Synthesis, Performance, Mechanism: A Hyperbranched Phase Reverse Nano-Demulsifier for Condensate Emulsion
Abstract
:1. Introduction
2. Results and Discussion
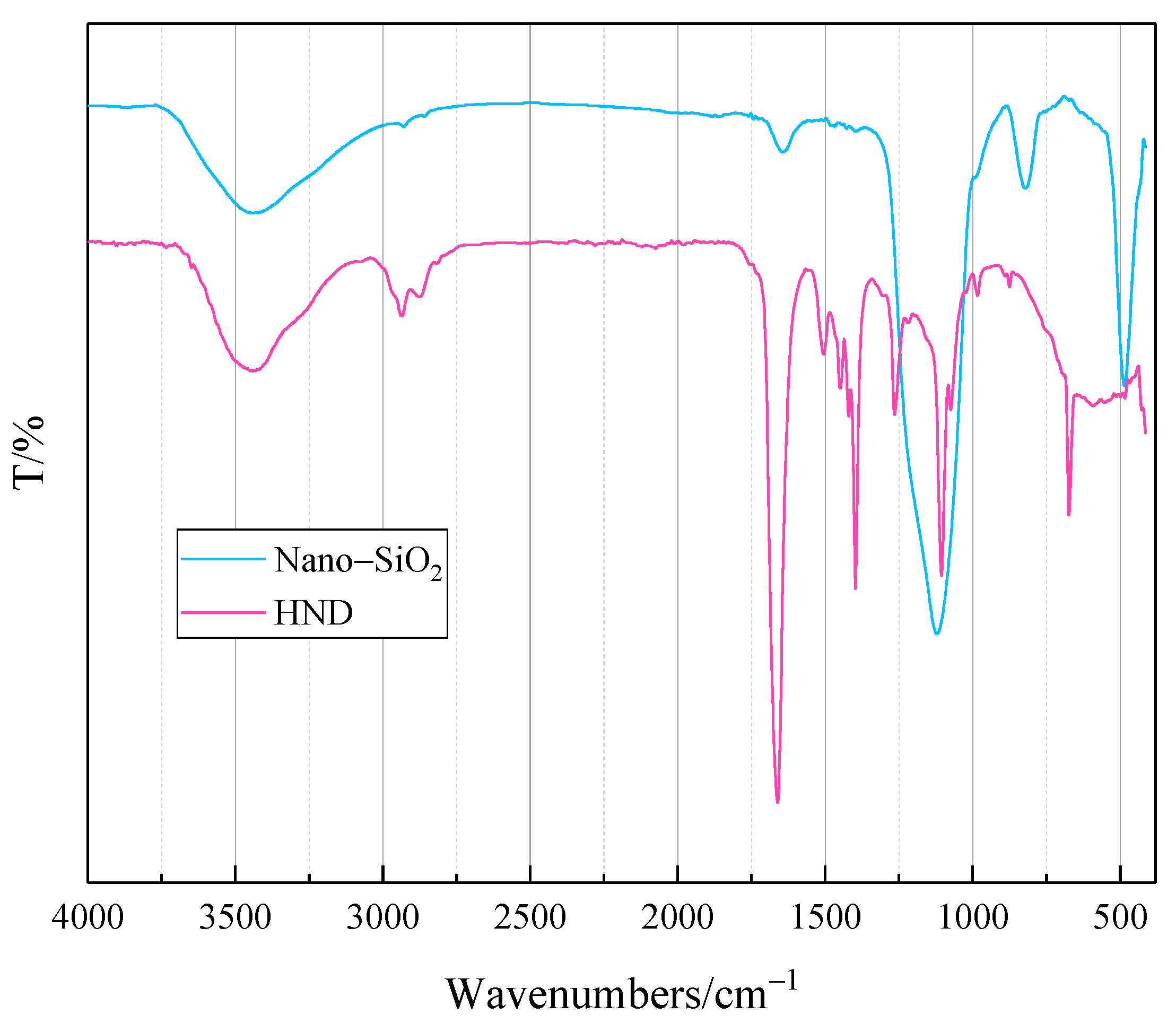
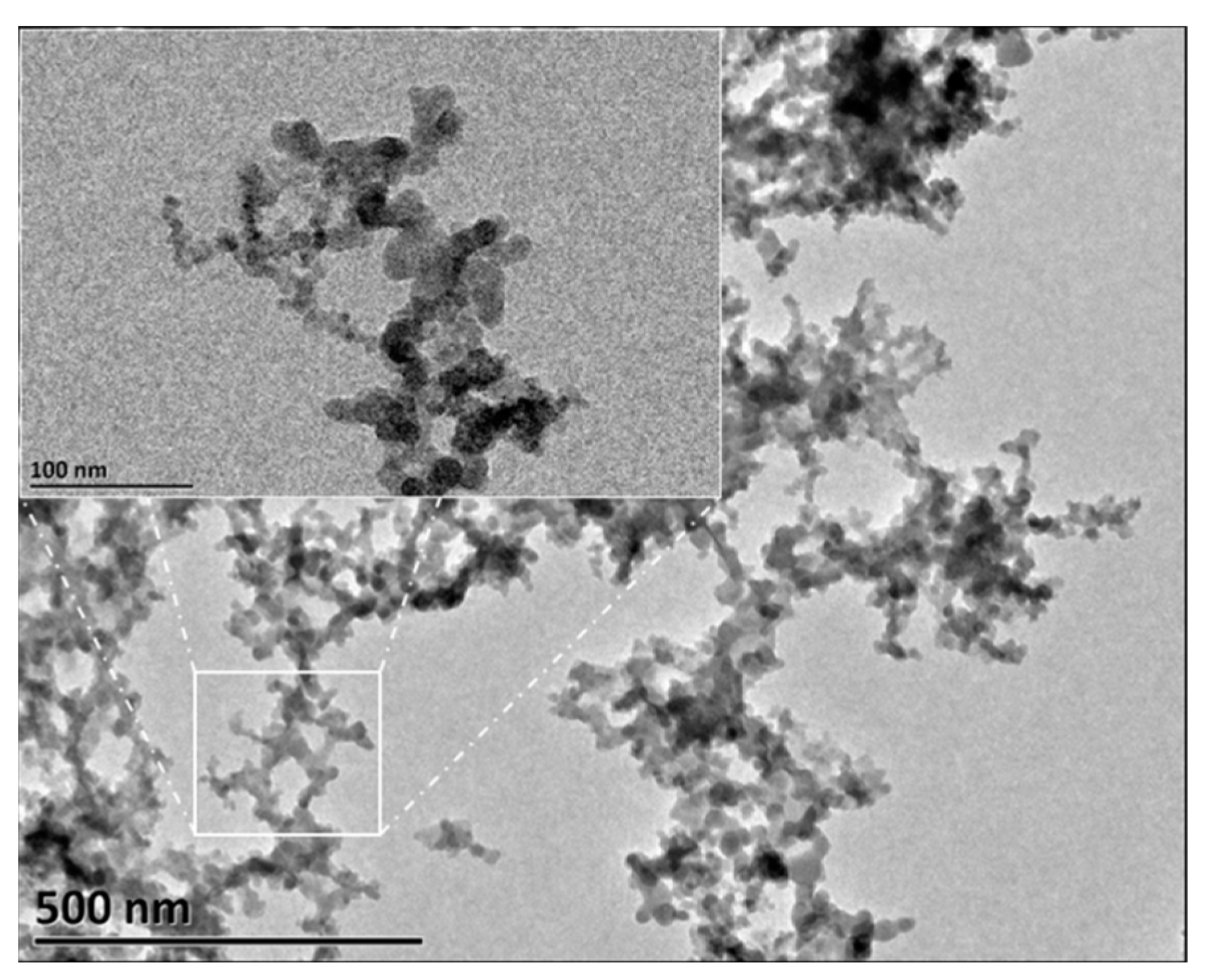
2.1. Characterization
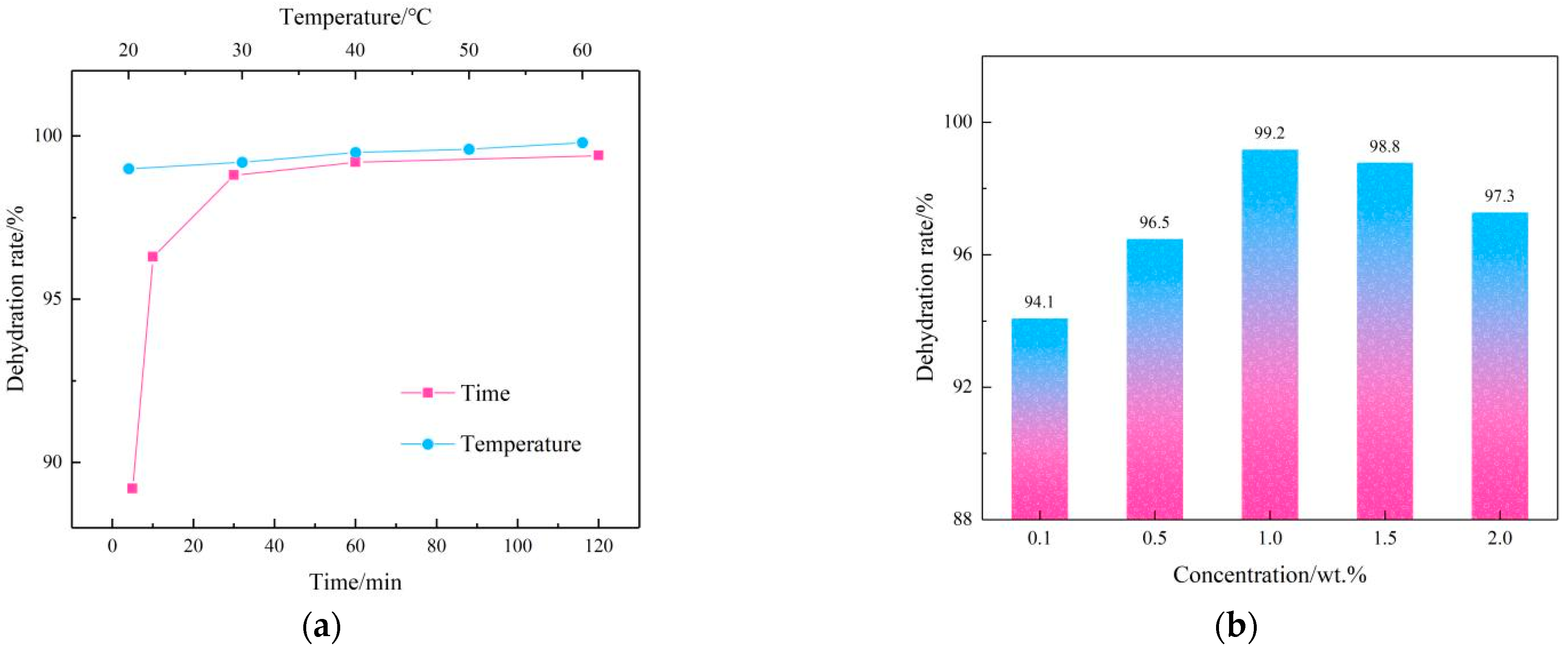
2.2. Performance
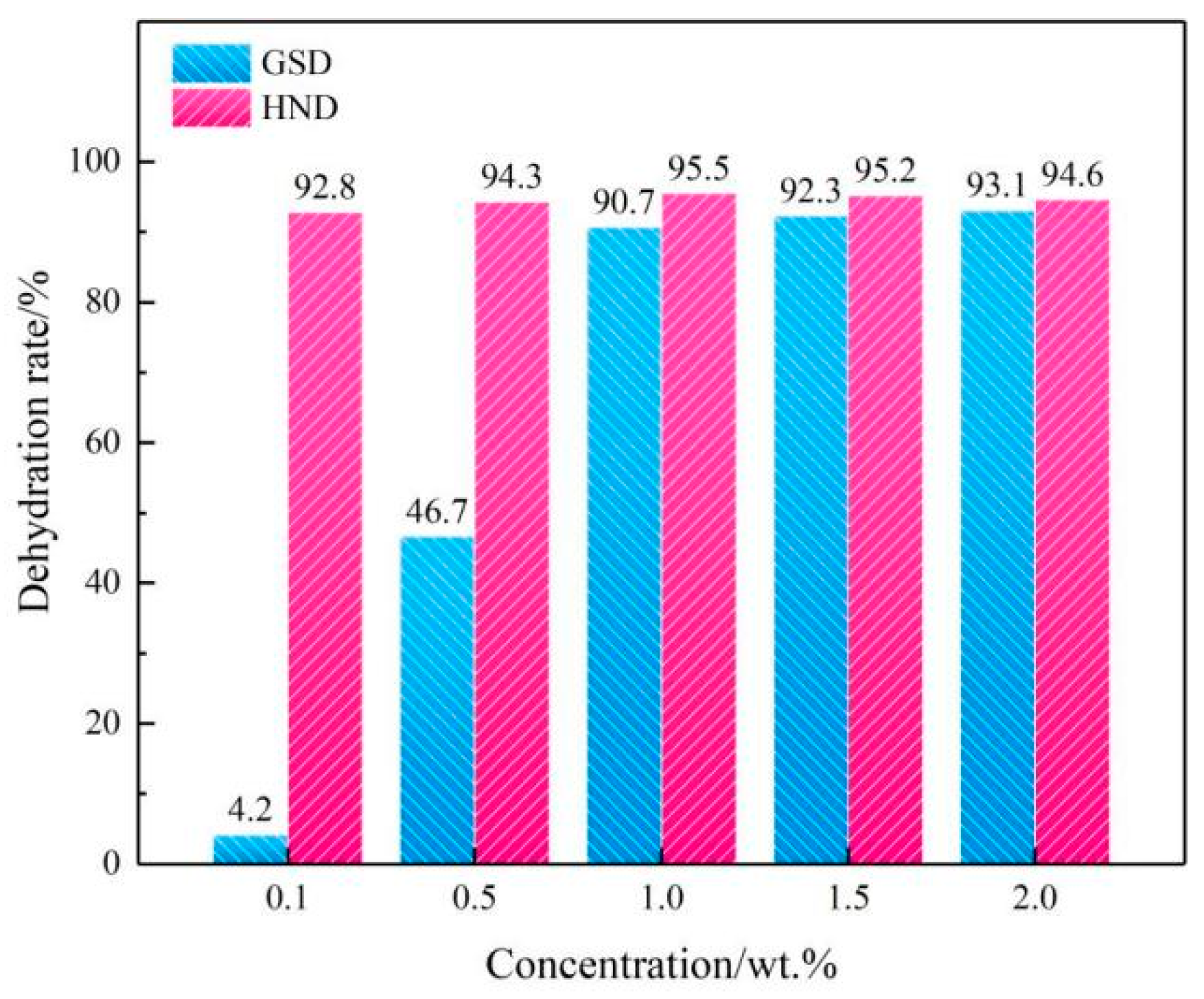
2.3. Applications
2.4. Mechanism
3. Experimental Method
3.1. Materials
3.2. Preparation
3.3. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masnadi, M.S.; El–Houjeiri, H.M.; Dominik, S.; Yunpo, L.; Englander, J.G.; Alhassan, B.; Jean–Christophe, M.; Anderson, J.E.; Wallington, T.J.; Bergerson, J.A. Global carbon intensity of crude oil production. Science 2018, 361, 851–853. [Google Scholar] [CrossRef]
- Punanova, S.A. Trace elements in naphthides and their use in the development of oil and gas–condensate fields. Pet. Chem. 2001, 41, 166–174. [Google Scholar]
- Guo, Z.; Long, B.; Gao, S.; Luo, J.; Gao, J. Carbon nanofiber based superhydrophobic foam composite for high performance oil/water separation. J. Hazard. Mater. 2021, 402, 123838. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Xu, Z.; Ban, X.; Lu, T. Study of Rheological Properties and Micro-mechanism of Heavy Oil Emulsion Prepared via Ultrasonic Dispersion. Energy Fuels 2020, 34, 15843–15854. [Google Scholar] [CrossRef]
