Norsesquiterpenes from the Latex of Euphorbia dentata and Their Chemical Defense Mechanisms against Helicoverpa armigera
Abstract
:1. Introduction
2. Results
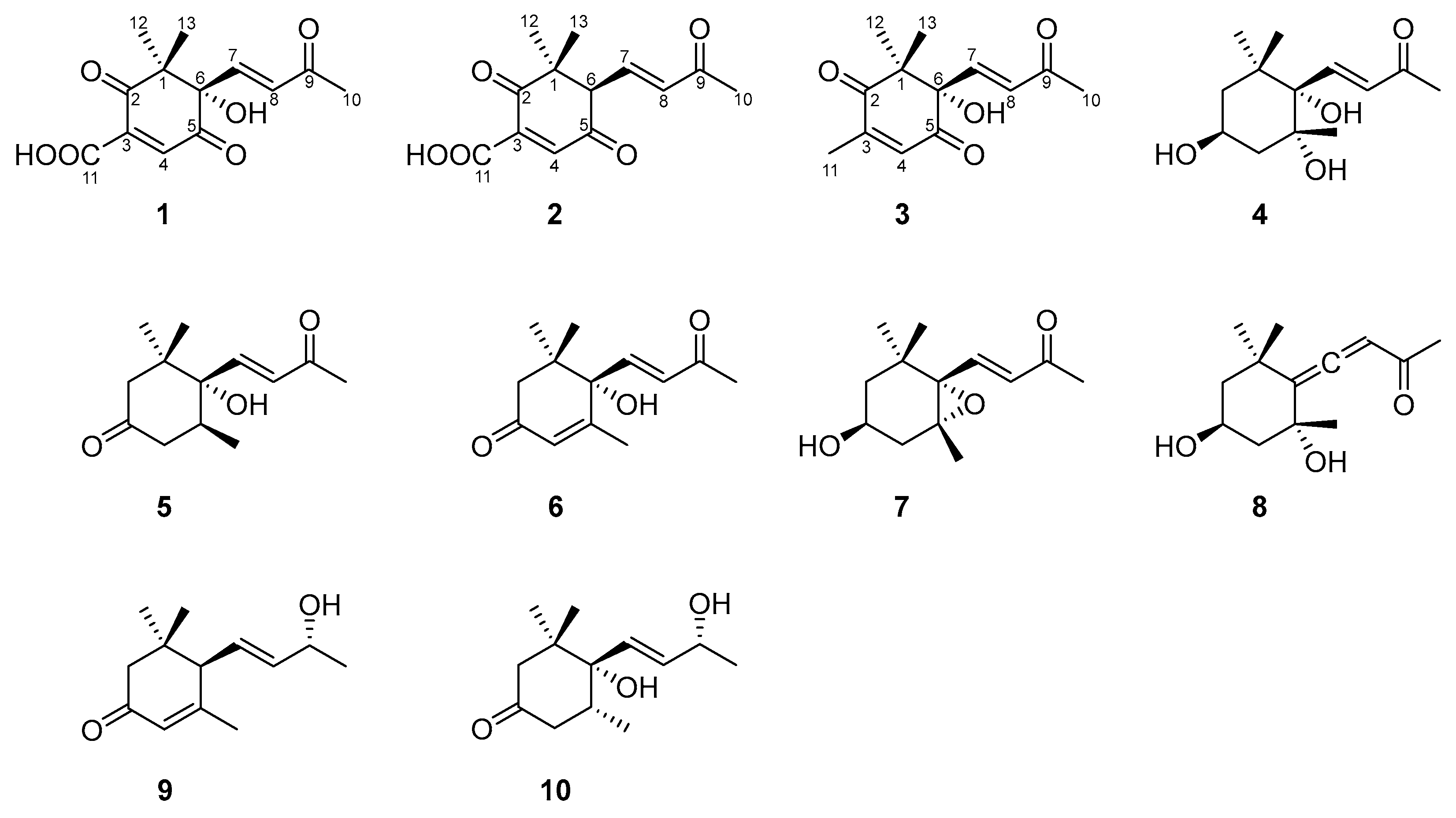
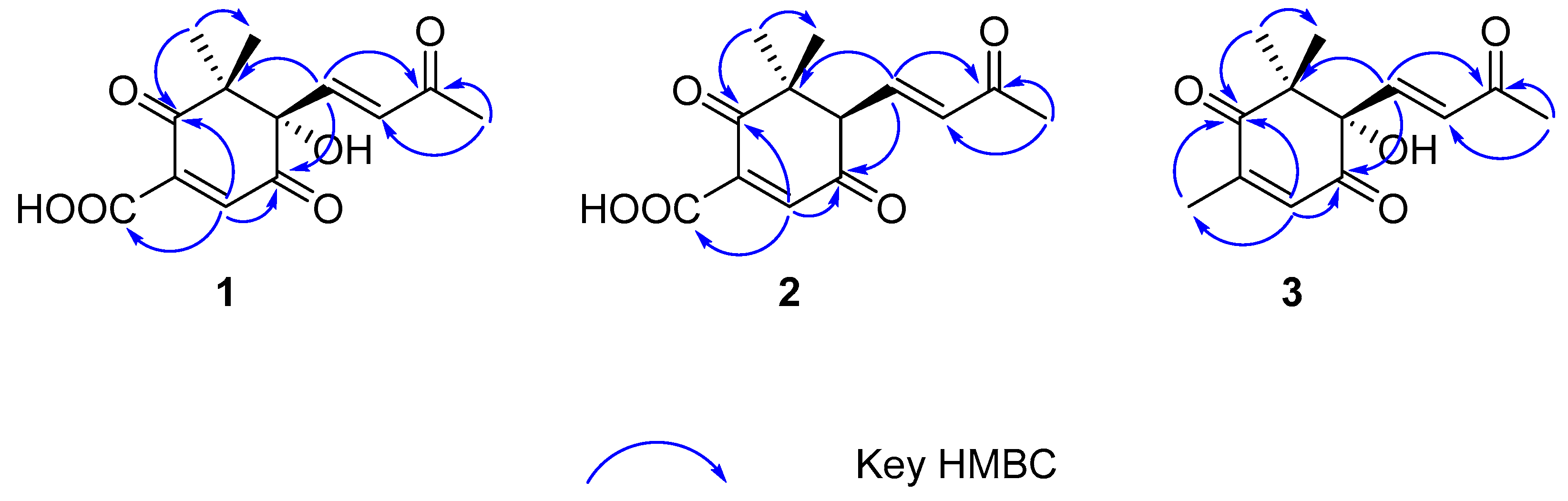
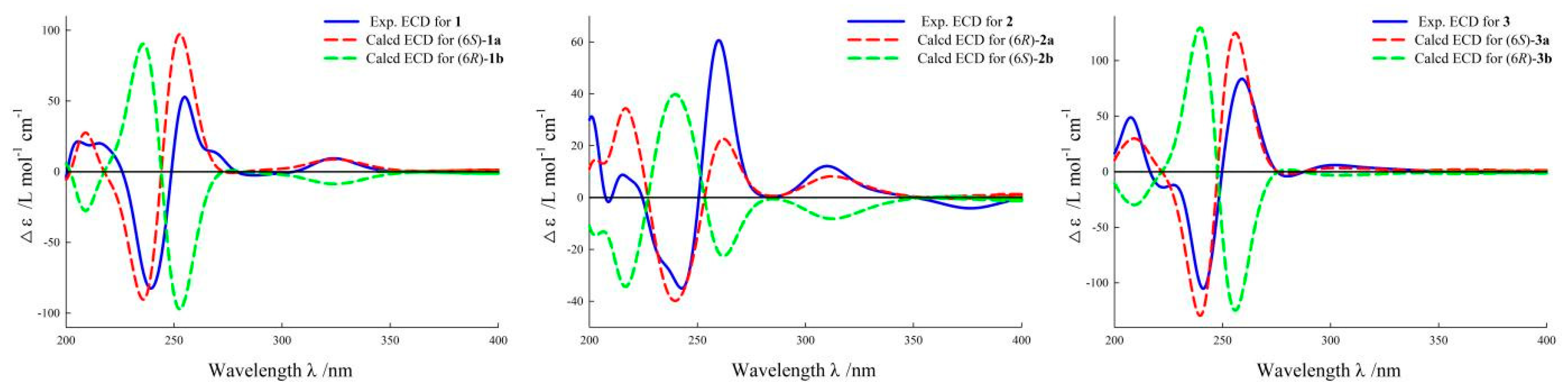
2.1. Structural Elucidation
2.2. Antifeedant and Growth-Inhibitory Effects
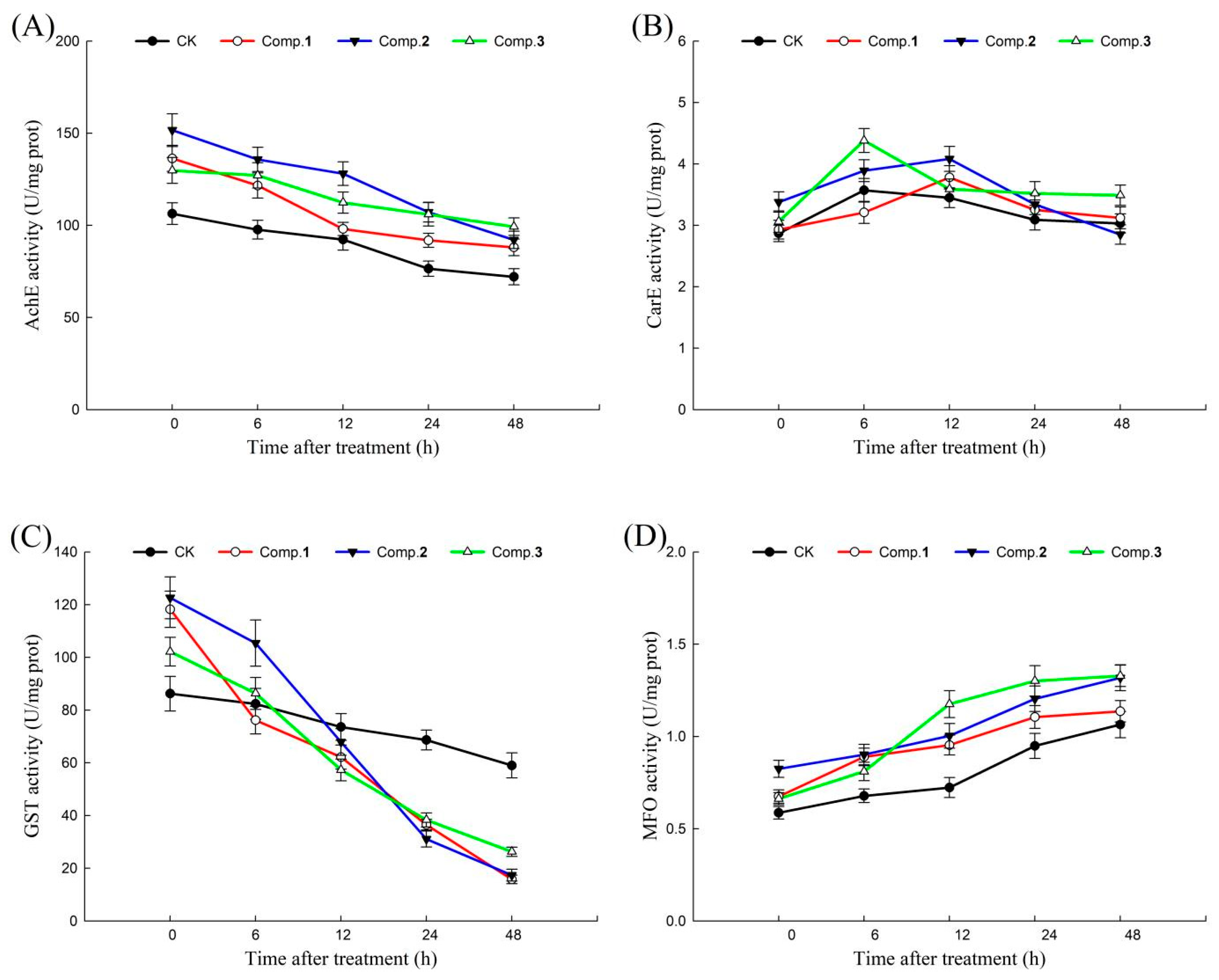
2.3. Detoxifying Enzymes Effects
2.4. Molecular Docking Analyses
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Insect Material
4.4. Extraction and Isolation
4.5. Spectroscopic Data
4.6. ECD Calculations
4.7. Antifeedant and Growth-Inhibitory Assay
4.8. Detoxifying Enzymes Assay
4.9. Molecular Docking Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Futuyma, D.J.; Agrawal, A.A. Macroevolution and the biological diversity of plants and herbivores. Proc. Natl. Acad. Sci. USA 2009, 106, 18054–18061. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; One, H.; Nakamura, M.; Tateishi, K.; Hirayama, C.; Tamura, Y.; Hattori, M.; Koyama, A.; Kohno, K. Mulberry latex rich in antidiabetic sugar-mimic alkaloids forces dieting on caterpillars. Proc. Natl. Acad. Sci. USA 2006, 103, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Salomé, A.; Luis, F.; Klinkhamer, P.G.L.; Choi, Y.H. Plant latex, from ecological interests to bioactive chemical resources. Phytochemistry 2019, 85, 856–868. [Google Scholar]
- Agrawal, A.A.; Konno, K. Latex: A model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 311–331. [Google Scholar] [CrossRef]
- Ramos, M.V.; Demarco, D.; Souza, I.C.; Freitas, C.D.T. Laticifers, latex, and their role in plant defense. Trends Plant Sci. 2019, 24, 553–567. [Google Scholar] [CrossRef]
