New Sustainable Solvent Extraction Pathways for Rare Earth Metals via Oximes Molecules
Abstract
1. Introduction
2. Results and Discussion
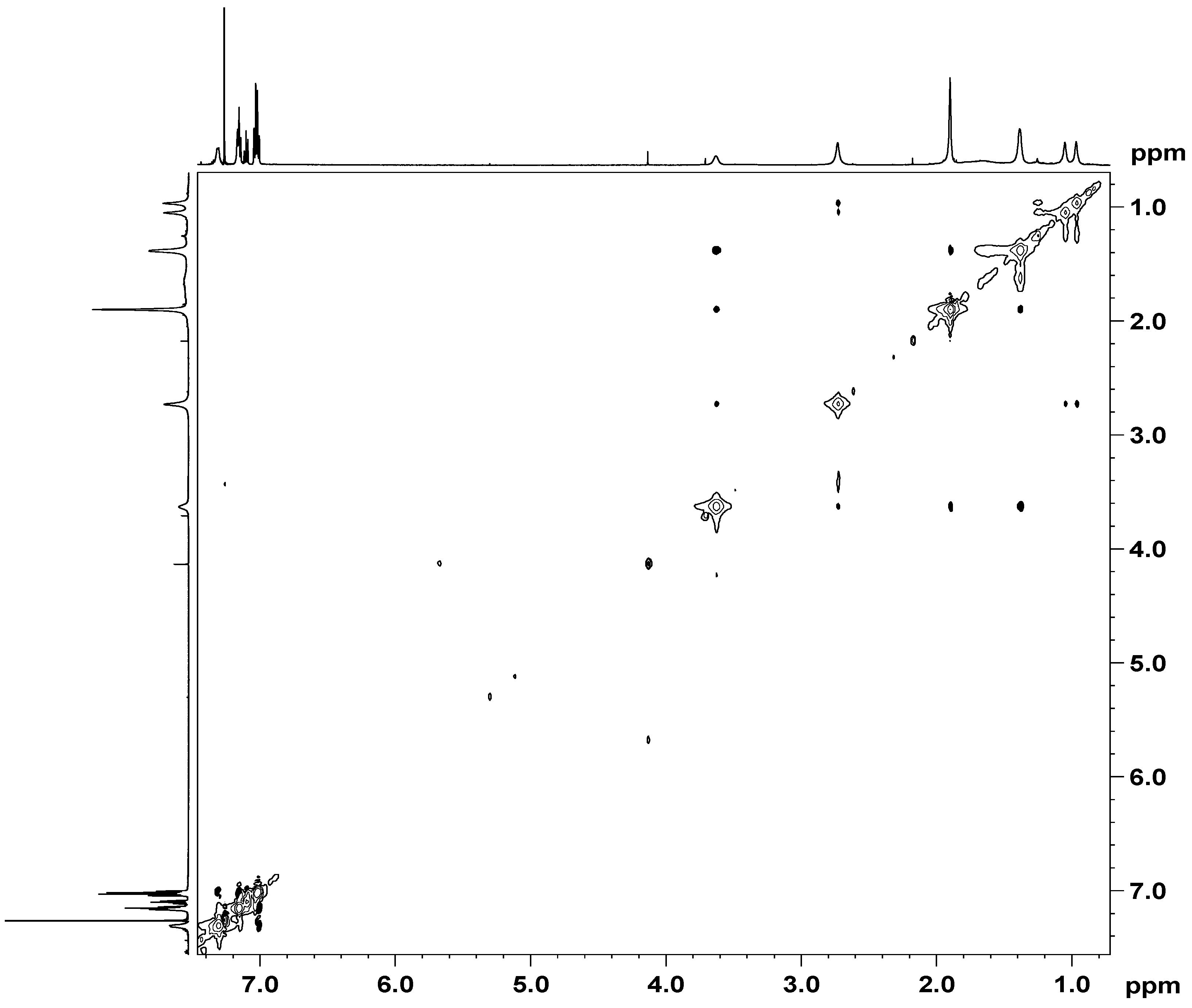
2.1. NMR Interactions Study between the HPBI Compound and the Two Synergistic Agents
2.2. Investigation of Synergism in IL Medium or CHCl3 Implementing Three Chelating Ligands (HL) in Combination with Two Oximes, i.e., HM-PAO or Pre-HM-PAO
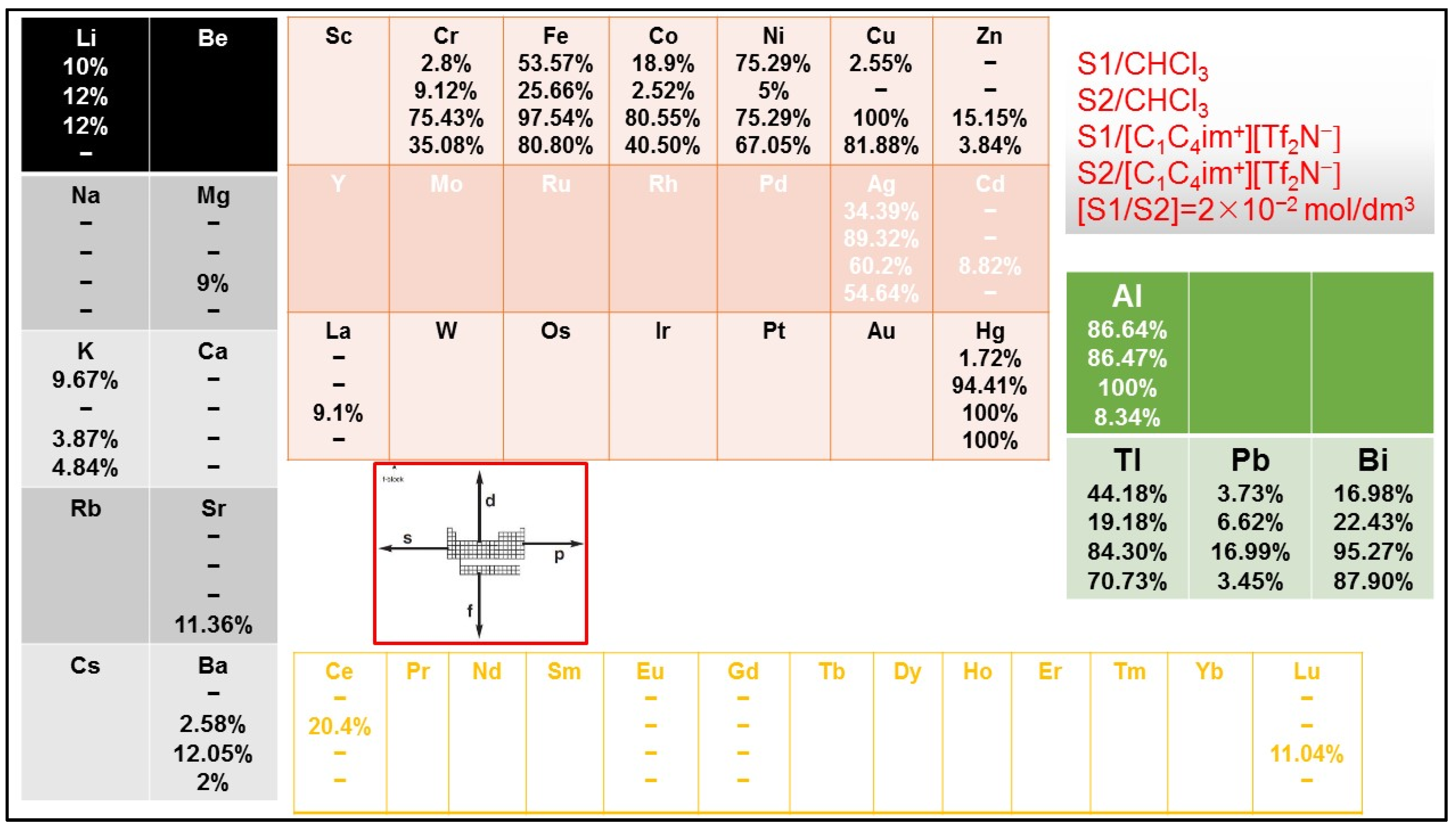
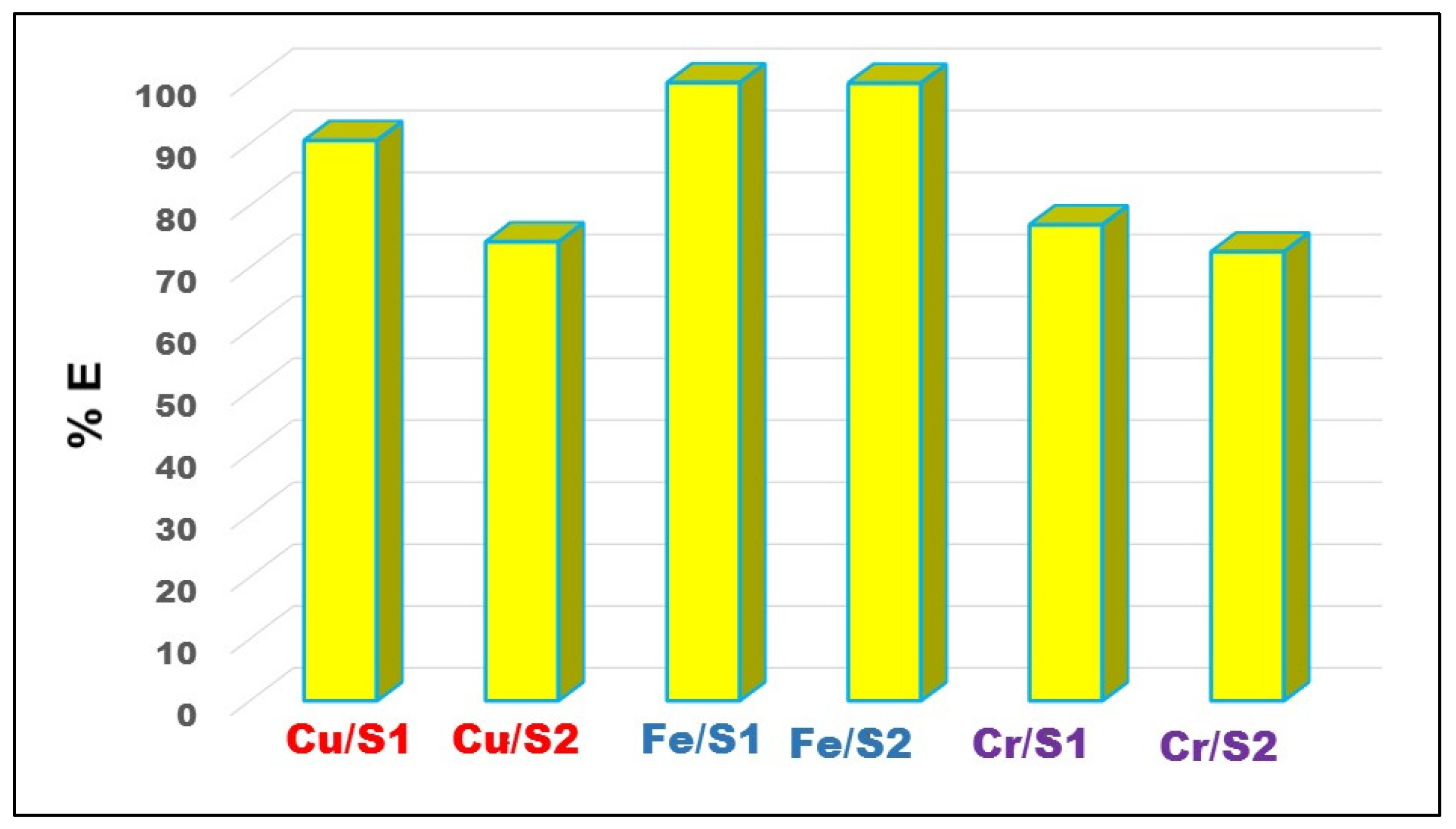
2.3. Solvent Extraction and Selectivity across the Periodic Table and 4f-Series
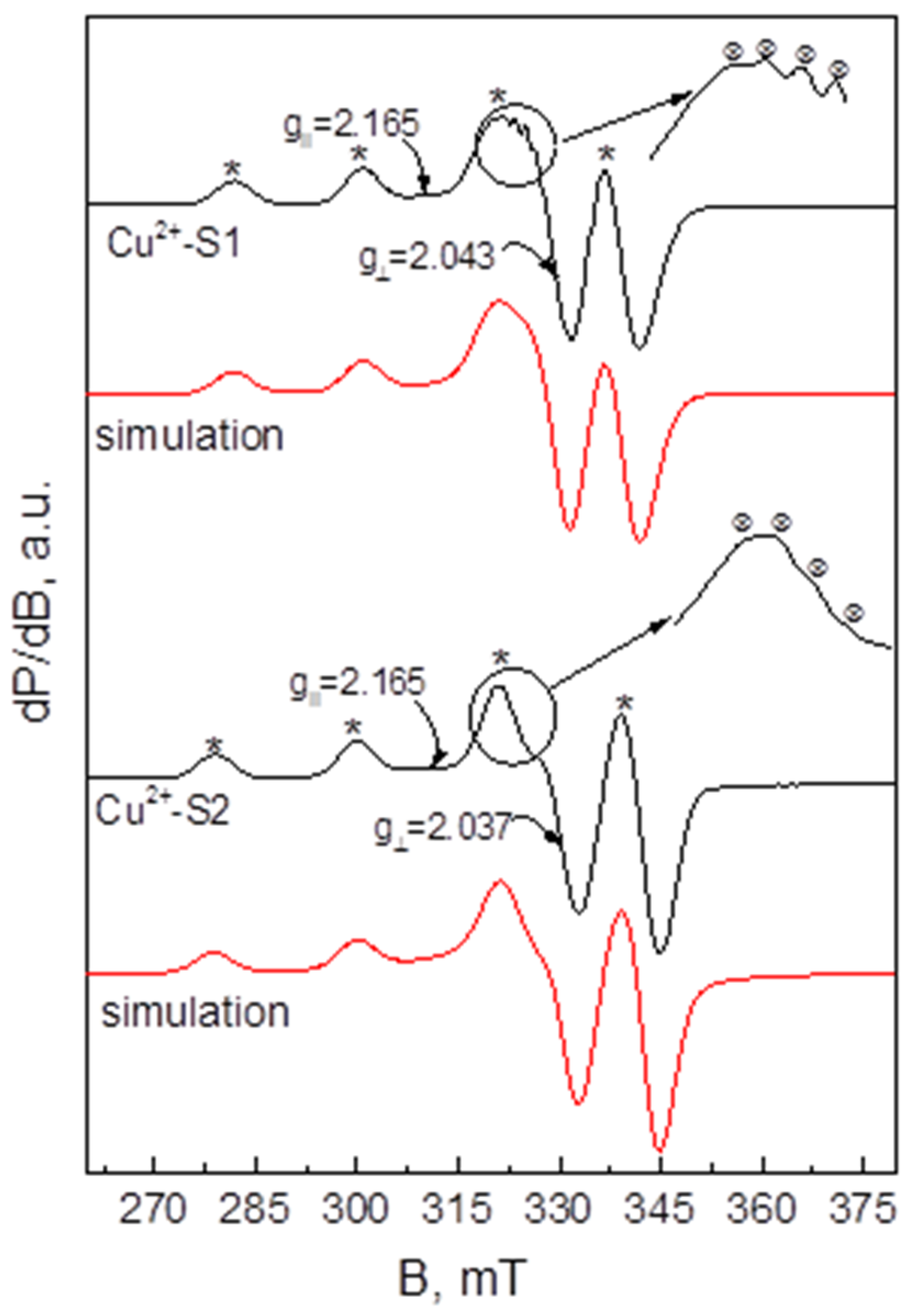
2.4. EPR Investigation of the Extracting Organic Phases
3. Experimental Part
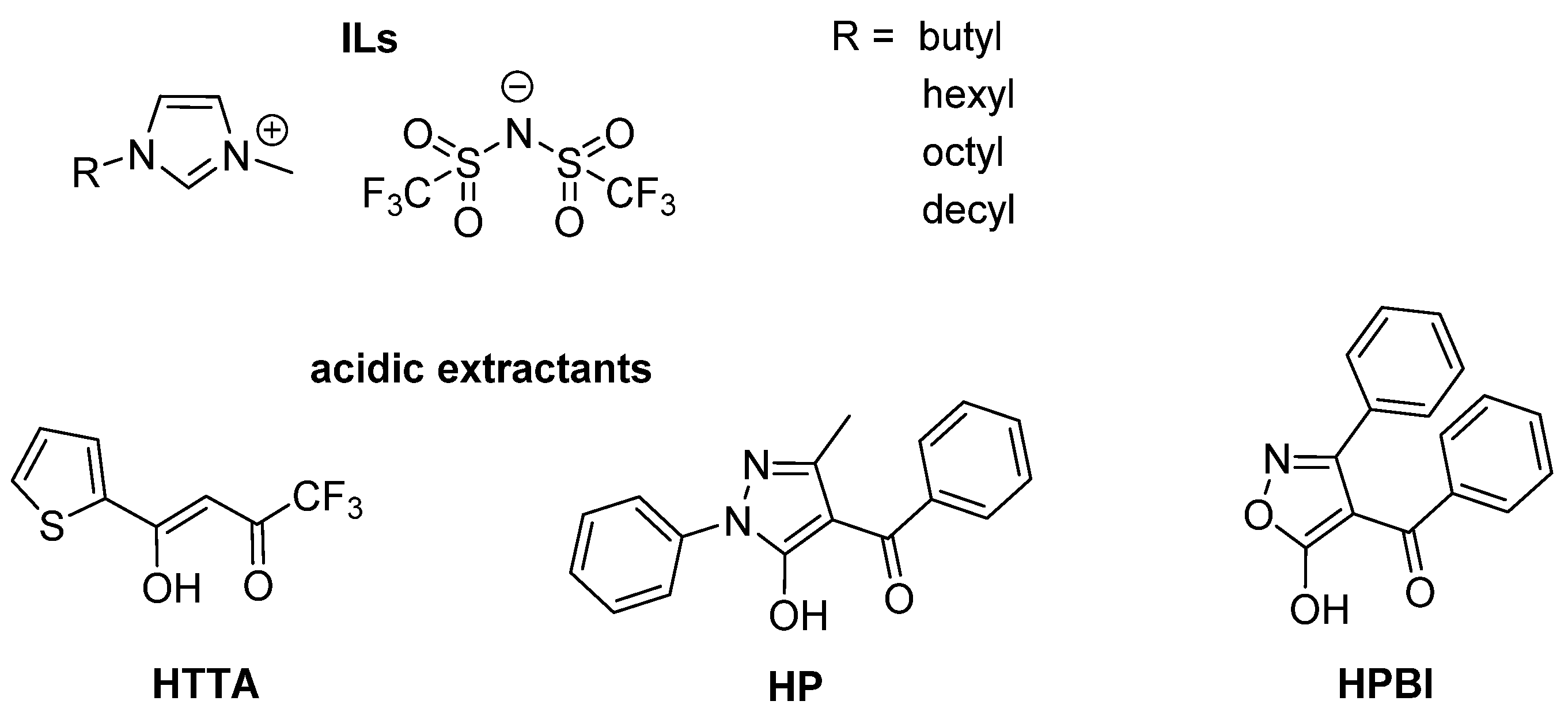
3.1. Reagents
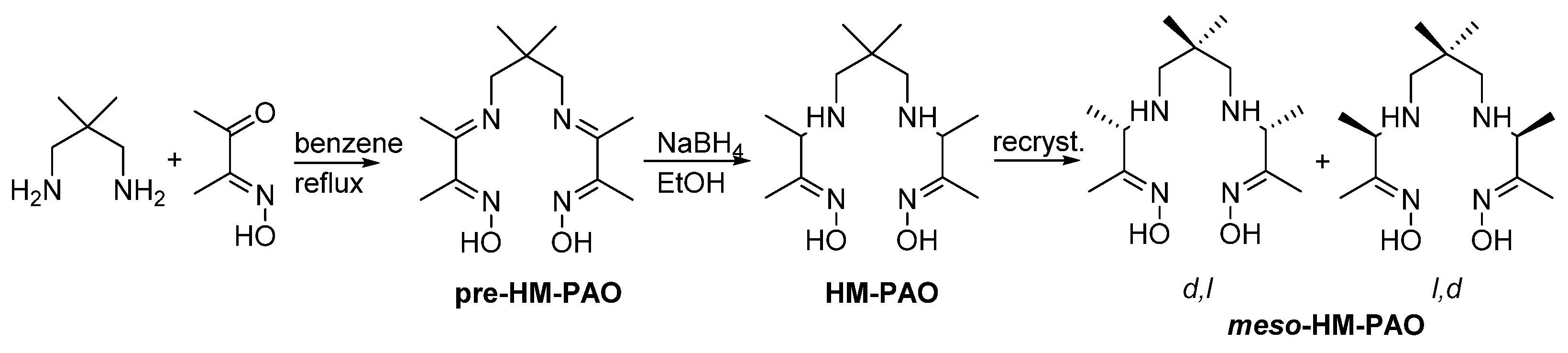
3.2. Synthesis of New Oxime Ligands in Solvent Extraction Chemistry
3.3. NMR Interaction Measurements
3.4. Solvent Extraction Experiments
3.5. EPR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adunka, R.; Orna, M. Carl Auer von Welsbach: Chemist, Inventor, Entrepreneur; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Cleaner Prod. 2013, 51, 1. [Google Scholar] [CrossRef]
- Liu, Z. (Ed.) Chemistry Research and Rare Earths, New Research Applications; Nova Publishers: New York, NY, USA, 2013. [Google Scholar]
- Greenwood, N.; Earnshaw, A. Chemistry of Elements; Butterworth-Heinemann: Oxford, UK, 1998. [Google Scholar]
- Huang, C. (Ed.) Rare Earth Coordination Chemistry, Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Atanassova, M.; Kurteva, V.; Billard, I. Coordination chemistry of europium(III) ion towards acylpyrazolone ligands. Anal. Sci. 2015, 31, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Marchetti, F.; Drozdov, A. β-Diketones and related ligands. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meye, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 1. [Google Scholar]
- Pettinary, C.; Merchetti, F.; Pettinari, R.; Drozdov, A.; Troyanov, S.; Voloshin, A.; Shavaleev, N. Synthesis, structure and luminescence properties of new rare earth metal complexes with 1-phenyl-3-methyl-4-acylpyrazol-5-ones. Dalton Trans. 2002, 7, 1409. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Acylpyrazolone ligands: Synthesis, structures, metal coordination chemistry and applications. Coord. Chem. Rev. 2005, 249, 2909. [Google Scholar] [CrossRef]
