A Fresh Perspective on the Impact of ZnTiO3 Coupling on the Microstructure and Photocatalytic Properties of TiO2 Fabricated at Varied Temperatures
Abstract
:1. Introduction
2. Results and Discussion
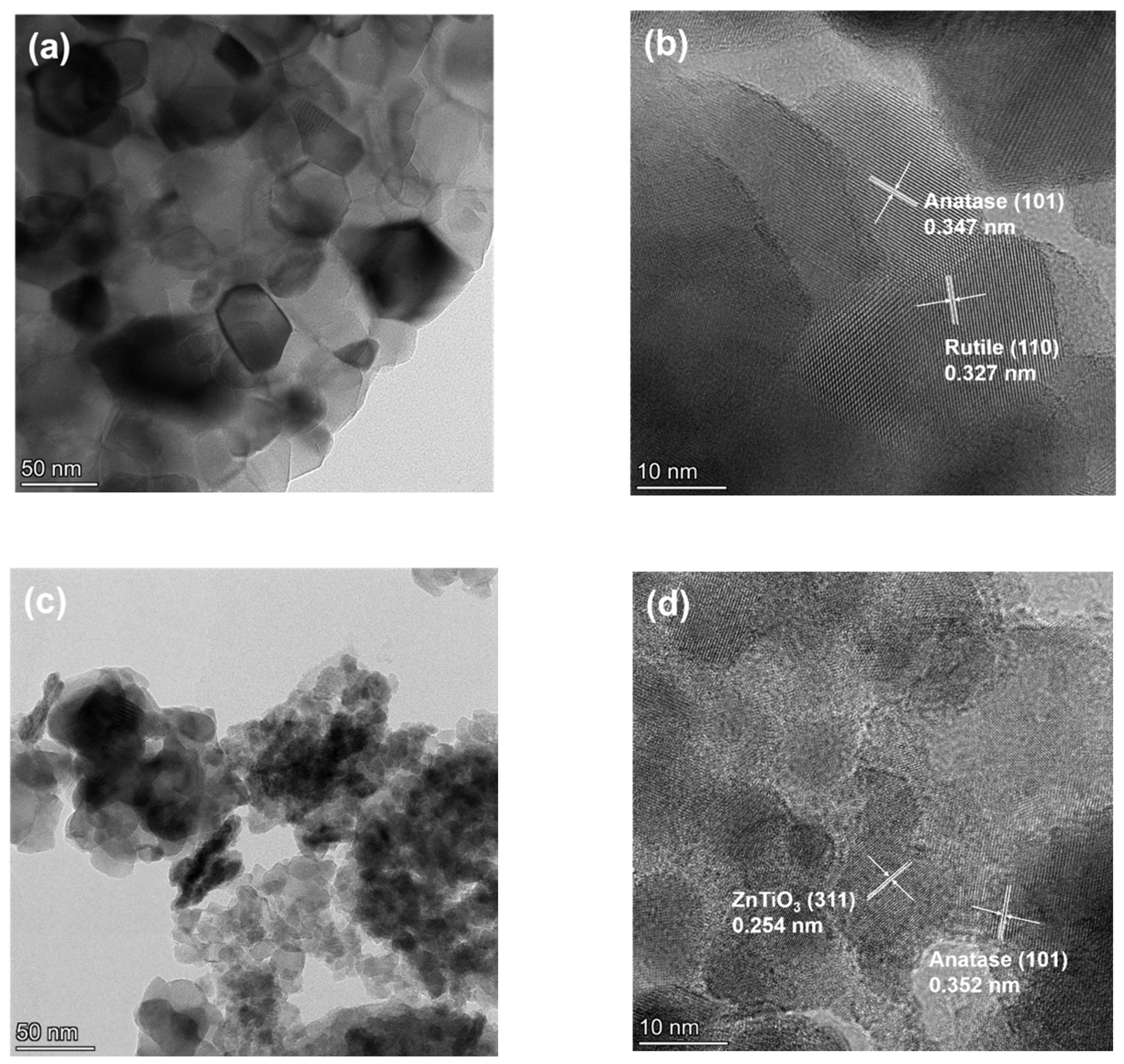
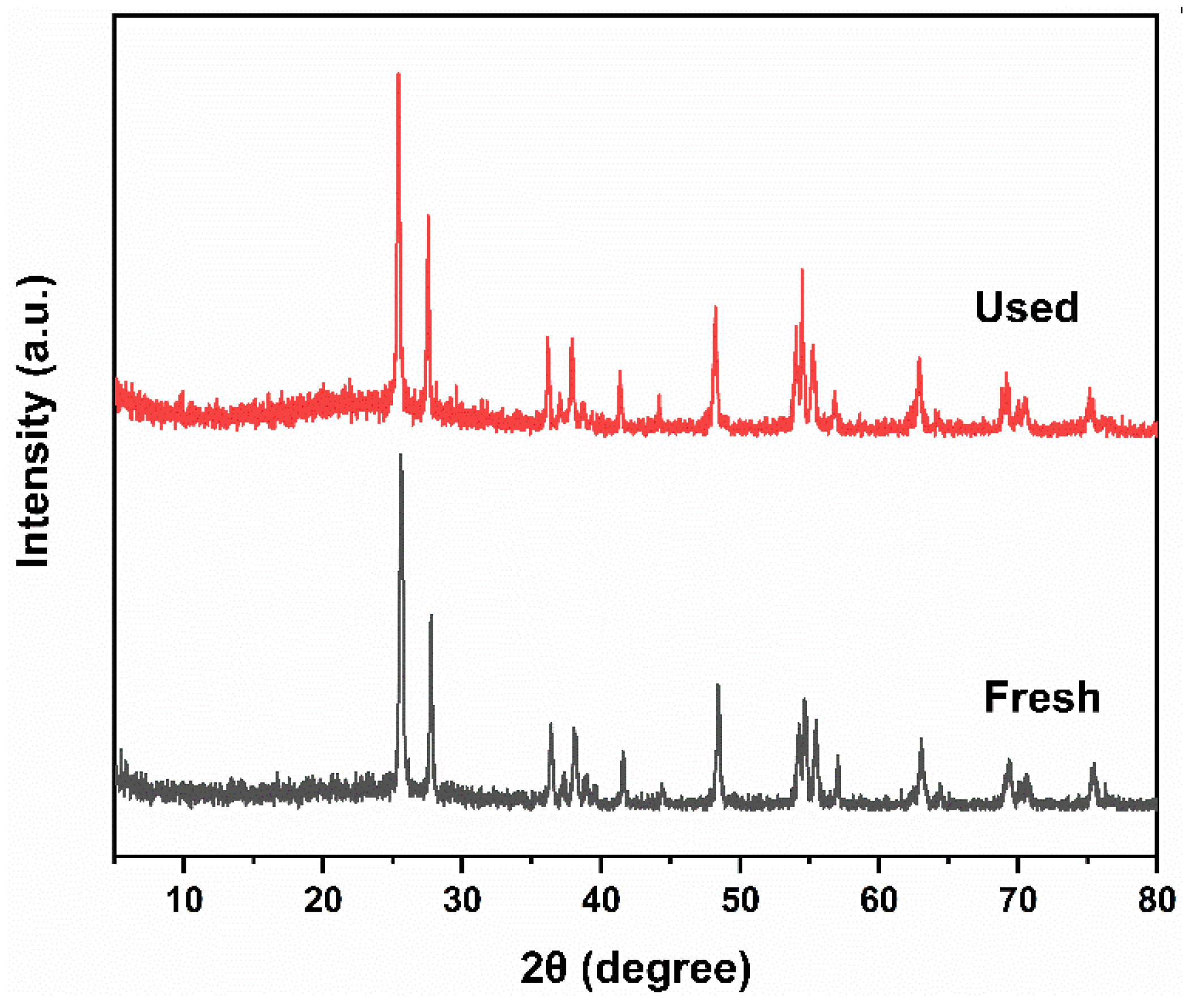
2.1. Phase Composition
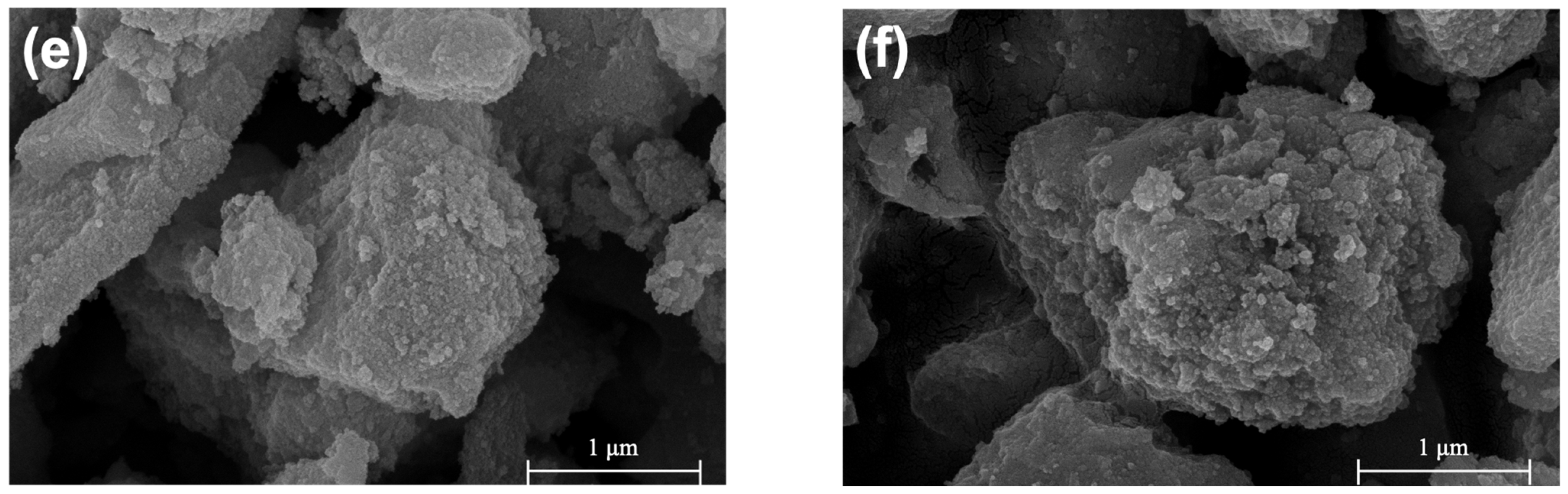
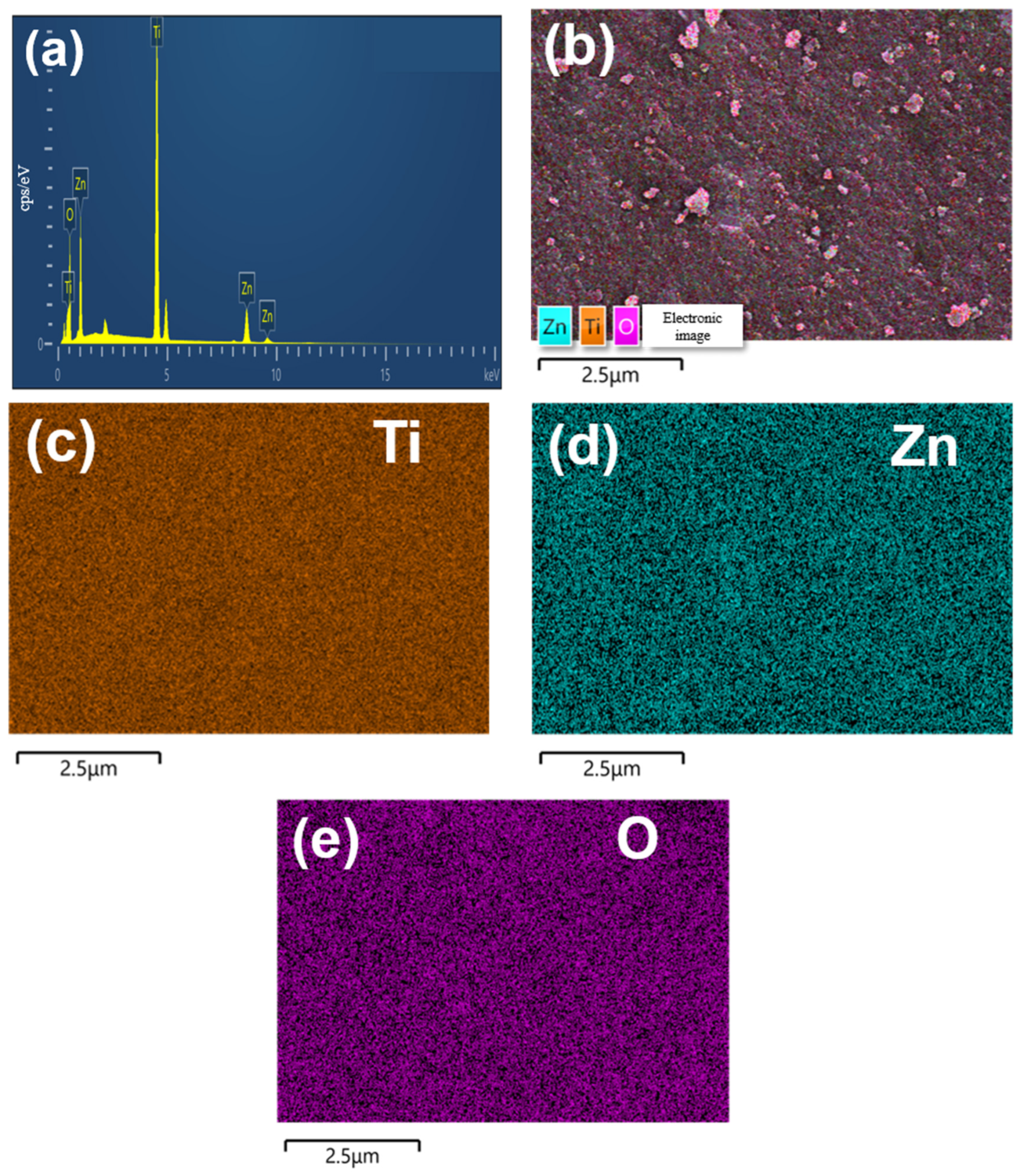
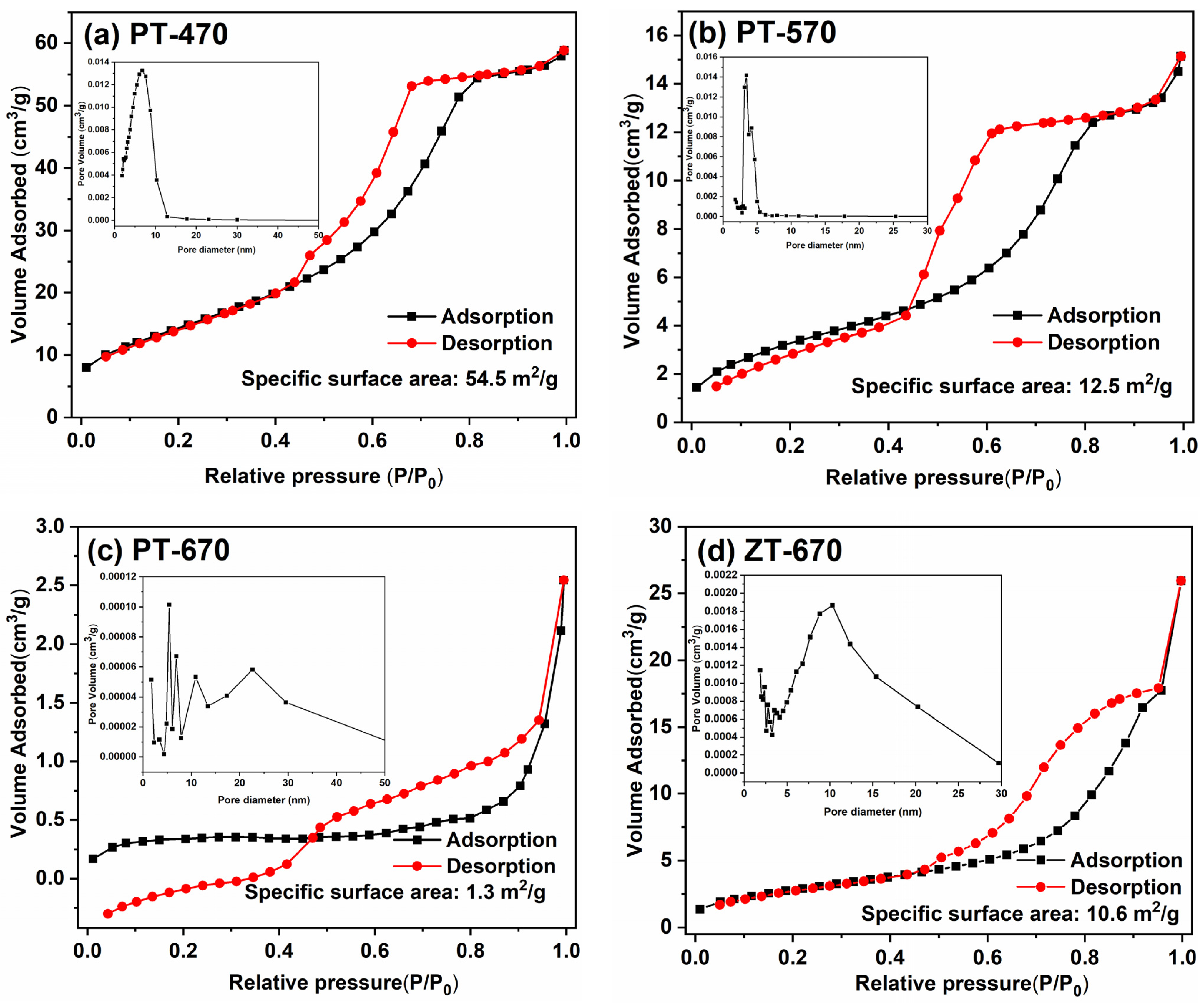
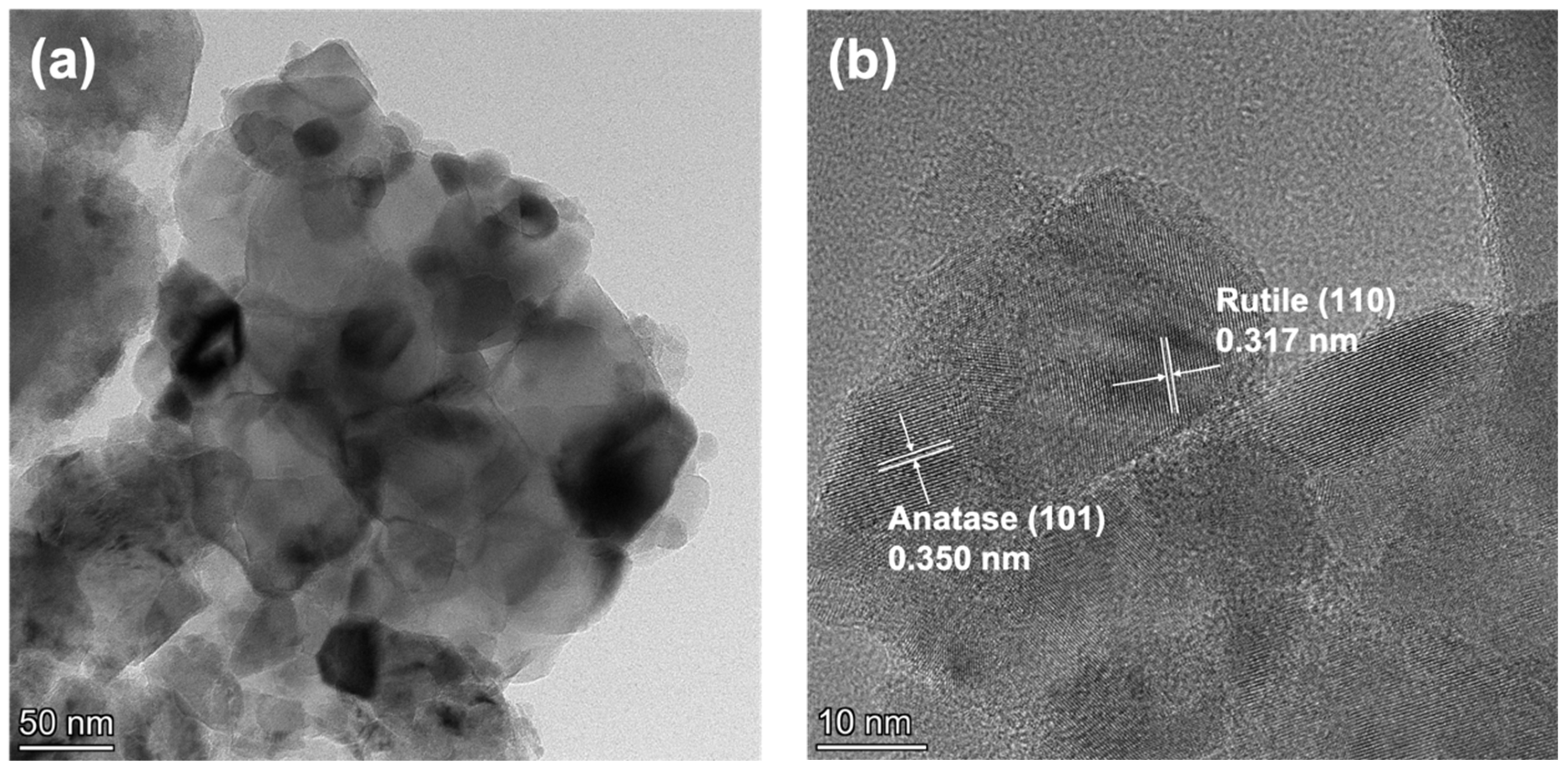
2.2. Morphology and BET Surface Area
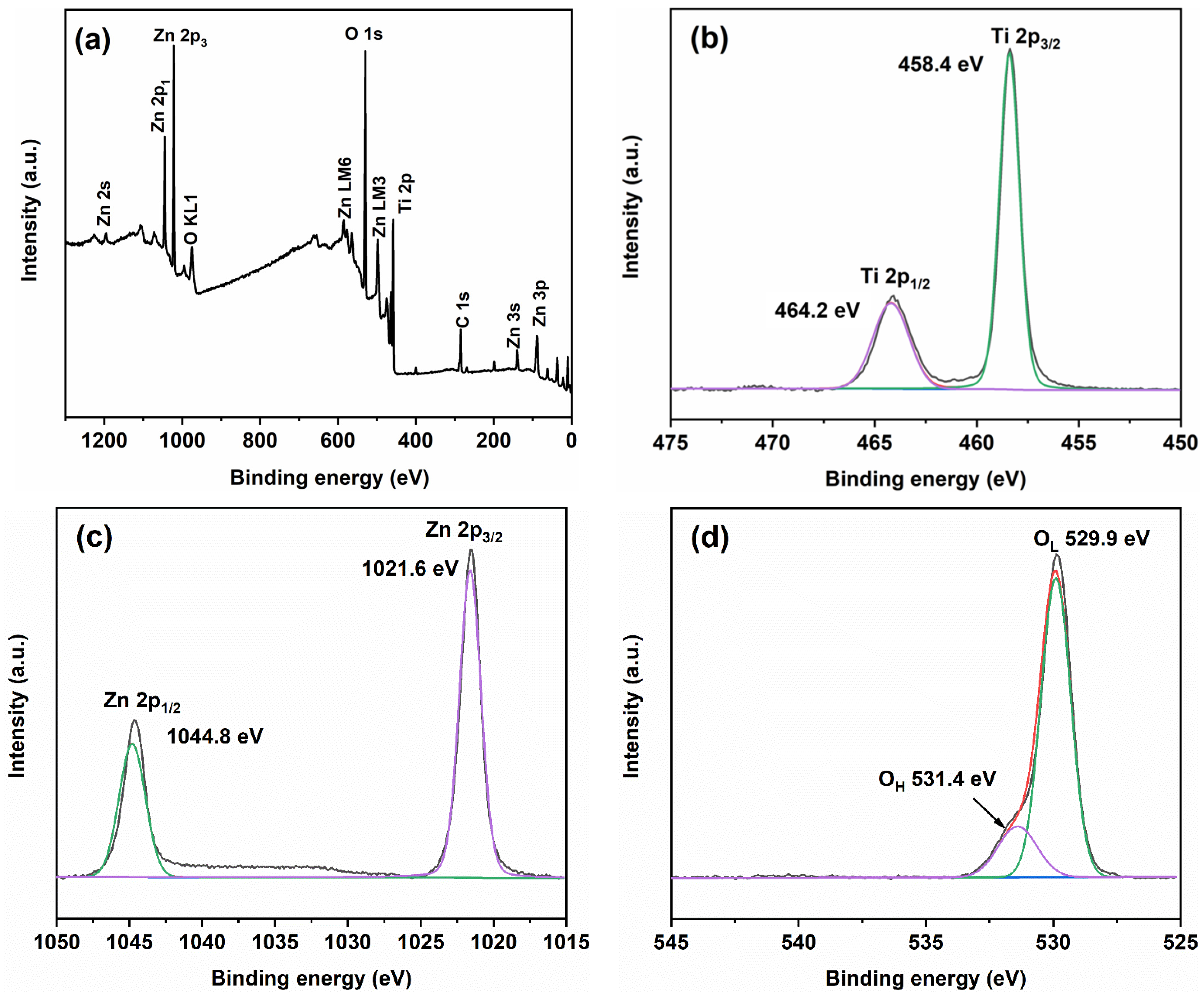
2.3. Element Valence State
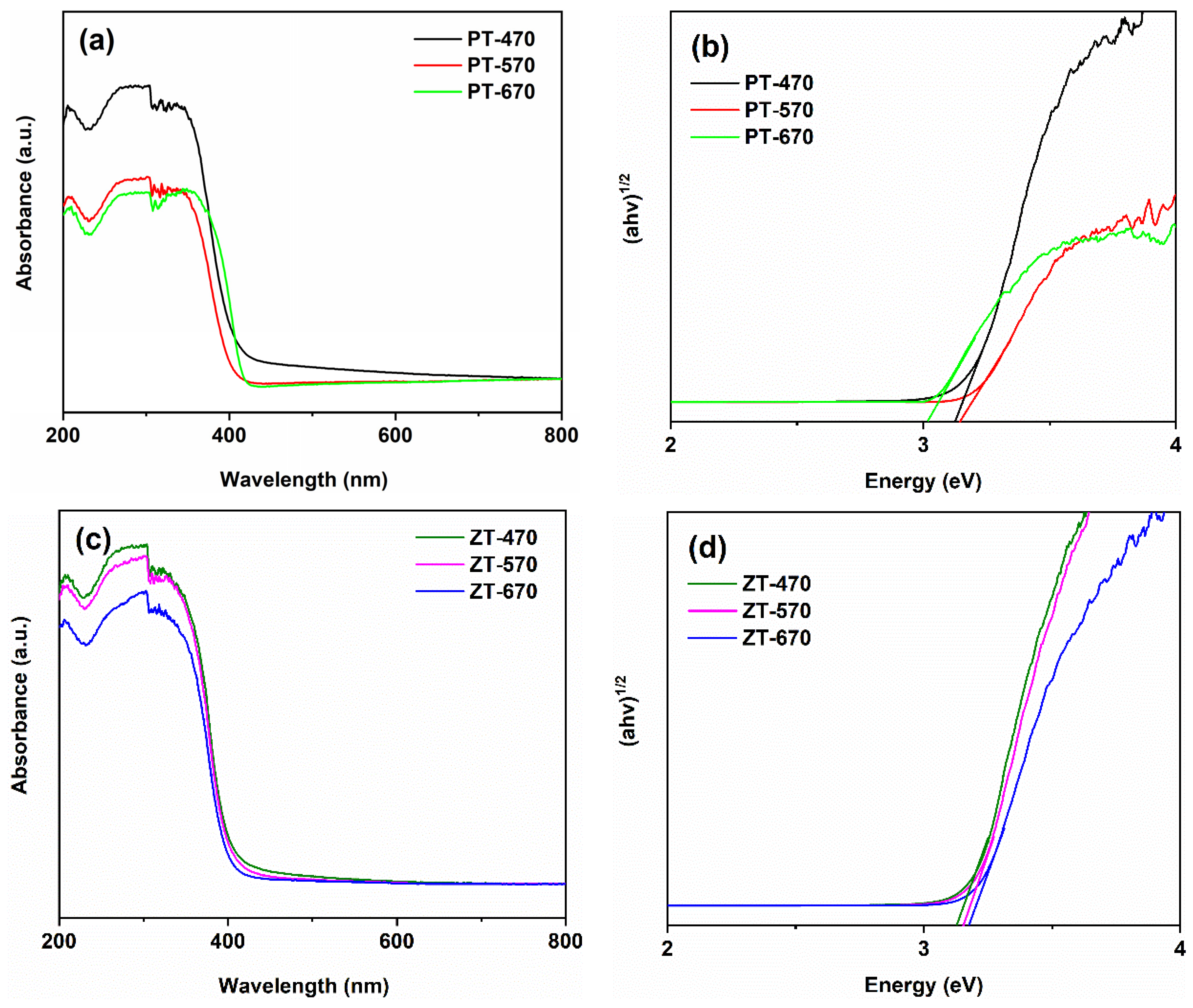
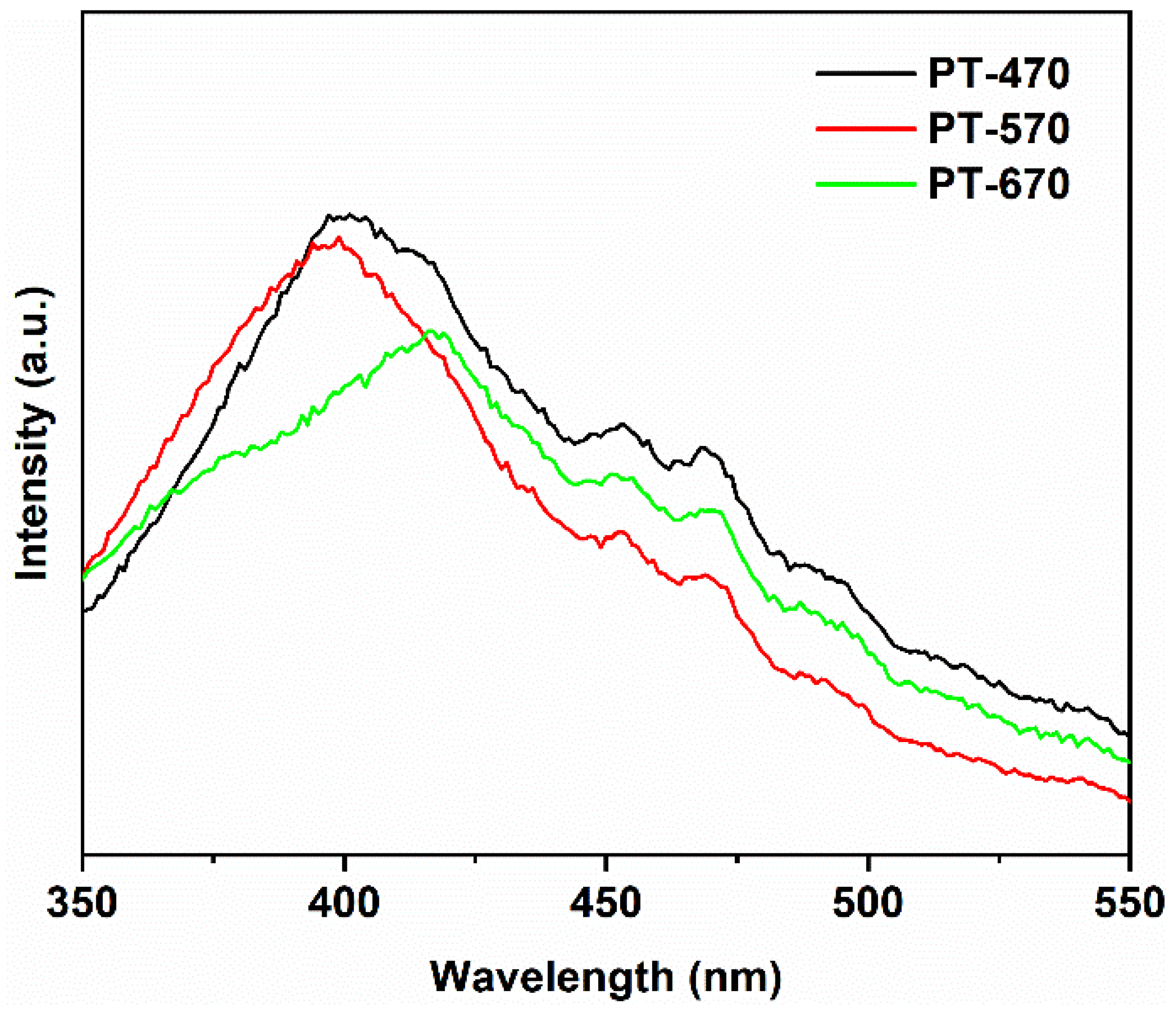
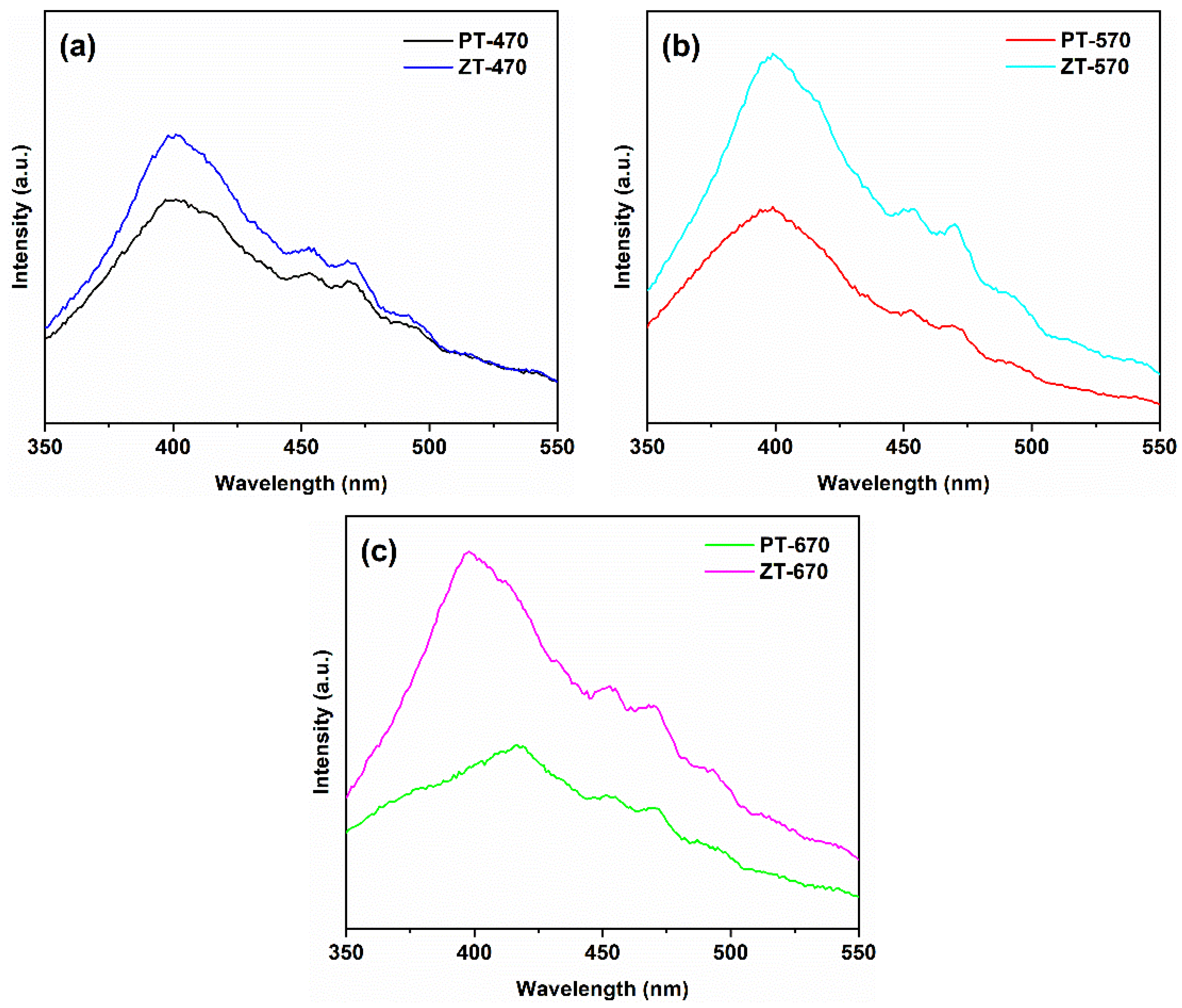
2.4. Optical Property
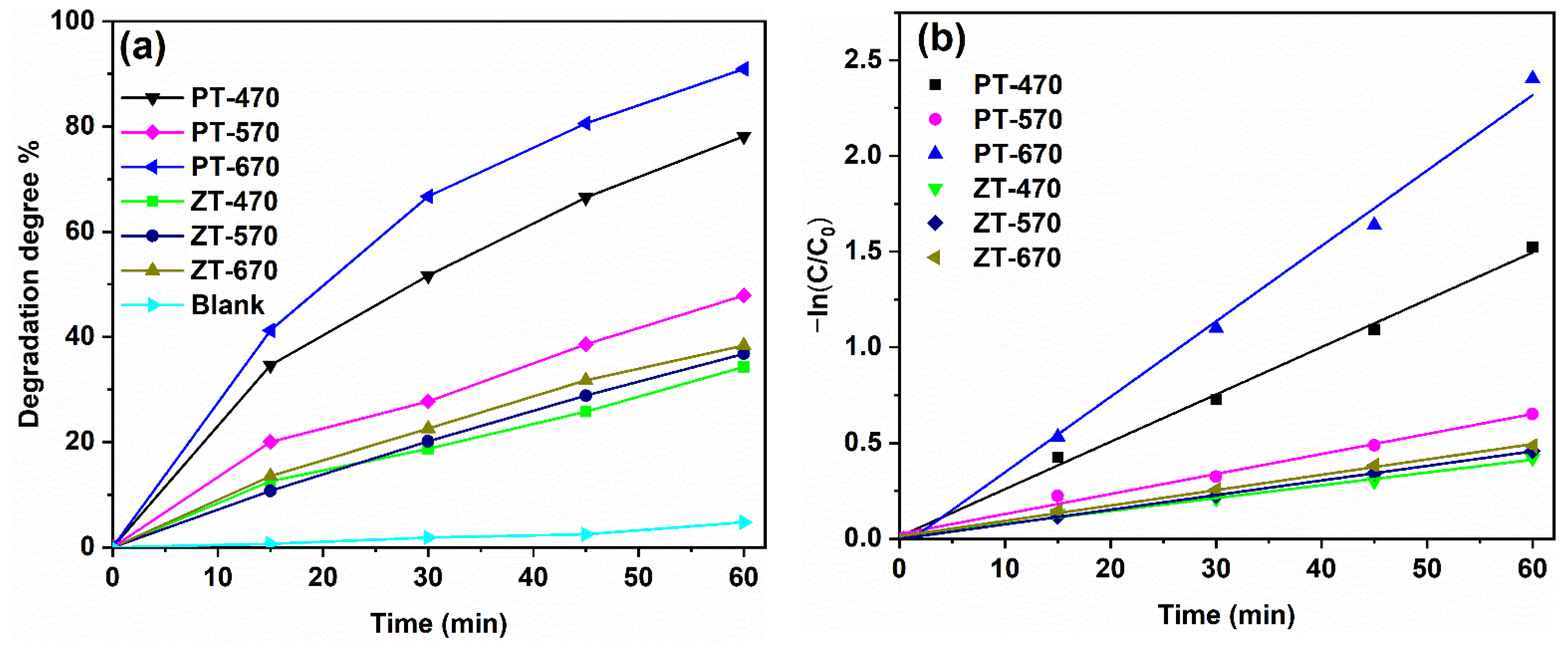
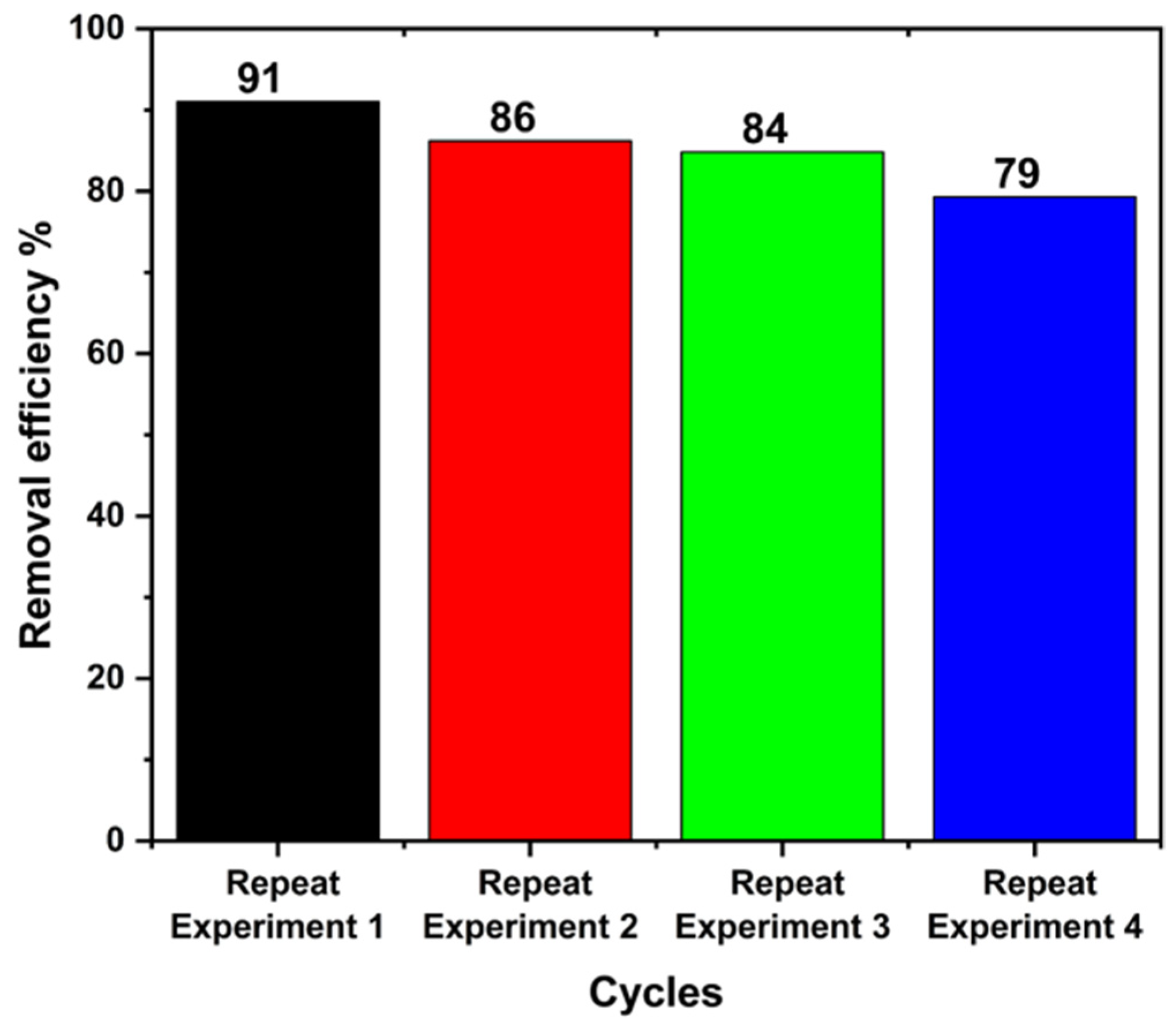
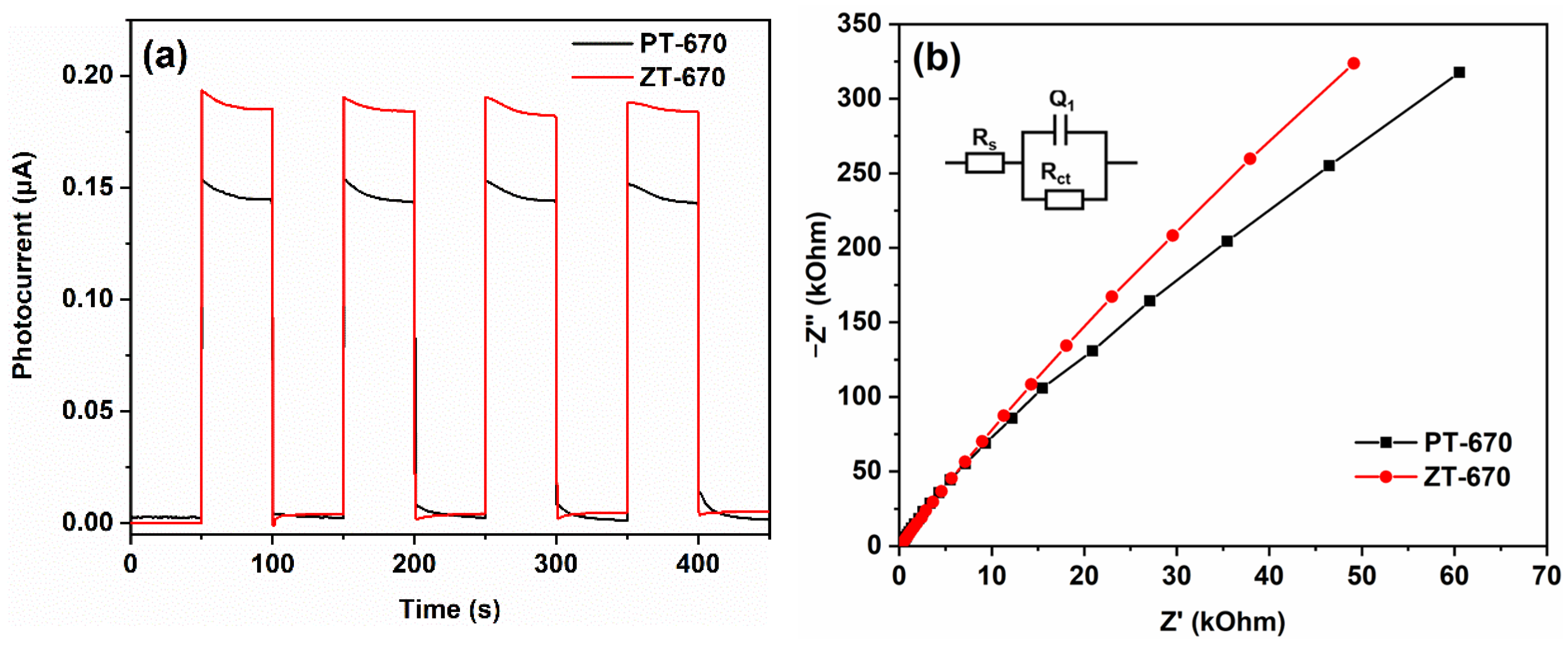
2.5. Photocatalytic Performance
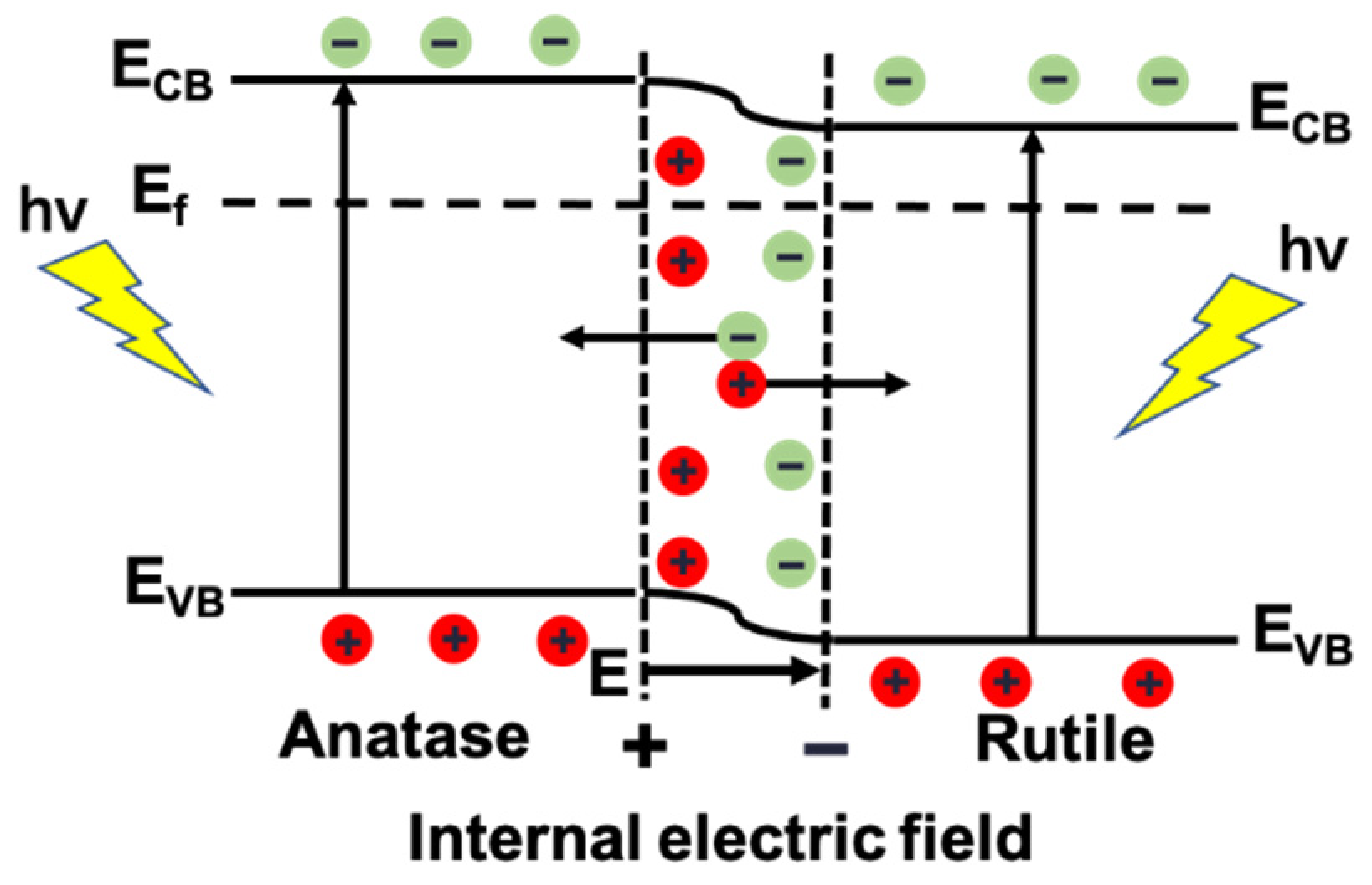
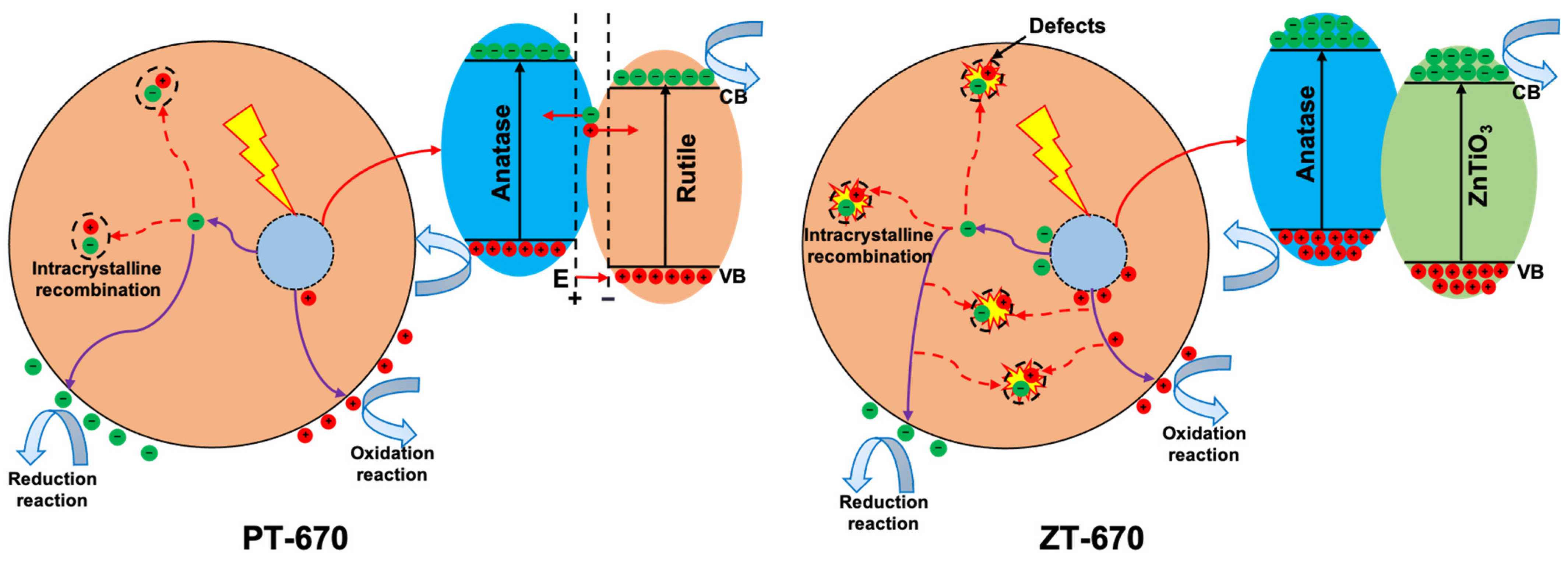
2.6. Photocatalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Sample Characterization
3.4. Photocatalysis Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dou, L.; Li, J.J.; Long, N.; Lai, C.X.; Zhong, J.B.; Li, J.Z.; Huang, S.T. Fabrication of 3D flower-like OVs-Bi2SiO5 hierarchical microstructures for visible light-driven removal of tetracycline. Surf. Interfaces 2022, 29, 101787. [Google Scholar] [CrossRef]
- Zhu, X.D.; Wang, J.; Yang, D.X.; Liu, J.W.; He, L.L.; Tang, M.; Feng, W.; Wu, X.Q. Fabrication, characterization and high photocatalytic activity of Ag-ZnO heterojunctions under UV-visible light. RSC Adv. 2021, 11, 27257. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.K.; Fu, F.; Yu, D.S.; Cao, J.L.; Sun, G. Facile synthesis and characterization of N-doped TiO2/C nanocomposites with enhanced visible-light photocatalytic performance. Appl. Surf. Sci. 2018, 430, 438–447. [Google Scholar] [CrossRef]
- Zhu, X.D.; Qin, F.Q.; He, L.L.; Jiao, Y.; Feng, W. Enhanced photocatalytic activity of anatase/rutile heterojunctions by Lanthanum and Tin co-doping. Int. J. Mol. Sci. 2022, 23, 11339. [Google Scholar] [CrossRef]
