) was also applicable to this transformation and provided the desired addition product, 4-(naphthalen-2-yloxy)-1,3-dioxolan-2-one (5), in 87% yield (Scheme 3, Equation (1)). Subsequently, instead of the benzene ring in substrate 1 studies addressed a pyridine ring and found that the reactions of 1H-pyrrolo[3,2-b]pyridine (6) and 1H-pyrrolo[2,3-b]pyridine (8) also proceeded smoothly to furnish the addition products 7 and 9 in 81% and 88% yields, respectively (Scheme 3, Equations (2) and (3)).
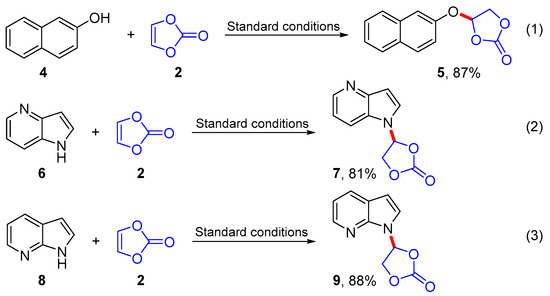
Scheme 3.
Substrate extension studies.
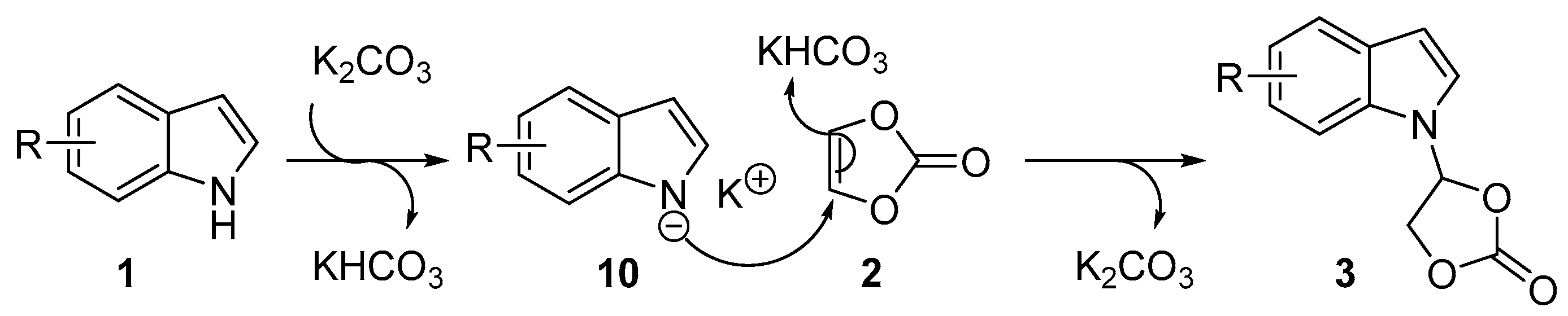
Based on the experimental results and previous reports [48,49,52], a plausible reaction mechanism for the synthesis of N-functionalized indoles is depicted in Scheme 4. Initially, indole nitrogen anion 10 was generated in situ through the deprotonation of indole in the presence of the base K2CO3. The indole nitrogen anion 10 then underwent nucleophilic addition to vinylene carbonate. Meanwhile, KHCO3 provided a proton to yield the addition product 3 and the regenerated base K2CO3.
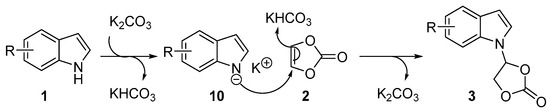
Scheme 4.
The plausible reaction mechanism.
3. Materials and Methods
3.1. General Information
Unless otherwise noted, all reactions were carried out in oven-dried 25-mL Schlenk tubes under a nitrogen atmosphere. An IKA plate was used as the heat source. All reagents and solvents were of pure analytical grade. Thin layer chromatography (TLC) was performed on HSGF254 silica gel, pre-coated on glass-backed plates coated with 0.2 mm silica and was revealed with either a UV lamp (λmax = 254 nm). The products were purified by using flash column chromatography on silica gel 200–300 mesh. 1H and 13C NMR spectra were recorded on a 400 MHz spectrometer (1H 400 MHz, 13C 101 MHz) using d6-DMSO or CDCl3 as the solvent with tetramethylsilane (TMS) as the internal standard at room temperature. The chemical shifts are reported in ppm downfield (δ) from TMS, and the coupling constants J are given in Hz. The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. The NMR spectra of all compounds are demonstrated in Supplementary Materials. High-resolution mass spectra were recorded on either Q-TOF mass spectrometry or LTQ Orbitrap XL mass spectrometry. X-ray crystallography analysis was performed on a Bruker D8 Quest X-ray diffractionmeter.
3.2. Synthetic Procedures
3.2.1. The Typical Procedure for the Synthesis of 4-Indolyl-1,3-dioxolanones 3
A mixture of indoles 1 (0.50 mmol), vinylene carbonate (172 mg, 2.0 mmol, 4.0 equiv), and K2CO3 (27.6 mg, 0.20 mmol, 40 mol%) in CH3CN (3 mL) was added into a Schlenk flask (25 mL) and stirred at 60 °C. After the reaction was finished, the solvent was evaporated under reduced pressure and the residue was purified by using column chromatography (petroleum ether/ethyl acetate 5:1 to 1:1) to provide the product 3.
