Molecular Hybridization of Alkaloids Using 1,2,3-Triazole-Based Click Chemistry
Abstract
1. Introduction
1.1. Natural Products as a Source of Bioactive Compounds
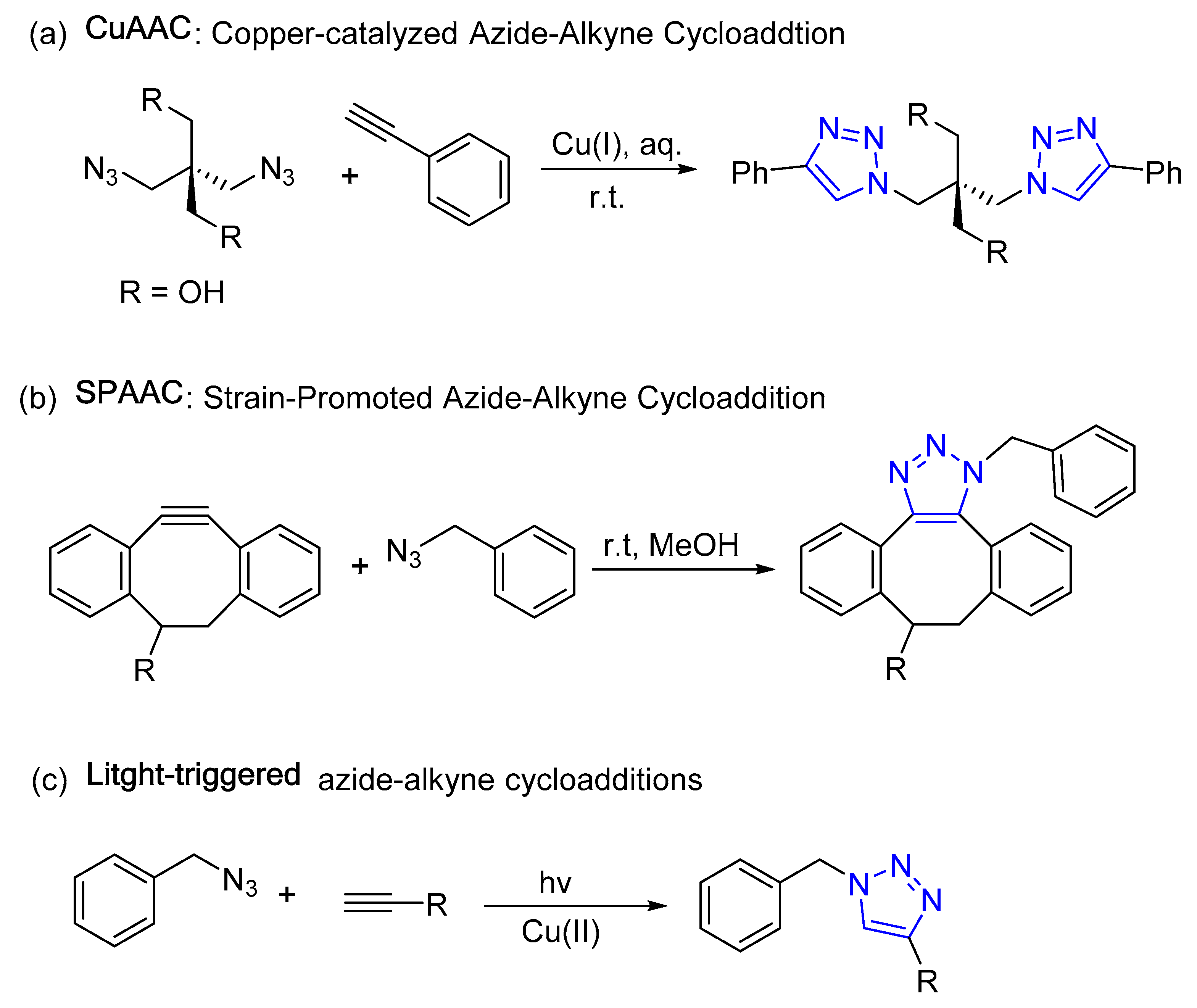
1.2. Click Chemistry: Principles and Applications
2. Application of Click Chemistry with Natural Product Hybridization
Molecular Hybridization of Alkaloids Using Click Chemistry

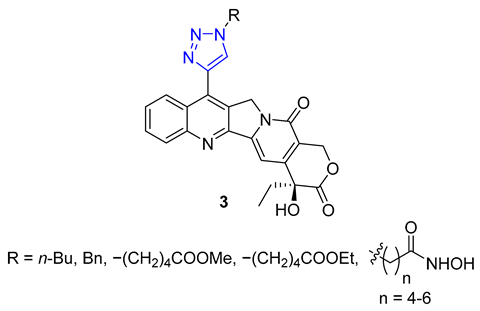
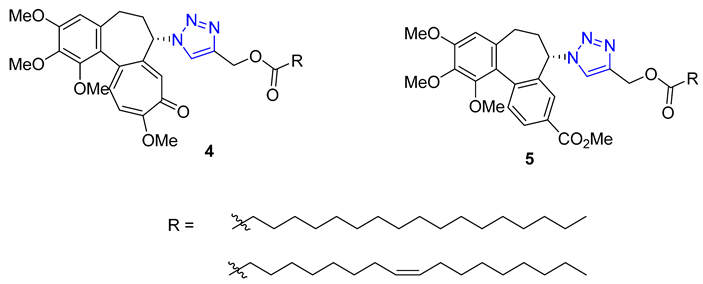
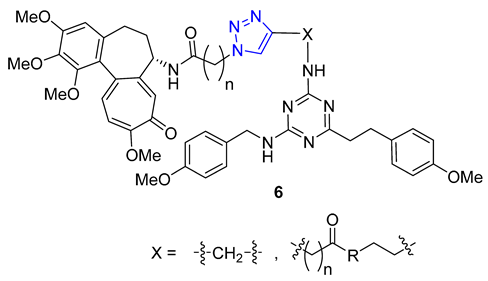
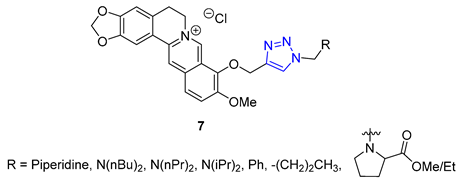


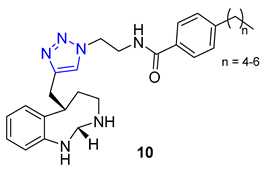
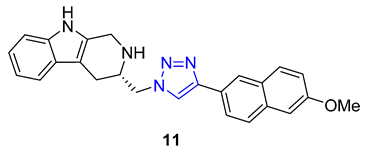
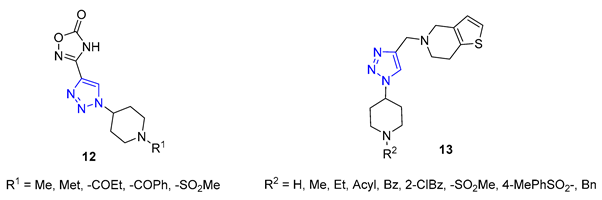
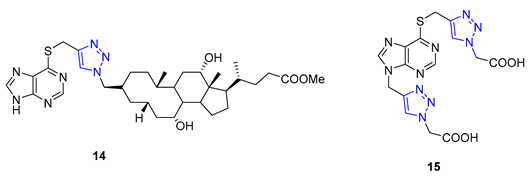
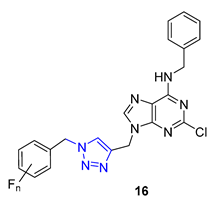
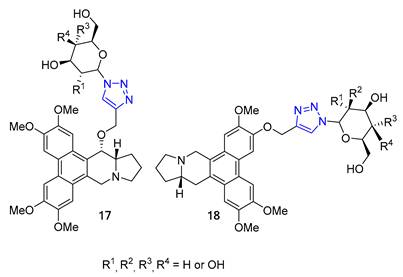
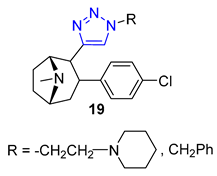
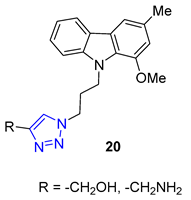
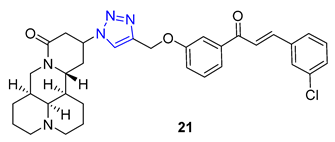
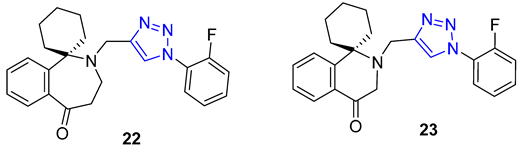
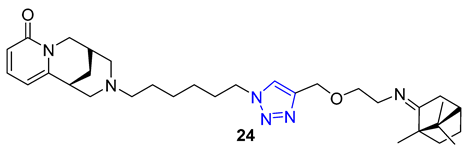
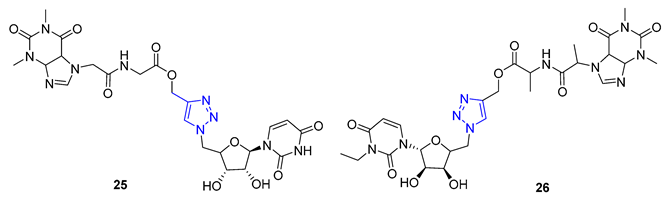
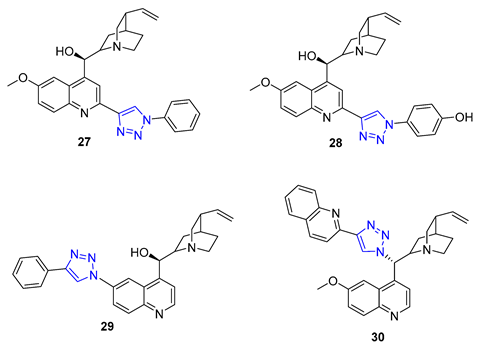
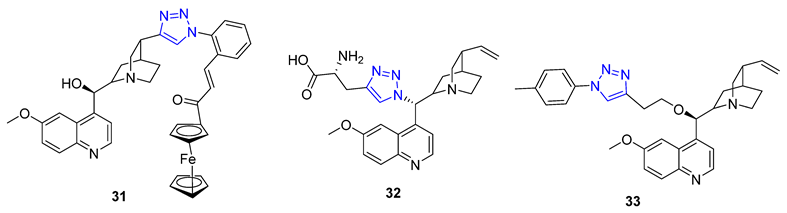

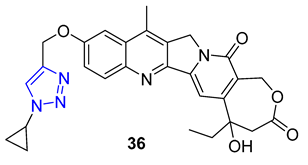
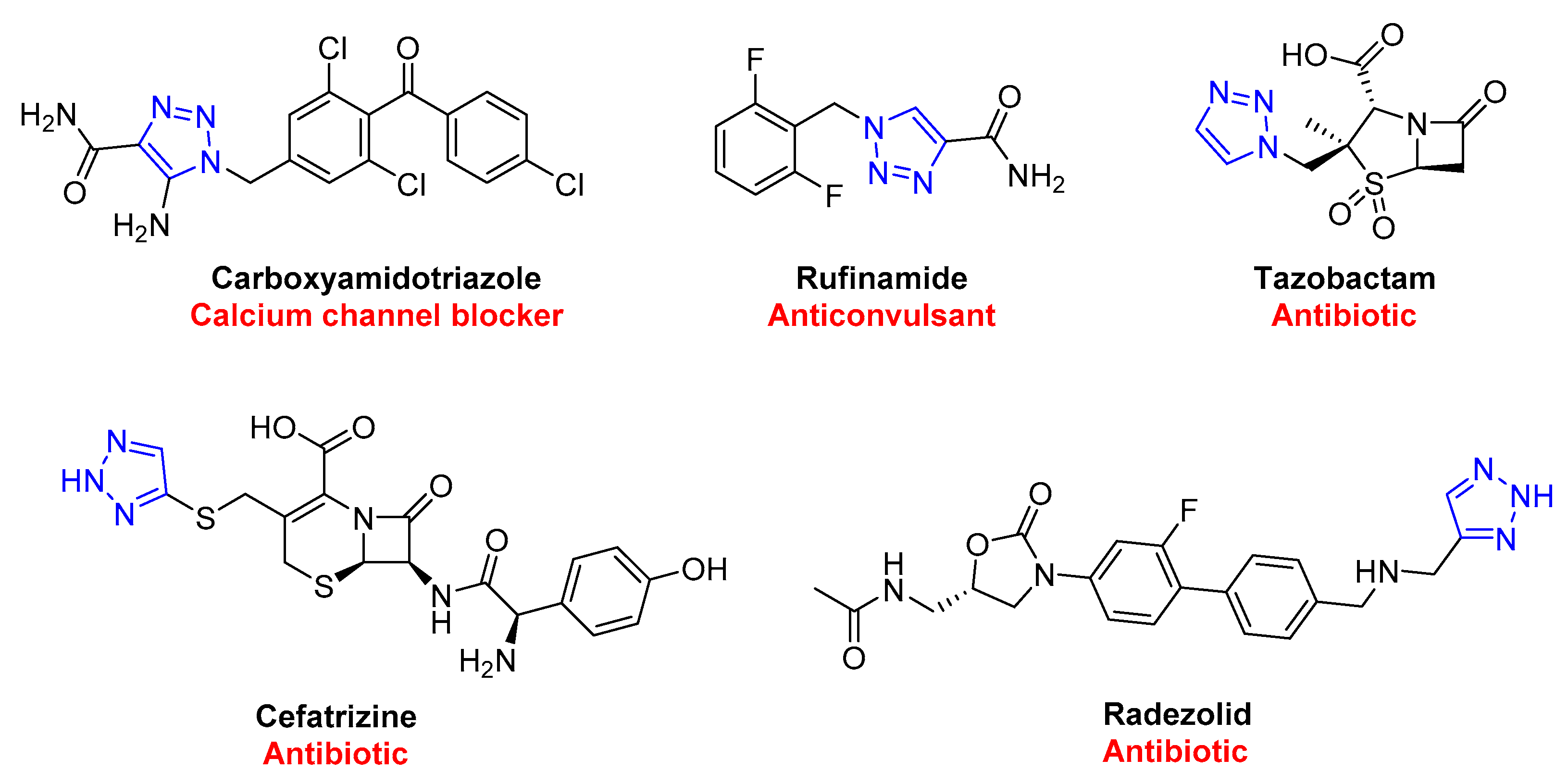
3. Drug-like Properties
4. Clinical Trials
5. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The international natural product sciences taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.; Zhou, Y.; Lian, X.; Yan, L.; Pan, T.; Jin, T.; Xie, H.; Liang, Z.; Qiu, W.; et al. NPCDR: Natural product-based drug combination and its disease-specific molecular regulation. Nucleic Acids Res. 2022, 50, D1324–D1333. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, , B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Barnes, E.C.; Kumar, R.; Davis, R.A. The use of isolated natural products as scaffolds for the generation of chemically diverse screening libraries for drug discovery. Nat. Prod. Rep. 2016, 33, 372–381. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Waltenberger, B.; Mocan, A.; Šmejkal, K.; Heiss, E.H.; Atanasov, A.G. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 2016, 21, 807. [Google Scholar] [CrossRef]
- Tintore, M.; Vidal-Jordana, A.; Sastre-Garriga, J. Treatment of multiple sclerosis—Success from bench to bedside. Nat. Rev. Neurol. 2019, 15, 53–58. [Google Scholar] [CrossRef]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.K. Biotechnology and Genetic Engineering for Alkaloid Production. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 213–250. [Google Scholar]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Smolke, C.D. Biosynthesis of medicinal tropane alkaloids in yeast. Nature 2020, 585, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Daley, S.K.; Cordell, G.A. Alkaloids in contemporary drug discovery to meet global disease needs. Molecules 2021, 26, 3800. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Sun, H.; Zhang, A.; Xu, H.; Yan, G.; Han, Y.; Wang, X. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids used as medicines: Structural phytochemistry meets biodiversity—An update and forward look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Olofinsan, K.; Abrahamse, H.; George, B.P. Therapeutic Role of Alkaloids and Alkaloid Derivatives in Cancer Management. Molecules 2023, 28, 5578. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Zhao, S.; Wang, X.; Liu, B.; Xu, H. Click Chemistry in Natural Product Modification. Front. Chem. 2021, 9, 774977. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Goncalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef]