- Dong, H.R.; Zeng, G.M.; Tang, L.; Fan, C.Z.; Zhang, C.; He, X.X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Borrego Perez, J.A.; Courel, M.; Pal, M.; Paraguay Delgado, F.; Mathews, N.R. Effect of ytterbium doping concentration on structural, optical and photocatalytic properties of TiO2 thin films. Ceram. Int. 2017, 43, 15777–15784. [Google Scholar] [CrossRef]
- Lang, X.L.; Gopalan, S.; Fu, W.L.; Ramakrishna, S. Photocatalytic water splitting utilizing electrospun semiconductors for solar hydrogen generation: Fabrication, modification and performance. Bull. Chem. Soc. Jpn. 2021, 94, 8–20. [Google Scholar] [CrossRef]
- Zhu, X.D.; Qin, F.Q.; Xia, Y.W.; Zhong, Y.Y.; Zhang, X.P.; Feng, W.; Jiao, Y. Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property. Nanotechnol. Rev. 2022, 11, 2916–2927. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Riyas, S.; Krishnan, G.; Mohan Das, P.N. Anatase–rutile transformation in doped titania under argon and hydrogen atmospheres. Adv. Appl. Ceram. 2007, 106, 255. [Google Scholar] [CrossRef]
- Elsellami, L.; Dappozze, F.; Fessi, N.; Houas, A.; Guillard, C. Highly photocatalytic activity of nanocrystalline TiO2 (anatase, rutile) powders prepared from TiCl4 by sol-gel method in aqueous solutions. Process. Saf. Environ. 2018, 113, 109–121. [Google Scholar] [CrossRef]
- Zhu, X.D.; Zhou, Q.; Xia, Y.W.; Wang, J.; Chen, H.J.; Xu, Q.; Liu, J.W.; Feng, W.; Chen, S.H. Preparation and characterization of Cu-doped TiO2 nanomaterials with anatase/rutile/brookite triphasic structure and their photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 21511–21524. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol-gel synthesis of mesoporous anatase-brookite and anatase-brookite-rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.L.; Wang, C.F.; Ni, M.; Leung, M.K.H.; Wang, X.X.; Fu, X.Z. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.; Erjavec, B.; Drazic, G.; Grdadolnik, J.; Pintar, A. Simple synthesis of anatase/rutile/brookite TiO2 nanocomposite with superior mineralization potential for photocatalytic degradation of water pollutants. Appl. Catal. B 2016, 181, 465–474. [Google Scholar] [CrossRef]