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Betancur-Galvis, L.; Palomares, E.; Marco, J.A.; Estornell, E. Tigliane diterpenes from the latex of Euphorbia obtusifolia with inhibitory activity on the mammalian mitochondrial respiratory chain. J. Ethnopharmacol. 2003, 85, 279–282. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of terpenoids: Monoterpenes, sesquiterpenes and diterpenes. Ann. Plant Rev. 2011, 40, 258–303. [Google Scholar]
- Barina, Z.; Shevera, M.; Sirbu, C.; Pinke, G. Current distribution and spreading of Euphorbia davidii (E. dentata agg.) in Europe. Cent. Eur. J. Biol. 2013, 8, 87–95. [Google Scholar] [CrossRef]
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Tay, W.T.; Soria, M.F.; Walsh, T.; Thomazoni, D.; Silvie, P.; Behere, G.T.; Anderson, C.; Downes, S. A brave new world for an old world pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS ONE 2013, 8, e80134. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.; Trujillo, D.; Bernardi, O.; Rodrigues, J.C.V.; Bailey, W.D.; Gilligan, T.M.; Carrillo, D. Comparative toxicity of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) to selected insecticides. Insects 2020, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Piao, X.M.; Zhang, L.X.; Song, S.Y.; Xu, Y.H. Ginsenosides from the stems and leaves of Panax ginseng show antifeedant activity against Plutella xylostella (Linnaeus). Ind. Crop. Prod. 2018, 124, 412–417. [Google Scholar] [CrossRef]