- 4-(1H-indol-1-yl)-1,3-dioxolan-2-one (3a): Yield: 80%, 80.9 mg, white solid, mp 132–134 °C, Rf = 0.41 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.71 (d, J = 3.4 Hz, 1H), 7.64 (d, J = 7.8 Hz, 1H), 7.59 (d, J = 8.2 Hz, 1H), 7.28 (t, J = 7.7 Hz, 1H), 7.23 (t, J = 6.3 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 6.69 (d, J = 3.3 Hz, 1H), 5.04 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 154.1, 136.0, 129.5, 126.2, 123.2, 121.6, 121.6, 110.4, 105.6, 82.3, 68.4. [M + H]+ calculated for C11H10NO3, 204.0661; found 204.0657.
- methyl 1-(2-oxo-1,3-dioxolan-4-yl)-1H-indole-3-carboxylate (3e): Yield: 66%, 86.6 mg, white solid, mp 206–208 °C, Rf = 0.32 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 8.53 (s, 1H), 8.10 (d, J = 7.8 Hz, 1H), 7.63 (d, J = 8.1 Hz, 1H), 7.42–7.33 (m, 2H), 7.28–7.21 (m, 1H), 5.17–4.99 (m, 2H), 3.86 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 164.5, 153.8, 136.0, 133.5, 126.9, 124.4, 123.5, 121.7, 111.2, 109.5, 82.4, 68.3, 51.6. HRMS (ESI) m/z: [M + H]+ calculated for C13H12NO5, 262.0715; found 262.0714.
- 4-(4-methyl-1H-indol-1-yl)-1,3-dioxolan-2-one (3f): Yield: 53%, 57.7 mg, white solid, mp 118–120 °C, Rf = 0.36 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.68 (d, J = 3.4 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.23–7.14 (m, 2H), 6.98 (d, J = 7.2 Hz, 1H), 6.72 (d, J = 3.3 Hz, 1H), 5.03 (d, J = 6.4 Hz, 2H), 2.49 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 154.1, 135.7, 130.6, 129.4, 125.6, 123.3, 121.7, 107.9, 104.1, 82.5, 68.4, 18.7. HRMS (ESI) m/z: [M + H]+ calculated for C12H12NO3, 218.0817; found 218.0815.
- 4-(4-methoxy-1H-indol-1-yl)-1,3-dioxolan-2-one (3g): Yield: 74%, 86.0 mg, white solid, mp 172–174 °C, Rf = 0.33 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.59 (d, J = 3.4 Hz, 1H), 7.23–7.15 (m, 3H), 6.69 (d, J = 7.6 Hz, 1H), 6.66 (d, J = 3.2 Hz, 1H), 5.01 (d, J = 6.4 Hz, 2H), 3.89 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 154.0, 153.4, 137.3, 124.7, 124.4, 119.7, 103.5, 102.6, 101.9, 82.5, 68.4, 55.6. HRMS (ESI) m/z: [M + H]+ calculated for C12H12NO4, 234.0766; found 234.0765.
- 4-(4-(benzyloxy)-1H-indol-1-yl)-1,3-dioxolan-2-one (3h): Yield: 65%, 100.3 mg, white solid, mp 146–148 °C, Rf = 0.32 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.61 (d, J = 3.4 Hz, 1H), 7.51 (d, J = 7.6 Hz, 2H), 7.41 (t, J = 7.4 Hz, 2H), 7.34 (t, J = 7.1 Hz, 1H), 7.21–7.14 (m, 3H), 6.78 (d, J = 6.5 Hz, 1H), 6.71 (d, J = 3.3 Hz, 1H), 5.26 (s, 2H), 5.02 (d, J = 6.4 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 154.0, 152.4, 137.8, 137.4, 128.9, 128.2, 127.9, 124.9, 124.3, 120.1, 103.7, 103.4, 102.6, 82.5, 69.6, 68.4. HRMS (ESI) m/z: [M + H]+ calculated for C18H16NO4, 310.1079; found 310.1075.
- 4-(4-chloro-1H-indol-1-yl)-1,3-dioxolan-2-one (3i): Yield: 84%, 100.0 mg, white solid, mp 130–132 °C, Rf = 0.48 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.86 (d, J = 3.5 Hz, 1H), 7.59 (d, J = 7.6 Hz, 1H), 7.33–7.22 (m, 3H), 6.73 (d, J = 3.3 Hz, 1H), 5.05 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.9, 136.8, 127.8, 127.5, 125.5, 124.2, 121.2, 109.7, 103.4, 82.3, 68.5. HRMS (ESI) m/z: [M + H]+ calculated for C11H9ClNO3, 238.0271; found 238.0266.
- 4-(4-bromo-1H-indol-1-yl)-1,3-dioxolan-2-one (3j): Yield: 87%, 123.0 mg, white solid, mp 154–156 °C, Rf = 0.36 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.87 (d, J = 3.4 Hz, 1H), 7.64 (d, J = 8.3 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 7.23 (t, J = 7.3 Hz, 2H), 6.65 (d, J = 3.4 Hz, 1H), 5.05 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.9, 136.4, 133.2, 129.7, 127.5, 124.5, 124.3, 114.5, 110.2, 105.1, 82.3, 68.5. HRMS (ESI) m/z: [M + H]+ calculated for C11H9BrNO3, 281.9766; found 281.9758.
- methyl 1-(2-oxo-1,3-dioxolan-4-yl)-1H-indole-4-carboxylate (3k): Yield: 91%, 119.0 mg, white solid, mp 146–148 °C, Rf = 0.39 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.92 (d, J = 7.5 Hz, 2H), 7.88 (d, J = 7.5 Hz, 1H), 7.41 (t, J = 7.9 Hz, 1H), 7.30 (t, J = 6.2 Hz, 1H), 7.19 (d, J = 3.3 Hz, 1H), 5.07 (dd, J = 8.6, 3.5 Hz, 2H), 3.92 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 167.1, 153.9, 136.9, 128.8, 128.3, 124.5, 122.7, 121.8, 115.6, 106.2, 82.0, 68.5, 52.4. HRMS (ESI) m/z: [M + H]+ calculated for C13H12NO5, 262.0715; found 262.0708.