- Ouyang, W.Y.; Munoz-Batista, M.J.; Kubacka, A.; Luque, R.; Fernandez-Garcia, M. Enhancing photocatalytic performance of TiO2 in H2 evolution via Ru co-catalyst deposition. Appl. Catal. B 2018, 238, 434–443. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Zeng, J.Y.; Guo, J.; Wang, H. Enhancing visible-light photocatalytic activity of Ag-TiO2 nanowire composites by one-step hydrothermal process. Mater. Lett. 2020, 279, 128506. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Li, Y.B.; Ding, Z.L.; Huang, J.G. A biotemplate synthesized hierarchical Sn-doped TiO2 with superior photocatalytic capacity under simulated solar light. Ceram. Int. 2021, 47, 8218–8227. [Google Scholar] [CrossRef]
- Vijayan, M.; Manikandan, V.; Rajkumar, C.; Hatamleh, A.A.; Alnafisi, B.K.; Easwaran, G.; Liu, X.H.; Sivakumar, K.; Kim, H. Constructing Z-scheme g-C3N4/TiO2 heterostructure for promoting degradation of the hazardous dye pollutants. Chemosphere 2023, 311, 136928. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Liang, Y.N. A novel electrostatic self-assembly method for preparation of TiO2@BiOI photocatalyst. Res. Chem. Intermed. 2018, 44, 7159–7171. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Zhao, B.S.; Xu, S.; Luo, C.H.; Zhao, Q. One-step hydrothermal preparation and characterization of ZnO-TiO2 nanocomposites for photocatalytic activity. Mater. Res. Express 2021, 7, 085010. [Google Scholar] [CrossRef]
- Shathy, R.A.; Fahim, S.A.; Sarker, M.; Quddus, M.S.; Moniruzzaman, M.; Masum, S.M.; Molla, M.A.I. Natural sunlight driven photocatalytic removal of toxic textile dyes in water using B-doped ZnO/TiO2 nanocomposites. Catalysts 2022, 12, 308. [Google Scholar] [CrossRef]
- Zhu, X.D.; Zhu, R.R.; Pei, L.X.; Liu, H.; Xu, L.; Wang, J.; Feng, W.; Jiao, Y.; Zhang, W.M. Fabrication, characterization, and photocatalytic activity of anatase/rutile/SnO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 21210–21218. [Google Scholar] [CrossRef]
- Zhu, X.D.; Qin, F.Q.; Xia, Y.W.; Yang, D.X.; Feng, W.; Jiao, Y. Three-phase mixed titania powder modified by silver and silver chloride with enhanced photocatalytic activity under UV-visible light. Nanomaterials 2022, 12, 1599. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, V.R.D.; Avansi, W., Jr.; Arenal, R.; Ribeiro, C. A building blocks strategy for preparing photocatalytically active anatase TiO2/rutile SnO2 heterostructures by hydrothermal annealing. J. Colloid Interface Sci. 2017, 505, 454–459. [Google Scholar] [CrossRef]
