Research Progress in Nanofluid-Enhanced Oil Recovery Technology and Mechanism
Abstract
:1. Introduction
2. Commonly Used Nanofluids
2.1. Inorganic Nanofluids
2.2. Organic Nanofluids
2.3. Organic–Inorganic Composite Nanofluid
3. Optimization and Modification Methods for Nanofluids
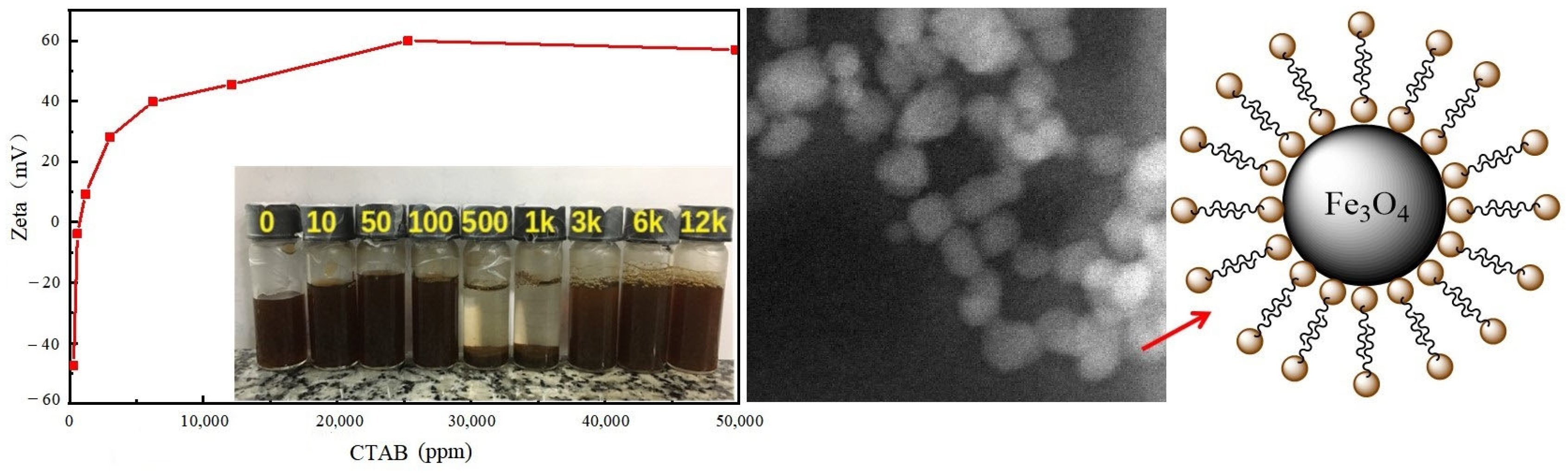
3.1. Physical Blending Modification
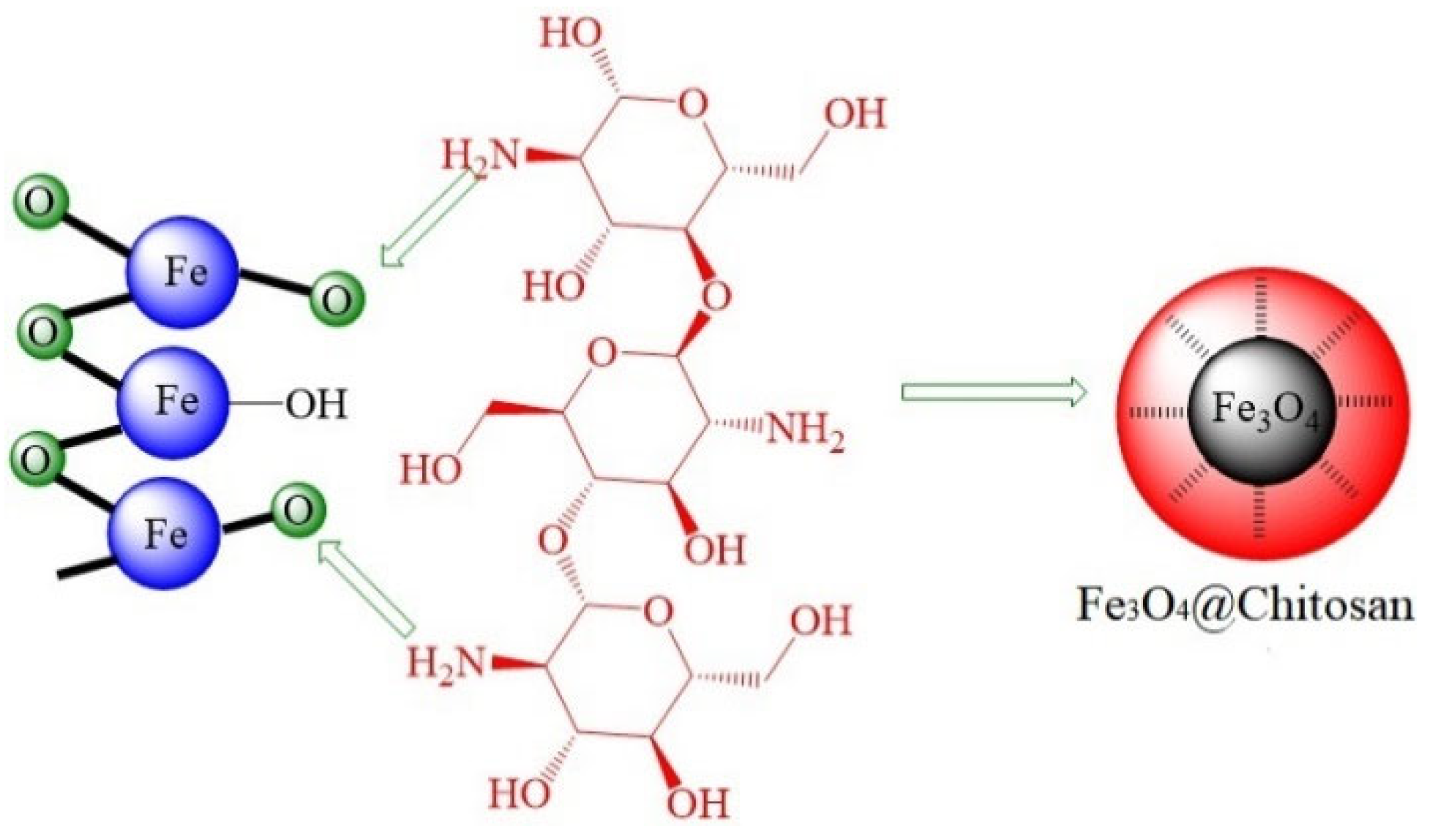
3.2. Chemical Coating Modification
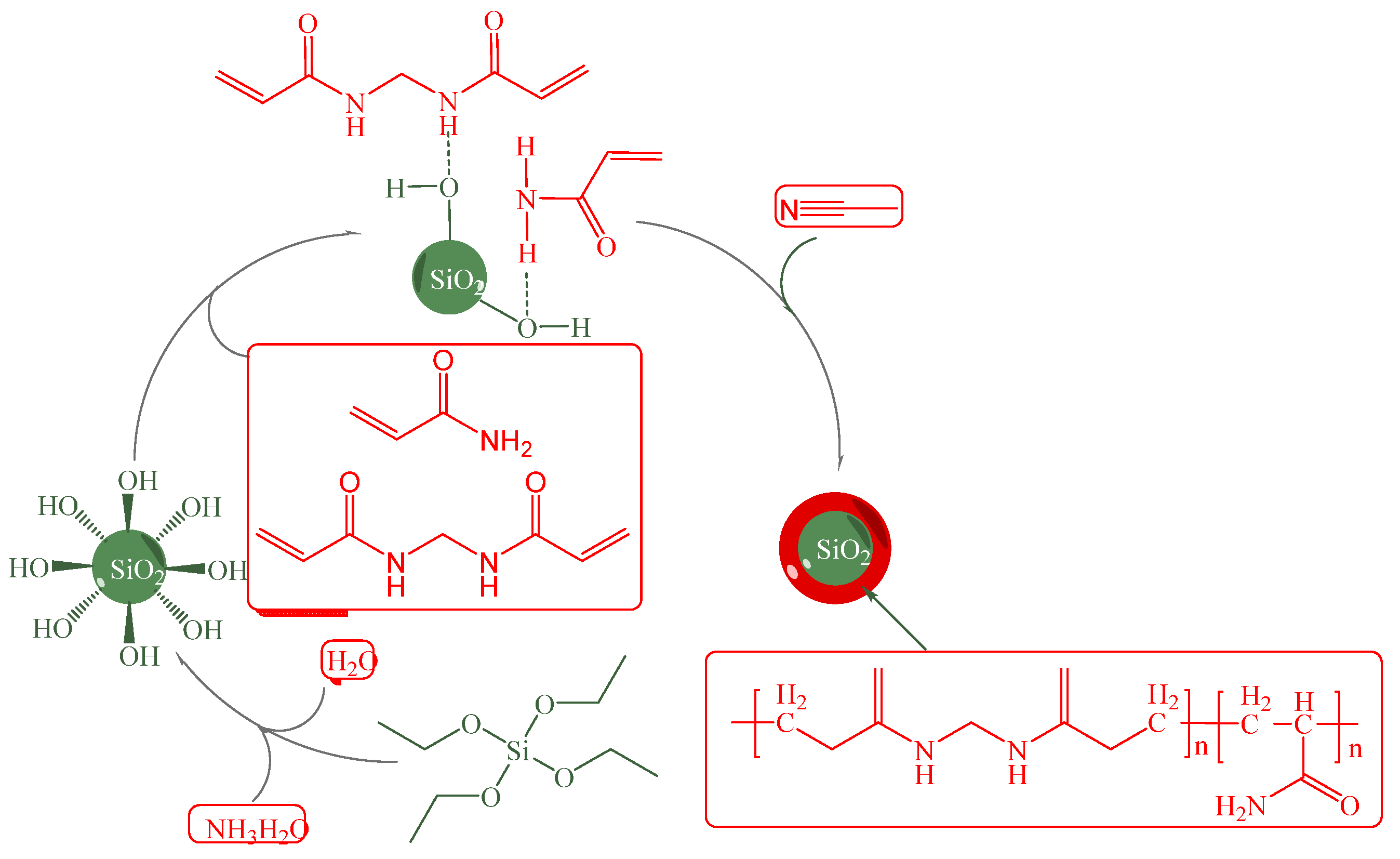
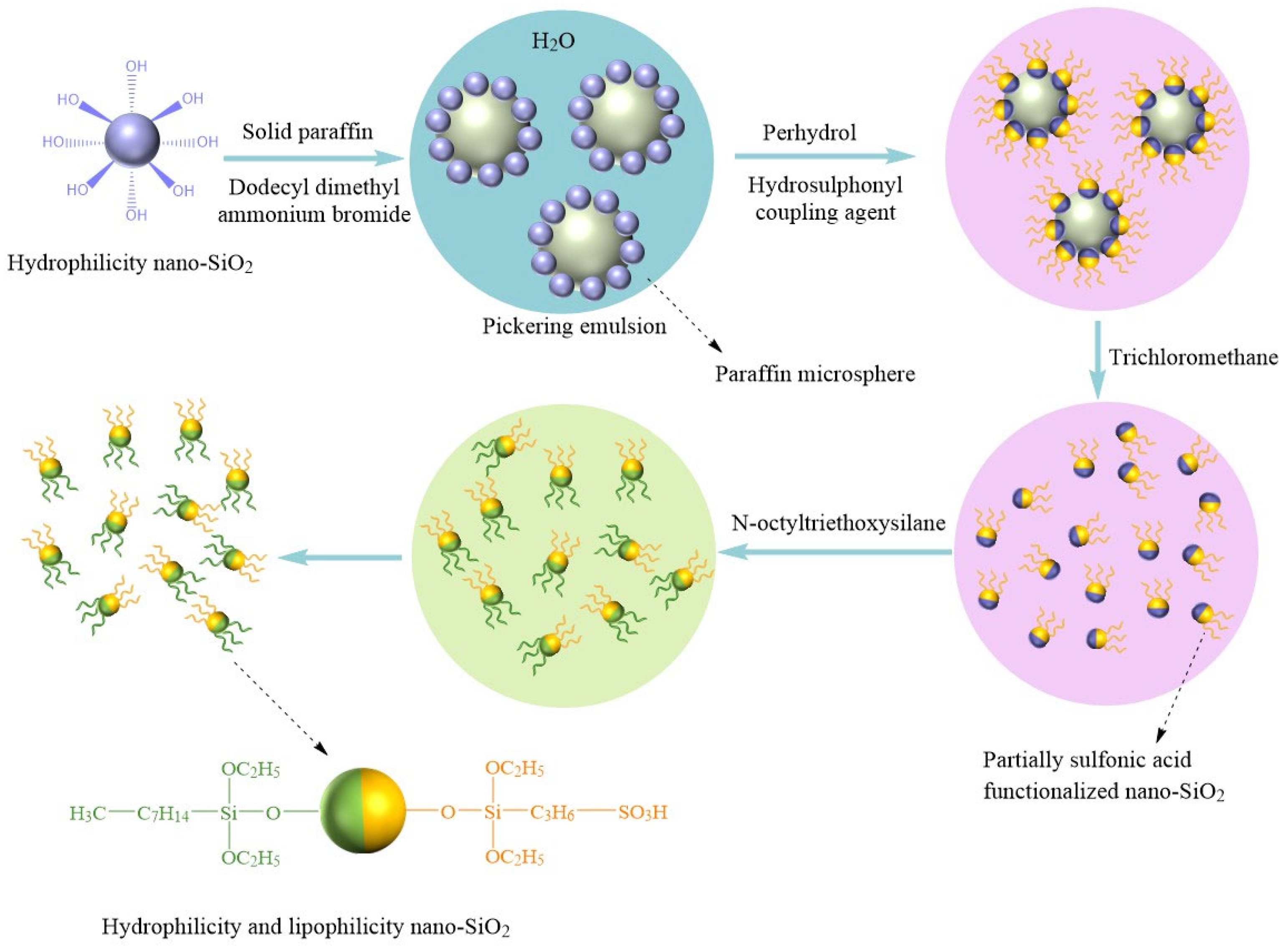
3.3. Surface Grafting Modification
4. Mechanism of Nanofluid EOR
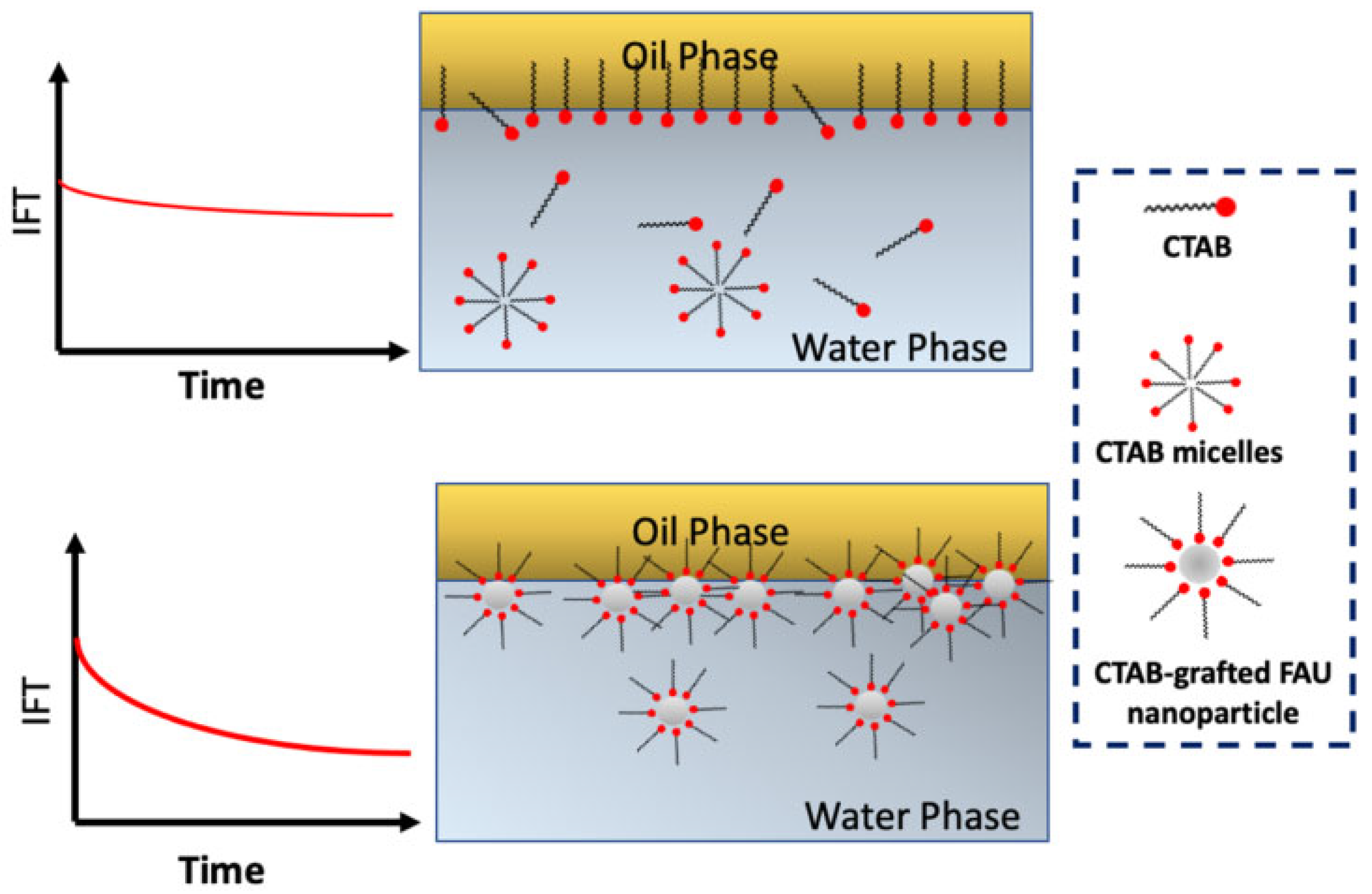
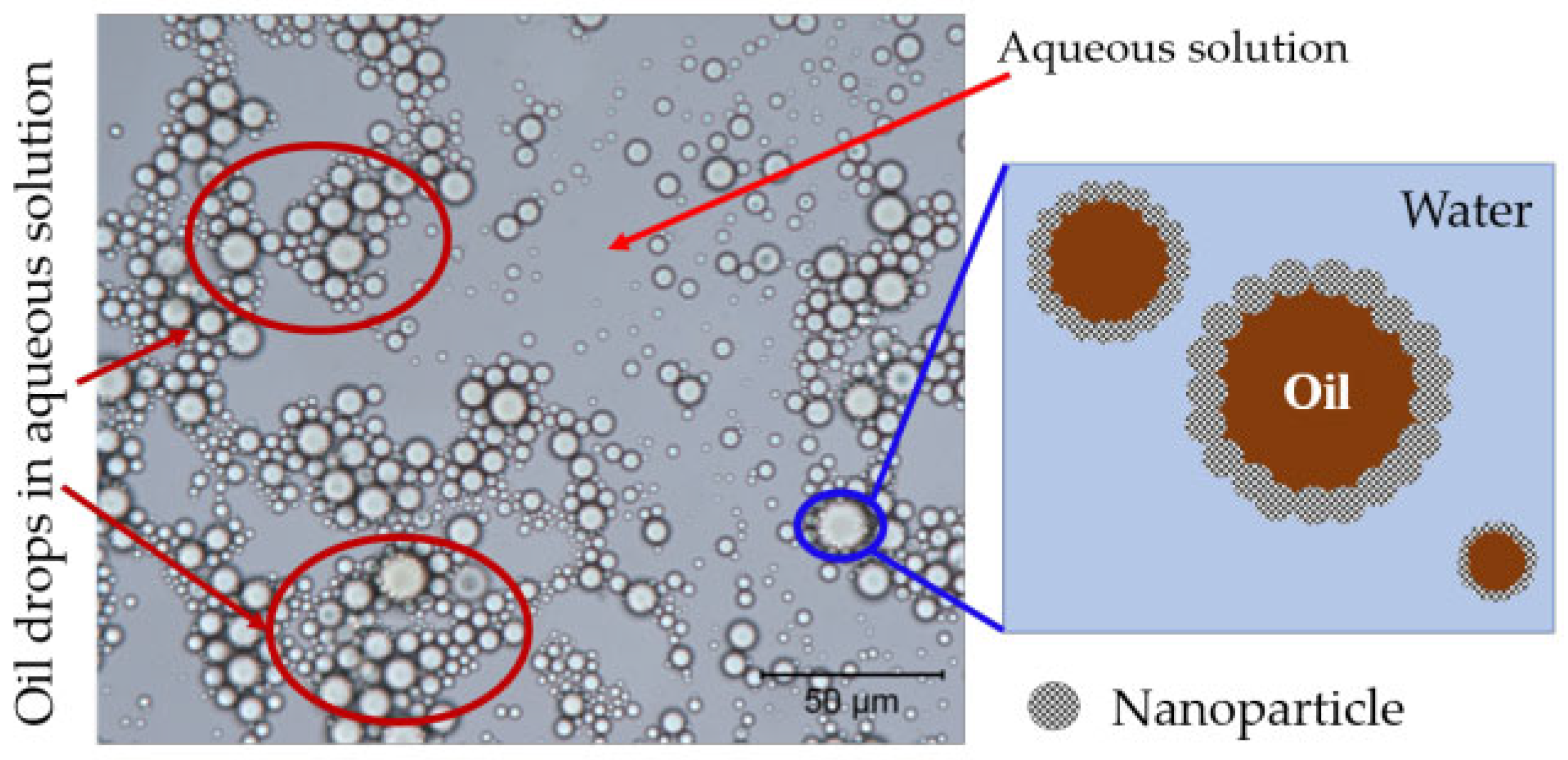
4.1. Reduce the Oil–Water IFT
4.2. Changing the Wettability of Rock Surfaces
4.3. Structural Separation Pressure
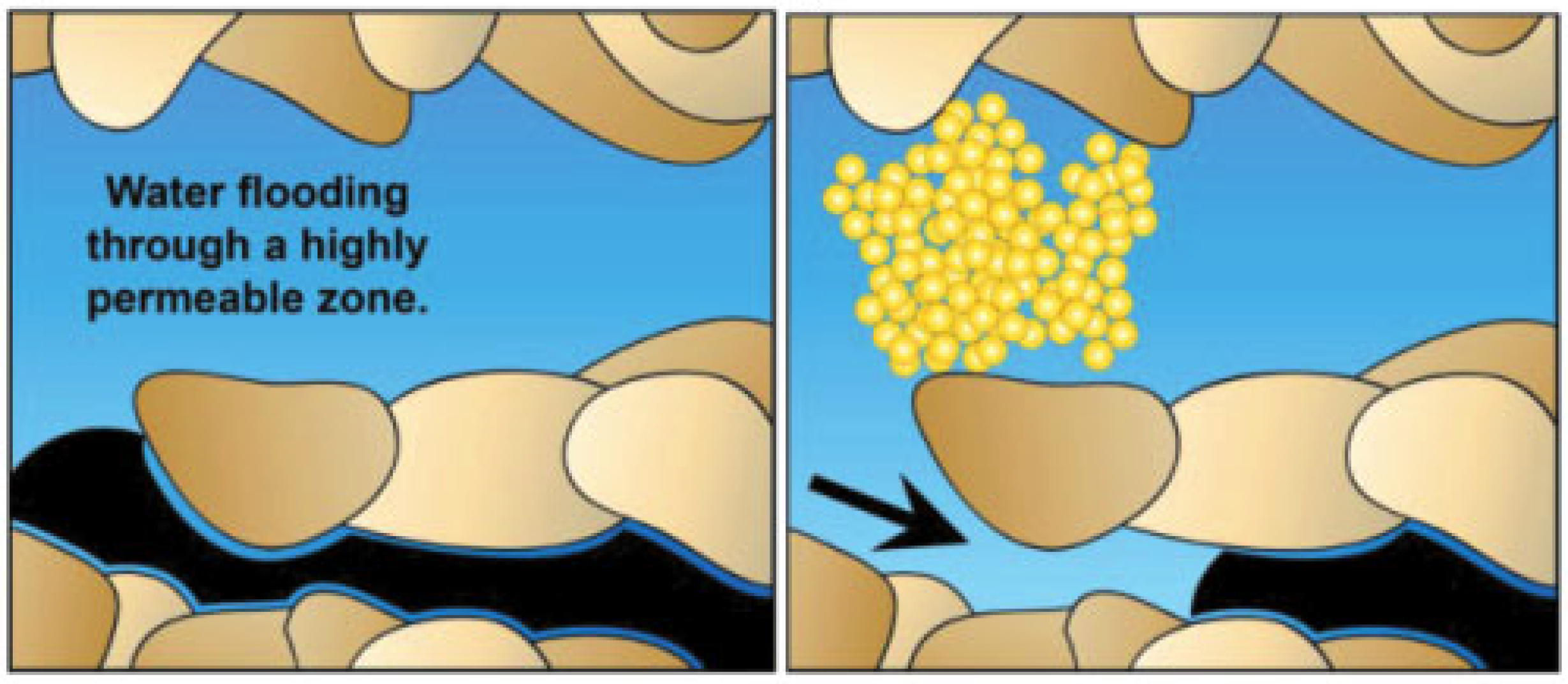
4.4. Improve the Flow Ratio
4.5. Improving Crude Oil Fluidity
- Thermal cracking for viscosity reduction
- Emulsification for viscosity reduction
5. Application of Nanofluid EOR Technology
6. Shortcomings and Future Development of Nanofluid EOR Technology
- Long term stability of nanofluids
- The Influence of Nanofluids on Reservoirs
- The Composition and Characteristics of Nanofluids
- The Transportation and Dispersion of Nanofluids
- The Economic Viability of Nanofluids
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. Asme Fed. 1995, 231, 99–105. [Google Scholar]
- Dolui, S.; Bhaumik, B.; De, S. Effect of a variable magnetic field on peristaltic slip flow of blood based hybrid nanofluid through a non-uniform annular channel. J. Mech. Med. Biol. 2023, 23, 2250070. [Google Scholar] [CrossRef]
- Baïri, A. Effects of ZnO-H2O nanofluid saturated porous medium on the thermal behavior of electronics contained in tilted hemispherical cavity. An experimental and numerical study. Appl. Therm. Eng. 2018, 138, S924–S933. [Google Scholar] [CrossRef]
- Sadabadi, H.; Sanati Nezhad, A. Nanofluids for performance improvement of heavy machinery journal bearings, A simulation study. Nanomaterials 2020, 10, 2120. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.U.; Bicer, Y. Performance Assessment of Spectrum Selective Nanofluid-Based Cooling for a Self-Sustaining Greenhouse. Energy Technol. 2021, 9, 2000875. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, L.; Luo, J.; Wang, P.; Ding, B.; Zeng, M.; Cheng, Z. A review of nanomaterials for nanofluid enhanced oil recovery. RSC Adv. 2017, 7, 32246–32254. [Google Scholar] [CrossRef]
- Hamza, M.F.; Soleimani, H.; Ridha, S.; Ahmed, A.A.; Sikiru, S. Double layer chemical encapsulation of SiO2 nanoparticles for interfacial tension reduction under low salinity condition. J. Mol. Liq. 2023, 371, 121100. [Google Scholar] [CrossRef]
- He, L.; Yang, L.; Zhang, L.; Wang, Z.; Shen, J. Removal of Ca2+ and Mg2+ from oilfield wastewater using reusable PEG/Fe3O4/GO-NH2 nanoadsorbents and its efficiency for oil recovery. J. Environ. Chem. Eng. 2020, 9, 104653. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Q. New progress and prospect of oilfields development technologies in China. Pet. Explor. Dev. 2018, 45, 698–711. [Google Scholar] [CrossRef]
- Luan, J.; Dong, P.; Zheng, J. Experimental studies on reaction laws during the process of air injection into the oil reservoirs with low permeability. J. Pet. Sci. Eng. 2020, 194, 107526. [Google Scholar] [CrossRef]