- 4-(4-nitro-1H-indol-1-yl)-1,3-dioxolan-2-one (3l): Yield: 90%, 111.7 mg, light yellow solid, mp 188–190 °C, Rf = 0.30 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 8.20 (d, J = 8.0 Hz, 1H), 8.15 (d, J = 8.5 Hz, 2H), 7.53 (t, J = 8.1 Hz, 1H), 7.36 (t, J = 6.2 Hz, 1H), 7.26 (d, J = 3.3 Hz, 1H), 5.13–5.04 (m, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.8, 140.2, 138.2, 131.0, 123.1, 122.8, 119.0, 118.1, 104.8, 81.9, 68.67. HRMS (ESI) m/z: [M + H]+ calculated for C11H9N2O5, 249.0511; found 249.0510.
- 1-(2-oxo-1,3-dioxolan-4-yl)-1H-indole-4-carbonitrile (3m): Yield: 91%, 112.2 mg, white solid, mp 166–168 °C, Rf = 0.35 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 8.05 (d, J = 3.4 Hz, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.72 (d, J = 7.4 Hz, 1H), 7.46 (t, J = 7.9 Hz, 1H), 7.30 (t, J = 6.3 Hz, 1H), 6.85 (d, J = 3.3 Hz, 1H), 5.06 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.8, 135.9, 130.4, 129.6, 126.9, 123.4, 118.4, 116.0, 103.5, 102.8, 82.0, 68.6. HRMS (ESI) m/z: [M + H]+ calculated for C12H9N2O3, 229.0613; found 229.0612.
- 4-(5-methyl-1H-indol-1-yl)-1,3-dioxolan-2-one (3n): Yield: 51%, 55.6 mg, light yellow solid, mp 112–114 °C, Rf = 0.36 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.64 (d, J = 3.4 Hz, 1H), 7.47–7.40 (m, 2H), 7.18 (t, J = 6.3 Hz, 1H), 7.10 (d, J = 8.4 Hz, 1H), 6.58 (d, J = 3.3 Hz, 1H), 5.02 (d, J = 6.3 Hz, 2H), 2.39 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 154.1, 134.3, 133.2, 130.3, 129.9, 126.3, 124.6, 121.2, 110.1, 105.1, 82.5, 68.3, 21.4. HRMS (ESI) m/z: [M + H]+ calculated for C12H12NO3, 218.0817; found 218.0810.
- 4-(5-methoxy-1H-indol-1-yl)-1,3-dioxolan-2-one (3o): Yield: 28%, 33.0 mg, white solid, mp 141–143 °C, Rf = 0.32 (H/E = 2:1). 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 8.6 Hz, 1H), 7.21 (d, J = 3.4 Hz, 1H), 7.15 (d, J = 1.9 Hz, 1H), 7.00 (dd, J = 8.9, 2.0 Hz, 1H), 6.73 (dd, J = 7.2, 5.1 Hz, 1H), 6.64 (d, J = 3.3 Hz, 1H), 4.98-4.85 (m, 2H), 3.91 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 155.5, 153.3, 130.5, 130.2, 124.9, 113.4, 110.1, 106.2, 103.7, 82.2, 67.9, 55.8. HRMS (ESI) m/z: [M + H]+ calculated for C12H12NO4, 234.0766; found 234.0758.
- 4-(5-(benzyloxy)-1H-indol-1-yl)-1,3-dioxolan-2-one (3p): Yield: 41%, 63.3 mg, white solid, mp 146–148 °C, Rf = 0.32 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.66 (d, J = 3.2 Hz, 1H), 7.47 (d, J = 8.1 Hz, 3H), 7.40 (t, J = 7.4 Hz, 2H), 7.33 (d, J = 6.9 Hz, 1H), 7.23 (s, 1H), 7.16 (t, J = 6.2 Hz, 1H), 7.00 (d, J = 8.9 Hz, 1H), 6.59 (d, J = 3.2 Hz, 1H), 5.13 (s, 2H), 5.01 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 154.10, 154.08, 137.9, 131.0, 130.2, 128.9, 128.2, 128.1, 126.9, 113.5, 111.1, 105.4, 105.0, 82.6, 70.1, 68.3. HRMS (ESI) m/z: [M + H]+ calculated for C18H16NO4, 310.1079; found 310.1078.
- 4-(5-chloro-1H-indol-1-yl)-1,3-dioxolan-2-one (3q): Yield: 85%, 100.4 mg, white solid, mp 127–129 °C, Rf = 0.37 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.81–7.79 (m, 1H), 7.70 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.30 (d, J = 8.7 Hz, 1H), 7.22 (t, J = 6.3 Hz, 1H), 6.68 (d, J = 3.4 Hz, 1H), 5.04 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 154.0, 134.6, 130.7, 127.8, 126.1, 123.1, 120.8, 112.0, 105.2, 82.2, 68.5. HRMS (ESI) m/z: [M + H]+ calculated for C11H9ClNO3, 238.0271; found 238.0265.
- 4-(5-bromo-1H-indol-1-yl)-1,3-dioxolan-2-one (3r): Yield: 80%, 112.5 mg, white solid, mp 108–110 °C, Rf = 0.37 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.85 (s, 1H), 7.79 (d, J = 3.3 Hz, 1H), 7.58 (d, J = 8.7 Hz, 1H), 7.42 (d, J = 8.6 Hz, 1H), 7.22 (t, J = 6.3 Hz, 1H), 6.68 (d, J = 3.2 Hz, 1H), 5.03 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.9, 134.9, 131.4, 127.7, 125.7, 123.8, 114.1, 112.5, 105.1, 82.2, 68.4. HRMS (ESI) m/z: [M + H]+ calculated for C11H9BrNO3, 281.9766; found 281.9757.