- Sherwood, J.D.; Atkinson, D.I.H. Venturi–assisted liquid removal from the sump of a gas well. Int. J. Multiph. Flow 2005, 31, 25–51. [Google Scholar] [CrossRef]
- Dousi, N.; Veeken, C.A.M.; Currie, P.K. Numerical and Analytical Modelling of the Gas Well Liquid Loading Process. SPE Prod. Oper. 2006, 21, 475–482. [Google Scholar] [CrossRef]
- Nakama, Y.; Siojima, Y.; Harusawa, F. O/W type emulsion using liquid crystal formed at oil/water interface: Emulsification method and adhesion of the emulsion droplets to hair. J. Oleo Sci. 1998, 47, 585–590, 625. [Google Scholar]
- Hongtao, L.; Zhang, W.; Bo, Z.; Hailong, G.; Lili, L.; Junyan, L.; Shuang, L.; Hankun, W. Effects of temperature and pressure on wax precipitation and melting characteristics of oil–gas condensate: An experimental and simulation study. Pet. Sci. Technol. 2023, 41, 978–998. [Google Scholar]
- Nakama, Y.; Shiojima, Y.; Harusawa, F. W/O emulsion preparation with liquid crystals formed at the oil/water interface: Preparation and mechanism for concentrated w/o emulsion formation. J. Oleo Sci. 1998, 47, 1331–1336. [Google Scholar]
- Lifeng, Z.; Ningning, Z.; Lijuan, Q.; Xingxing, Z.; Lishan, Z.; Houkai, T.; Wenjun, F. Methacrylated hyperbranched polyglycerol demulsifier for O/W emulsion. Chem. Ind. Eng. Prog. 2021, 40, 3434–3443. [Google Scholar]