- Kresinski, R.; Lees, A.; Platt, A. Structural variations in a series of lanthanide nitrate complexes with an unsymmetrical diphosphonate ligand (MeO)2P(O)C(CH2)CH2P(O)(OMe)2. Polyhedron 2012, 33, 341. [Google Scholar] [CrossRef]
- Gupta, C.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Moyer, B. (Ed.) Ion Exchange and Solvent Extraction. A Series of Advances; Taylor and Francis Group LLC: Abingdon, UK, 2010; Volume 19. [Google Scholar]
- Foulds, G. Nickel 1986. Coord. Chem. Rev. 1997, 162, 75–154. [Google Scholar] [CrossRef]
- Liu, M.; Zou, D.; Yu, D.; Xiang, S.; Deng, Y.; Chen, J.; Xiao, J. Synthesis of new extractant P113 for cerium(IV) extraction and higher separation over thorium from bastnaesite. Sep. Purif. Technol. 2023, 317, 123909. [Google Scholar] [CrossRef]
- Kolodguska, D.; Burdzy, K.; Hunger, S.; Aurich, H.; Ju, Y. Green extractants in assisting recovery of REEs: A case study. Molecules 2023, 28, 965. [Google Scholar] [CrossRef]
- Lukomska, A.; Wisniewska, A.; Dabrowski, Z.; Lach, J.; Wrobel, K.; Kolosa, D.; Domanska, U. Recovery of metals from electronic waste-printed circuit boards by ionic liquids, DESs and organophosphorus-based acid extraction. Molecules 2022, 27, 4984. [Google Scholar] [CrossRef]
- Simonnet, M.; Kobayashi, T.; Shimojo, K.; Yokoyama, K.; Yaita, T. Study on phenanthroline carboxamide for lanthanide separation: Influence of amide substituents. Inorg. Chem. 2021, 60, 13409–13418. [Google Scholar] [CrossRef]
- Shimojo, K.; Suzuki, H.; Yokoyama, K.; Yaita, T.; Ikeda-Ohno, A. Solvent extraction of technetium(VII) and rhenium(VII) using a hexaoctylnitritotriacetamide extractant. Anal. Sci. 2020, 36, 1435–1437. [Google Scholar] [CrossRef]
- Atanassova, M.; Todorova, S.; Kurteva, V.; Todorova, N. Insights into the synergistic selectivity of 4f-ions implementing 4-acyl-5-pyrazolone and two new unsymmetrical NH-urea containing ring molecules in an ionic liquid. Sep. Purif. Technol. 2018, 204, 328–335. [Google Scholar] [CrossRef]