- Abd EI-Lateef, H.M.; Khalaf, M.M. Corrosion resistance of ZrO2-TiO2 nanocomposite multilayer thin films coated on carbon steel in hydrochloric acid solution. Mater. Charact. 2015, 108, 29–41. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.S.; Li, Y.; Guo, X.L. Preparation of nano TiO2/SiO2 photocatalysts by electrostatic self-assembly method. Chin. J. Inorg. Chem. 2011, 27, 2239–2244. [Google Scholar]
- Wu, L.X.; Wu, P.X.; Zhu, Y.J.; Zhu, N.W.; Dang, Z. Preparation and characterization of ZnTiO3-TiO2/pillared montmorillonite composite catalyst for enhanced photocatalytic activity. Res. Chem. Intermed. 2016, 42, 5253–5268. [Google Scholar] [CrossRef]
- Bhagwat, U.O.; Wu, J.J.; Asiri, A.M.; Anandan, S. Synthesis of ZnTiO3@TiO2 heterostructure nanomaterial as a visible light photocatalyst. Chem. Select. 2019, 4, 6106–6112. [Google Scholar] [CrossRef]
- Fahim, S.A.; Zahan, N.; Shathy, R.A.; Quddus, M.S.; Moniruzzaman, M.; Masum, S.M.; Molla, M.A.I. B–Sn/TiO2 nanoparticles for photodegradation of metronidazole antibiotics under different lights. Mater. Chem. Phys. 2023, 305, 127937. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ahmed, A.I.; Mannaa, M.A. Structural, photocatalytic, biological and catalytic properties of SnO2/TiO2 nanoparticles. Ceram. Int. 2018, 44, 6201–6211. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charact. 2007, 58, 883–891. [Google Scholar] [CrossRef]
- Padayachee, D.; Mahomed, A.S.; Singh, S.; Friedrich, H.B. Effect of the TiO2 anatase/rutile ratio and interface for the oxidative activation of n-octane. ACS Catal. 2020, 10, 2211–2220. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, L.L.; Yu, S.G.; Jiang, H.Y.; Yun, Y.L.; Sun, Y.W.; Shi, J.S. Ag-induced synthesis of three dimensionally ordered macroporous anatase/rutile homojunction for solar light-driven Z-scheme photocatalysis. Sol. Energy 2018, 174, 770–779. [Google Scholar] [CrossRef]