- Harrison, J.R.; Brand, S.; Smith, V.; Robinson, D.A.; Thompson, S.; Smith, A.; Davies, K.; Mok, N.; Torrie, L.S.; Collie, I.; et al. A molecular hybridization approach for the design of potent, highly selective, and brain-penetrant n-myristoyltransferase inhibitors. J. Med. Chem. 2018, 61, 8374–8389. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, H.M.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef] [PubMed]
- Bokhtia, R.M.; Girgis, A.S.; Ibrahim, T.S.; Rasslan, F.; Nossier, E.S.; Barghash, R.F.; Sakhuja, R.; Abdel-Aal, E.H.; Panda, S.S.; Al-Mahmoudy, A.M.M. Synthesis, antibacterial evaluation, and computational studies of a diverse set of linezolid conjugates. Pharmaceuticals 2022, 15, 191. [Google Scholar] [CrossRef]
- Panda, S.S.; Tran, Q.L.; Rajpurohit, P.; Pillai, G.G.; Thomas, S.J.; Bridges, A.E.; Capito, J.E.; Thangaraju, M.; Lokeshwar, B.L. Design, synthesis, and molecular docking studies of curcumin hybrid conjugates as potential therapeutics for breast cancer. Pharmaceuticals 2022, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Thangaraju, M.; Lokeshwar, B.L. Ursolic acid analogs as potential therapeutics for cancer. Molecules 2022, 27, 8981. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.L.; Hansen, D.W.; Brown, L.D.; Stewart, L.E.; Ortiz, E.; Panda, S.S. Modified curcumins as potential drug candidates for breast cancer: An overview. Molecules 2022, 27, 8891. [Google Scholar] [CrossRef]
- Bokhtia, R.M.; Panda, S.S.; Girgis, A.S.; Samir, N.; Said, M.F.; Abdelnaser, A.; Nasr, S.; Bekheit, M.S.; Dawood, A.S.; Sharma, H.; et al. New NSAID conjugates as potent and selective COX-2 inhibitors: Synthesis, molecular modeling and biological investigation. Molecules 2023, 28, 1945. [Google Scholar] [CrossRef]
- Wyman, K.A.; Girgis, A.S.; Surapaneni, P.S.; Moore, J.M.; Abo Shama, N.M.; Mahmoud, S.H.; Mostafa, A.; Barghash, R.F.; Juan, Z.; Dobaria, R.D.; et al. Synthesis of potential antiviral agents for SARS-CoV-2 using molecular hybridization approach. Molecules 2022, 27, 5923. [Google Scholar] [CrossRef]
- Ghanim, A.M.; Girgis, A.S.; Kariuki, B.M.; Samir, N.; Said, M.F.; Abdelnaser, A.; Nasr, S.; Bekheit, M.S.; Abdelhameed, M.F.; Almalki, A.J.; et al. Design and synthesis of ibuprofen-quinoline conjugates as potential anti-inflammatory and analgesic drug candidates. Bioorg. Chem. 2022, 119, 105557. [Google Scholar] [CrossRef]
- Panda, S.S.; Girgis, A.S.; Mishra, B.B.; Elagawany, M.; Devarapalli, V.; Littlefield, W.F.; Samir, A.; Fayad, W.; Fawzy, N.G.; Srour, A.M.; et al. Synthesis, computational studies, antimycobacterial and antibacterial properties of pyrazinoic acid-isoniazid hybrid conjugates. RSC Adv. 2019, 9, 20450–20462. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Saxena, M.; Rishi, N. An overview of recent advances in biomedical applications of click chemistry. Bioconjugate Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Kondengadan, S.M.; Bansal, S.; Yang, C.; Liu, D.; Fultz, Z.; Wang, B. Click chemistry and drug delivery: A bird’s-eye view. Acta Pharm. Sin. B 2023, 13, 1990–2016. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Finn, M.G. Introduction: Click chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef]