- Shimojo, K.; Fujiwara, I.; Fujisawa, K.; Okamura, H.; Sugita, T.; Oshima, T.; Baba, Y.; Naganawa, H. Extraction behavior of rare-earth elements using a mono-alkylated diglycolamic acid extractant. Solvent Extr. Res. Dev. Jpn. 2016, 23, 151–159. [Google Scholar] [CrossRef]
- Guo, Z.; Chu, T. Separation of Mo(VI) from U(VI) and other fission elements in synthetic solutions by di-quinolinol chelating ligand in [C4mim][NTf2] ionic liquid. Hydrometallurgy 2023, 215, 105968. [Google Scholar] [CrossRef]
- Ueda, Y.; Morisada, S.; Kawakita, H.; Ohto, K. Solvent extraction behavior of divalent palladium ion with phenylurea derivative of trident molecule. Solvent Extr. Res. Dev. Jpn. 2014, 21, 9–19. [Google Scholar] [CrossRef][Green Version]
- Kitiphaisalnot, P.; Thohinung, S.; Hanmungtum, P.; Chaichit, N.; Patrarakorn, S.; Siripaisarnpipet, S. Effect of metal ions with length of alkylene bridge on the strength of hydrogen bonds in diiminedioxime cobalt(III) and nickel(III) complexes. Polyhedron 2006, 25, 2710–2716. [Google Scholar] [CrossRef]
- Pal, J.; Murmann, R.; Schlemper, E.; Kay Fair, C.; Hussain, M. Intramolecular hydrogen bonding: Synthesis and crystal structure of nickel(II) and copper(II) complexes of a tetradentate amine oxime. Inorg. Chim. Acta 1986, 115, 153–161. [Google Scholar] [CrossRef]
- Premužić, D.; Korabik, M.; Holyńska, M. Structure and magnetic properties of a new binuclear copper(II) complex of a tripodal oxime. J. Mol. Struct. 2014, 1059, 265–270. [Google Scholar] [CrossRef]
- El-Nadi, Y.A. Solvent extraction and its applications on ore processing and recovery of metals: Classical approach. Sep. Purif. Rev. 2017, 46, 195. [Google Scholar] [CrossRef]
- Turanov, A.N.; Matveeva, A.G.; Kudryavtsev, I.Y.; Pasechnik, M.P.; Matveev, S.V.; Godovikova, M.I.; Baulina, T.V.; Karandashev, V.K.; Brel, V.K. Tripodal organophosphorus ligands as synergistic agents in the solvent extraction of lanthanides(III). Structure of mixed complexes and effect of diluents. Polyhedron 2019, 161, 276. [Google Scholar] [CrossRef]
- Moeller, T.; Martin, D.; Thompson, L.; Ferrús, R.; Feistel, G.; Randall, W. The coordination chemistry of yttrium and the rare earth metal ions. Chem. Rev. 1965, 65, 1. [Google Scholar] [CrossRef]
- Kajan, I.; Florianova, M.; Ekberg, C.; Matyskin, A. Effect of diluents on the extraction of europium(III0 and americium(III) with N,N,N’,N’-tetraoctyldiglycolamide(TODGA). RSC Adv. 2021, 11, 36707. [Google Scholar] [CrossRef]