- Karunakaran, C.; Vinayagamoorthy, P.; Jayabharathi, J. Electrical, optical, photocatalytic, and bactericidal properties of polyethylene glycol-assisted sol-gel synthesized ZnTiO3-implanted ZnO nanoparticles. Mater. Res. Express 2014, 1, 045019. [Google Scholar] [CrossRef]
- Chen, S.F.; Liu, W.; Zhang, S.J.; Chen, Y.H. Preparation and activity evaluation of relative p-n junction photocatalyst Co-TiO2/TiO2. J. Sol-Gel Sci. Technol. 2010, 54, 258–267. [Google Scholar]
- Meng, Q.Y.; Liu, B.C.; Liu, H.J.; Cai, Y.D.; Dong, L. Effects of S and Ta codoping on photocatalytic activity of rutile TiO2. J. Sol-gel Sci. Technol. 2018, 86, 631–639. [Google Scholar] [CrossRef]
- Dette, C.; Perez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, K.N.; Wu, X.F.; Wang, N.; Li, X.F.; Li, Q.; Li, L.; Lv, K.L. Dramatic promotion of visible-light photoreactivity of TiO2 hollow microspheres towards NO oxidation by introduction of oxygen vacancy. Appl. Catal. B 2019, 256, 117860. [Google Scholar] [CrossRef]
- Khaidukov, N.M.; Makhov, V.N.; Zhang, Q.H.; Shi, R.; Liang, H.B. Extended broadband luminescence of dodecahedral multisite Ce3+ ions in garnets {Y3}[MgA](BAlSi)O12 (A = Sc, Ga, Al; B = Ga, Al). Dyes Pigm. 2017, 142, 524–529. [Google Scholar] [CrossRef]
- Fan, X.; Wan, J.; Liu, E.Z.; Sun, L.; Hu, Y.; Li, H.; Hu, X.Y.; Fan, J. High-efficiency photoelectrocatalytic hydrogen generation enabled by Ag deposited and Ce doped TiO2 nanotube arrays. Ceram. Int. 2015, 41, 5107–5116. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H. Preparation and characterization of Ag-doped TiO2 nanomaterials and their photocatalytic reduction of Cr (VI) under visible light. Appl. Surf. Sci. 2014, 321, 396–403. [Google Scholar] [CrossRef]
- Chin, Y.H.; Sin, J.C.; Lam, S.M.; Zeng, H.H.; Lin, H.; Li, H.X.; Huang, L.L.; Mohamed, A.R. 3-D/3-D Z-scheme heterojunction composite formed by marimo-like Bi2WO6 and mammillaria-like ZnO for expeditious sunlight photodegradation of dimethyl phthalate. Catalysts 2022, 17, 1427. [Google Scholar] [CrossRef]
- Appavu, B.; Thiripuranthagan, S. Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes. J. Photochem. Photobiol. A 2017, 340, 146–156. [Google Scholar]
- Dhal, J.P.; Sahoo, S.K.; Padhiari, S.; Dash, T.; Hota, G. In-situ synthesis of mixed phase electrospun TiO2 nanofibers: A novel visible light photocatalyst. SN Appl. Sci. 2019, 1, 243. [Google Scholar] [CrossRef]