- 4-(5-iodo-1H-indol-1-yl)-1,3-dioxolan-2-one (3s): Yield: 86%, 141.6 mg, white solid, mp 134–136 °C, Rf = 0.36 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 8.02 (s, 1H), 7.73 (d, J = 3.3 Hz, 1H), 7.55 (d, J = 8.6 Hz, 1H), 7.45 (d, J = 8.7 Hz, 1H), 7.20 (t, J = 6.3 Hz, 1H), 6.65 (d, J = 3.2 Hz, 1H), 5.02 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.9, 135.3, 132.1, 131.1, 130.0, 127.2, 112.9, 104.8, 85.6, 82.1, 68.4. HRMS (ESI) m/z: [M + H]+ calculated for C11H9INO3, 329.9627; found 329.9619.
- methyl 1-(2-oxo-1,3-dioxolan-4-yl)-1H-indole-5-carboxylate (3t): Yield: 82%, 107.2 mg, white solid, mp 164–166 °C, Rf = 0.34 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 8.33 (s, 1H), 7.90 (d, J = 8.7 Hz, 1H), 7.86 (d, J = 3.3 Hz, 1H), 7.71 (d, J = 8.7 Hz, 1H), 7.28 (t, J = 6.3 Hz, 1H), 6.85 (d, J = 3.3 Hz, 1H), 5.05 (d, J = 6.2 Hz, 2H), 3.87 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 167.2, 153.9, 138.7, 129.2, 127.7, 124.0, 123.8, 123.1, 110.6, 106.7, 82.1, 68.6, 52.4. HRMS (ESI) m/z: [M + H]+ calculated for C13H12NO5, 262.0715; found 262.0708.
- 4-(5-nitro-1H-indol-1-yl)-1,3-dioxolan-2-one (3u): Yield: 91%, 112.5 mg, light yellow solid, mp 188–190 °C, Rf = 0.40 (H/E = 1:1). 1H NMR (400 MHz, d6-DMSO) δ 8.64 (s, 1H), 8.18 (d, J = 9.1 Hz, 1H), 8.00 (d, J = 3.4 Hz, 1H), 7.83 (d, J = 9.1 Hz, 1H), 7.32 (t, J = 6.3 Hz, 1H), 6.97 (d, J = 3.4 Hz, 1H), 5.12–4.99 (m, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.8, 142.6, 139.2, 129.5, 128.9, 118.4, 118.3, 111.2, 107.6, 81.9, 68.7. HRMS (ESI) m/z: [M + H]+ calculated for C11H9N2O5, 249.0511; found 249.0503.
- 1-(2-oxo-1,3-dioxolan-4-yl)-1H-indole-5-carbonitrile (3v): Yield: 97%, 111.0 mg, white solid, mp 186–188 °C, Rf = 0.40 (H/E = 1:1). 1H NMR (400 MHz, d6-DMSO) δ 8.19 (s, 1H), 7.95 (d, J = 3.4 Hz, 1H), 7.81 (d, J = 8.6 Hz, 1H), 7.67 (d, J = 8.6 Hz, 1H), 7.30 (t, J = 6.2 Hz, 1H), 6.83 (d, J = 3.3 Hz, 1H), 5.05 (d, J = 5.8 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.8, 137.9, 129.3, 128.6, 127.0, 126.1, 120.5, 111.9, 106.2, 103.9, 81.9, 68.6. HRMS (ESI) m/z: [M + H]+ calculated for C12H9N2O3, 229.0613; found 229.0607.
- 4-(6-methyl-1H-indol-1-yl)-1,3-dioxolan-2-one (3w): Yield: 47%, 51.2 mg, white solid, mp 130–132 °C, Rf = 0.36 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.61 (d, J = 3.4 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.38 (s, 1H), 7.18 (t, J = 6.3 Hz, 1H), 7.01 (d, J = 8.1 Hz, 1H), 6.61 (d, J = 3.2 Hz, 1H), 5.02 (d, J = 6.3 Hz, 2H), 2.44 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 154.1, 133.2, 132.5, 127.3, 125.4, 123.2, 121.2, 110.3, 105.5, 82.3, 68.3, 22.0. [M + H]+ calcd for C12H12NO3, 218.0817; found 218.0810.
- 4-(6-methoxy-1H-indol-1-yl)-1,3-dioxolan-2-one (3x): Yield: 55%, 63.9 mg, white solid, mp 152–154 °C, Rf = 0.34 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.54 (d, J = 3.5 Hz, 1H), 7.49 (d, J = 8.6 Hz, 1H), 7.23 (t, J = 6.3 Hz, 1H), 7.19 (s, 1H), 6.82 (d, J = 8.6 Hz, 1H), 6.60 (d, J = 3.3 Hz, 1H), 5.02 (d, J = 6.3 Hz, 2H), 3.81 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 157.0, 154.1, 137.2, 124.4, 123.2, 122.0, 111.2, 105.7, 94.4, 82.1, 68.4, 55.9. HRMS (ESI) m/z: [M + H]+ calculated for C12H12NO4, 234.0766; found 234.0765.
