Halogen-Dependent Diversity and Weak Interactions in the Heterometallic Ni/Cd Complex Solids: Structural and Theoretical Investigation
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Spectroscopic Characterization
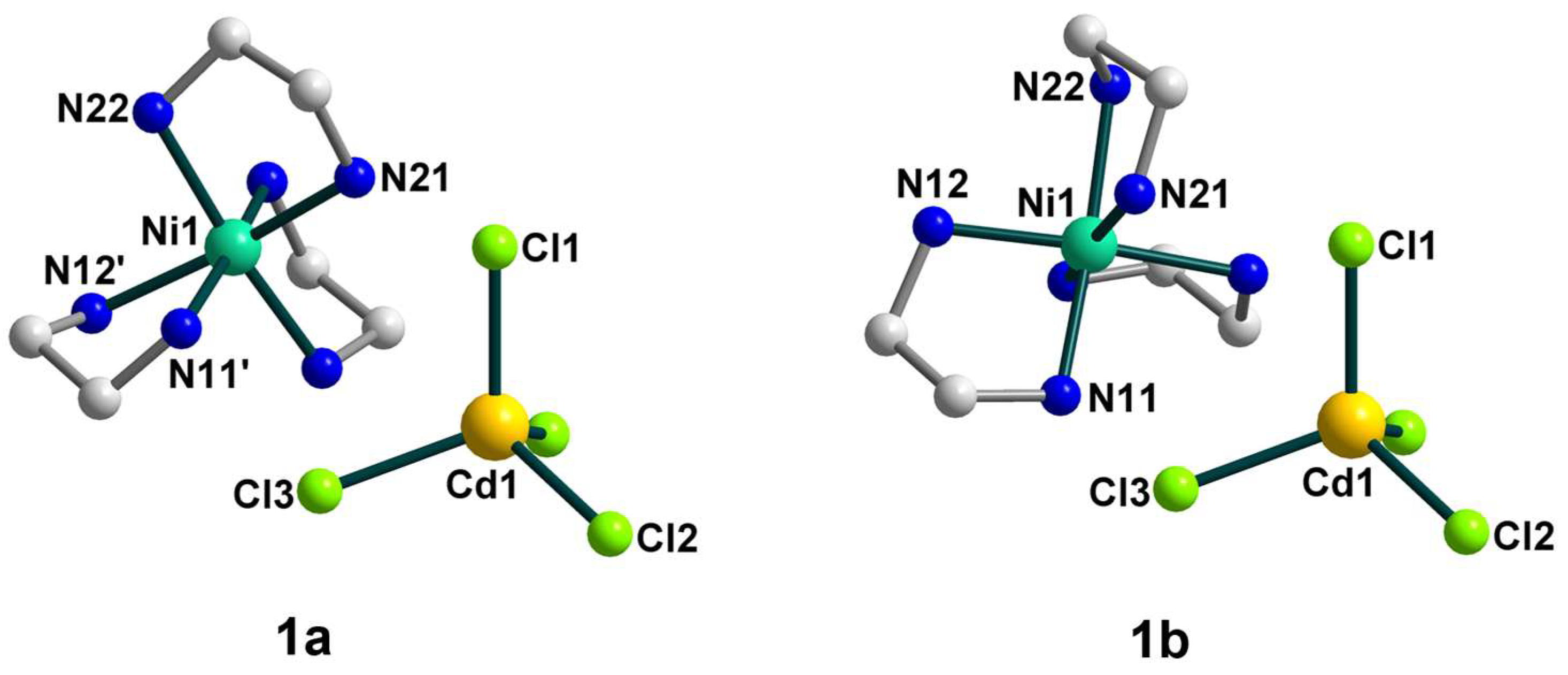
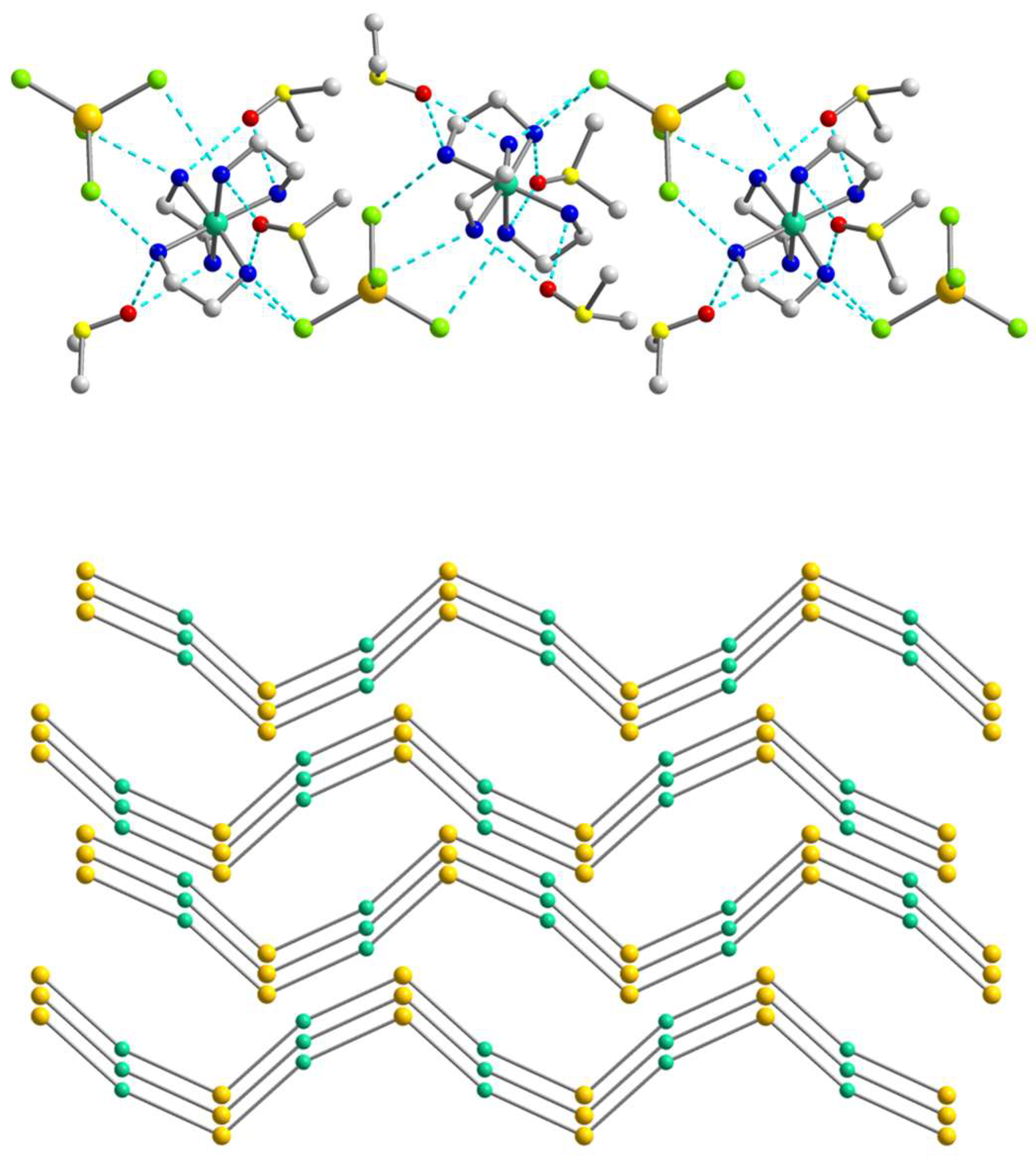
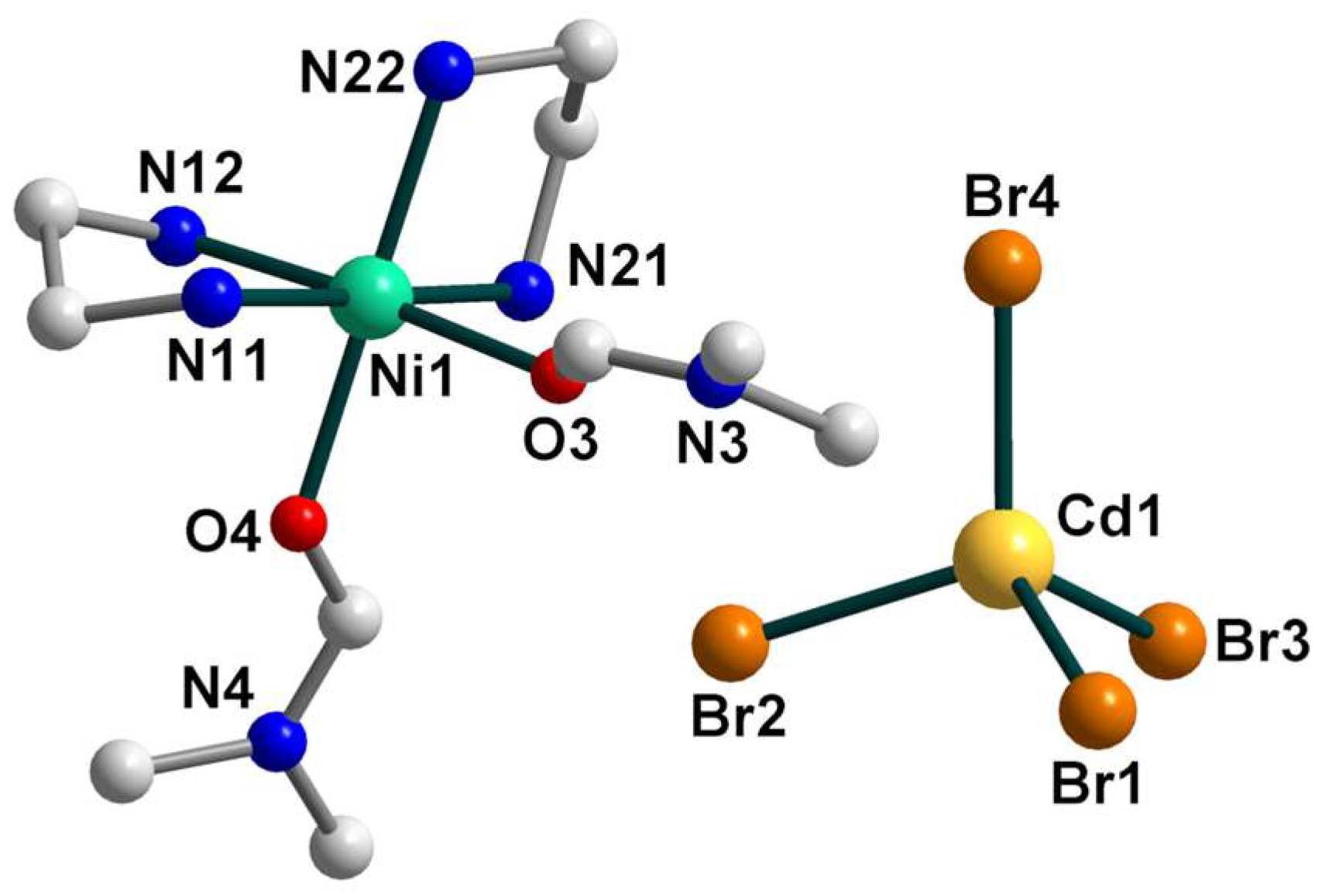
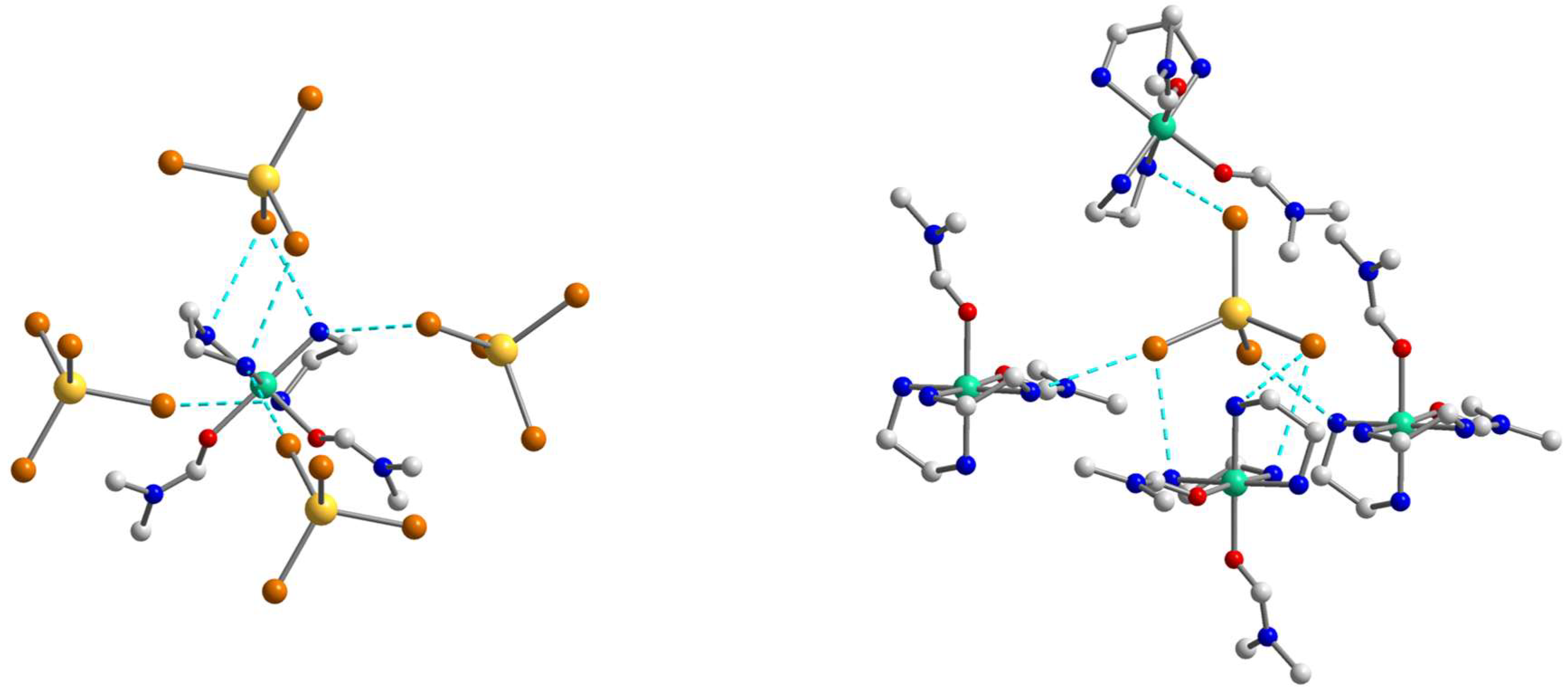
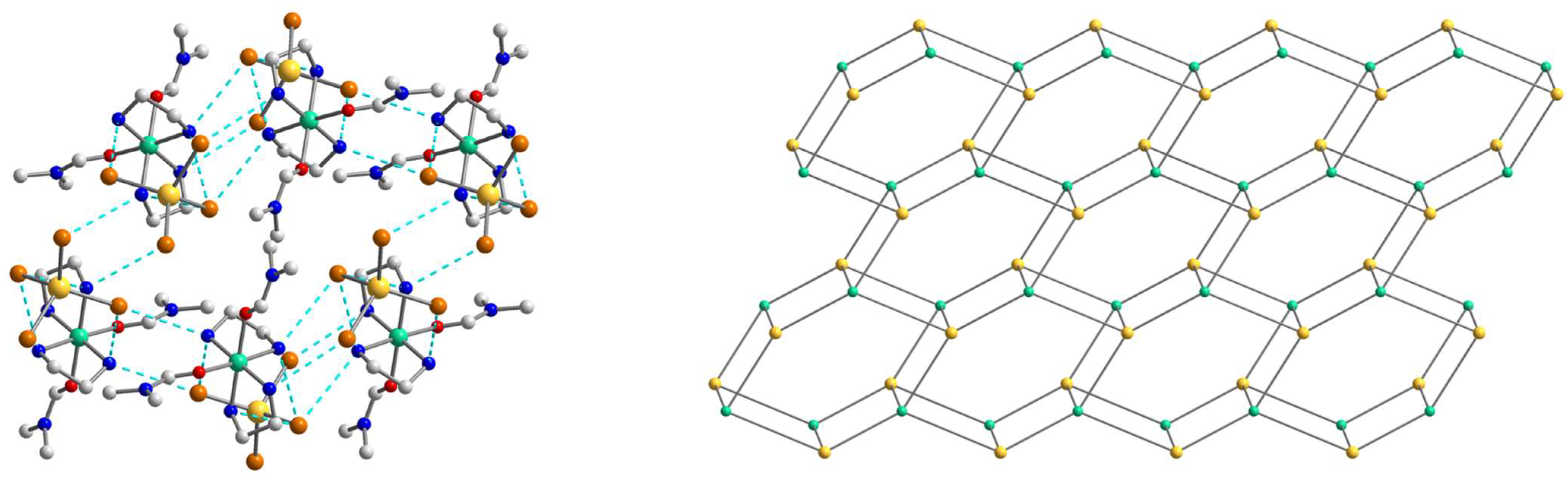
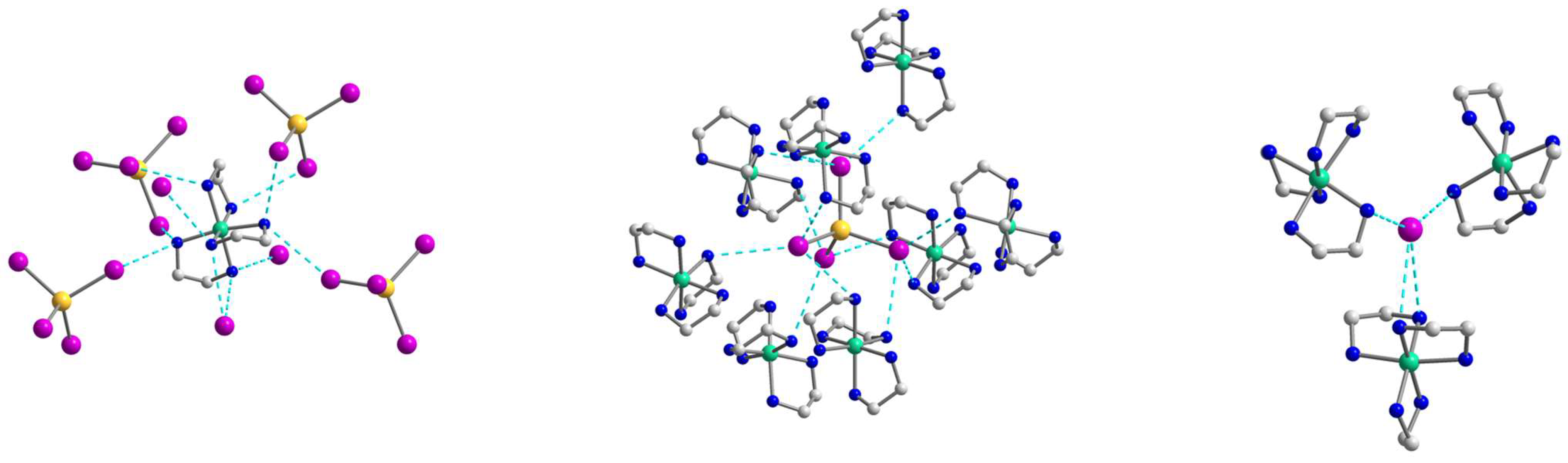
2.2. Crystal Structures
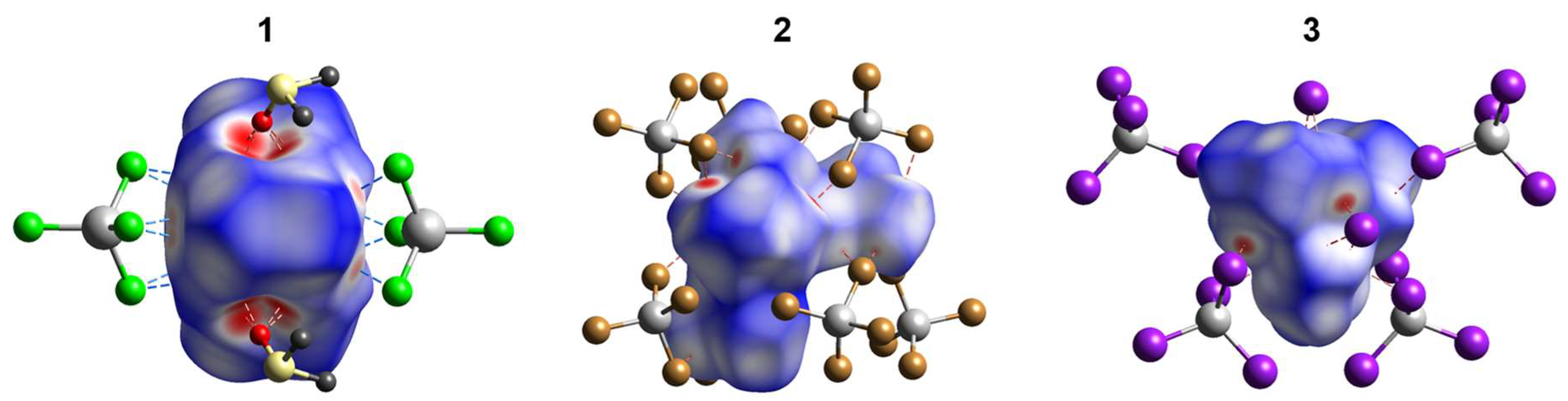
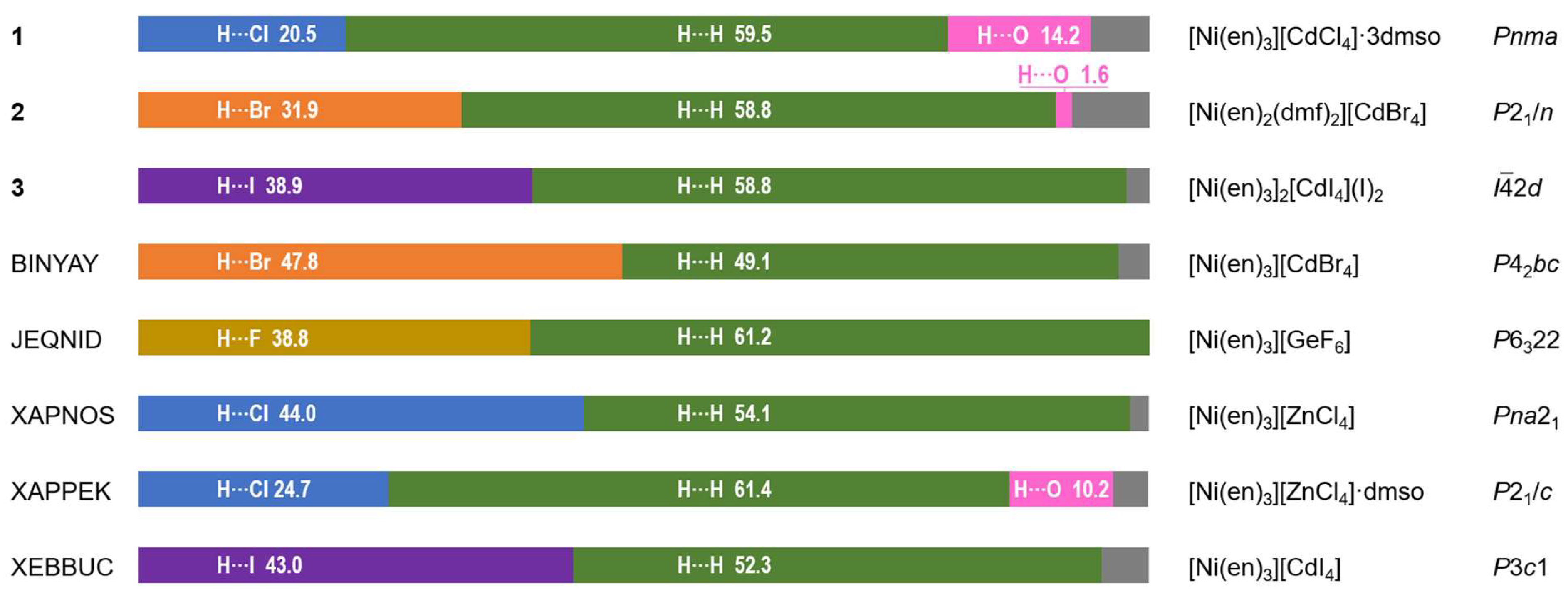
2.3. Hirshfeld Surface Analysis
2.4. Theoretical Studies
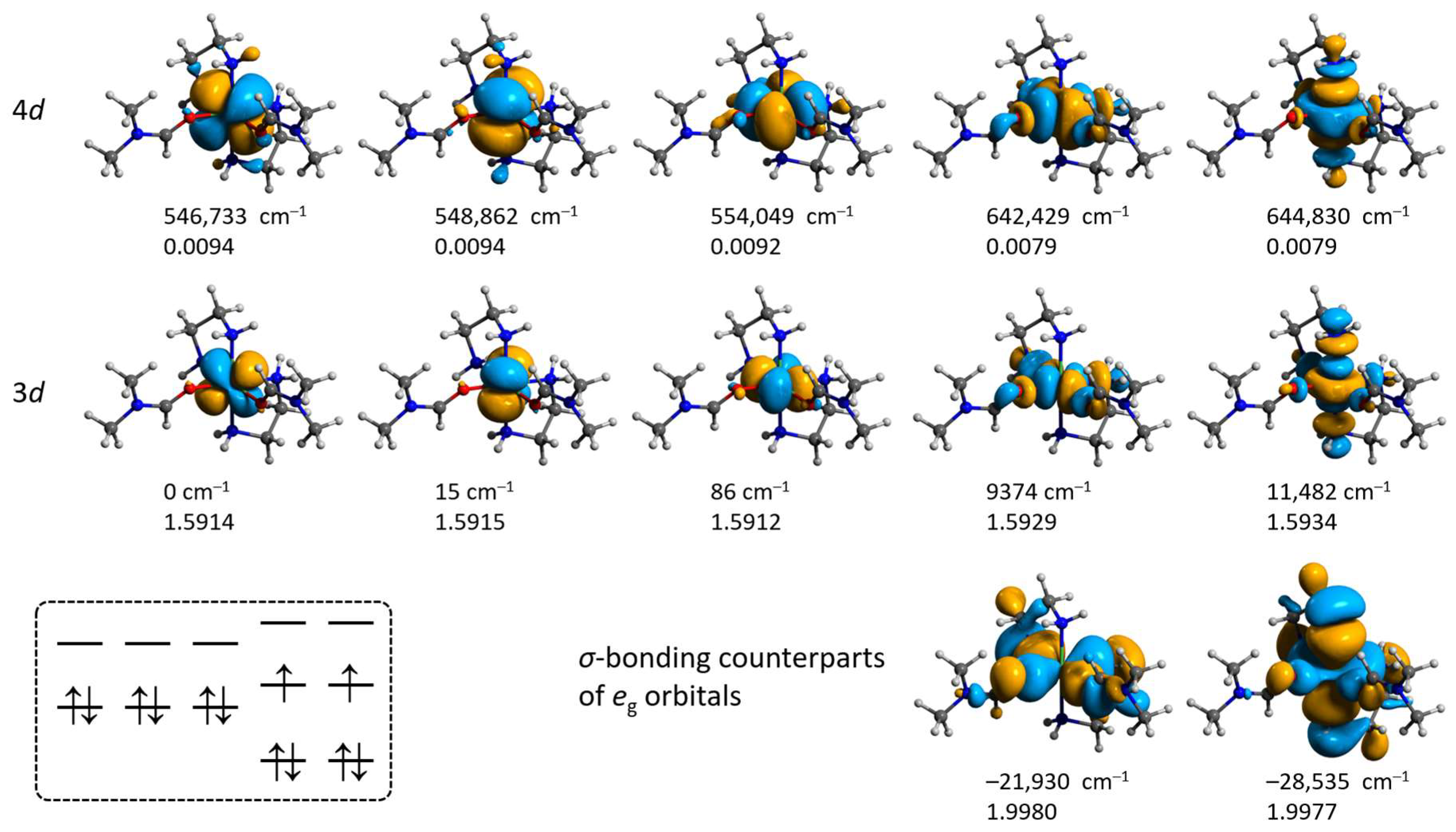
2.4.1. Electronic Structures of [Ni(en)3]2+ and [Ni(en)2(dmf)2]2+ Cations
2.4.2. Non-Covalent Interactions in the Lattices of 1–3
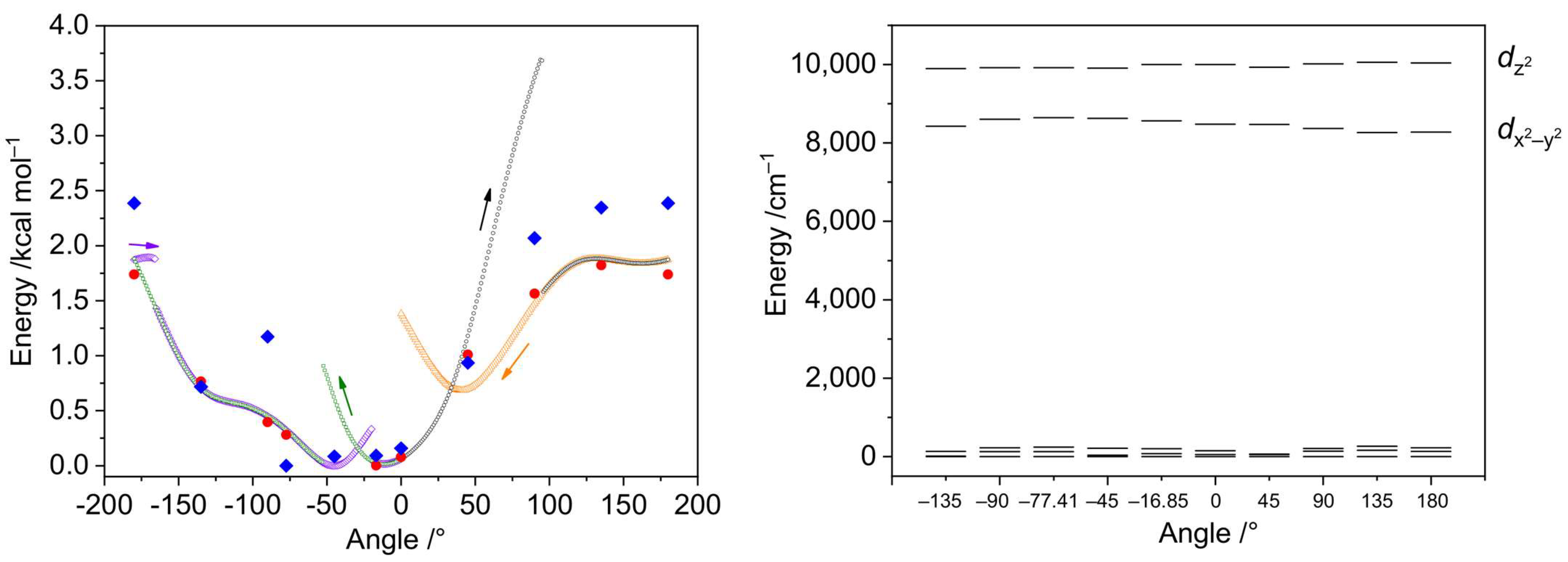
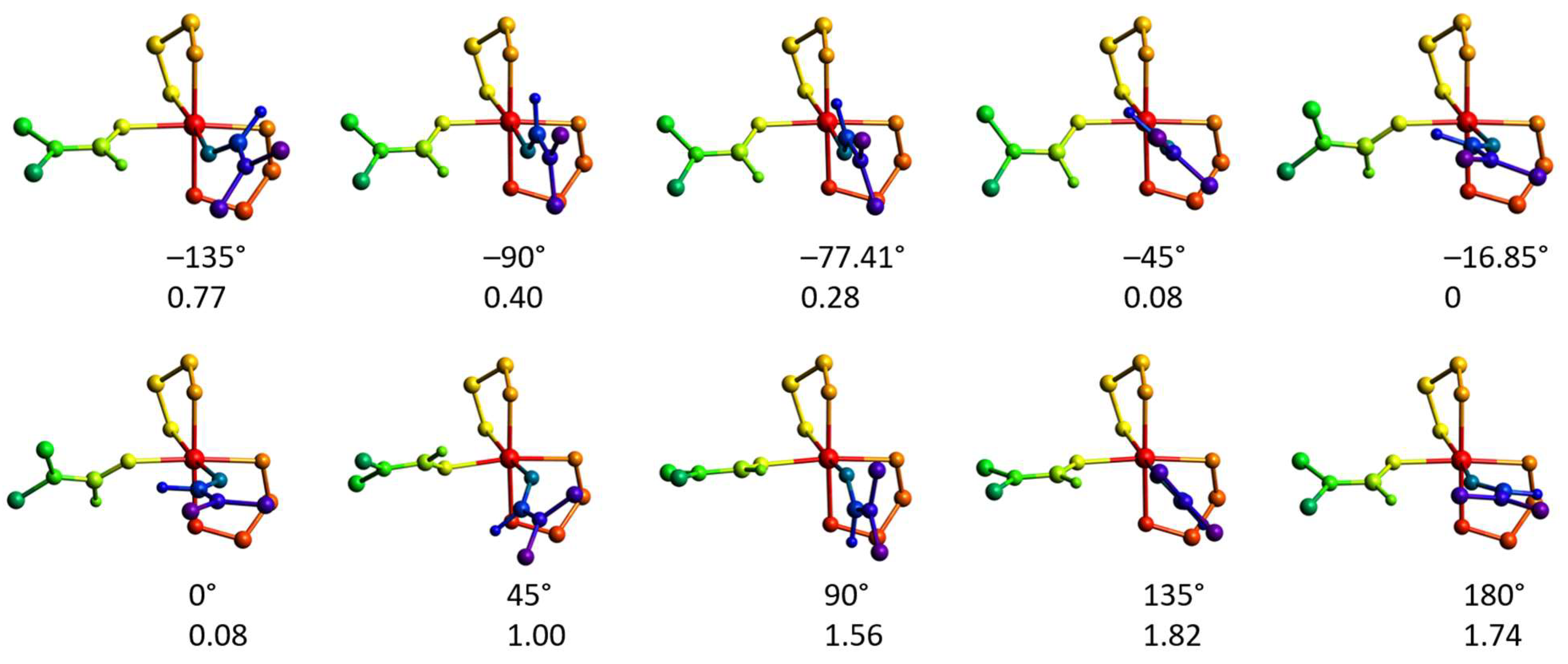
2.4.3. Rotation of the Coordinated dmf Ligand in 2′
2.4.4. The Mechanism of Interconversion of [Ni(en)2(dmf)2]2+ and [Ni(en)3]2+

3. Materials and Methods
3.1. Synthesis of [Ni(en)3][CdCl4]∙3dmso (1)
3.2. Synthesis of [Ni(en)2(dmf)2][CdBr4] (2)
3.3. Synthesis of [Ni(en)3]2[CdI4](I)2 (3)
3.4. Crystallography
3.5. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, R.B.; He, Y.B.; Li, P.; Wang, H.L.; Zhou, W.; Chen, B.L. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef]
- Li, P.H.; Ryder, M.R.; Stoddart, J.F. Hydrogen-Bonded Organic Frameworks: A Rising Class of Porous Molecular Materials. Acc. Mat. Res. 2020, 1, 77–87. [Google Scholar] [CrossRef]
- Wehner, M.; Würthner, F. Supramolecular polymerization through kinetic pathway control and living chain growth. Nat. Rev. Chem. 2020, 4, 38–53. [Google Scholar] [CrossRef]
- Deng, J.H.; Luo, J.; Mao, Y.L.; Lai, S.; Gong, Y.N.; Zhong, D.C.; Lu, T.B. π-π stacking interactions: Non-negligible forces for stabilizing porous supramolecular frameworks. Sci. Adv. 2020, 6, eaax9976. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Powell, J.A.; Li, E.R.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.Y.; Xiao, Z.F.; Zhu, C.F.; Zhang, L.L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, L.B.; Lin, R.B.; Zhou, W.; Zhang, Z.J.; Xiang, S.C.; Chen, B.L. Porous metal-organic frameworks for gas storage and separation: Status and challenges. Energychem. 2019, 1, 100006. [Google Scholar] [CrossRef]