- Best, M.D. Click chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochemistry 2009, 48, 6571–6584. [Google Scholar] [CrossRef]
- Bahia, S.B.B.B.; Reis, W.J.; Jardim, G.A.M.; Souto, F.T.; De Simone, C.A.; Gatto, C.C.; Menna-Barreto, R.F.; de Castro, S.L.; Cavalcanti, B.C.; Pessoa, C.; et al. Molecular hybridization as a powerful tool towards multitarget quinoidal systems: Synthesis, trypanocidal and antitumor activities of naphthoquinone-based 5-iodo-1,4-disubstituted-, 1,4- and 1,5-disubstituted-1,2,3-triazoles. Med. Chem. Commun. 2016, 7, 1555–1563. [Google Scholar] [CrossRef]
- Thomopoulou, P.; Sachs, J.; Teusch, N.; Mariappan, A.; Gopalakrishnan, J.; Schmalz, H.G. New colchicine-derived triazoles and their influence on cytotoxicity and microtubule morphology. ACS Med. Chem. Lett. 2016, 7, 188–191. [Google Scholar] [CrossRef]
- Li, H.-N.; Wang, H.; Wang, Z.-P.; Yan, H.-N.; Zhang, M.; Liu, Y.; Cheng, M.-S. Synthesis, antitumor activity evaluation and mechanistic study of novel hederacolchiside a1 derivatives bearing an aryl triazole moiety. Bioorg. Med. Chem. 2018, 26, 4025–4033. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Sinha, S.; Kumaran, A.P.; Mishra, D.; Paira, P. Synthesis and cytotoxicity study of novel 3-(triazolyl)coumarins based fluorescent scaffolds. Bioorg. Med. Chem. Lett. 2016, 26, 5557–5561. [Google Scholar] [CrossRef]
- Mbua, N.E.; Guo, J.; Wolfert, M.A.; Steet, R.; Boons, G.J. Strain-promoted alkyne-azide cycloadditions (SPAAC) reveal new features of glycoconjugate biosynthesis. ChemBioChem 2011, 12, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Qing, L. Light-Triggered Click Chemistry. Chem. Rev. 2021, 121, 6991–7031. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Balderas, F.; Ortega-Muñoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F.G.; Calvo-Asín, J.A.; Isac-García, J.; Santoyo-González, F. Multivalent Neoglycoconjugates by Regiospecific Cycloaddition of Alkynes and Azides Using Organic-Soluble Copper Catalysts. Org. Lett. 2003, 5, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Acik, G.; Tasdelen, M.A. The emerging applications of click chemistry reactions in the modification of industrial polymers. Polym. Chem. 2019, 10, 3806–3821. [Google Scholar] [CrossRef]
- Tietze, L.F.; Bell, H.P.; Chandrasekhar, S. Natural product hybrids as new leads for drug discovery. Angew. Chem. Int. Ed. 2003, 42, 3996–4028. [Google Scholar] [CrossRef]
- Mehta, G.; Singh, V. Hybrid systems through natural product leads: An approach towards new molecular entities. Chem. Soc. Rev. 2002, 31, 324–334. [Google Scholar] [CrossRef]
- Tomohara, K.; Ohashi, N.; Uchida, T.; Nose, T. Synthesis of natural product hybrids by the Ugi reaction in complex media containing plant extracts. Sci. Rep. 2022, 12, 15568. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- O’Connor, S.E.; Maresh, J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006, 23, 532–547. [Google Scholar] [CrossRef]