- 4-(6-(benzyloxy)-1H-indol-1-yl)-1,3-dioxolan-2-one (3y): Yield: 55%, 85.0 mg, white solid, mp 139–141 °C, Rf = 0.36 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.55 (d, J = 3.4 Hz, 1H), 7.52–7.48 (m, 3H), 7.41 (t, J = 7.4 Hz, 2H), 7.37–7.31 (m, 2H), 7.21 (t, J = 6.3 Hz, 1H), 6.90 (d, J = 8.7 Hz, 1H), 6.60 (d, J = 3.3 Hz, 1H), 5.19-5.12 (m, 2H), 5.02 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 156.0, 154.1, 137.6, 137.2, 133.2, 128.9, 128.3, 128.2, 124.6, 123.5, 122.1, 111.7, 105.7, 95.8, 82.1, 70.2, 68.3. HRMS (ESI) m/z: [M + H]+ calculated for C18H16NO4, 310.1079; found 310.1071.
- methyl 1-(2-oxo-1,3-dioxolan-4-yl)-1H-indole-6-carboxylate (3z): Yield: 90%, 116.9 mg, white solid, mp 140–142 °C, Rf = 0.37 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 8.28 (s, 1H), 7.98 (d, J = 3.3 Hz, 1H), 7.80-7.73 (m, 2H), 7.38 (t, J = 6.3 Hz, 1H), 6.80 (d, J = 3.3 Hz, 1H), 5.16–4.92 (m, 2H), 3.89 (s, 3H). 13C NMR (101 MHz, d6-DMSO) δ 167.3, 153.9, 135.6, 133.2, 129.7, 124.3, 122.2, 121.5, 112.2, 105.87, 82.0, 68.5, 52.5. HRMS (ESI) m/z: [M + H]+ calculated for C13H12NO5, 262.0715; found 262.0712.
- 4-(6-chloro-1H-indol-1-yl)-1,3-dioxolan-2-one (3aa): Yield: 92%, 109.0 mg, white solid, mp 152–154 °C, Rf = 0.38 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.76 (s, 2H), 7.64 (d, J = 8.4 Hz, 1H), 7.30–7.17 (m, 2H), 6.72 (d, J = 3.3 Hz, 1H), 5.03 (d, J = 6.0 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.9, 136.7, 133.2, 128.1, 128.0, 126.9, 122.9, 121.9, 110.6, 105.9, 82.0, 68.5. HRMS (ESI) m/z: [M + H]+ calculated for C11H9ClNO3, 238.0271; found 238.0264.
3.2.2. The Typical Procedure for the Synthesis of 5
To an oven-dried 25 mL Schlenk tube equipped with a magnetic stir bar was added 2-naphthol substrate 4 (0.50 mmol, 1.0 equiv.), vinylene carbonate (172 mg, 2.0 mmol, 4.0 equiv.), K2CO3 (27.6 mg, 0.20 mmol, 40 mol%), and CH3CN (3 mL) under an air atmosphere. The reaction mixture was stirred at 60 °C for 24 h and then cooled to room temperature. The solvent was removed under reduced pressure and the crude product was purified by using silica gel column chromatography to afford the desired products 5 as a white solid (100.0 mg, yield: 87%).
- 4-(naphthalen-2-yloxy)-1,3-dioxolan-2-one (5): Yield: 87%, 100.0 mg, white solid, mp 106–108 °C, Rf = 0.45 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 7.96 (d, J = 8.9 Hz, 1H), 7.92 (d, J = 8.2 Hz, 2H), 7.59 (s, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.46 (t, J = 7.5 Hz, 1H), 7.32 (dd, J = 8.9, 2.3 Hz, 1H), 6.71 (d, J = 3.9 Hz, 1H), 4.88 (dd, J = 9.9, 5.5 Hz, 1H), 4.66 (d, J = 10.0 Hz, 1H). 13C NMR (101 MHz, d6-DMSO) δ 154.1, 153.3, 134.1, 130.5, 130.2, 128.1, 127.7, 127.3, 125.4, 118.9, 111.3, 98.1, 70.9. HRMS (ESI) m/z: [M + H]+ calculated for C13H11O4, 231.0657; found 231.0654.
3.2.3. The Typical Procedure for the Synthesis of 7 and 9
To an oven-dried 25 mL Schlenk tube equipped with a magnetic stir bar was added pyrrolo-pyridine substrate 6 or 8 (0.50 mmol, 1.0 equiv.), vinylene carbonate (172 mg, 2.0 mmol, 4.0 equiv.), K2CO3 (27.6 mg, 0.20 mmol, 40 mol%), and CH3CN (3 mL) under an air atmosphere. The reaction mixture was stirred at 60 °C for 24 h, and then cooled to room temperature. The solvent was removed under reduced pressure and the crude product was purified by using silica gel column chromatography to afford the desired products 7 or 9.
- 4-(1H-pyrrolo [3,2-b]pyridin-1-yl)-1,3-dioxolan-2-one (7): Yield: 81%, 82.2 mg, white solid, mp 176–178 °C, Rf = 0.31 (EtOAc). 1H NMR (400 MHz, d6-DMSO) δ 8.47 (d, J = 4.7 Hz, 1H), 8.05 (d, J = 3.5 Hz, 1H), 8.00 (d, J = 8.3 Hz, 1H), 7.29 (dd, J = 8.3, 4.6 Hz, 1H), 7.23 (t, J = 6.3 Hz, 1H), 6.81 (d, J = 3.4 Hz, 1H), 5.05 (d, J = 6.2 Hz, 2H). 13C NMR (101 MHz, d6-DMSO) δ 153.9, 147.6, 144.8, 129.8, 128.9, 118.0, 117.9, 106.1, 82.3, 68.4. HRMS (ESI) m/z: [M + H]+ calculated for C10H9N2O3, 205.0613; found 205.0613.