- Nikolov, A.; Zhang, H. The dynamics of capillary-driven two-phase flow, The role of nanofluid structural forces. J. Colloid Interface 2015, 449, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, X.; Li, W.; Wang, L.; Ning, H.; Zhong, S. Monodisperse SiO2 Microspheres with Large Specific Surface Area, Preparation and Particle Size Control. Res. Appl. Mater. Sci. 2021, 3, 17–23. [Google Scholar]
- Song, W.; Hatzignatiou, D.G. On the reduction of the residual oil saturation through the injection of polymer and nanoparticle solutions. J. Pet. Sci. Eng. 2021, 208, 109430. [Google Scholar] [CrossRef]
- Matteo, C.; Candido, P.; Vera, R.; Francesca, V. Current and future nanotech applications in the oil industry. Am. J. Appl. Sci. 2012, 9, 784. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.K.; Lee, J.K.; Jeong, Y.M.; Cheong, S.I.; Ahn, Y.C.; Kim, S. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Manshad, A.K.; Mohammadi, A.H. Effects of concentration and size of TiO2 nano-particles on the performance of smart water in wettability alteration and oil production under spontaneous imbibition. J. Pet. Sci. Eng. 2019, 183, 106357. [Google Scholar] [CrossRef]
- Ziaraty, A.; Saboori, R.; Sabbaghi, S.; Rasouli, K. Investigation of the effect of Fe3O4/SiO2 nanofluid on asphaltene adsorption and wettability alteration in hydrocarbon reservoirs, Optimization of nanocomposite composition and nanofluid concentration. Chem. Eng. Res. Des. 2023, 194, 810–828. [Google Scholar] [CrossRef]
- Haeri, F.; Rao, D.N. Precise Wettability Characterization of Carbonate Rocks to Evaluate Oil Recovery Using Surfactant-Based Nanofluids. Energy Fuels 2019, 33, 8289–8301. [Google Scholar] [CrossRef]
- Zuniga, C.A.; Goods, J.B.; Cox, J.R.; Swager, T.M. Long-term high-temperature stability of functionalized graphene oxide nanoplatelets in Arab-D and API brine. ACS Appl. Mater. Interfaces 2016, 8, 1780–1785. [Google Scholar] [CrossRef]
- Esmaeilnezhad, E.; Van, S.L.; Chon, B.H.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M. An experimental study on enhanced oil recovery utilizing nanoparticle ferrofluid through the application of a magnetic field. J. Ind. Eng. Chem. 2018, 58, 319–327. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Khazaei, M.; Misaghi, M.; Koosheshi, M.H. Improving the stability of nanofluids via surface-modified titanium dioxide nanoparticles for wettability alteration of oil-wet carbonate reservoirs. Mater. Res. Express 2022, 9, 035005. [Google Scholar] [CrossRef]
- Keykhosravi, A.; Vanani, M.B.; Aghayari, C. TiO2 nanoparticle-induced Xanthan Gum Polymer for EOR, Assessing the underlying mechanisms in oil-wet carbonates. J. Pet. Sci. Eng. 2021, 204, 108756. [Google Scholar] [CrossRef]
- Samba, M.A.; Hassan, H.A.; Munayr, M.S.; Yusef, M.; Eschweido, A.; Burkan, H.; Elsharafi, M.O. Nanoparticles EOR aluminum oxide (Al2O3) used as a spontaneous imbibition test for sandstone core. ASME International Mechanical Engineering Congress and Exposition. Am. Soc. Mech. Eng. 2019, 59438, V006T06A054. [Google Scholar]
- Mansouri, M.; Nakhaee, A.; Pourafshary, P. Effect of SiO2 nanoparticles on fines stabilization during low salinity water flooding in sandstones. J. Pet. Sci. Eng. 2019, 174, 637–648. [Google Scholar] [CrossRef]