- Wu, G.; Miyata, T. Susceptibilities to methamidophos and enzymatic characteristics in 18 species of pest insects and their natural enemies in crucifer vegetable crops. Pestic. Biochem. Phys. 2005, 82, 79–93. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Varela, R.M.; Torres, A.; Oliva, R.M.; Molinillo, J.M.G. Bioactive norsesquiterpenes from Helianthus annuus with potential allelopathic activity. Phytochemistry 1998, 48, 631–636. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, D.G.; Yeon, S.W.; Kwon, H.S.; Ko, J.H.; Shin, D.J.; Park, H.S.; Kim, Y.S.; Bang, M.H.; Baek, N.I. Isolation of megastigmane sesquiterpenes from the silkworm (Bombyx mori L.) droppings and their promotion activity on HO-1 and SIRT1. Arch. Pharm. Res. 2011, 34, 533–542. [Google Scholar] [CrossRef]
- Hossen, K.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Identification of four allelopathic compounds including a novel compound from Elaeocarpus floribundus Blume and determination of their allelopathic activity. J. Environ. Manag. 2023, 326, 116728. [Google Scholar] [CrossRef]
- Yuan, Z.G.; Zheng, X.W.; Zhao, Y.; Liu, Y.; Zhou, S.X.; Wei, C.X.; Hu, Y.X.; Shao, H. Phytotoxic compounds isolated from leaves of the invasive weed Xanthium spinosum. Molecules 2018, 23, 2840. [Google Scholar] [CrossRef]
- Kim, I.; Chin, Y.W.; Lim, S.W.; Kim, Y.C.; Kim, J. Norisoprenoids and hepatoprotective flavone glycosides from the aerial parts of Beta vulgaris var. cicla. Arch. Pharm. Res. 2004, 27, 600–603. [Google Scholar] [CrossRef]
- Kuang, H.X.; Yang, B.Y.; Xia, Y.G.; Feng, W.S. Chemical constituents from the flower of Datura metel L. Arch. Pharm. Res. 2008, 31, 1094–1097. [Google Scholar] [CrossRef]