- Cui, Y.J.; Li, B.; He, H.J.; Zhou, W.; Chen, B.L.; Qian, G.D. Metal-Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef]
- Buchwalter, P.; Rose, J.; Braunstein, P. Multimetallic Catalysis Based on Heterometallic Complexes and Clusters. Chem. Rev. 2015, 115, 28–126. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and heterometallic polynuclear transition metal catalysts for alkane C-H bonds oxidative functionalization: Recent advances. Coord. Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Andruh, M. Heterotrimetallic complexes in molecular magnetism. Chem. Commun. 2018, 54, 3559–3577. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Kokozay, V.N.; Pombeiro, A.J.L. Polynuclear Heterometallic Complexes from Metal Powders: The “Direct Synthesis” Approach. Eur. J. Inorg. Chem. 2014, 2014, 4496–4517. [Google Scholar] [CrossRef]
- Semenaka, V.V.; Nesterova, O.V.; Kokozay, V.N.; Zybatyuk, R.I.; Shishkin, O.V.; Boca, R.; Gomez-Garcia, C.J.; Clemente-Juan, J.M.; Jezierska, J. Structural and magnetic studies of tetranuclear heterometallic M/Cr (M=Co, Mn) complexes self-assembled from zerovalent cobalt or manganese, Reineckes salt and diethanolamine. Polyhedron 2010, 29, 1326–1336. [Google Scholar] [CrossRef]
- Nikitina, V.M.; Nesterova, O.V.; Kokozay, V.N.; Dyakonenko, V.V.; Shishkin, O.V.; Jezierska, J. Novel heterometallic Cu(II)/Cr(III) complex with unique open-chain N-ligand produced in conditions of direct template synthesis. Inorg. Chem. Commun. 2009, 12, 101–104. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Kasyanova, K.V.; Buvaylo, E.A.; Vassilyeva, O.Y.; Skelton, B.W.; Nesterov, D.S.; Pombeiro, A.J.L. Heterometallic CoIIIZnII Schiff Base Catalyst for Mild Hydroxylation of C(sp3)-H Bonds of Unactivated Alkanes: Evidence for Dual Mechanism Controlled by the Promoter. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Lipetskaya, A.V.; Petrusenko, S.R.; Kokozay, V.N.; Skelton, B.W.; Jezierska, J. Novel 1D and 2D heterometallic Cu/Cd complexes comprising unique mixed-anion Cd(µ-Cl)(µ-O2CMe)Cl(O2CMe)2−, Cd(µ-O2CMe)2I(O2CMe)2−, Cd(µ-I)(µ-O2CMe)I(O2CMe)2− and Cd2(NCS)2I(O2CMe)22− building blocks: Synthesis from elemental copper, structure and magnetism. Polyhedron 2005, 24, 1425–1434. [Google Scholar] [CrossRef]
