Proliferation, Migration and Invasion of Breast Cancer Cell Lines Are Inhibited by 1,5-Disubstituted Tetrazol-1,2,3-triazole Hybrids through Interaction with p53
Abstract
:1. Introduction
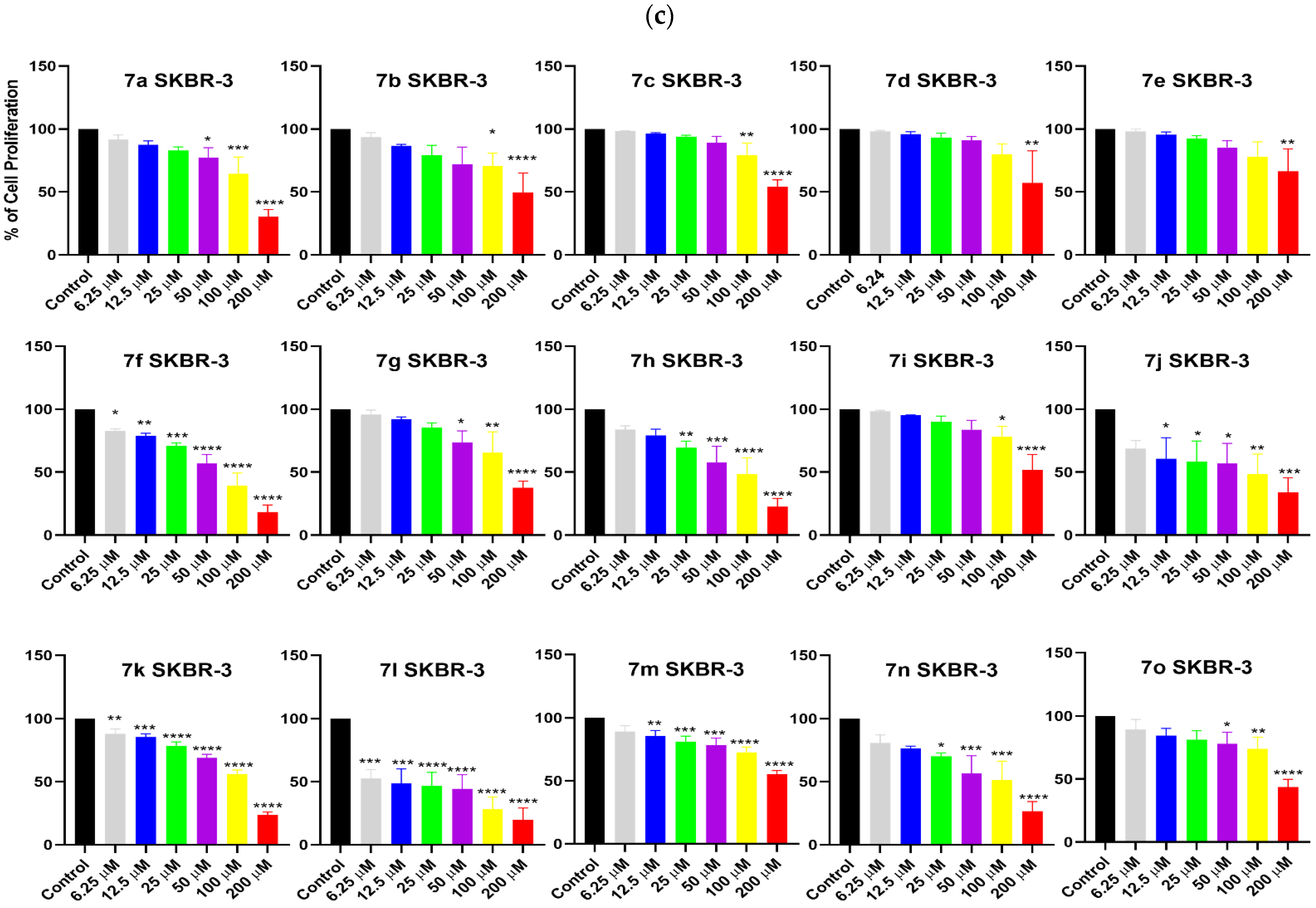
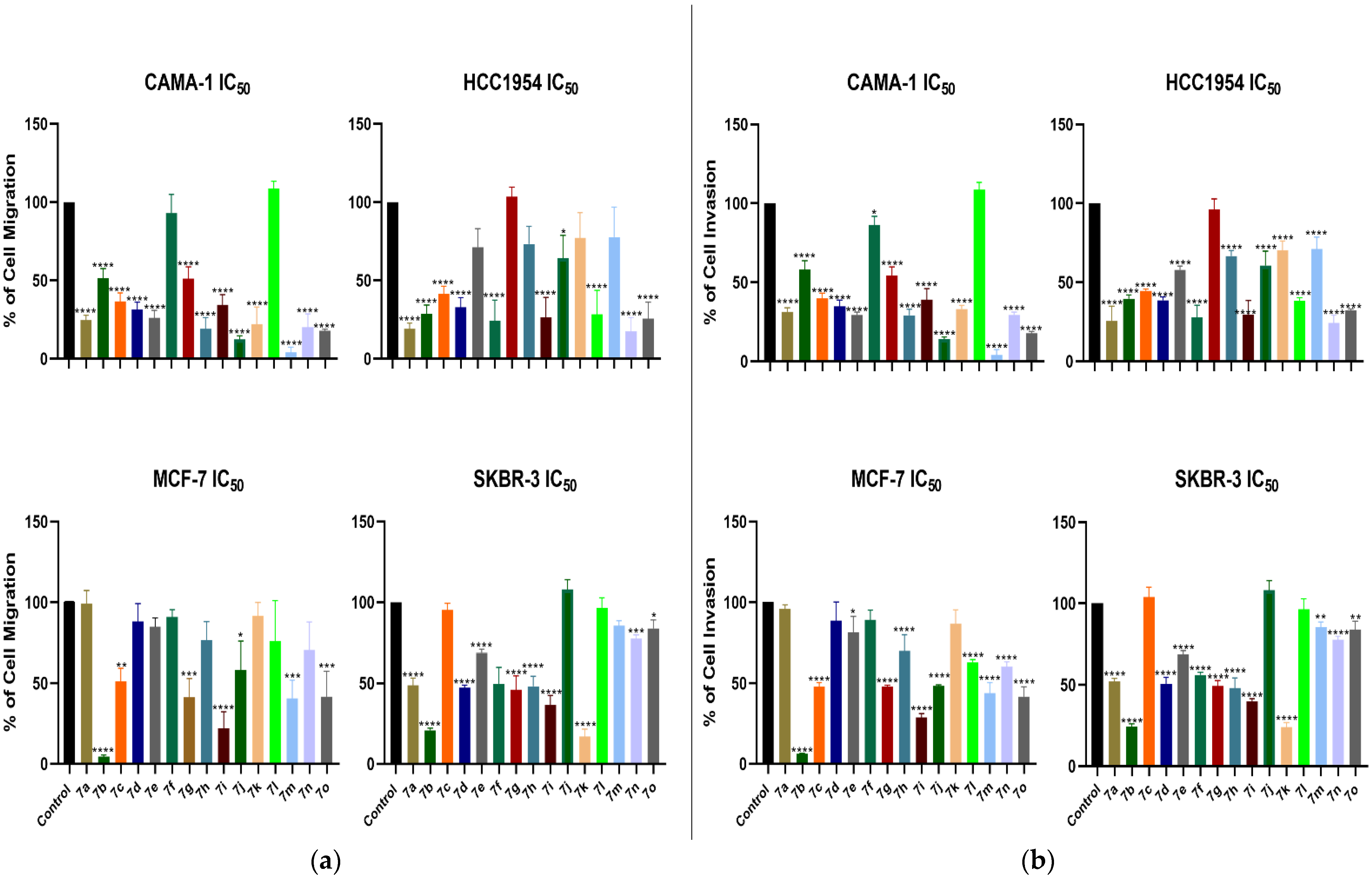
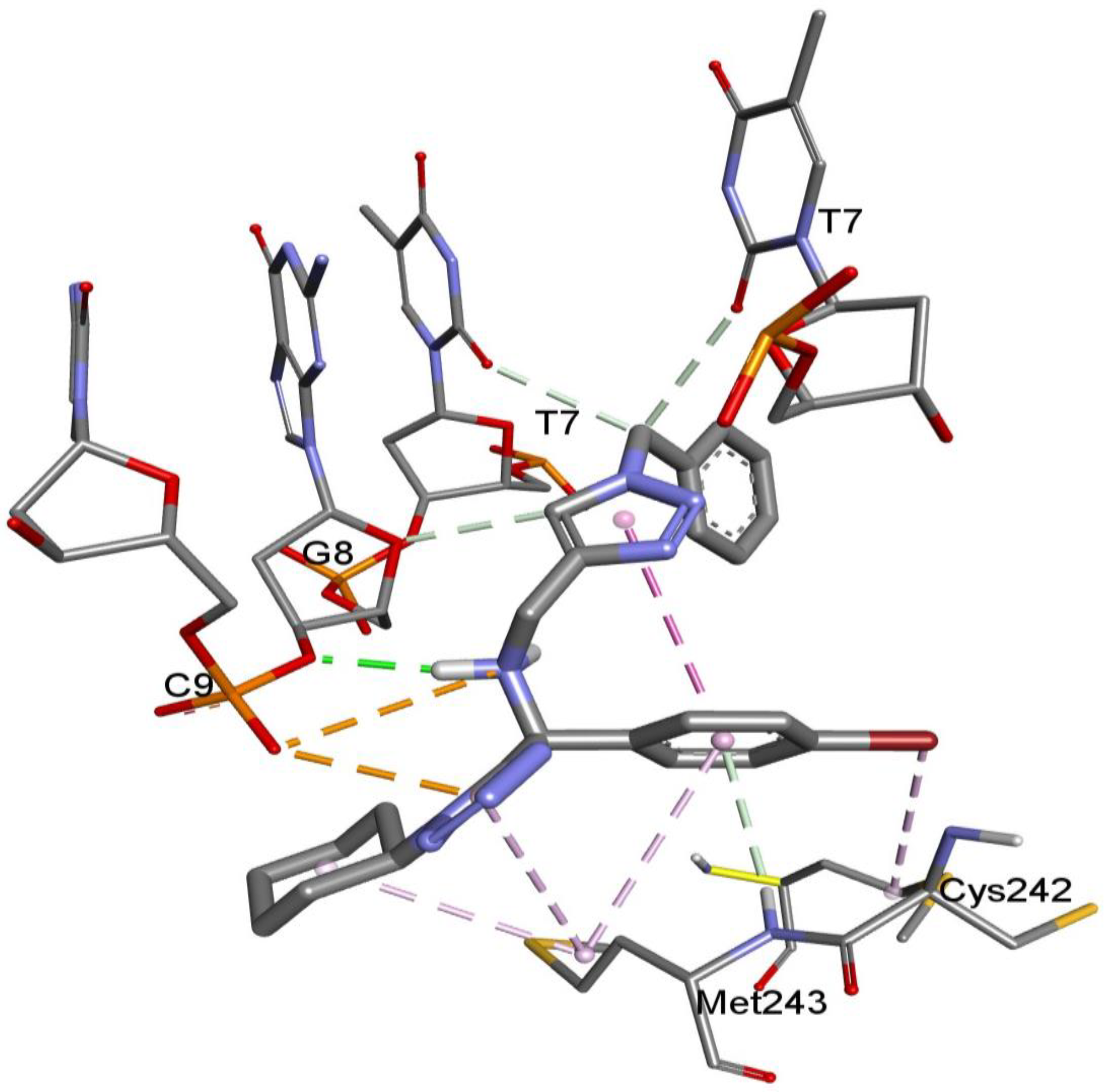
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
General Information
3.2. Cell Lines
3.3. Cell Proliferation Analysis
3.4. Cell Migration Analysis
3.5. Cell Invasion Analysis
3.6. Molecular Docking Studies
- Ligand preparation
- Receptor Preparation
- Docking Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, A.; Kaur, B.; Venugopal, S.; Wadhwa, P.; Sahu, S.; Kaur, P.; Kumar, D.; Sharma, A. Tetrazole: A privileged scaffold for the discovery of anticancer agents. Chem. Biol. Drug. Des. 2022, 100, 419–442. [Google Scholar] [CrossRef]
- Lengerli, D.; Ibis, K.; Nural, Y.; Banoglu, E. The 1,2,3-triazole ‘all-in-one’ ring system in drug discovery: A good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool. Expert. Opin. Drug. Discov. 2022, 17, 1209–1236. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, B.; Mehra, V.; Kumar, V. Recent accomplishments on the synthetic/biological facets of pharmacologically active 1H-1,2,3-triazoles. Eur. J. Med. Chem. 2021, 212, 113069. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Ryabukhin, S.V.; Judd, D.B. The Symbiotic Relationship Between Drug Discovery and Organic Chemistry. Chemistry 2020, 26, 1196–1237. [Google Scholar] [CrossRef]
- Galloway, W.R.; Isidro-Llobet, A.; Spring, D.R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010, 1, 80. [Google Scholar] [CrossRef]
- Aguilar-Morales, C.M.; Río, R.; Islas-Jácome, A.; Gámez-Montaño, R.; Chacón-García, L.; Cortés-García, C. Synthesis of 1,5-disubstituted tetrazole-1,2,3 triazoles hybrids via Ugi-azide/CuAAC. Synth. Commun. 2022, 53, 127–134. [Google Scholar] [CrossRef]