- Faisal, S.; Badshah, S.L.; Kubra, B.; Emwas, A.; Jaremko, M. Alkaloids as potential antivirals. A comprehensive review. Nat. Prod. Bioprospect. 2023, 13, 4. [Google Scholar] [CrossRef]
- Li, J.; Li, J.-X.; Jiang, H.; Li, M.; Chen, L.; Wang, Y.-Y.; Wang, L.; Zhang, N.; Guo, H.-Z.; Ma, K.-L. Phytochemistry and biological activities of corynanthe alkaloids. Phytochemistry 2023, 213, 113786. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, Y.; Wang, Y.; Jiang, M.; Yao, L. The recent developments of camptothecin and its derivatives as potential anti-tumor agents. Eur. J. Med. Chem. 2023, 260, 115710. [Google Scholar] [CrossRef]
- Islam, F.; Dehbia, Z.; Zehravi, M.; Das, R.; Sivakumar, M.; Krishnan, K.; Billah, A.A.M.; Bose, B.; Ghosh, A.; Paul, S.; et al. Indole alkaloids from marine resources: Understandings from therapeutic point of view to treat cancers. Chem. Biol. Interact. 2023, 383, 110682. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Q.; Chen, S.; Wang, S.; Lu, J.; Gao, X.; Zhang, D.; Jin, X. Natural epidithiodiketopiperazine alkaloids as potential anticancer agents: Recent mechanisms of action, structural modification, and synthetic strategies. Bioorg. Chem. 2023, 137, 106642. [Google Scholar] [CrossRef] [PubMed]
- Skiera, I.; Antoszczak, M.; Trynda, J.; Wietrzyk, J.; Boratynśki, P.; Kacprzak, K.; Huczynśki, A. Antiproliferative activity of polyether antibiotic—Cinchona alkaloid conjugates obtained via click chemistry. Chem. Biol. Drug Des. 2015, 86, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, W.; Zhang, J.; Tong, L.; Luo, Y.; Chen, Y.; Lu, W.; Huang, Q. Synthesis of 7-triazole-substituted camptothecin via click chemistry and evaluation of in vitro antitumor activity. Chin. J. Chem. 2014, 32, 157–162. [Google Scholar] [CrossRef]
- Nicolaus, N.; Zapke, J.; Riesterer, P.; Neudörfl, J.-M.; Prokop, A.; Oschkinat, H.; Schmalz, H.-G. azides derived from colchicine and their use in library synthesis: A practical entry to new bioactive derivatives of an old natural drug. Chem. Med. Chem. 2010, 5, 661–665. [Google Scholar] [CrossRef]
- Kuznetsova, N.R.; Svirshchevskaya, E.V.; Sitnikov, N.S.; Abodo, L.; Sutorius, H.; Zapke, J.; Velder, J.; Thomopoulou, P.; Oschkinat, H.; Prokop, A.; et al. Lipophilic prodrugs of a triazole-containing colchicine analogue in liposomes: Biological effects on human tumor cells. Russ. J. Bioorg. Chem. 2013, 39, 543–552. [Google Scholar] [CrossRef]
- Malysheva, Y.B.; Combes, S.; Allegro, D.; Peyrot, V.; Knochel, P.; Gavryushin, A.E.; Fedorov, A.Y. Synthesis and biological evaluation of novel anticancer bivalent colchicine-tubulizine hybrids. Bioorg. Med. Chem. 2012, 20, 4271–4278. [Google Scholar] [CrossRef]
- Shi, A.; Huang, L.; Lu, C.; He, F.; Li, X. Synthesis, biological evaluation and molecular modeling of novel triazole-containing berberine derivatives as acetylcholinesterase and β-amyloid aggregation inhibitors. Bioorg. Med. Chem. 2011, 19, 2298–2305. [Google Scholar] [CrossRef]