- Jørgensen, C. Structure and Bonding. Rare Earths; Springer: Berlin/Heidelberg, Germany, 1976. [Google Scholar]
- Romano, P.; Rahmati, S.; Adanodi, R.; Birloaya, I.; Veglio, F. Leaching of rare earth elements from permanent magnet swarf in citric acid: Effects of acid concentration on extraction kinetics. Metals 2023, 13, 1801. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Chen, W.; Long, F.; Chen, Z.; Chi, R. Effects of ammonium salts on rare earth leaching process of weathered crust elution-deposited rare earth ores. Metals 2023, 13, 1112. [Google Scholar] [CrossRef]
- Xiao, F.; Hu, W.; Zhao, J.; Zhu, H. Technologies of recycling REES and iron from NdFeB scrap. Metals 2023, 13, 779. [Google Scholar] [CrossRef]
- Spooren, J.; Binnemans, K. Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: Technology development trends. Resour. Conserv. Recycl. 2020, 160, 104919. [Google Scholar] [CrossRef]
- Reck, B.; Graedel, T. Challenges in metal recycling. Science 2012, 337, 690. [Google Scholar] [CrossRef]
- Gunn, G. (Ed.) Critical Metals Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Petrova, M. The Crucial Performance of Mutual Solubility among Water and Ionic Liquids in the Time of Liquid-Liquid Extraction of Metallic Species; Academic Solutions: Sofia, Bulgaria, 2020. [Google Scholar]
- Atanassova, M.; Kurteva, V.; Dukov, I. The interaction of extractants during synergistic solvent extraction of metals. Is it an important reaction? RSC Adv. 2016, 6, 8125–81265. [Google Scholar] [CrossRef]
- Sekine, T.; Saitou, T.; Iga, H. Association of β-diketones with trioctylphosphine oxide in solvent extraction systems. Bull. Chem. Soc. Jpn. 1983, 56, 700–704. [Google Scholar] [CrossRef]
- Hokura, A.; Sekine, T. Gradual destruction of synergistic enhancement of copper(II) extraction into carbon tetrachloride with 2-thenoyltrifluoroacetone and trioctylphosphine oxide. Solvent Extr. Res. Dev. Jpn 1995, 2, 102–111. [Google Scholar]
- Marcus, Y.; Kertes, A.S. Ion Exchange and Solvent Extraction of Metal Complexes; Willey Interscience: New York, NY, USA, 1969; p. 815. [Google Scholar]