- Niño-Pantoja, I.; Gallardo-Alfonzo, A.; Solis-Santos, M.; Ordoñez, M.; Contreras-Celedón, C.; Islas-Jácome, A.; Cha-cón-García, L.; Cortés-García, C.J. Synthesis of 1,5-Disubstituted Tetrazole−Indolizine Bis-Heterocycles and Their Copper (II) Recognizing Properties. Eur. J. Org. Chem. 2022, 2022, e202200230. [Google Scholar] [CrossRef]
- Aguilar-Morales, C.M.; Araujo-Huitrado, J.G.; Lopez-Hernandez, Y.; Contreras-Celedon, C.; Islas-Jacome, A.; Granados-Lopez, A.J.; Solorio-Alvarado, C.R.; Lopez, J.A.; Chacon-Garcia, L.; Cortes-Garcia, C.J. A One-Pot Six-Component Reaction for the Synthesis of 1,5-Disubstituted Tetrazol-1,2,3-Triazole Hybrids and Their Cytotoxic Activity against the MCF-7 Cell Line. Molecules 2021, 26, 6104. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Ataollahi, M.R.; Sharifi, J.; Paknahad, M.R.; Paknahad, A. Breast cancer and associated factors: A review. J. Med. Life 2015, 8, 6–11. [Google Scholar]
- Mittendorf, E.A.; Ardavanis, A.; Litton, J.K.; Shumway, N.M.; Hale, D.F.; Murray, J.L.; Perez, S.A.; Ponniah, S.; Baxevanis, C.N.; Papamichail, M.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 2016, 7, 66192–66201. [Google Scholar] [CrossRef]
- Redmond, K.M.; Wilson, T.R.; Johnston, P.G.; Longley, D.B. Resistance mechanisms to cancer chemotherapy. Front. Biosci. 2008, 13, 5138–5154. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Boichuk, S.; Galembikova, A.; Sitenkov, A.; Khusnutdinov, R.; Dunaev, P.; Valeeva, E.; Usolova, N. Establishment and characterization of a triple negative basal-like breast cancer cell line with multi-drug resistance. Oncol. Lett. 2017, 14, 5039–5045. [Google Scholar] [CrossRef]