- Jin, X.; Yan, T.-H.; Yan, L.; Li, Q.; Wang, R.-L.; Hu, Z.-L.; Jiang, Y.-Y.; Sun, Q.-Y.; Cao, Y.-B. Design, synthesis, and anticancer activity of novel berberine derivatives prepared via cuaac ′′click′′ chemistry as potential anticancer agents. Drug. Des. Dev. Ther. 2014, 8, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Design, synthesis and molecular docking studies of novel n-benzenesulfonyl-1,2,3,4-tetrahydroisoquinoline-based triazoles with potential anticancer activity. Eur. J. Med. Chem. 2014, 81, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Bunders, C.; Cavanagh, J.; Melander, C. flustramine inspired synthesis and biological evaluation of pyrroloindoline triazole amides as novel inhibitors of bacterial biofilms. Org. Biomol. Chem. 2011, 9, 5476–5481. [Google Scholar] [CrossRef]
- Jiaranaikulwanitch, J.; Boonyarat, C.; Fokin, V.V.; Vajragupta, O. Triazolyl tryptoline derivatives as β-secretase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6572–6576. [Google Scholar] [CrossRef] [PubMed]
- Sangshetti, J.N.; Nagawade, R.R.; Shinde, D.B. synthesis of novel 3-(1-(1-substituted piperidin-4-yl)-1h-1,2,3-triazol-4-yl)-1,2,4- oxadiazol-5(4h)-one as antifungal agents. Bioorg. Med. Chem. Lett. 2009, 19, 3564–3567. [Google Scholar] [CrossRef] [PubMed]
- Darandale, S.N.; Mulla, N.A.; Pansare, D.N.; Sangshetti, J.N.; Shinde, D.B. A novel amalgamation of 1,2,3-triazoles, piperidines and thieno pyridine rings and evaluation of their antifungal activity. Eur. J. Med. Chem. 2013, 65, 527–532. [Google Scholar] [CrossRef]
- Corrales, R.C.N.R.; de Souza, N.B.; Pinheiro, L.S.; Abramo, C.; Coimbra, E.S.; Da Silva, A.D. Thiopurine derivatives containing triazole and steroid: Synthesis, antimalarial and antileishmanial activities. Biomed. Pharmacother. 2011, 65, 198–203. [Google Scholar] [CrossRef]
- Nair, N.; Kudo, W.; Smith, M.A.; Abrol, R.; Goddard, W.A., III; Reddy, V.P. Novel purine-based fluoroaryl-1,2,3-triazoles as neuro- protecting agents: Synthesis, neuronal cell culture investigations, and cdk5 docking studies. Bioorg. Med. Chem. Lett. 2011, 21, 3957–3961. [Google Scholar] [CrossRef]
- Wu, M.; Han, G.; Meng, C.; Wang, Z.; Liu, Y.; Wang, Q. Design, synthesis, and anti-tobacco mosaic virus (tmv) activity of glycoconjugates of phenanthroindolizidines alkaloids. Mol. Divers. 2014, 18, 25–37. [Google Scholar] [CrossRef]
- Jin, C.; Navarro, H.A.; Carroll, F.I. Synthesis and receptor binding properties of 2β-alkynyl and 2β-(1,2,3-triazol)substituted 3β- (substituted phenyl)tropane derivatives. Bioorg. Med. Chem. 2008, 16, 5529–5535. [Google Scholar] [CrossRef]
- Thuy, T.T.T.; Cuong, N.M.; Toan, T.Q.; Thang, N.N.; Tai, B.H.; Nhiem, N.X.; Hong, H.-J.; Kim, S.; Legoupy, S.; Koh, Y.S.; et al. Synthesis of novel derivatives of murrayafoline a and their inhibitory effect on lps-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. Arch. Pharma. Res. 2013, 36, 832–839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, L.; Mao, L.; Hong, G.; Yang, X.; Liu, T. Design, synthesis and anticancer activity of matrine-1h-1,2,3-triazole-chalcone conjugates. Bioorg. Med. Chem. Lett. 2015, 25, 2540–2544. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.-Y.; Zhang, T.-J.; Kamara, M.O.; Liang, J.-W.; Zhu, J.; Meng, F.-H. Design, synthesis and biological evaluation of homoerythrina alkaloid derivatives bearing a triazole moiety as parp-1 inhibitors and as potential antitumor drugs. Bioorg. Chem. 2020, 94, 103385. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.-Y.; Zhang, T.J.; Zhu, J.; Xue, W.-H.; Qian, X.-H.; Meng, F.-H. Design, synthesis and biological evaluation of erythrina derivatives bearing a 1,2,3-triazole moiety as parp-1 inhibitors. Bioorg. Chem. 2020, 96, 103575. [Google Scholar] [CrossRef]