- Mollick, M.M.R.; Bhowmick, B.; Mondal, D.; Maity, D.; Rana, D.; Dash, S.K.; Chattopadhyay, S.; Roy, S.; Sarkar, J.; Acharya, K.; et al. Anticancer (in vitro) and antimicrobial effect of gold nanoparticles synthesized using Abelmoschus esculentus (L.) pulp extract via a green route. RSC Adv. 2014, 4, 37838–37848. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, F.; Wang, Q.; Qu, X.; Yang, Z. Flexible responsive Janus nanosheets. Chem. Commun. 2015, 51, 3562–3565. [Google Scholar] [CrossRef]
- Zaid, H.M.; Radzi, N.S.A.; Latiff, N.R.A.; Shafie, A. Effect of morphology of aluminium oxide nanoparticles on viscosity and interfacial tension (IFT) and the recovery efficiency in enhanced oil recovery (EOR). AIP Conf. Proc. 2014, 1621, 705–710. [Google Scholar]
- Pranav, T.; Navpreet, K.; Vinay, S.; Mobin, S.M. High-yield graphene produced from the synergistic effect of inflated temperature and gelatin offers high stability and cellular compatibility. Phys. Chem. Chem. Phys. 2018, 20, 20096–20107. [Google Scholar]
- Raj, I.; Liang, T.; Qu, M.; Xiao, L.; Xian, C. An experimental investigation of MoS2 nanosheets stabilized foams for enhanced oil recovery application. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125420. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Manshad, A.K.; Mohammadi, A.H. Effects of TiO2, MgO, and γ-Al2O3 nano-particles in carbonated water on water-oil interfacial tension (IFT) reduction in chemical enhanced oil recovery (CEOR) process. J. Mol. Liq. 2019, 292, 111348. [Google Scholar] [CrossRef]
- Tajmiri, M.; Ehsani, M.R. Water Saving by using Nanoparticles in Heavy Oil Reservoir through Thermal EOR Method, Special Pertaining to ZnO & CuO. Ambient Sci. 2017, 4, 7–12. [Google Scholar]
- Barahoei, M.; Hezave, Z.; Sabbaghi, S.; Ayatollahi, S. Copper oxide nano-fluid stabilized by ionic liquid for enhancing thermal conductivity of reservoir formation, Applicable for thermal Enhanced Oil Recovery processes. Chem. Ind. Chem. Eng. Q. 2016, 22, 211–225. [Google Scholar] [CrossRef]
- Mortimer, M.; Kasemets, K.; Heinlaan, M.; Kurvet, I.; Kahru, A. High throughput kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles. Toxicol. Vitr. 2008, 22, 1412–1417. [Google Scholar] [CrossRef]
- Tangestani, E.; Vafaie, S.M.; Shadman, M.M.; Kazemi, T.E.; Ahmadi, S. Performance of low-salinity water flooding for enhanced oil recovery improved by SiO2 nanoparticles. Pet. Sci. 2019, 16, 357–365. [Google Scholar]
- Sun, Z.; Wang, X.; Kang, X. The Reservoir Adaptability Evaluation and Oil Displacement Mechanism of a Novel Particle-Type Polymer Flooding Technology. Soc. Sci. Electron. Publ. 2021, 3898483. [Google Scholar] [CrossRef]
- Caplan, S.P.C.; Silva, T.B.G.; Franscisco, A.D.S.; Lachter, E.R.; Nascimento, R.S.V. Sulfonated Polystyrene Nanoparticles as Oleic Acid Diethanolamide Surfactant Nanocarriers for Enhanced Oil Recovery Processes. Polymers 2019, 11, 1513. [Google Scholar] [CrossRef]
- Esfahlan, M.S.; Khodapanah, E.; Tabatabaei-Nezhad, S.A. Fabrication, optimization and characterization of preformed-particle-gel containing nanogel particles for conformance control in oil reservoirs. Polym. Bull. 2022, 79, 7137–7159. [Google Scholar] [CrossRef]
- Aadland, R.C.; Akarri, S.; Heggset, E.B.; Syverud, K.; Torster, O. A core flood and microfluidics investigation of nanocellulose as a chemical additive to water flooding for EOR. Nanomaterials 2020, 10, 1296. [Google Scholar] [CrossRef]
- Huang, P.; Jia, H.; Wang, T.; Xu, Y.; Liu, D. Effects of modification degrees on the colloidal stability of amphiphilic Janus graphene oxide in aqueous solution with and without electrolytes. Langmuir 2021, 37, 10061–10070. [Google Scholar] [CrossRef]