- Hui, X.; Qing, W.; Lifeng, Z.; Hui, W. Synthesis and properties of a hyperbranched polymer reverse demulsifier. Spec. Petrochem. 2019, 36, 46–49. [Google Scholar]
- Khormali, A. Effect of water cut on the performance of an asphaltene inhibitor package: Experimental and modeling analysis. Petrol. Sci. Technol. 2022, 40, 2890–2906. [Google Scholar] [CrossRef]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: A review. J. Petrol. Explor. Prod. Technol. 2020, 10, 125–137. [Google Scholar] [CrossRef]
- Alzarieni, K.Z.; Zhang, Y.; Niyonsaba, E.; Wehde, K.E.; Johnston, C.T.; Kilaz, G.; Kenttamaa, H.I. Determination of the Chemical Compositions of Condensate–like Oils with Different API Gravities by Using the Distillation, Precipitation, Fractionation Mass Spectrometry (DPF MS) Method. Energy Fuels 2021, 35, 8646–8656. [Google Scholar] [CrossRef]
- Durand, J.P.; Fafet, A.; Barreau, A. Direct and automatic capillary GC analysis for molecular weight determination and distribution in crude oils and condensates up to C20. J. High Resolut. Chromatogr. 1989, 12, 230–233. [Google Scholar] [CrossRef]
- Dmitrievskii, A.N.; Skibitskaya, N.A.; Zekel’, L.A.; Pribylov, A.A.; Navrotskii, O.K.; Domanova, N.V.K.G. Composition and properties of the natural high–molecular–weight components of gas condensate and oil–gas condensate fields. Solid Fuel Chem. 2010, 4, 203–212. [Google Scholar] [CrossRef]