- Zhang, X.; Hofmann, S.; Harbeck, N.; Jeschke, U.; Sixou, S. Impact of Etoposide on BRCA1 Expression in Various Breast Cancer Cell Lines. Drugs R D 2017, 17, 569–583. [Google Scholar] [CrossRef]
- Abzianidze, V.; Beltyukov, P.; Zakharenkova, S.; Moiseeva, N.; Mejia, J.; Holder, A.; Trishin, Y.; Berestetskiy, A.; Kuznetsov, V. Synthesis and Biological Evaluation of Phaeosphaeride A Derivatives as Antitumor Agents. Molecules 2018, 23, 3043. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Ashmawy, A.M.; Ahmed, D.M.; Wessjohann, L.A. Combinatorial synthesis, in silico, molecular and biochemical studies of tetrazole-derived organic selenides with increased selectivity against hepatocellular carcinoma. Eur. J. Med. Chem. 2016, 122, 55–71. [Google Scholar] [CrossRef]
- Vanaparthi, S.; Bantu, R.; Jain, N.; Janardhan, S.; Nagarapu, L. Synthesis and anti-proliferative activity of a novel 1,2,3-triazole tethered chalcone acetamide derivatives. Bioorg Med. Chem. Lett. 2020, 30, 127304. [Google Scholar] [CrossRef]
- Lambert, P.A.; Somers, K.D.; Kohn, E.C.; Perry, R.R. Antiproliferative and antiinvasive effects of carboxyamido-triazole on breast cancer cell lines. Surgery 1997, 122, 372–378; discussion 378–379. [Google Scholar] [CrossRef]
- Dhiman, N.; Kaur, K.; Jaitak, V. Tetrazoles as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Bioorg Med. Chem. 2020, 28, 115599. [Google Scholar] [CrossRef]
- Filho, R.I.; Gonzaga, D.T.G.; Demaria, T.M.; Leandro, J.G.B.; Costa, D.C.S.; Ferreira, V.F.; Sola-Penna, M.; de C. da Silva, F.; Zancan, P. A Novel Triazole Derivative Drug Presenting In Vitro and In Vivo Anticancer Properties. Curr. Top. Med. Chem. 2018, 18, 1483–1493. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Duan, Y.C.; Ma, J.L.; Xu, R.M.; Zi, X.; Lv, W.L.; Wang, M.M.; Ye, X.W.; Zhu, S.; Mobley, D.; et al. Triazole-dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J. Med. Chem. 2013, 56, 8543–8560. [Google Scholar] [CrossRef]