- Jafarbeigi, E.; Salimi, F.; Kamari, E.; Mansouri, M. Effects of modified graphene oxide (GO) nanofluid on wettability and IFT changes, Experimental study for EOR applications. Pet. Sci. 2022, 19, 1779–1792. [Google Scholar] [CrossRef]
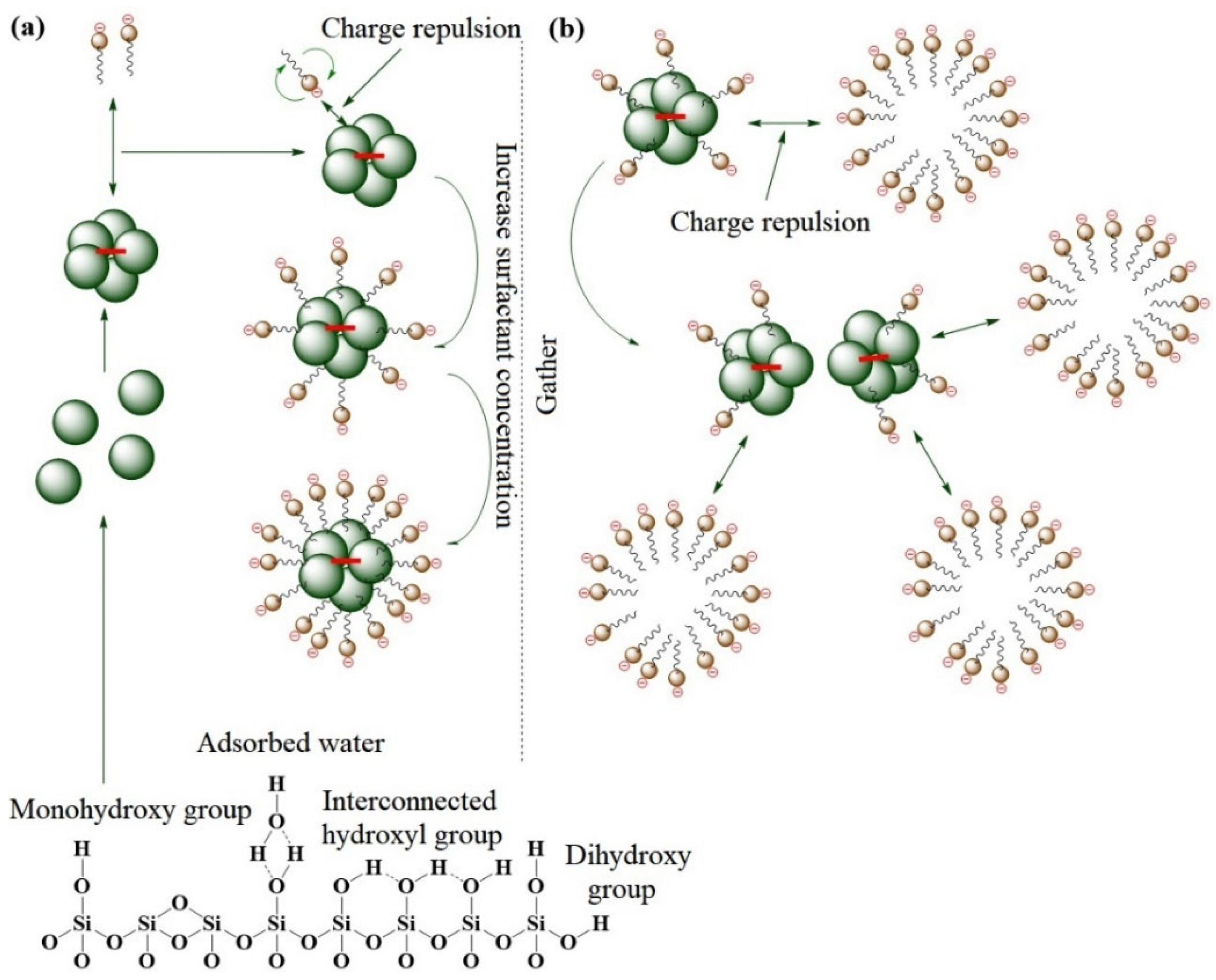
- Wang, B.; Hu, J.; Liu, K.; Zhang, L.; Jiang, H.; Li, C. Reinforcement mechanism of silica surface hydroxyl, The opposite effect. Appl. Surf. Sci. 2023, 623, 157000. [Google Scholar] [CrossRef]
- Zhou, R.; Ma, H.; Zhou, Z.; Xu, W.; Ren, F.; Li, C. Preparation of SiO2 particles with silicone-methoxy groups on surface and its co-curing hydroxyl silicone oil. Mater. Res. Express 2020, 7, 065309. [Google Scholar] [CrossRef]
- Afifi, H.R.; Mohammadi, S.; Derazi, A.M.; Alemi, F.M. Enhancement of smart water-based foam characteristics by SiO2 nanoparticles for EOR applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127143. [Google Scholar] [CrossRef]
- Jia, H.; Dai, J.; Miao, L.; Wei, X.; Tang, H.; Huang, P.; Liu, D. Potential application of novel amphiphilic Janus-SiO2 nanoparticles stabilized O/W/O emulsion for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126658. [Google Scholar] [CrossRef]
- Ojo, O.F.; Farinmade, A.; John, V.; Nguyen, D. A nanocomposite of halloysite/surfactant/wax to inhibit surfactant adsorption onto reservoir rock surfaces for improved oil recovery. Energy Fuels 2020, 34, 8074–8084. [Google Scholar] [CrossRef]
- Asl, F.O.; Zargar, G.; Manshad, A.K.; Iglauer, S.; Keshavarz, A. Experimental investigation and simulation for hybrid of nanocomposite and surfactant as EOR process in carbonate oil reservoirs. Fuel 2022, 319, 123591. [Google Scholar] [CrossRef]
- Mahdavinezhad, M.; Kazemi-Beydokhti, A.; Sanati, A.; Malayeri, M.R. Efficacy of f-MWCNT-CTAB nano-complex in low-salinity seawater EOR operation. J. Pet. Sci. Eng. 2022, 208, 109532. [Google Scholar] [CrossRef]
- Liang, T.; Hou, J.; Xi, J. Mechanisms of nanofluid based modification MoS2 nanosheet for enhanced oil recovery in terms of interfacial tension, wettability alteration and emulsion stability. J. Dispers. Sci. Technol. 2023, 44, 26–37. [Google Scholar] [CrossRef]
- Hu, J.; Chen, M.; Wu, L. Organic-inorganic nanocomposites synthesized viaminiemulsion polymerization. Polym. Chem. 2011, 2, 760–772. [Google Scholar] [CrossRef]
- Visco, A.; Yousef, S.; Galtieri, G.; Nocita, D.; Pistone, A.; Njuguna, J. Thermal, Mechanical and Rheological Behaviors of Nanocomposites Based on UHMWPE/Paraffin Oil/Carbon Nanofiller Obtained by Using Different Dispersion Techniques. JOM 2016, 68, 1078–1089. [Google Scholar] [CrossRef]
- Wang, D.; Sun, S.; Sha, T.; Liu, T.; Dong, H.; Cui, K.; Li, H.; Gong, Y.; Hou, J.; Zhang, Z.; et al. Synergistic Effect of Silica Nanoparticles and Rhamnolipid on Wettability Alteration of Low Permeability Sandstone Rocks. Energy Fuels 2018, 32, 8098–8107. [Google Scholar] [CrossRef]
- Pereira, M.L.D.O.; Maia, K.C.; Silva, W.C.; Leite, A.C.; Francisco, A.D.D.S.; Vasconcelos, T.L.; Regina, S.N.; Grasseschi, D. Fe3O4 nanoparticles as surfactant carriers for enhanced oil recovery and scale prevention. ACS Appl. Nano Mater. 2020, 3, 5762–5772. [Google Scholar] [CrossRef]