- Pryma, O.V.; Petrusenko, S.R.; Kokozay, V.N.; Shishkin, O.V.; Zhigalko, M.V. Novel heterometallic Cu/Cd complex containing a unique polymeric ladder-like anion [Cd2(O2CMe)6]2−n derived from elemental copper and cadmium oxide. Inorg. Chem. Commun. 2003, 6, 896–899. [Google Scholar] [CrossRef]
- Pryma, O.V.; Petrusenko, S.R.; Kokozay, V.N.; Shishkin, O.V.; Zhigalko, M.V.; Linert, W. New one- and two-dimensional heterometallic Cu/Cd halogeno or thiocyanato bridged coordination polymers synthesized directly from elemental copper and cadmium oxide in the presence of ethylenediamine. Z. Naturforsch. B J. Chem. Sci. 2003, 58, 1117–1123. [Google Scholar]
- Nesterova, O.V.; Kasyanova, K.V.; Makhankova, V.G.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W.; Nesterov, D.S.; Pombeiro, A.J.L. Stereospecific sp3 C-H oxidation with m-CPBA: A CoIII Schiff base complex as pre-catalyst vs. its CoIIICdII heterometallic derivative. Appl. Catal. A Gen. 2018, 560, 171–184. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Kokozay, V.N.; Skelton, B.W. A Pentanuclear Cu/Co/Ni Complex with 2-(Dimethylamino)ethanol—Observation of a Rare Molecular Structure Type and Its Place in General Structural Types: An Analysis of the Cambridge Structural Database. Eur. J. Inorg. Chem. 2009, 2009, 5469–5473. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Makhankova, V.G.; Kokozay, V.N.; Skelton, B.W. Direct synthesis and crystal structures of new heteropolynuclear complexes containing aminoalcohol ligands: From heterobi- (Co/Zn) to heterotrimetallic (Cu/Co/Zn) compounds. Inorg. Chim. Acta 2005, 358, 4519–4526. [Google Scholar] [CrossRef]
- Krzystek, J.; Ozarowski, A.; Telser, J. Multi-frequency, high-field EPR as a powerful tool to accurately determine zero-field splitting in high-spin transition metal coordination complexes. Coord. Chem. Rev. 2006, 250, 2308–2324. [Google Scholar] [CrossRef]
- Pladzyk, A.; Ozarowski, A.; Ponikiewski, L. Crystal and electronic structures of Ni(II) silanethiolates containing flexible diamine ligands. Inorg. Chim. Acta 2016, 440, 84–93. [Google Scholar] [CrossRef]