- Choppin, G.; Morgenstern, A. Thermodynamics of solvent extraction. Solvent Extr. Ion Exch. 2000, 18, 1029. [Google Scholar] [CrossRef]
- Choppin, G. Studies of the synergistic effect. Sep. Sci. Technol. 1981, 16, 1113. [Google Scholar] [CrossRef]
- Janssen, C.; Macias-Ruvalcaba, N.; Aguilar-Martinez, M.; Kobrak, M. Metal extraction to ionic liquids: The relationship between structure, mechanism and application. Int. Revs. Phys. Chem. 2015, 34, 591–622. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V. Synergism as a phenomenon in solvent extraction of 4f-elements with calixarenes. RSC Adv. 2016, 6, 11303–11324. [Google Scholar] [CrossRef]
- Atanassova, M. Worthy extraction and uncommon selectivity of 4f-ions in IL medium: 4-acylpyrazolones and CMPO. ACS Sustainable Chem. Eng. 2016, 4, 2366–2375. [Google Scholar]
- Hirayama, N.; Okamura, H.; Kidani, K.; Imura, H. Ionic liquid synergistic cation-exchange system for the selective extraction of lanthanum(III) using 2-thenoyltrifluoroacetone and 18-crown-6. Anal. Sci. 2008, 24, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Neuefeind, J.; Beitz, J.; Skanthamukar, S.; Soderholm, L. Mechanism of metal ion transfer into room-temperature ionic liquids: The role of anion exchange. J. Am. Chem. Soc. 2003, 125, 15466–15473. [Google Scholar] [CrossRef]
- Wu, R.; Liu, R.; Liu, X.; Zhang, J.; Xue, W.; Yang, Y. P350-N235 synergistic extraction system used for the recovery of Nd(III) from waste NdFeB magnets. Sep. Purif. Technol. 2023, 319, 124042. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V. Peculiar synergistic extraction behavior of Eu(III) in ionic liquids: Benzoylacetone and CMPO fusion. Sep. Purif. Technol. 2017, 183, 226. [Google Scholar] [CrossRef]
- Okamura, H.; Takagi, H.; Isomura, T.; Morita, K.; Nagatani, H.; Imura, H. Highly selective synergism for the extraction of lanthanoid (III) ions with β-diketones and triocylphosphine oxide in an ionic liquid. Anal. Sci. 2014, 30, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Billard, I. Comparing extraction, synergism and separation of lanthanoids using acidic and neutral compounds in chloroform and one ionic liquid: Is the letter always “better”? RSC Adv. 2014, 4, 38820–38829. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, J.; Li, Y.; Mehio, N.; Qi, Y.; Liu, H.; Dai, S. An ionic liquid-based synergistic extraction strategy for rare earths. Green Chem. 2015, 17, 2981–2993. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Billard, I. Solvent extraction and separation of light lanthanoids with mixtures of two chelating extractants: Benzene vs. ionic liquids. Sep. Sci. Technol. 2016, 52, 290–299. [Google Scholar] [CrossRef]
- Okamura, H.; Aoyagi, N.; Shimojo, K.; Naganawa, H.; Imura, H. Role of Tf2N− anions in the ionic liquid-water distribution of europium(III) chelates. RSC Adv. 2017, 7, 7610–7618. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Ding, Y. Recovery of rare earths in different media with novel dicarboxylate IL compound and application to recycle SmCo magnets. Hydrometallurgy 2022, 210, 105844. [Google Scholar] [CrossRef]
- Liang, X.; Zeng, Q. Recovery of samarium(III) and cobalt(II) in synthetic nitric acid solutions using carboxyl functionalized ionic liquids and application to recycling SmCo permanent magnets. Hydrometallurgy 2023, 216, 106016. [Google Scholar] [CrossRef]
- Hu, K.; Xing, L.; Nie, Y.; Li, X.; Dong, H.; Gao, H. Removal of aluminum to obtain high purity gadolinium with pyridinium-based ionic liquids. Hydrometallurgy 2022, 213, 105930. [Google Scholar] [CrossRef]
- Emam, S.; El-Hefny, N.E. Bi-functional ionic liquid based on Aliquat 336 and Cyanex 572 for effective separation of Nd(III) and Ni(II) from chloride solution and recover Nd2O3 from waste Nd-Ni-Fe magnets. Hydrometallurgy 2023, 219, 106064. [Google Scholar] [CrossRef]
- Bezdomnikov, A.; Kostikova, G.; Baulin, D.; Tsivadze, A. Liquid extraction of lithium using a mixture of alkyl salicylate and tri-n-octylphosphine oxide. Sep. Purif. Technol. 2023, 320, 124137. [Google Scholar] [CrossRef]
- Lv, C.; Fu, J.; Wang, S.-L.; Zhang, G.-H.; Guo, S.-J.; Tao, L.G.-H. Separation and recovery of Th(IV) from rare earth and other cation solutions using pH-responsive ionic liquids at high acidity condition of 1M HNO3. Hydrometallurgy 2022, 214, 105953. [Google Scholar] [CrossRef]
- Atanassova, M. Synergistic solvent extraction and separation of lanthanide(III) ions with 4-benzoyl-3-phenyl-5-isoxazolone and the quaternary ammonium salt. Solvent Extr. Ion Exch. 2009, 27, 159–171. [Google Scholar] [CrossRef]