- Azizpour, H.; Talebi, M.; Tichelaar, F.D.; Sotudeh-Gharebagh, R.; Guo, J.; Van Ommen, J.R.; Mostoufi, N. Effective coating of titania nanoparticles with alumina via atomic layer deposition. Appl. Surf. Sci. 2017, 426, 480–496. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Mahdavian, M.; Karimi, E. Evaluation of the corrosion protection properties of an epoxy coating containing sol–gel surface modified nano-zirconia on mild steel. Rsc Adv. 2015, 5, 28769–28777. [Google Scholar] [CrossRef]
- Pu, H.; Xu, L. Molecularly Imprinted Nanoparticles Synthesized by Electrochemically Mediated Atom Transfer Radical Precipitation Polymerization. Macromol. Chem. Phys. 2022, 223, 2100478. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Liu, S. Synthesis of OCPs Composite Spheres Using Core-shell PS Templates with Microchannels in Shell. J. Funct. Mater. 2013, 44, 3331–3334. [Google Scholar]
- Rezvani, H.; Riazi, M.; Tabaei, M.; Kazemzadeh, Y.; Sharifi, M. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4@Chitosan nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 15–27. [Google Scholar] [CrossRef]
- Long, Y.; Huang, X.; Gao, Y.; Chen, L.; Song, F.; Zhang, H. Swelling Mechanism of Core–Shell Polymeric Nanoparticles and Their Application in Enhanced Oil Recovery for Low-Permeability Reservoirs. Energy Fuels 2019, 33, 3077–3088. [Google Scholar] [CrossRef]
- Radnia, H.; Rashidi, A.; Nazar, A.R.S.; Eskandari, M.M.; Jalilian, M. A novel nanofluid based on sulfonated graphene for enhanced oil recovery. J. Mol. Liq. 2018, 271, 795–806. [Google Scholar] [CrossRef]
- Bai, Y.; Pu, C.; Liu, S.; Fan, Q.; Zhang, C.; Chen, Z. Preparation and Enhanced Imbibition Recovery Factor Performance of Amphiphilic Nano-SiO2 Particles. Fine Chem. 2022, 39, 828–836. [Google Scholar]
- Fan, H.; Striolo, A. Nanoparticle effects on the water-oil interfacial tension. Phys. Rev. E 2012, 86, 051610. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Bai, B. The synergistic effects of nanoparticle-surfactant nanofluids in EOR applications. J. Pet. Sci. Eng. 2018, 171, 196–210. [Google Scholar]
- Natalya, S.A.C.; Kadja, G.T.M.; Azhari, N.J. Two-dimensional (2D) nanomaterials for enhanced oil recovery (EOR), A review. FlatChem 2022, 34, 100383. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Wu, T.; Zhao, Y.; Zhou, D.; Liu, S. Pore-Scale Investigation on the Plugging Behavior of Submicron-Sized Microspheres for Heterogeneous Porous Media with Higher Permeability. Geofluids 2020, 2020, 8869760. [Google Scholar] [CrossRef]
- Combariza, M.Y.; Gonzalez, M.M.; Blanco-Tirado, C. Nanocellulose as an inhibitor of water-in-crude oil emulsion formation. Fuel 2020, 264, 116830. [Google Scholar]
- Michels, R.; Siebert, D.N.; Santos, L.O.E.D. Investigation on the influence of capillary number on the immiscible displacement using a lattice boltzmann method. J. Pet. Sci. Eng. 2021, 205, 108918. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Chen, S.; Mbia, E.; Wu, K. The Second Critical Capillary Number for Chemical Flooding in Low Permeability Reservoirs: Experimental and Numerical Investigations. Chem. Eng. Sci. 2019, 196, 202–213. [Google Scholar] [CrossRef]
- Hethnawi, A.; Ashoorian, S.; Hashlamoun, K.; Contrerasmateus, M.; Sagala, F.; Nassar, N.N. Influence of CTAB-Grafted Faujasite Nanoparticles on the Dynamic Interfacial Tension of Oil/Water Systems. Energy Fuels 2022, 36, 5666–5680. [Google Scholar] [CrossRef]