- Karmalkar, D.G.; Larson, V.A.; Malik, D.D.; Lee, Y.M.; Seo, M.S.; Kim, J.; Vasiliauskas, D.; Shearer, J.; Lehnert, N.; Nam, W. Preparation and Characterization of a Formally NiIV-Oxo Complex with a Triplet Ground State and Application in Oxidation Reactions. J. Am. Chem. Soc. 2022, 144, 22698–22712. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Cho, K.B.; Lee, Y.M.; Hong, S.; Nam, W. Mechanistic dichotomies in redox reactions of mononuclear metal-oxygen intermediates. Chem. Soc. Rev. 2020, 49, 8988–9027. [Google Scholar] [CrossRef] [PubMed]
- Nesterov, D.S.; Nesterova, O.V. Catalytic Oxidations with Meta-Chloroperoxybenzoic Acid (m-CPBA) and Mono- and Polynuclear Complexes of Nickel: A Mechanistic Outlook. Catalysts 2021, 11, 1148. [Google Scholar] [CrossRef]
- Bok, K.H.; Lee, M.M.; You, G.R.; Ahn, H.M.; Ryu, K.Y.; Kim, S.-J.; Kim, Y.; Kim, C. Synthesis, Characterization, and Catalytic Activities of A Nickel(II) Monoamido-Tetradentate Complex: Evidence For NiIII-Oxo and NiIV-Oxo Species. Chem. Eur. J. 2017, 23, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Jeong, A.R.; Shin, J.W.; Jeong, J.H.; Bok, K.H.; Kim, C.; Jeong, D.; Cho, J.; Hayami, S.; Min, K.S. Dinuclear Iron(III) and Nickel(II) Complexes Containing N-(2-Pyridylmethyl)-N’-(2-hydroxyethyl)ethylenediamine: Catalytic Oxidation and Magnetic Properties. Chem. Eur. J. 2017, 23, 3023–3033. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci.Cryst. Eng. Mat. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- According to the Cambridge Structural Database Version 5.43. Available online: https://software.chem.ucla.edu/csd/ (accessed on 14 November 2023).
- Wieczorrek, C.; Tebbe, K.F. Bis tris(1,2-ethandiamin)cadmium -tetra-iodocadmat-diiodid. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 1804–1807. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Petrusenko, S.R.; Dyakonenko, V.V.; Shishkin, O.V.; Linert, W. A three-dimensional framework of bis[tris(ethylenediamine)zinc] tetraiodocadmate diiodide assisted by N-H center dot center dot center dot I hydrogen bonds. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, M281–M283. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Petrusenko, S.R.; Kokozay, V.N.; Skelton, B.W.; Bjernemose, J.K.; Raithby, P.R. Heterometallic Ni/Zn amine complexes possessing extended 2D and 3D hydrogen-bonded networks prepared from zinc oxide. Inorg. Chim. Acta 2005, 358, 2725–2738. [Google Scholar] [CrossRef]