- Lv, N.; Li, Y.Y.; Huang, Z.L.; Li, T.; Ye, S.Y.; Dionysiou, D.D.; Song, X.L. Synthesis of GO/TiO2/Bi2WO6 nanocomposites with enhanced visible light photocatalytic degradation of ethylene. Appl. Catal. B 2019, 246, 303–311. [Google Scholar] [CrossRef]
- Han, Z.Y.; Zhang, J.C.; Yang, X.Y.; Zhu, H.; Cao, W.L. Synthesis and photoelectric property of poly (3-octylthiophene)/zinc oxide complexes. Sol. Energy Mater. Sol. Cells 2010, 94, 194–200. [Google Scholar] [CrossRef]
- Oveisi, M.; Mahmoodi, N.M.; Asli, M.A. Facile and green synthesis of metal-organic framework/inorganic nanofiber using electrospinning for recyclable visible-light photocatalysis. J. Clean. Prod. 2019, 222, 669–684. [Google Scholar] [CrossRef]
- Ashwini, S.; Prashantha, S.C.; Naik, R.; Naik, Y.V.; Nagabhushana, H.; Narasimhamurthy, K.N. Photoluminescence and photocatalytic properties of novel Bi2O3:Sm3+ nanophosphor. J. Sci. Adv. Mater. Devices 2019, 4, 531–537. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.L.; Lu, Z.D.; Xu, G.L.; Wang, X.Y.; Qian, L.S.; Wang, H.B.; Zhang, E.P.; Xi, J.H.; Ji, Z.G. One-step synthesis of rutile nano-TiO2 with exposed {111} facets for high photocatalytic activity. J. Alloys Compd. 2015, 632, 133–139. [Google Scholar] [CrossRef]
- Gu, X.T.; Qin, N.; Zhang, P.; Hu, Y.Q.; Zhang, Y.N.; Zhao, G.H. In-situ synthesis of {111} TiO2/Ti photoelectrode to boost efficient removal of dimethyl phthalate based on a bi-functional interface. Chem. Eng. J. 2021, 422, 129980. [Google Scholar] [CrossRef]
- Liu, S.L.; Bu, Y.F.; Cheng, S.; Tao, R.R. Synthesis of TiO2/g-C3N5 heterojunction for photocatalytic degradation of methylene blue wastewater under visible light irradiation: Mechanism analysis. Diam. Relat. Mater. 2023, 136, 110062. [Google Scholar] [CrossRef]
- Yu, H.H.; Li, S.Y.; Peng, S.Y.; Yu, Z.L.; Chen, F.Y.; Liu, X.T.; Guo, J.L.; Zhu, B.L.; Huang, W.P.; Zhang, S.M. Construction of rutile/anatase TiO2 homojunction and metal-support interaction in Au/TiO2 for visible photocatalytic water splitting and degradation of methylene blue. Int. J. Hydrogen Energy 2023, 48, 975–990. [Google Scholar] [CrossRef]