- Grandane, A.; Tanc, M.; Zalubovskis, R.; Supuran, C.T. Synthesis of 6-tetrazolyl-substituted sulfocoumarins acting as highly potent and selective inhibitors of the tumor-associated carbonic anhydrase isoforms IX and XII. Bioorg. Med. Chem. 2014, 22, 1522–1528. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, L.; Li, Z.; Shen, Q.; Xu, Q.; Li, W.; Liu, Y.; Gong, P. Design, synthesis and biological evaluation of novel 7-amino-[1,2,4]triazolo[4,3-f]pteridinone, and 7-aminotetrazolo[1,5-f]pteridinone derivative as potent antitumor agents. Eur. J. Med. Chem. 2019, 163, 690–709. [Google Scholar] [CrossRef]
- Yu, Y.; Lv, F.; Liang, D.; Yang, Q.; Zhang, B.; Lin, H.; Wang, X.; Qian, G.; Xu, J.; You, W. HOTAIR may regulate proliferation, apoptosis, migration and invasion of MCF-7 cells through regulating the P53/Akt/JNK signaling pathway. Biomed. Pharmacother. 2017, 90, 555–561. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, M.; Hao, M.; Diao, K.; Wang, J.; Li, S.; Cao, Q.; Mi, X. FAM53A Affects Breast Cancer Cell Proliferation, Migration, and Invasion in a p53-Dependent Manner. Front. Oncol. 2019, 9, 1244. [Google Scholar] [CrossRef]
- Milne, G.W.A. Software Review of ChemBioDraw 12.0. J. Chem. Inf. Model. 2010, 50, 2053. [Google Scholar] [CrossRef]
- SPARTAN, version 14; Wavefunction, Inc.: Irvine, CA, USA, 2016.
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Biovia, D.S. Discovery Studio Visualizer v21. 1.0. 20298; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]


| Product | IC50(µM) | |||
|---|---|---|---|---|
| CAMA-1 | MCF-7 | HCC1954 | SKBR-3 | |
| 7a | 90.94 | - | 15.58 | 131.8 |
| 7b | 68.91 | 31.63 | 10.77 | 174 |