- Atanassova, M.; Billard, I. Determination of pKaIL values of three chelating extractants in ILs: Consequences for the extraction of 4f elements. J. Solution Chem. 2015, 44, 606–620. [Google Scholar] [CrossRef]
- Ramachandra Reddy, B.; Kumar, J.; Varade Reddy, A. 3-Phenyl-4-acyl-isoxazolones as reagents for liquid-liquid extraction of tetravalent zirconium and hafnium from acidic chloride solutions. J. Braz. Chem. Scoc. 2006, 17, 780–784. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Xu, L.; Liu, C.; Jin, C.; Zhang, A.; Xiao, C. Extraction and complexation mechanism of uranium(VI) and thorium(IV) by tetradentate phenanthroline phosphonate (POPhen) ligands. Sep. Purif. Technol. 2023, 320, 124124. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.E. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch. Biochem. Biophys. 1974, 165, 691–708. [Google Scholar] [CrossRef] [PubMed]
- Holyńska, M. Structural variety and magnetic properties of oxime-bridged copper(II) complexes. J. Mol. Struct. 2015, 1098, 175–180. [Google Scholar] [CrossRef]
- Neirinckx, R.D.; Canning, L.R.; Piper, I.M. Technetium-99m d,l-HM-PAO: A new radiopharmaceutical for SPECT imaging of regional cerebral blood perfusion. J. Nucl. Med. 1987, 28, 191–202. [Google Scholar]
- Kurteva, V.; Simova, S. Determination of the diastereoisomeric purity of D,L- and meso-HM-PAO by 13C NMR spectroscopy. Eur. J. Med. Chem. 2003, 38, 219–222. [Google Scholar] [CrossRef]
- Savvin, S.B. Arsenazo III; Atomizdat: Moscow, Russia, 1966; p. 177. [Google Scholar]



| Solvent Mixture | SC |
|---|---|
| HPBI + S1 | −0.010 |
| HPBI + S2 | −0.005 |
| HP + S1 | 0.029 |
| HP + S2 | 0.26 |
| HTTA + S1 | 0.47 |
| HTTA + S2 | 1.78 |
| Diluent | Sc | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | U | Th |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn+ in | 13.01 ± 0.44 | 12.91 ± 0.22 | 12.65 ± 0.22 | 12.86 ± 0.26 | 12.79 ± 0.32 | 13.23 ± 0.26 | 12.94 ± 0.22 | 13.05 ± 0.22 | 13.30 ± 0.40 | 13.07 ± 0.26 | 12.82 ± 0.34 | 13.14 ± 0.35 | 12.67 ± 0.20 | 13.01 ± 0.21 | 13.04 ± 0.40 | 12.95 ± 0.30 | 13.08 ± 0.28 | 12.95 ± 0.60 |
| CHCl3 | 65.2% | 1.6% | − | − | − | 1.9% | 2.6% | 3.7% | − | 1.8% | − | 3.8% | 8.9% | 6.8% | 3.3% | 2.5% | 3.5% | 73.3% |