- Rashid, J.; Saleem, S.; Awan, S.U.; Iqbal, A.; Kumar, R.; Barakat, M.A.; Arshad, M.; Zaheer, M.; Rafique, M.; Awad, M. Stabilized fabrication of anatase-TiO2/FeS2 (pyrite) semiconductor composite nanocrystals for enhanced solar light-mediated photocatalytic degradation of methylene blue. RSC Adv. 2018, 8, 11935. [Google Scholar] [CrossRef] [PubMed]
- Haghighatzadeh, A.; Hosseini, M.; Mazinani, B.; Shokouhimehr, M. Improved photocatalytic activity of ZnO-TiO2 nanocomposite catalysts by modulating TiO2 thickness. Mater. Res. Express 2019, 6, 115060. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Zeng, H.H.; Lin, H.; Li, H.X.; Huang, L.L.; Tham, K.O.; Mohamed, A.R.; Lim, J.W. Enhanced synchronous photocatalytic 4-chlorophenol degradation and Cr (VI) reduction by novel magnetic separable visible-light-driven Z-scheme CoFe2O4/P-doped BiOBr heterojunction nanocomposites. Environ. Res. 2022, 212, 113394. [Google Scholar] [CrossRef]
- Zhao, L.; Lam, S.M.; Ong, Y.T.; Sin, J.C.; Zeng, H.H.; Xie, Q.D.; Lim, J.W. Fe2WO6 coupling on cube-like SrTiO3 as a highly active S-scheme heterojunction composite for visible light photocatalysis and antibacterial applications. Environ. Technol. Innov. 2022, 28, 102941. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dong, S. Challenges in band alignment between semiconducting materials: A case of rutile and anatase TiO2. Prog. Nat. Sci. Mater. Int. 2019, 29, 277–284. [Google Scholar] [CrossRef]
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Kou, J.H.; Lu, C.H.; Wang, J.; Chen, Y.K.; Xu, Z.Z.; Varma, R.S. Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y.J. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893. [Google Scholar] [CrossRef] [PubMed]
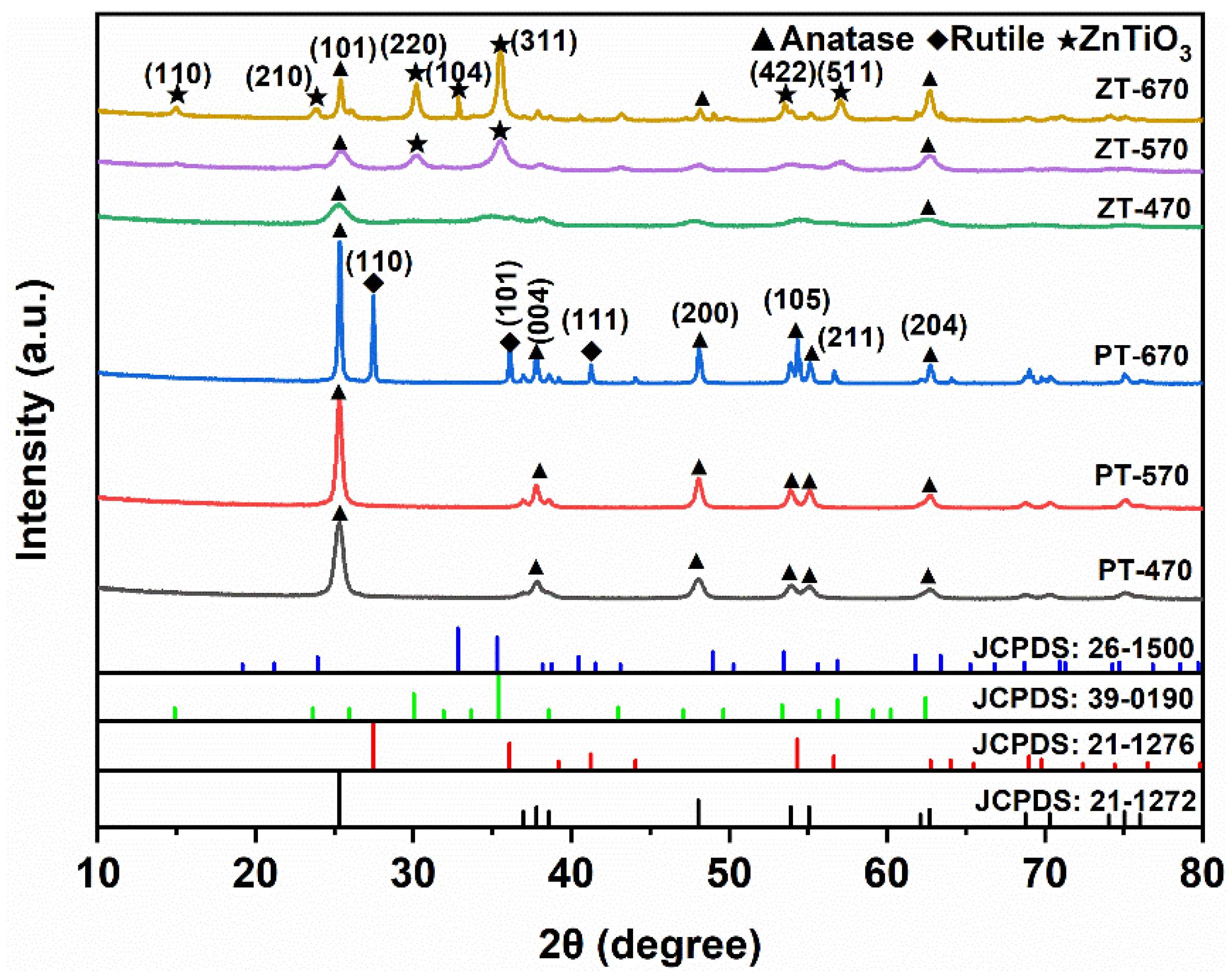

| Sample | 2θ | FWHM | D (nm) |
|---|---|---|---|
| PT-470 | 25.3° | 0.565 | 14.3 nm |
| PT-570 | 25.4° | 0.387 | 20.8 nm |
| PT-670 Anatase (56.3%) | 25.3° | 0.228 | 35.3 nm |
| PT-670 Rutile (43.7%) | 27.4° | 0.150 | 53.6 nm |
| ZT-470 | 25.3° | 0.863 | 9.3 nm |
| ZT-570 | 25.3° | 0.724 | 11.1 nm |
| ZT-670 | 25.4° | 0.298 | 27.0 nm |
| Sample | Specific Surface Area |
|---|---|
| PT-470 | 54.5 m2/g |
| PT-570 | 12.5 m2/g |