- Chesnut, D.J.; Haushalter, R.C.; Zubieta, J. The hydrothermal synthesis and structural characterization of a new class of compounds: Nickel organoamine-halocadmates. Inorg. Chim. Acta 1999, 292, 41–51. [Google Scholar] [CrossRef]
- Allen, F.H.; Bruno, I.J. Bond lengths in organic and metal-organic compounds revisited: X-H bond lengths from neutron diffraction data. Acta Crystallogr. Sect. B Struct. Sci.Cryst. Eng. Mat. 2010, 66, 380–386. [Google Scholar] [CrossRef]
- Weller, M.T.; Henry, P.F.; Ting, V.P.; Wilson, C.C. Crystallography of hydrogen-containing compounds: Realizing the potential of neutron powder diffraction. Chem. Commun. 2009, 2973–2989. [Google Scholar] [CrossRef]
- Nolet, M.C.; Beaulac, R.; Boulanger, A.M.; Reber, C. Allowed and forbidden d-d bands in octahedral coordination compounds: Intensity borrowing and interference dips in absorption spectra. In Optical Spectra and Chemical Bonding in Transition Metal Complexes; Schonherr, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 107, pp. 145–158. [Google Scholar]
- González, E.; Rodrigue-Witchel, A.; Reber, C. Absorption spectroscopy of octahedral nickel(II) complexes:: A case study of interactions between multiple electronic excited states. Coord. Chem. Rev. 2007, 251, 351–363. [Google Scholar] [CrossRef]
- Eftimie, E.L.A.; Avram, C.N.; Brik, M.G.; Chernyshev, V.A.; Avram, N.M. Ab initio analysis of the optical spectra and EPR parameters of Ni2+ ions in CaF2 and CdF2 crystals. J. Lumin. 2019, 214, 116577. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W.T. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Edit. 2022, 61, e202205735. [Google Scholar] [CrossRef]
- Riplinger, C.; Pinski, P.; Becker, U.; Valeev, E.F.; Neese, F. Sparse maps-A systematic infrastructure for reduced-scaling electronic structure methods. II. Linear scaling domain based pair natural orbital coupled cluster theory. J. Chem. Phys. 2016, 144, 024109. [Google Scholar] [CrossRef] [PubMed]