- Riediker, S.; Suter, J.F.; Giger, W. Benzene- and naphthalenesulfonates in leachates and plumes of landfills. Water Res. 2000, 34, 2069–2079. [Google Scholar] [CrossRef]
- Guoyan, Z.; Mei, C.; Jibin, Z.; Baofeng, H.; Huai, Y.; Bai, Y. Effective increase in the refractive index of novel transparent silicone hybrid films by introduction of functionalized silicon nanoparticles. RSC Adv. 2015, 5, 62128–62133. [Google Scholar]
- Zhang, F.; Wang, F.; Zhang, J.O. The Development and Application of a Demulsifier Used for ASP Flooding–Produced Liquid from the Xing 2 Area of the Daqing Oilfield. Pet. Sci. Technol. 2011, 29, 69–78. [Google Scholar] [CrossRef]
- Liang, L.; Wang, Y.; Liu, B.; Gong, J.; Shi, W.; Liang, S. Fluoropolymer-coated sio2 nanoparticle-based nanofluids for oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 12842. [Google Scholar] [CrossRef]
- Ratajczak, I.; Rzepecka, E.; Magdalena, W.; Szentner, K.; Bartłomiej, M. The effect of alkyd resin on the stability of binding (3–aminopropyl) triethoxysilane with cellulose and wood. Drewno 2015, 58, 137857848. [Google Scholar]