- Al-Yaseri, A.Z.; Roshan, H.; Lebedev, M.; Barifcani, A.; Iglauer, S. Dependence of quartz wettability on fluid density. Geophys. Res. Lett. 2016, 43, 3771–3776. [Google Scholar] [CrossRef]
- Olugbenga, F.; Edo, M. Wettability Effects on Capillary Pressure, Relative Permeability, and Irredcucible Saturation Using Porous Plate. J. Pet. Eng. 2014, 2014, 465418. [Google Scholar]
- Wang, T.; Zhang, Y.; Li, L.; Yang, Z.; Liu, Y.; Fang, J.; Dai, C.; You, Q. Experimental study on pressure-decreasing performance and mechanism of nanoparticles in low permeability reservoir. J. Pet. Sci. Eng. 2018, 166, 693–703. [Google Scholar] [CrossRef]
- Tetteh, J.; Bai, S.; Kubelka, J.; Piri, M. Wettability reversal on oil-wet calcite surfaces, Experimental and computational investigations of the effect of the hydrophobic chain length of cationic surfactants. J. Colloid Interface Sci. 2022, 619, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Jia, R.; Fu, M.; Wang, Y.; Huang, Y. Wettability alteration of oil-wet carbonate surface induced by self-dispersing silica nanoparticles, Mechanism and monovalent metal ion’s effect. J. Mol. Liq. 2019, 294, 111601. [Google Scholar] [CrossRef]
- Sneha, M.; Ng, Z.Y.; Sheard, G.J. Stability of flow in a channel with repeated flow-facing wedge-shaped protrusions. J. Fluid Mech. 2022, 941, A59. [Google Scholar]
- Kondiparty, K.; Nikolov, A.; Wu, S.; Wasan, D. Wetting and spreading of nanofluids on solid surfaces driven by the structural disjoining pressure, statics analysis and experiments. Langmuir ACS J. Surf. Colloids 2011, 27, 3324–3335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ramakrishnan, T.S.; Nikolov, A.; Wasan, D. Enhanced oil recovery driven by nanofilm structural disjoining pressure, flooding experiments and microvisualization. Energy Fuels 2016, 30, 2771–2779. [Google Scholar] [CrossRef]
- Henderson, D. An explicit expression for the solvent contribution to the force between colloidal particles using a hard sphere model. J. Colloid Interface Sci. 1988, 121, 486–490. [Google Scholar] [CrossRef]
- Trokhymchuk, A.; Henderson, D.; Nikolov, A.; Wasan, D.T. A Simple Calculation of Structural and Depletion Forces for Fluids/Suspensions Confined in a Film. Langmuir 2001, 17, 4940–4947. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Ali, M.; Memon, S.; Bhatti, M.A.; Lagat, C.; Sarmadivaleh, M. Reversible and irreversible adsorption of bare and hybrid silica nanoparticles onto carbonate surface at reservoir condition. Petroleum 2020, 6, 277–285. [Google Scholar]
- Kułynycz, V.; Pikłowska, A.; Kulynych, O. Overview of the nanoparticles application for reservoir engineering and Enhanced Oil Recovery (EOR) methods. AGH Drill. Oil Gas 2018, 35, 107–124. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K.; Chauhan, P.S. Nanoparticles applications for hydraulic fracturing of unconventional reservoirs, A comprehensive review of recent advances and prospects. J. Pet. Sci. Eng. 2019, 178, 41–73. [Google Scholar] [CrossRef]
- Lin, W.Z.; Lai, Y.S.; Hao, L.; Zhan, M.; Lu, W.; Ying, L. Influence of polymer injecting occasion on the sweep efficiency and sweep front in reservoirs with thick heterogeneous oil layers. Sci. Technol. Eng. 2017, 17, 197–202. [Google Scholar]
- Pasrija, R.; Gupta, S. Modified model for the effective thermal conductivity of metal oxide nanofluids. Mater. Today Proc. 2021, 34, 621–625. [Google Scholar] [CrossRef]