| CCl4 | 100% | 59.5% | 18.8% | 16.2% | 18.6% | 29.8% | 54.8% | 64.2% | 68.4% | 76.1% | 79% | 78.2% | 99.2% | 78.7% | 76% | 69.7% | 50.8% | 80.2% |
| C6H6 | 96.2% | 3.7% | − | − | 0.7% | 2.0% | 3.8% | 4.7% | 3.2% | 4.2% | 8.2% | 6.2% | 12.4% | 9.3% | 4.8% | 5.7% | 6.4% | 76.3% |
| C2H4Cl2 | 53.9% | − | − | − | − | − | 0.7% | 0.6% | − | − | − | 5.7% | 3.8% | − | 1.5% | 1% | 75% | |
| [C1C4im+] | 78% | 22.1% | − | − | − | 6.6% | 20.7% | 26.9% | 28.1% | 34.3% | 40.7% | 42.2% | 62.6% | 59.5% | 69% | 64% | 23% | 75% |
| [C1C6im+] | 78.7% | 39.5% | − | − | 3.4% | 13.45% | 34.6% | 40% | 47.6% | 53.3% | 58.8% | 59.9% | 81.8% | 71.4% | 60.9% | 73.4% | 34% | 77.4% |
| [C1C8im+] | 79.4% | 47.5% | − | − | 6.7% | 15.6% | 40.8% | 48.6% | 56.8% | 60.9% | 66.7% | 67.4% | 91.5% | 79.4% | 68% | 77.8% | 39% | 77.3% |
| [C1C10im+] | 81.7% | 54% | 5.0% | 7.7% | 11.3% | 23.5% | 49.3% | 56.4% | 62.9% | 67.6% | 71.9% | 71.7% | 95.4% | 75.2% | 71.2% | 81.2% | 46.2% | 78.9% |
| [C1C4pyr+] | 85.4% | 19.82% | − | − | 3.0% | 10.3% | 22.4% | 22.9% | 25.5% | 31.8% | 36.5% | 35.8% | 54.6% | 43.5% | 33.8% | 51.5% | 22.2% | 77.6% |
| Diluent | Lu/Eu | U/Eu | Th/Eu | Gd/Eu | Lu/La |
|---|---|---|---|---|---|
| CHCl3 | 0.64 | 0.93 | 70.58 | 0.37 | |
| [C1C4im+][Tf2N−] | 4.82 | 0.81 | 8.18 | 1.06 | |
| [C1C10im+][Tf2N−] | 3.33 | 0.66 | 2.89 | 1.31 | 80.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanassova, M.; Kukeva, R.; Kurteva, V. New Sustainable Solvent Extraction Pathways for Rare Earth Metals via Oximes Molecules. Molecules 2023, 28, 7467. https://doi.org/10.3390/molecules28227467
Atanassova M, Kukeva R, Kurteva V. New Sustainable Solvent Extraction Pathways for Rare Earth Metals via Oximes Molecules. Molecules. 2023; 28(22):7467. https://doi.org/10.3390/molecules28227467
Chicago/Turabian StyleAtanassova, Maria, Rositsa Kukeva, and Vanya Kurteva. 2023. "New Sustainable Solvent Extraction Pathways for Rare Earth Metals via Oximes Molecules" Molecules 28, no. 22: 7467. https://doi.org/10.3390/molecules28227467
APA StyleAtanassova, M., Kukeva, R., & Kurteva, V. (2023). New Sustainable Solvent Extraction Pathways for Rare Earth Metals via Oximes Molecules. Molecules, 28(22), 7467. https://doi.org/10.3390/molecules28227467