- Shimura, T.; Akai, J.; Lazic, B.; Armbruster, T.; Shimizu, M.; Kamei, A.; Tsukada, K.; Owada, M.; Yuhara, M. Magnesiohogbomite–2N4S: A new polysome from the central Sor Rondane Mountains, East Antarctica. Am. Mineral. 2012, 97, 268–280. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.F.; Hou, X.D.; Deng, D.W. Synthesis and tribological properties of diamond–like carbon films by electrochemical anode deposition. Appl. Surf. Sci. 2012, 258, 6527–6530. [Google Scholar] [CrossRef]
- Kuang, J.; Jiang, X.; Mi, Y.; Ye, F.; Xie, F. Demulsification of oil-in-water emulsions using hyperbranched poly(amido amine) demulsifiers with 4,4-diaminodiphenyl methane as initial cores. J. Appl. Polym. Sci. 2020, 137, 48846. [Google Scholar] [CrossRef]
- Yan, S.; He, G.; Ye, D.; Guo, Y.; Fang, W. Amphiphilic hyperbranched polyethyleneimine for highly efficient oil–water separation. J. Mater. Chem. A 2020, 8, 2412–2423. [Google Scholar] [CrossRef]
- Zhang, L.; He, G.; Ye, D.; Zhan, N.; Guo, Y.; Fang, W. Methacrylated Hyperbranched Polyglycerol as a High–Efficiency Demulsifier for Oil–in–Water Emulsions. Energy Fuels 2016, 30, 9939–9946. [Google Scholar] [CrossRef]
- Zhang, L.; Ying, H.; Yan, S.; Zhan, N.; Guo, Y.; Fang, W. Hyperbranched poly(amido amine) demulsifiers with ethylenediamine/1,3–propanediamine as an initiator for oil–in–water emulsions with microdroplets. Fuel 2018, 226, 381–388. [Google Scholar] [CrossRef]