- De Marino, S.; Borbone, N.; Zollo, N.; Ianaro, A.; Di Meglio, P.; Iorizzi, M. Megastigmane and phenolic components from Laurus nobilis L. leaves and their inhibitory effects on nitric oxide production. J. Agric. Food Chem. 2004, 52, 7525–7531. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Liu, Y.; Xiao, C.J.; Jing, S.X.; Luo, S.H.; Li, S.H. Chemical profile and defensive function of the latex of Euphorbia peplus. Phytochemistry 2017, 136, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.H.; Hua, J.; Niu, X.M.; Liu, Y.; Li, C.H.; Zhou, Y.Y.; Jing, S.X.; Zhao, X.; Li, S.H. Defense sesterterpenoid lactones from Leucosceptrum canum. Phytochemistry 2013, 86, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Wang, M.Q.; Tian, M.X.; Yuan, L.L.; Yu, B.M.; Qu, B.; An, T.; Feng, Y.L. Pyrrole alkaloids from Solanum rostratum and their chemical defense function against Henosepilachna vigintioctomaculata. Fitoterapia 2021, 155, 105031. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, J.L.; An, T.; Lyu, F.N.; Jia, P.F.; Zhou, M.J.; Liu, Z.X.; Feng, Y.L. Secondary metabolites from Solanum rostratum and their antifeedant defense mechanisms against Helicoverpa armigera. J. Agric. Food Chem. 2020, 68, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.K.; Desiraju, G.R. Strong and weak hydrogen bonds in theprotein-ligand interface. Proteins 2007, 67, 128–141. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Luo, S.H.; Yi, T.S.; Li, C.H.; Luo, Q.; Hua, J.; Liu, Y.; Li, S.H. Secondary metabolites from Glycine soja and their growth inhibitory effect against Spodoptera litura. J. Agric. Food Chem. 2011, 59, 6004–6010. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ren, L.L.; Chen, F.; Feng, Y.Q.; Luo, Y.Q. Antifeedant activity of Ginkgo biloba secondary metabolites against Hyphantria cunea Larvae: Mechanisms and application. PLoS ONE 2016, 11, e0155682. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 1962, 8, 401–416. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [PubMed]
- Shang, C.C.; Soderlund, D.M. Monooxygenase activity of tobacco budworm (Heliothis virescens F.) larvae: Tissue distribution and optimal assay conditions for the gut activity. Comp. Biochem. Phys. B 1984, 79, 407–411. [Google Scholar] [CrossRef]

| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 51.7 | 44.9 | 52.4 | |||
| 2 | 210.5 | 212.3 | 207.1 | |||
| 3 | 142.1 | 140.5 | 144.2 | |||
| 4 | 160.8 | 7.82 (1H, s) | 161.4 | 7.75 (1H, s) | 136.0 | 6.77 (1H, s) |
| 5 | 192.3 | 194.0 | 195.6 | |||
| 6 | 97.2 | 55.8 | 2.96 (1H, d, 8.9) | 99.3 | ||
| 7 | 147.6 | 7.24 (1H, d, 15.8) | 144.2 | 7.28 (1H, dd, 15.3, 8.9) | 146.2 | 7.22 (1H, d, 16.1) |
| 8 | 132.5 | 6.37 (1H, d, 15.8) | 136.0 | 6.28 (1H, d, 15.3) | 134.1 | 6.41 (1H, d, 16.1) |
| 9 | 200.4 | 204.5 | 202.6 | |||
| 10 | 27.2 | 2.34 (3H, s) | 27.3 | 2.37 (3H, s) | 27.5 | 2.42 (3H, s) |
| 11 | 171.3 | 172.6 | 14.7 | 2.26 (3H, s) | ||
| 12 | 25.3 | 1.17 (3H, s) | 28.2 | 1.19 (3H, s) | 25.7 | 1.18 (3H, s) |
| 13 | 23.1 | 0.96 (3H, s) | 26.1 | 0.98 (3H, s) | 23.9 | 0.95 (3H, s) |
| Comp. | Antifeedant Rate (%) | Growth-Inhibitory Rate (%) | ||||
|---|---|---|---|---|---|---|
| 100 µg/mL | 50 µg/mL | 25 µg/mL | 100 µg/mL (24 h) | 100 µg/mL (48 h) | 100 µg/mL (72 h) | |
| 1 | 85.16 ± 7.44 | 68.14 ± 7.82 | 42.19 ± 3.67 | 33.18 ± 2.73 | 50.31 ± 4.79 | 74.28 ± 8.35 |