- Artyushin, O.I.; Moiseeva, A.A.; Zarubaev, V.V.; Slita, A.V.; Galochkina, A.V.; Muryleva, A.A. Synthesis of camphecene and cytisine conjugates using click chemistry methodology and study of their antiviral activity. Chem. Biodivers. 2019, 16, e1900340. [Google Scholar] [CrossRef] [PubMed]
- Ruddarraju, R.R.; Murugulla, A.C.; Kotla, R.; Tirumalasetty, M.C.B.; Wudayagiri, R.; Donthabakthuni, S. Design, synthesis, anticancer activity and docking studies of theophylline containing 1,2,3-triazoles with variant amide derivatives. Med. Chem. Commun. 2017, 8, 176–183. [Google Scholar] [CrossRef]
- Ruddarraju, R.R.; Murugulla, A.C.; Kotla, R.; Chandra Babu Tirumalasetty, M.; Wudayagiri, R.; Donthabakthuni, S. Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of theophylline containing acetylenes and theophylline containing 1,2,3-triazoles with variant nucleoside derivatives. Eur. J. Med. Chem. 2016, 123, 379–396. [Google Scholar] [CrossRef]
- Boratyński, P.J.; Gałęzowska, J.; Turkowiak, K.; Anisiewicz, A.; Kowalczyk, R.; Wietrzyk, J. Triazole biheterocycles fromcinchonaalkaloids: Coordination and antiproliferative properties. ChemistrySelect 2018, 3, 9368–9373. [Google Scholar] [CrossRef]
- Podolski-Renić, A.; Bősze, S.; Dinić, J.; Kocsis, L.; Hudecz, F.; Csámpai, A.; Pešić, M. Ferrocene-cinchona hybrids with triazolyl-chalcone linkers act as pro-oxidants and sensitize human cancer cell lines to paclitaxel. Metallomics 2017, 9, 1132–1141. [Google Scholar] [CrossRef]
- Sahu, A.; Agrawal, R.K.; Pandey, R. synthesis and systemic toxicity assessment of quinine-triazole scaffold with antiprotozoal potency. Bioorg. Chem. 2019, 88, 102939. [Google Scholar] [CrossRef]
- Faidallah, H.M.; Panda, S.S.; Serrano, J.C.; Girgis, A.S.; Khan, K.A.; Alamry, K.A.; Therathanakorn, T.; Meyers, M.J.; Sverdrup, F.M.; Eickhoff, C.S.; et al. Synthesis, antimalarial properties and 2d-qsar studies of novel triazole-quinine conjugates. Bioorg. Med. Chem. 2016, 24, 3527–3539. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yan, L.; Li, H.-j.; Wang, R.-L.; Hu, Z.-L.; Jiang, Y.-Y.; Cao, Y.-B.; Yan, T.-H.; Sun, Q.-Y. Novel triazolyl berberine derivatives prepared via cuaac click chemistry: Synthesis, anticancer activity and structure-activity relationships. Anticancer Agents Med. Chem. 2014, 15, 89–98. [Google Scholar] [CrossRef]
- Batra, N.; Rajendran, V.; Agarwal, D.; Wadi, I.; Ghosh, P.C.; Gupta, R.D.; Nath, M. Synthesis and antimalarial evaluation of [1, 2,3]-triazole-tethered sulfonamide-berberine hybrids. ChemistrySelect 2018, 3, 9790–9793. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Liu, W.; Sheng, C.; Yao, J.; Dong, G.; Fang, K.; Li, J.; Yu, Z.; Min, X.; et al. Discovery of 7-methyl-10-hydroxyhomocamptothecins with 1,2,3-triazole moiety as potent topoisomerase I inhibitors. Chem. Biol. Drug Des. 2016, 88, 398–403. [Google Scholar] [CrossRef]
- Konan, K.V.; Le, T.C.; Mateescu, M.A. Enhanced solubility of alkaloids by complexation with polycarboxylic materials for controlled release formulations: Case of peschiera fuchsiaefolia. AAPS PharmSciTech 2019, 20, 108. [Google Scholar] [CrossRef]