| 7c | - | - | 137.6 | - |
| 7d | 55.48 | 50.97 | ||
| 7e | - | 62.73 | 12.16 | - |
| 7f | 33.34 | 19.76 | 14.47 | 57.29 |
| 7g | 122.3 | - | 10.28 | 148 |
| 7h | 94.05 | 41.67 | 11.72 | 66.12 |
| 7i | - | - | 109.4 | - |
| 7j | 83.07 | 44.51 | 43.96 | 127.1 |
| 7k | 105.8 | 60.77 | 59.21 | 95.32 |
| 7l | 64.34 | 22.84 | 49.82 | 18.63 |
| 7m | 180.9 | - | 180.6 | - |
| 7n | 180.1 | 29.25 | 44.91 | 68.87 |
| 7o | 182.7 | - | - | - |
| Compound | p53 | p58 | p38 | JNK1 | ||||
| ΔG (kcal/mol) | ki (nM) | ΔG (kcal/mol) | ki (nM) | ΔG (kcal/mol) | ki (nM) | ΔG (kcal/mol) | ki (nM) | |
| 7a | −8.78 | 365.03 | −8.89 | 304.09 | −9.37 | 135 | −8.5 | 586.06 |
| 7b | −10.33 | 26.79 | −8.39 | 712.37 | −9.74 | 72.93 | −9.23 | 170.25 |
| 7c | −11.26 | 5.58 | −10.82 | 11.78 | −9.19 | 183.41 | −10.07 | 41.67 |
| 7d | −11.26 | 5.54 | −9.42 | 124.96 | −8.33 | 789.01 | −9.66 | 83.65 |
| 7e | −9.79 | 66.55 | −8.92 | 290.05 | −8.42 | 674.06 | −8.7 | 419.39 |
| 7f | −10.66 | 15.35 | −10.02 | 45.22 | −9.94 | 51.79 | −10 | 46.69 |
| 7g | −10.71 | 14.12 | −8.89 | 305.1 | −8.48 | 608.53 | −9.04 | 236.53 |
| 7h | −9.76 | 68.89 | −9.21 | 178.9 | −8.62 | 476.64 | −9.03 | 239.07 |
| 7i | −9.59 | 94.19 | −8.52 | 570.85 | −8.22 | 938.79 | −8.65 | 455.06 |
| 7j | −10.11 | 38.96 | −8.89 | 306.44 | −8.91 | 292.7 | −9.35 | 139.73 |
| 7k | −9.59 | 93.26 | −8.45 | 641.24 | −8.16 | 104 | −9.22 | 174.08 |
| 7l | −9.9 | 55.54 | −8.93 | 286.6 | −8.52 | 564.82 | −9.56 | 84.25 |
| 7m | −9.51 | 106.9 | −8.35 | 756.33 | −8.16 | 104 | −8.92 | 287.06 |
| 7n | −10.7 | 14.42 | −10.03 | 44.63 | −9.02 | 245.3 | −9.47 | 113.53 |