| 2 | 80.62 ± 6.55 | 73.20 ± 7.11 | 40.17 ± 4.69 | 24.37 ± 2.81 | 44.15 ± 3.93 | 78.11 ± 6.26 |
| 3 | 57.14 ± 6.28 | 42.63 ± 5.02 | 35.89 ± 4.22 | 45.16 ± 6.02 | 53.19 ± 5.71 | 60.43 ± 5.79 |
| 4 | 33.15 ± 4.90 | 28.12 ± 3.16 | 12.18 ± 1.73 | 6.32 ± 0.89 | 17.25 ± 1.94 | 29.15 ± 3.43 |
| 5 | 19.51 ± 2.16 | 13.70 ± 1.12 | 10.04 ± 0.87 | ND b | ND b | ND b |
| 6 | 17.27 ± 1.19 | 15.46 ± 1.45 | 9.17 ± 1.02 | 4.15 ± 0.26 | 13.04 ± 1.56 | 15.24 ± 2.01 |
| 7 | 14.55 ± 2.01 | 10.57 ± 1.82 | 4.26 ± 0.53 | 3.57 ± 0.43 | 7.29 ± 0.81 | 11.65 ± 1.32 |
| 8 | ND b | ND b | ND b | ND b | ND b | ND b |
| 9 | 23.35 ± 1.96 | 14.18 ± 2.03 | 8.77 ± 0.85 | 4.75 ± 0.68 | 10.12 ± 1.35 | 24.21 ± 1.94 |
| 10 | 34.25 ± 2.98 | 13.90 ± 1.17 | 5.96 ± 0.64 | 6.12 ± 0.83 | 13.48 ± 1.57 | 21.80 ± 1.95 |
| Neem oil | 92.28 ± 7.11 | 86.45 ± 6.93 | 62.17 ± 3.85 | ND a | ND a | ND a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, T.; Cao, D.; Zhang, Y.; Han, X.; Yu, Z.; Liu, Z. Norsesquiterpenes from the Latex of Euphorbia dentata and Their Chemical Defense Mechanisms against Helicoverpa armigera. Molecules 2023, 28, 7681. https://doi.org/10.3390/molecules28237681
An T, Cao D, Zhang Y, Han X, Yu Z, Liu Z. Norsesquiterpenes from the Latex of Euphorbia dentata and Their Chemical Defense Mechanisms against Helicoverpa armigera. Molecules. 2023; 28(23):7681. https://doi.org/10.3390/molecules28237681
Chicago/Turabian StyleAn, Tong, Dongxu Cao, Yangyang Zhang, Xiamei Han, Zhiguo Yu, and Zhixiang Liu. 2023. "Norsesquiterpenes from the Latex of Euphorbia dentata and Their Chemical Defense Mechanisms against Helicoverpa armigera" Molecules 28, no. 23: 7681. https://doi.org/10.3390/molecules28237681