- Riplinger, C.; Sandhoefer, B.; Hansen, A.; Neese, F. Natural triple excitations in local coupled cluster calculations with pair natural orbitals. J. Chem. Phys. 2013, 139, 134101. [Google Scholar] [CrossRef]
- Cohen, A.J.; Mori-Sanchez, P.; Yang, W.T. Challenges for Density Functional Theory. Chem. Rev. 2012, 112, 289–320. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Pombeiro, A.J.L.; Nesterov, D.S. Tetranuclear Copper Complexes with Bulky Aminoalcohol Ligands as Catalysts for Oxidative Phenoxazinone Synthase-like Coupling of Aminophenol: A Combined Experimental and Theoretical Study. Catalysts 2022, 12, 1408. [Google Scholar] [CrossRef]
- Richens, D.T. Ligand substitution reactions at inorganic centers. Chem. Rev. 2005, 105, 1961–2002. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wil. Interdiscipl. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wil. Interdiscipl. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef] [PubMed]
- Najibi, A.; Goerigk, L. DFT-D4 counterparts of leading meta-generalized-gradient approximation and hybrid density functionals for energetics and geometries. J. Comput. Chem. 2020, 41, 2562–2572. [Google Scholar] [CrossRef]
- Zheng, J.J.; Xu, X.F.; Truhlar, D.G. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 2011, 128, 295–305. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms In Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Pantazis, D.A.; Neese, F. All-electron scalar relativistic basis sets for the 6p elements. Theor. Chem. Acc. 2012, 131, 1292. [Google Scholar] [CrossRef]
- Singh, S.K.; Eng, J.; Atanasov, M.; Neese, F. Covalency and chemical bonding in transition metal complexes: An ab initio based ligand field perspective. Coord. Chem. Rev. 2017, 344, 2–25. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab-initio calculation of vibrational absorption and circular-dichroism spectra using density-functional force-fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Hertwig, R.H.; Koch, W. On the parameterization of the local correlation functional. What is Becke-3-LYP? Chem. Phys. Lett. 1997, 268, 345–351. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Bannwarth, C.; Grimme, S. Extension of the D3 dispersion coefficient model. J. Chem. Phys. 2017, 147, 034112. [Google Scholar] [CrossRef]