- Bila, A.; Torster, O. Experimental Investigation of Polymer-Coated Silica Nanoparticles for EOR under Harsh Reservoir Conditions of High Temperature and Salinity. Nanomaterials 2021, 11, 765. [Google Scholar] [CrossRef]
- Dai, C.; Wang, T.; Zhao, M.; Sun, X.; Gao, M.; Xu, Z.; Guan, B.; Liu, P. Impairment mechanism of thickened supercritical carbon dioxide fracturing fluid in tight sandstone gas reservoirs. Fuel 2018, 211, 60–66. [Google Scholar] [CrossRef]
- Safran, S.E.; Kok, M.V. Nanoparticle-stabilized CO2 foam to improve conventional CO2 EOR process and recovery at Batı Raman oil field, Turkey. J. Pet. Sci. Eng. 2022, 208, 109547. [Google Scholar] [CrossRef]
- Ortega, D.J.S. Alteration of the Wetting Character of a Composite Rock, through the Use of Nanoparticles as an Enhanced Oil Recovery Method, Ben Nevis Formation, Hebron Field, Jeanne d’Arc Basin, Offshore Newfoundland, Canada. Ph.D. Dissertation, Memorial University of Newfoundland, St. John’s, NL, Canada, 2018. [Google Scholar]
- Wu, T.; Zhao, Y.; Cheng, C.; Cao, R. Evaluation and Application of Polymer Microsphere and Surfactant Compound Profile Control and Flooding System. Oilfield Chem. 2022, 39, 46–50+81. [Google Scholar]
- Chen, G.; Wang, W.; Zhu, J.; Dai, G. Research and Application of Nano Flooding Technology—Taking QSS-46 Well Group as An Example. Drill. Prod. Technol. 2021, 44, 110–113. [Google Scholar]
- Hou, J.; Wen, Y.; Qu, M.; Liang, T.; Wu, W.; Ding, Y. Research and Application of Nano–materials to Enhance Oil and Gas Recovery Technology. Spec. Oil Gas Reserv. 2020, 27, 47–53. [Google Scholar]




| Nanofluids | Main Components | Mechanism and Effect | Ref |
|---|---|---|---|
| Fe3O4 | Fe3O4 NPs aqueous solution | Increase oil recovery by 14% under the magnetic field. | [20] |
| TiO2 | TiO2 NPs aqueous solution | Reduce the forward and backward angles of water from 165.1° and 166.2° to 37.6° and 48.2°. | [21] |
| TiO2 NPs; xanthan gum solution | Increase the CA of oil from 21° to 148°, increase oil recovery by 25%. | [22] | |
| Al2O3 | Spherical Al2O3 NPs aqueous solution | Reduce crude oil viscosity, increase 3.1% oil recovery. | [23] |
| Sheet-like Al2O3 NPs aqueous solution | Reduce the IFT, increase 10% oil recovery. | [27] | |
| MgO | MgO NPs aqueous solution | Reduce the IFT to 3.7 under high temperature and pressure. | [30] |
| ZnO | ZnO NPs; Steam | Reduce crude oil viscosity; the efficiency of steam flooding has increased by 35.5%. | [31] |
| ZnO@PAM nanocomposites; cationic surfactants solution | Reduced the IFT from 29.16 to 0.176 mN/m; decreased the CA of water from 145.86° to 12.79°; increase 30% oil recovery. | [47] | |
| CuO | CuO NPs; Surfactant solution | Increase the thermal conductivity of rocks to 33%, thereby reducing the viscosity of crude oil. | [32] |
| SiO2 | SiO2 NPs; KCl solution. | Controlling fines migration and reducing the pressure drop in the porous media. | [34] |
| Janus C12 aqueous solution | The Janus-C12 stabilized multiple O/W/O Pickering emulsions improve the oil recovery by 27.2%. | [44] | |
| MoS2 | MoS2 nanosheets; AOS solution | The addition of modified nanosheets to the foam generation leads to 12.1% of increased oil recovery. | [29] |
| KH-550-MoS2 aqueous solution | Reduce the IFT to 2.6 mN/m, reduce the CA of water from 131.2° to 51.7°; increase 14% oil recovery. | [48] | |
| Polymer NPs | Nanogel microsphere aqueous solution | High water absorption and expansion performance under high temperature and high-salinity-conditions. | [37] |