- Alshamrani, M.; Khan, M.K.; Khan, B.A.; Salawi, A.; Almoshari, Y. Technologies for solubility, dissolution and permeation enhancement of natural compounds. Pharmaceuticals 2022, 15, 653. [Google Scholar] [CrossRef]
- Venditti, A. Artifacts in natural products studies. An old and underestimated re-emerging problem. Nat. Prod. Res. 2018, 32, i–ii. [Google Scholar] [CrossRef]
- van de Waterbeemd, H.; Gifford, E. ADMET in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef]
- Flores-Holguin, N.; Frau, J.; Glossman-Mitnik, D. computational pharmacokinetics report, admet study and conceptual dft-based estimation of the chemical reactivity properties of marine cyclopeptides. ChemistryOpen 2021, 10, 1142–1149. [Google Scholar] [CrossRef]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score—A comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 2019, 10, 148–157. [Google Scholar] [CrossRef]
- Duran-Iturbide, N.A.; Diaz-Eufracio, B.I.; Medina-Franco, J.L. In silico adme/tox profiling of natural products: A focus on BIOFACQUIM. ACS Omega 2020, 5, 16076–16084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, T.; Fan, X.; Ai, N. In silico modeling on ADME properties of natural products: Classification models for blood-brain barrier permeability, its application to traditional Chinese medicine and in vitro experimental validation. J. Mol. Graph. Model. 2017, 75, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sahakian, D.C.; de Morais, S.M.F.; Xu, J.J.; Polzer, R.J.; Winter, S.M. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr. Top. Med. Chem. 2003, 3, 1125–1154. [Google Scholar] [CrossRef]
- Tibbitts, J.; Canter, D.; Graff, R.; Smith, A.; Khawli, L.A. Key factors influencing ADME properties of therapeutic proteins: A need for ADME characterization in drug discovery and development. MAbs 2016, 8, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Mullard, A. Re-assessing the rule of 5, two decades on. Nat. Rev. Drug Discov. 2018, 17, 777. [Google Scholar] [CrossRef]
- Gomis-Tena, J.; Brown, B.M.; Cano, J.; Trenor, B.; Yang, P.C.; Saiz, J.; Clancy, C.E.; Romero, L. When does the IC50 accurately assess the blocking potency of a drug? J. Chem. Inf. Model. 2020, 60, 1779–1790. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Lehmann, D.F.; Eggleston, W.D.; Wang, D. Validation and clinical utility of the herg IC50:cmax ratio to determine the risk of drug-induced torsades de pointes: A meta-analysis. Pharmacotherapy 2018, 38, 341–348. [Google Scholar] [CrossRef]
- Stergiopoulos, C.; Tsopelas, F.; Valko, K. Prediction of hERG inhibition of drug discovery compounds using biomimetic HPLC measurements. ADMET DMPK 2021, 9, 191–207. [Google Scholar] [CrossRef]
- Creanza, T.M.; Delre, P.; Ancona, N.; Lentini, G.; Saviano, M.; Mangiatordi, G.F. Structure-based prediction of herg-related cardiotoxicity: A benchmark study. J. Chem. Inf. Model. 2021, 61, 4758–4770. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Lepailleur, A.; Mignani, S.M.; Dallemagne, P.; Rochais, C. hERG toxicity assessment: Useful guidelines for drug design. Eur. J. Med. Chem. 2020, 195, 112290. [Google Scholar] [CrossRef] [PubMed]
- Small Molecule Drug Discovery & Data Visualizations. Available online: https://optibrium.com/stardrop/ (accessed on 20 October 2023).