- Stoychev, G.L.; Auer, A.A.; Neese, F. Automatic Generation of Auxiliary Basis Sets. J. Chem. Theor. Comput. 2017, 13, 554–562. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Hirata, S.; Head-Gordon, M. Time-dependent density functional theory within the Tamm-Dancoff approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.P. Introduction of n-electron valence states for multireference perturbation theory. J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.P. n-electron valence state perturbation theory: A spinless formulation and an efficient implementation of the strongly contracted and of the partially contracted variants. J. Chem. Phys. 2002, 117, 9138–9153. [Google Scholar] [CrossRef]
- Weigend, F.; Kattannek, M.; Ahlrichs, R. Approximated electron repulsion integrals: Cholesky decomposition versus resolution of the identity methods. J. Chem. Phys. 2009, 130, 164106. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree-Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree-Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Asgeirsson, V.; Birgisson, B.O.; Bjornsson, R.; Becker, U.; Neese, F.; Riplinger, C.; Jonsson, H. Nudged Elastic Band Method for Molecular Reactions Using Energy-Weighted Springs Combined with Eigenvector Following. J. Chem. Theor. Comput. 2021, 17, 4929–4945. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis o molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]





| 1 | 2 | 3 | |
|---|---|---|---|
| Empirical formula | C12H42CdCl4N6NiO3S3 | C10H30Br4CdN6NiO2 | C12H48CdI6N12Ni2 |
| Formula weight | 727.60 | 757.15 | 1351.84 |
| Temperature, K | 150(2) | 150(2) | 150(2) |
| Wavelength, Å | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Orthorhombic | Monoclinic | Tetragonal |
| Space group | Pnma | P21/n | I2d |
| a, Å | 17.298(4) | 12.3920(12) | 14.6310(10) |
| b, Å | 13.884(3) | 11.3388(11) | 14.6310(10) |
| c, Å | 12.558(3) | 17.672(2) | 16.824(2) |
| α, ° | 90 | 90 | 90 |
| β, ° | 90 | 108.043(2) | 90 |
| γ, ° | 90 | 90 | 90 |
| Volume, Å3 | 3016.0(12) | 2361.0(4) | 3601.4(7) |
| Z | 4 | 4 | 4 |
| Density (calculated), Mg/m3 | 1.602 | 2.130 | 2.493 |
| μ, mm−1 | 1.915 | 8.482 | 6.787 |
| F(000) | 1488 | 1456 | 2504 |
| Crystal size, mm3 | 0.450 × 0.180 × 0.060 | 0.550 × 0.240 × 0.220 | 0.160 × 0.160 × 0.130 |