| Cellulose nanocrystals | CNCs; T-CNFs; LSW | CNCs can reduce the IFT; T-CNFs can change the wettability of rocks; produced 5.8% of OOIP more oil than LSW. | [38] |
| Graphene Oxide | Aqueous solution of GO-Su-HMDS | Reduce the IFT from 18.45 to 8.8, change the wettability of rocks; increase oil recovery by 20%. | [40] |
| Halloysite nanotubes | Aqueous solution of halloysite nanotubes containing surfactants inside | Improved oil displacement by 16% compared to using surfactants alone. | [45] |
| Carbon nanotubes | f-MWCNT-CTAB; LSSW | Reduced the IFT to 0.3 mN/m, changing the dolomite slabs wettability from an oil-wet toward a neutral-wet state (128–105°), result in a 21% increase in oil recovery after secondary water injection. | [47] |
| Modification Method | Operation Method | Advantages And Performance Improvement Mechanism | Difficulties and Challenges |
|---|---|---|---|
| Physical blending modification | mechanical mixing, ultrasonic blending, magnetic stirring. | Synergy occurs between NPs and surfactants or polymers. This synergistic effect has positive effects on enhanced oil recovery, such as improved surfactant interfacial activity and reduced shear dilution of polymers. | The interaction mechanism between NPs and their blends is not yet clear, and the risk of reservoir damage increases when multiple substances are mixed. |
| Chemical coating modification | deposition, sol-gel, electroplating], and polymer encapsulation. | Composite NPs are commonly prepared using the method that combines the advantages of two or more different substances. Additionally, the particle size of these NPs can be adjusted to adapt to reservoir conditions with varying permeabilities. | Achieving precise control over the nanoparticle size can be challenging, and the dispersion index of particle sizes tends to remain relatively wide even after coating modification. |
| Surface grafting modification | solution immersion and interfacial polymerization. | Surface grafting modification involves altering the surface chemistry of particles by grafting new compounds through condensation reactions. This process aims to improve particle dispersion in the fluid, enhancing their compatibility with reservoir conditions. | It is difficult to accurately quantify the characteristic groups on the surface of NPs, making it difficult to control the proportion of reactants. This inaccuracy leads to the production of by-products during the reaction process, which not only affects the modification efficiency but also increases the cost of purification. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Q.; Fan, Z.; Liu, Q.; Qiao, S.; Cai, L.; Fu, Y.; Zhang, X.; Sun, A. Research Progress in Nanofluid-Enhanced Oil Recovery Technology and Mechanism. Molecules 2023, 28, 7478. https://doi.org/10.3390/molecules28227478
Tong Q, Fan Z, Liu Q, Qiao S, Cai L, Fu Y, Zhang X, Sun A. Research Progress in Nanofluid-Enhanced Oil Recovery Technology and Mechanism. Molecules. 2023; 28(22):7478. https://doi.org/10.3390/molecules28227478
Chicago/Turabian StyleTong, Qilei, Zhenzhong Fan, Qingwang Liu, Sanyuan Qiao, Li Cai, Yuanfeng Fu, Xuesong Zhang, and Ao Sun. 2023. "Research Progress in Nanofluid-Enhanced Oil Recovery Technology and Mechanism" Molecules 28, no. 22: 7478. https://doi.org/10.3390/molecules28227478