- 4-(1H-pyrrolo [2,3-b]pyridin-1-yl)-1,3-dioxolan-2-one (9): Yield: 88%, 89.3 mg, white solid, mp 169-171 °C, Rf = 0.37 (H/E = 2:1). 1H NMR (400 MHz, d6-DMSO) δ 8.33 (d, J = 4.7 Hz, 1H), 8.07 (d, J = 7.8 Hz, 1H), 7.82 (d, J = 3.7 Hz, 1H), 7.28-7.12 (m, 2H), 6.66 (d, J = 3.7 Hz, 1H), 5.08-4.98 (m, 2H). 13C NMR (101 MHz, d6-DMSO) δ 154.3, 147.5, 143.8, 130.0, 128.0, 121.9, 118.0, 102.9, 81.6, 68.4. HRMS (ESI) m/z: [M + H]+ calculated for C10H9N2O3, 205.0613; found 205.0608.
3.3. X-ray Crystallographic Analysis
The structure of 3a was determined based on single-crystal X-ray analysis. The detailed procedure was as follows: The 3a solid was dissolved in AcOEt (1 mL). Then, the solvent was placed in the inner tube, and n-hexane (5 mL) was placed in the outer container. The crystals of 3a were grown from solution, which is suitable for X-ray diffraction analysis.
CCDC No. 2299714 (3a) contains the supplementary crystallographic data for this paper. The crystal data can be obtained free of charge from the Cambridge Crystallographic Data Centre through www.ccdc.cam.ac.uk/datarequest/cif (accessed on 7 October 2023).
4. Conclusions
In summary, employing a base-catalyzed transition-metal-free strategy, we have successfully developed a convenient and alternative method to achieve N-functionalization of indoles with high regioselectivity. The nucleophilic addition reaction of various N-H indoles with vinylene carbonate proceeded smoothly to produce 4-indolyl-1,3-dioxolanones in satisfactory to excellent yields (up to >97% yield). These 4-Indolyl-1,3-dioxolanones, as novel indolyl-containing skeletons, are a type of indolyl-containing ethylene carbonate which could undergo further transformation to obtain structurally diverse bioactive molecules containing indole moiety. The readily available starting materials, the mild reaction conditions, and the experimental simplicity make the present methodology highly useful in the synthesis of 4-indolyl-1,3-dioxolanones. Based on the universality and significance of indole moiety in drugs, functional materials, and bioactive molecules, we believe that both this methodology and the synthesized indolyl-containing skeletons could be of interest to organic chemists and provide many medicinally active scaffolds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28217450/s1: Crystallographic data and copies of NMR spectra.
Author Contributions
Conceptualization, X.-Y.Z.; methodology, X.C.; investigation, X.C., X.-Y.Z. and M.B.; writing—original draft preparation, X.C.; writing—review and editing, X.-Y.Z. and M.B.; supervision, X.-Y.Z. and M.B.; project administration, X.-Y.Z. and M.B.; funding acquisition, X.-Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 22062012, 22262019 and 22172014), and the Natural Science Foundation of Guizhou Province (No. qiankehejichu-ZK [2023]zhongdian048) is also thanked for its financial support. This work was also supported by the Foundation of Guizhou Educational Committee (No. qianjiaoji [2023]088) and Scientific Research Projects of Liupanshui Normal University (LPSSYZDZK202201).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data obtained in this project are contained within this article and are available upon request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dadashpour, S.; Emami, S. Indole in the target-based design of anticancer agents: A versatile scaffold with diverse mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-H.; Shi, F. Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 2022, 55, 2562–2580. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The importance of indole and azaindole scaffold in the development ofantitumor agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Singh, O.M. Recent Progress in Biological Activities of Indole and Indole Alkaloids. Mini-Rev. Med. Chem. 2018, 18, 9–25. [Google Scholar] [CrossRef]
- Goel, B.; Jaiswal, S.; Jain, S.K. Indole derivatives targeting colchicine bindingsite as potential anticancer agents. Arch. Pharm. 2023, 356, e2300210. [Google Scholar] [CrossRef]
- Chadha, N.; Silakari, O. Indoles as therapeutics of interest in medicinal chemistry: Bird’s eye view. Eur. J. Med. Chem. 2017, 134, 159–184. [Google Scholar] [CrossRef]
- Neto, J.S.S.; Zeni, G. Recent advances in the synthesis of indoles from alkynes and nitrogen sources. Org. Chem. Front. 2020, 7, 155–210. [Google Scholar] [CrossRef]
- Ye, Z.-S.; Li, J.-C.; Wang, G. Transition-Metal-Catalyzed Enantioselective Synthesis of Indoles from 2-Alkynylanilines. Synthesis 2022, 54, 2133–2147. [Google Scholar] [CrossRef]
- Bandini, M.; Eichholzer, A. Catalytic Functionalization of Indoles in a New Dimension. Angew. Chem. Int. Ed. 2009, 48, 9608–9644. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Y.; Qian, B.; Yang, L.; Xia, C.; Huang, H. Enantioselective N–H Functionalization of Indoles with α,β-Unsaturated γ-Lactams Catalyzed by Chiral Brønsted Acids. Angew. Chem. Int. Ed. 2011, 50, 5682–5686. [Google Scholar] [CrossRef]