| 7o | −8.51 | 575.22 | −8.48 | 613.02 | −8.92 | 291.79 | −8.69 | 427.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Perea, M.; Suárez-Castro, A.; Fraire-Soto, I.; Sifuentes-Padilla, J.L.; Gutiérrez-Hernández, R.; Reyes-Estrada, C.A.; López-Hernández, Y.; Cortés-García, C.J.; Chacón-García, L.; Granados-López, A.J.; et al. Proliferation, Migration and Invasion of Breast Cancer Cell Lines Are Inhibited by 1,5-Disubstituted Tetrazol-1,2,3-triazole Hybrids through Interaction with p53. Molecules 2023, 28, 7600. https://doi.org/10.3390/molecules28227600
Moreno-Perea M, Suárez-Castro A, Fraire-Soto I, Sifuentes-Padilla JL, Gutiérrez-Hernández R, Reyes-Estrada CA, López-Hernández Y, Cortés-García CJ, Chacón-García L, Granados-López AJ, et al. Proliferation, Migration and Invasion of Breast Cancer Cell Lines Are Inhibited by 1,5-Disubstituted Tetrazol-1,2,3-triazole Hybrids through Interaction with p53. Molecules. 2023; 28(22):7600. https://doi.org/10.3390/molecules28227600
Chicago/Turabian StyleMoreno-Perea, Marisol, Abel Suárez-Castro, Ixamail Fraire-Soto, Jessica Lizbeth Sifuentes-Padilla, Rosalinda Gutiérrez-Hernández, Claudia Araceli Reyes-Estrada, Yamilé López-Hernández, Carlos J. Cortés-García, Luis Chacón-García, Angelica Judith Granados-López, and et al. 2023. "Proliferation, Migration and Invasion of Breast Cancer Cell Lines Are Inhibited by 1,5-Disubstituted Tetrazol-1,2,3-triazole Hybrids through Interaction with p53" Molecules 28, no. 22: 7600. https://doi.org/10.3390/molecules28227600
APA StyleMoreno-Perea, M., Suárez-Castro, A., Fraire-Soto, I., Sifuentes-Padilla, J. L., Gutiérrez-Hernández, R., Reyes-Estrada, C. A., López-Hernández, Y., Cortés-García, C. J., Chacón-García, L., Granados-López, A. J., & López, J. A. (2023). Proliferation, Migration and Invasion of Breast Cancer Cell Lines Are Inhibited by 1,5-Disubstituted Tetrazol-1,2,3-triazole Hybrids through Interaction with p53. Molecules, 28(22), 7600. https://doi.org/10.3390/molecules28227600