| PT-670 | 1.3 m2/g |
| ZT-670 | 10.6 m2/g |
| Sample | 470 °C | 570 °C | 670 °C |
|---|---|---|---|
| PT | 3.20 eV | 3.22 eV | 3.06 eV |
| ZT | 3.21 eV | 3.21 eV | 3.23 eV |
| Sample | k/min−1 | R2 |
|---|---|---|
| PT-470 | 0.025 | 0.997 |
| PT-570 | 0.010 | 0.990 |
| PT-670 | 0.039 | 0.995 |
| ZT-470 | 0.007 | 0.991 |
| ZT-570 | 0.008 | 0.999 |
| ZT-670 | 0.008 | 0.996 |
| Photocatalyst | Catalyst g·L−1 | CMB mg·L−1 | Light Source | Decolorization Degree | Ref. |
|---|---|---|---|---|---|
| TiO2/g-C3N5 | 0.2 | 20 | Xenon lamp (500 W) | 97.4% in 180 min | [52] |
| Au/TiO2 | 0.5 | 10 | Xenon lamp (300 W) | 69.7% in 240 min | [53] |
| SnO2/TiO2 | 0.05 | 10 | Halogen lamp (400 W) | 100% in 180 min | [31] |
| TiO2/FeS2 | 1 | 25 | Cool white lamp (104 W) | 100% in 180 min | [54] |
| ZnO-TiO2 | 0.04 | 10 | UV lamp (18 W) | 59.6% in 120 min | [55] |
| PT-670 | 1 | 10 | Xenon lamp (250 W) | 91.0% in 60 min | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Zhang, X.; Xia, Y.; Zhang, L.; Xu, Q.; Zhu, X.; Feng, W.; Qin, Q. A Fresh Perspective on the Impact of ZnTiO3 Coupling on the Microstructure and Photocatalytic Properties of TiO2 Fabricated at Varied Temperatures. Molecules 2023, 28, 7626. https://doi.org/10.3390/molecules28227626
Zhong Y, Zhang X, Xia Y, Zhang L, Xu Q, Zhu X, Feng W, Qin Q. A Fresh Perspective on the Impact of ZnTiO3 Coupling on the Microstructure and Photocatalytic Properties of TiO2 Fabricated at Varied Temperatures. Molecules. 2023; 28(22):7626. https://doi.org/10.3390/molecules28227626
Chicago/Turabian StyleZhong, Yuanyuan, Xiuping Zhang, Yangwen Xia, Ling Zhang, Qiao Xu, Xiaodong Zhu, Wei Feng, and Qin Qin. 2023. "A Fresh Perspective on the Impact of ZnTiO3 Coupling on the Microstructure and Photocatalytic Properties of TiO2 Fabricated at Varied Temperatures" Molecules 28, no. 22: 7626. https://doi.org/10.3390/molecules28227626
APA StyleZhong, Y., Zhang, X., Xia, Y., Zhang, L., Xu, Q., Zhu, X., Feng, W., & Qin, Q. (2023). A Fresh Perspective on the Impact of ZnTiO3 Coupling on the Microstructure and Photocatalytic Properties of TiO2 Fabricated at Varied Temperatures. Molecules, 28(22), 7626. https://doi.org/10.3390/molecules28227626