- Chen, J.B.; Jia, Y.X. Recent Progress in Transition-Metal-Catalyzed Enantioselective Indole Functionalizations. Org. Biomol. Chem. 2017, 15, 3550–3567. [Google Scholar] [CrossRef]
- Cruz, F.A.; Zhu, Y.; Tercenio, Q.D.; Shen, Z.; Dong, V.M. Alkyne Hydroheteroarylation: Enantioselective Coupling of Indoles and Alkynes via Rh-Hydride Catalysis. J. Am. Chem. Soc. 2017, 139, 10641–10644. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Kim, S.-T.; Jeong, J.; Baik, M.-H.; Buchwald, S.L. CuH-Catalyzed Enantioselective Alkylation of Indole Derivatives with Ligand-Controlled Regiodivergence. J. Am. Chem. Soc. 2019, 141, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Fandrick, K.R.; Song, H.-J. Enantioselective Friedel-Crafts Alkylations of α,β-Unsaturated 2-Acyl Imidazoles Catalyzed by Bis(oxazolinyl)pyridine-Scandium(III) Triflate Complexes. J. Am. Chem. Soc. 2005, 127, 8942–8943. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Fandrick, K.R.; Song, H.-J.; Scheidt, K.A.; Xu, R. Enantioselective Friedel-Crafts Alkylations Catalyzed by Bis(oxazolinyl)pyridine-Scandium(III) Triflate Complexes. J. Am. Chem. Soc. 2007, 129, 10029–10041. [Google Scholar] [CrossRef]
- Rueping, M.; Nachtsheim, B.J.; Moreth, S.A.; Bolte, M. Asymmetric Brønsted Acid Catalysis: Enantioselective Nucleophilic Substitutions and 1,4-Additions. Angew. Chem. Int. Ed. 2008, 47, 593–596. [Google Scholar] [CrossRef]
- Ganesh, M.; Seidel, D. Catalytic Enantioselective Additions of Indoles to Nitroalkenes. J. Am. Chem. Soc. 2008, 130, 16464–16465. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, V.K. Highly Enantioselective Friedel-Crafts Reaction of Indoles with 2-EnoylPyridine 1-Oxides Catalyzed by Chiral Pyridine 2,6-Bis(5′,5′-diphenyloxazoline)-Cu(II) Complexes. Org. Lett. 2008, 10, 4121–4124. [Google Scholar] [CrossRef]
- Trubitsõn, D.; Kanger, T. Enantioselective Catalytic Synthesis of N-alkylated Indoles. Symmetry 2020, 12, 1184. [Google Scholar] [CrossRef]
- Clanton, N.A.; Spiller, T.E.; Ortiz, E.; Gao, Z.; Rodriguez-Poirier, J.M.; DelMonte, A.J.; Frantz, D.E. A Metal-Free Reductive N-Alkylation of Indoles with Aldehydes. Org. Lett. 2021, 23, 3233–3236. [Google Scholar] [CrossRef]
- Cui, H.-L.; Feng, X.; Peng, J.; Lei, J.; Jiang, K.; Chen, Y.-C. Chemoselective Asymmetric N-Allylic Alkylation of Indoles with Morita-Baylis-Hillman Carbonates. Angew. Chem. Int. Ed. 2009, 48, 5737–5740. [Google Scholar] [CrossRef] [PubMed]
- Stanley, L.M.; Hartwig, J.F. Iridium-Catalyzed Regio- and Enantioselective N-Allylation of Indoles. Angew. Chem. Int. Ed. 2009, 48, 7841–7844. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Sun, W.-S.; Liu, C.-X.; Wang, L.; Wang, R. Asymmetric Organocatalytic N- Alkylation of Indole-2-carbaldehydes with α,β-Unsaturated Aldehydes: One-Pot Synthesis of Chiral Pyrrolo[1,2-a]indole-2-carbaldehydes. Chem. Eur. J. 2010, 16, 440–444. [Google Scholar] [CrossRef]
- Bandini, M.; Bottoni, A.; Eichholzer, A.; Miscione, G.P.; Stenta, M. Asymmetric Phase-Transfer-Catalyzed Intramolecular N-Alkylation of Indoles and Pyrroles: A Combined Experimental and Theoretical Investigation. Chem. Eur. J. 2010, 16, 12462–12473. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Yu, X.-Y.; Chen, J.-R.; Feng, B.; Zhang, H.; Qi, Y.-H.; Xiao, W.-J. Enantioselective Direct Functionalization of Indoles by Pd/Sulfoxide-Phosphine-Catalyzed N-Allylic Alkylation. Org. Lett. 2015, 17, 1381–1384. [Google Scholar] [CrossRef]
- Yagil, G. The proton dissociation constant of pyrrole, indole and related compounds. Tetrahedron 1967, 23, 2855–2861. [Google Scholar] [CrossRef]
- Ghosh, K.; Nishii, Y.; Miura, M. Rhodium-Catalyzed Annulative Coupling Using Vinylene Carbonate as an Oxidizing Acetylene Surrogate. ACS Catal. 2019, 9, 11455–11460. [Google Scholar] [CrossRef]
- Hara, H.; Hirano, M.; Tanaka, K. A New Route to Substituted Phenols by Cationic Rhodium(I)/BINAP Complex-Catalyzed Decarboxylative [2+2+2] Cycloaddition. Org. Lett. 2009, 11, 1337–1340. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, F.; Hayashi, T. Synthesis of Arylacetaldehydes by Iridium-Catalyzed Arylation of Vinylene Carbonate with Arylboronic Acids. Angew. Chem. Int. Ed. 2019, 58, 11054–11057. [Google Scholar] [CrossRef]
- Kato, M.; Ghosh, K.; Nishii, Y.; Miura, M. Rhodium-Catalyzed Direct Formylmethylation Using Vinylene Carbonate and Sequential Dehydrogenative Esterification. Chem. Commun. 2021, 57, 8280–8283. [Google Scholar] [CrossRef]
- Hu, W.; Wang, X.; Yu, X.; Zhu, X.; Hao, X.; Song, M. Rh(III)-Catalyzed Divergent C2-carboxymethylation of Indoles and C7-formylmethylation of Indolines with Vinylene Carbonate. Asian J. Org. Chem. 2021, 10, 2557–2561. [Google Scholar] [CrossRef]