| θ range for data collection, ° | 2.004 to 25.174 | 1.777 to 27.00 | 1.845 to 37.552 |
| Index ranges | −20 ≤ h ≤ 20, −16 ≤ k ≤ 16, −14 ≤ l ≤ 14 | −16 ≤ h ≤ 16, −15 ≤ k ≤ 15, −24 ≤ l ≤ 24 | −25 ≤ h ≤ 25, −25 ≤ k ≤ 25, −28 ≤ l ≤ 28 |
| Reflections collected | 22,087 | 22,374 | 35,196 |
| Independent reflections | 2818 [R(int) = 0.069] | 5146 [R(int) = 0.052] | 4747 [R(int) = 0.033] |
| Completeness to θ = 25.174° (for 1), 25.242° (for 2) and 25.242° (for 3), % | 99.5 | 99.9 | 100.0 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2818/0/191 | 5146/0/221 | 4747/0/76 |
| Goodness-of-fit on F2 | 1.091 | 0.989 | 1.113 |
| Final R indices [I > 2σ(I)] | R1 = 0.0434, wR2 = 0.1003 | R1 = 0.0336, wR2 = 0.0818 | R1 = 0.0198, wR2 = 0.0391 |
| R indices (all data) | R1 = 0.0785, wR2 = 0.1241 | R1 = 0.0480, wR2 = 0.0873 | R1 = 0.0221, wR2 = 0.0397 |
| Largest diff. peak and hole, e Å−3 | 1.121 and −0.564 | 1.421 and −1.094 | 1.125 and −0.412 |
| Absolute structure parameter | – | – | 0.002(11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterova, O.V.; Petrusenko, S.R.; Skelton, B.W.; Nesterov, D.S. Halogen-Dependent Diversity and Weak Interactions in the Heterometallic Ni/Cd Complex Solids: Structural and Theoretical Investigation. Molecules 2023, 28, 7652. https://doi.org/10.3390/molecules28227652
Nesterova OV, Petrusenko SR, Skelton BW, Nesterov DS. Halogen-Dependent Diversity and Weak Interactions in the Heterometallic Ni/Cd Complex Solids: Structural and Theoretical Investigation. Molecules. 2023; 28(22):7652. https://doi.org/10.3390/molecules28227652
Chicago/Turabian StyleNesterova, Oksana V., Svitlana R. Petrusenko, Brian W. Skelton, and Dmytro S. Nesterov. 2023. "Halogen-Dependent Diversity and Weak Interactions in the Heterometallic Ni/Cd Complex Solids: Structural and Theoretical Investigation" Molecules 28, no. 22: 7652. https://doi.org/10.3390/molecules28227652
APA StyleNesterova, O. V., Petrusenko, S. R., Skelton, B. W., & Nesterov, D. S. (2023). Halogen-Dependent Diversity and Weak Interactions in the Heterometallic Ni/Cd Complex Solids: Structural and Theoretical Investigation. Molecules, 28(22), 7652. https://doi.org/10.3390/molecules28227652