- ADME Properties. Available online: http://www.swissadme.ch/ (accessed on 20 October 2023).
- Clinical Trial Studies. Available online: https://clinicaltrials.gov (accessed on 20 October 2023).

| Compd. | logP | MW | HBD | HBA | TPSA | Rotatable Bonds | hERG pIC50 | BBB | HIA |
|---|---|---|---|---|---|---|---|---|---|
| 8 | 6.11 | 549.6 | 0 | 8 | 71.5 | 7 | 6.47 | - | + |
| 11 | 4.21 | 409.5 | 2 | 6 | 67.8 | 4 | 6.26 | - | + |
| 14 | 5.25 | 653.9 | 3 | 11 | 151.9 | 10 | 5.82 | - | - |
| 15 | 0.36 | 430.4 | 2 | 14 | 179.6 | 9 | 3.62 | - | - |
| 20a | 3.00 | 350.4 | 1 | 6 | 65.1 | 6 | 5.99 | - | + |
| 20b | 2.72 | 349.4 | 1 | 6 | 70.9 | 6 | 5.80 | - | + |
| 21 | 5.01 | 586.1 | 0 | 8 | 80.6 | 7 | 6.74 | - | + |
| 22 | 3.69 | 404.5 | 0 | 5 | 51.0 | 3 | 6.77 | - | + |
| 23 | 3.54 | 390.5 | 0 | 5 | 51.0 | 3 | 6.64 | - | + |
| 24 | 5.05 | 548.8 | 0 | 8 | 77.5 | 12 | 6.58 | - | + |
| 25 | −0.77 | 660.6 | 3 | 20 | 236 | 12 | 3.11 | - | + |
| 26 | −2.17 | 604.5 | 4 | 20 | 246.9 | 11 | 2.76 | - | + |
| 27 | 3.70 | 467.6 | 1 | 7 | 76.3 | 6 | 6.59 | - | + |
| 28 | 3.28 | 483.6 | 2 | 8 | 96.5 | 6 | 6.59 | - | + |
| 29 | 4.27 | 436.5 | 1 | 5 | 54.2 | 5 | 6.74 | - | + |
| 30 | 4.50 | 502.6 | 0 | 7 | 68.9 | 6 | 7.47 | - | + |
| 32 | 0.20 | 462.5 | 2 | 9 | 119.4 | 8 | 5.07 | - | - |
| 33 | 5.18 | 509.6 | 0 | 7 | 65.3 | 9 | 7.41 | - | + |
| 34 | 6.26 | 539.6 | 0 | 7 | 62.28 | 6 | 6.60 | - | - |
| 35 | 5.51 | 639.1 | 1 | 10 | 108.4 | 7 | 7.09 | - | + |
| 36 | 2.51 | 513.5 | 1 | 10 | 121.4 | 5 | 5.01 | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchanan, D.; Pham, A.M.; Singh, S.K.; Panda, S.S. Molecular Hybridization of Alkaloids Using 1,2,3-Triazole-Based Click Chemistry. Molecules 2023, 28, 7593. https://doi.org/10.3390/molecules28227593
Buchanan D, Pham AM, Singh SK, Panda SS. Molecular Hybridization of Alkaloids Using 1,2,3-Triazole-Based Click Chemistry. Molecules. 2023; 28(22):7593. https://doi.org/10.3390/molecules28227593
Chicago/Turabian StyleBuchanan, Devan, Ashley M. Pham, Sandeep K. Singh, and Siva S. Panda. 2023. "Molecular Hybridization of Alkaloids Using 1,2,3-Triazole-Based Click Chemistry" Molecules 28, no. 22: 7593. https://doi.org/10.3390/molecules28227593
APA StyleBuchanan, D., Pham, A. M., Singh, S. K., & Panda, S. S. (2023). Molecular Hybridization of Alkaloids Using 1,2,3-Triazole-Based Click Chemistry. Molecules, 28(22), 7593. https://doi.org/10.3390/molecules28227593