- Nishii, Y.; Miura, M. Cp*M-Catalyzed Direct Annulation with Terminal Alkynes and Their Surrogates for the Construction of Multi-Ring Systems. ACS Catal. 2020, 10, 9747–9757. [Google Scholar] [CrossRef]
- Mihara, G.; Ghosh, K.; Nishii, Y.; Miura, M. Concise Synthesis of Isocoumarins through Rh-Catalyzed Direct Vinylene Annulation: Scope and Mechanistic Insight. Org. Lett. 2020, 22, 5706–5711. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Ma, Q.; Yin, J.; Liang, C.; Tian, L.; Ma, Y. RhIII-Catalyzed formal [5+1] cyclization of 2-pyrrolyl/indolylanilines using vinylene carbonate as a C1 synthon. Org. Chem. Front. 2021, 8, 1764–1769. [Google Scholar] [CrossRef]
- Wang, C.; Fan, X.; Chen, F.; Qian, P.-C.; Cheng, J. Vinylene carbonate: Beyond the ethyne surrogate in rhodium-catalyzed annulation with amidines toward 4-methylquinazolines. Chem. Commun. 2021, 57, 3929–3932. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, H.K.; Kang, J.Y.; Mishra, N.K.; Kim, I.S. Assembly of the Hydroxycinnoline Core via Hydrazide-Assisted Rh(III)-Catalyzed C–H Functionalization and Annulation. Synthesis 2022, 54, 4461–4471. [Google Scholar] [CrossRef]
- Shivarkar, A.B.; Gupte, S.P.; Chaudhari, R.V. Synthesis of β-Amino Alcohols from Aromatic Amines and Alkylene Carbonates Using Na-Y Zeolite Catalyst. Synlett 2006, 9, 1374–1378. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, L.; Xia, C. A method for the synthesis of 2-oxazolidinones and 2-imidazolidinones from five-membered cyclic carbonates and β-aminoalcohols or 1,2-diamines. Green Chem. 2007, 9, 369–372. [Google Scholar] [CrossRef]
- Gong, H.; Yang, N.-F.; Deng, G.-J.; Xu, G.-Y. An Eco-friendly, Convenient, and Practical Conversion of Arylamines to Oxazolidinones. Chem. Lett. 2009, 38, 584–589. [Google Scholar] [CrossRef]
- Mei, C.; Zhao, Y.; Zou, K.; Cao, C.; Pang, G.; Shi, Y. Synthesis of N-aryl-2-oxazolidinones from cyclic carbonates and aromatic amines catalyzed by bio-catalyst. Res. Chem. Intermed. 2018, 44, 2179–2194. [Google Scholar] [CrossRef]
- Chong, S.Y.; Wang, T.T.; Cheng, L.C.; Lv, H.Y.; Ji, M. Metal–Organic Framework MIL-101-NH2-Supported Acetate-Based Butylimidazolium Ionic Liquid as a Highly Efficient Heterogeneous Catalyst for the Synthesis of 3-Aryl-2-oxazolidinones. Langmuir 2019, 35, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, X.-Y. Ruthenium-Catalyzed Regio-Selective Synthesis of C3-Alkylated Indoles following Transfer Hydrogenation or Borrowing Hydrogen Strategy. Synthesis 2023, 55, 1460–1466. [Google Scholar]
- Zhou, X.-Y.; Chen, X. Halogen Bond-Catalyzed Friedel-Crafts Alkylation of Indole with Ketones and Aldehydes for the Synthesis of Symmetrical 3,3’-diindolylmethanes Using Simple Halogen Donor Catalyst. Lett. Org. Chem. 2021, 18, 604–610. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Chen, X. Pd/C-Catalyzed transfer hydrogenation of N–H indoles with trifluoroethanol and tetrahydroxydiboron as the hydrogen source. Org. Biomol. Chem. 2021, 19, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Y.; Chen, X.; Liu, H.-L. Ru-catalyzed oxidative dearomatization-hydroxylation of N-Boc indoles. Syn. Commun. 2021, 51, 453–460. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.-Y.; Feng, X.-J.; Bao, M. Ruthenium-Catalyzed Oxidative Dearomatization of N-Boc Indoles. Synthesis 2021, 53, 1121–1126. [Google Scholar]
- Zhou, X.-Y.; Chen, X. Ru-catalyzed oxidation and C–C bond formation of indoles for the synthesis of 2-indolyl indolin-3-ones under mild reaction conditions. Can. J. Chem. 2020, 98, 667–669. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Chen, X. An Easy-to-Operate N-Carbonylation of Indoles with Diaryl Carbonates as Reagent and Na2CO3 as Catalyst. Syn. Commun. 2020, 80, 1854–1862. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Chen, X. Na2CO3-Catalyzed N-Acylation of Indoles with Alkenyl Carboxylates. Synthesis 2019, 51, 516–521. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Chen, X.; Wang, L.-G.; Yang, D.; Li, J.-H. Ruthenium-Catalyzed Oxidative Dearomatization of Indoles for the Construction of C2-Quaternary Indolin-3-ones. Synlett 2018, 29, 835–839. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Chen, X.; Wang, L.-G.; Yang, D.; Li, Z. Palladium-catalyzed Oxidation-hydroxylation and -methoxylation of N-Boc Indoles for the Synthesis of 3-Oxoindolines. Synthesis 2017, 49, 3662–3669. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, H.; Meng, X.; Cao, Z.; Chen, G.; Tian, L.; Sun, X.; You, J. Base-Mediated Domino Reaction of ortho-Carbonylated Alkynyl-Substituted Arenealdehydes with Indoles: Access to Indole-Functionalized Isobenzofurans. Eur. J. Org. Chem. 2017, 2017, 2615–2620. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).