- Fortuny, M.; Oliveira, C.B.Z.; Melo, R.L.F.V.; Márcio, N.; Coutinho, R.C.C.; Santos, A.F. Effect of Salinity, Temperature, Water Content, and pH on the Microwave Demulsification of Crude Oil Emulsions. Energy Fuels 2007, 21, 1358–1364. [Google Scholar] [CrossRef]
- Harada, T.; Yokomizo, K. Demulsification of oil–in–water emulsion under freezing conditions: Effect of crystal structure modifier. J. Am. Oil Chem. Soc. 2000, 77, 859–864. [Google Scholar] [CrossRef]
- Littlefield, D.L.; Garcia, R.M.; Bless, S.J. The effect of offset on the performance of segmented penetrators. Int. J. Impact Eng. 1999, 23, 547–560. [Google Scholar] [CrossRef]
- Roques-Carmes, T.; Monnier, H.; Portha, J.F.; Marchal, P.; Falk, L. Influence of the plate-type continuous micro-separator dimensions on the efficiency of demulsification of oil-in-water emulsion. Chem. Eng. Res. Des. 2014, 92, 2758–2769. [Google Scholar] [CrossRef]
- Ferreira, B.M.S.; Ramalho, J.O.B.V.S.; Lucas, E.F. Demulsification of Water–in–Crude Oil Emulsions by Microwave Radiation: Effect of Aging, Demulsifier Addition, and Selective Heating. Energy Fuels 2013, 27, 615–621. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, R.; Pu, W.; Liu, R.; Fang, S. Coalescence and separation of surfactant-stabilized water-in-oil emulsion via membrane coalescer functionalized by demulsifier. J. Clean. Prod. 2022, 330, 129945. [Google Scholar] [CrossRef]
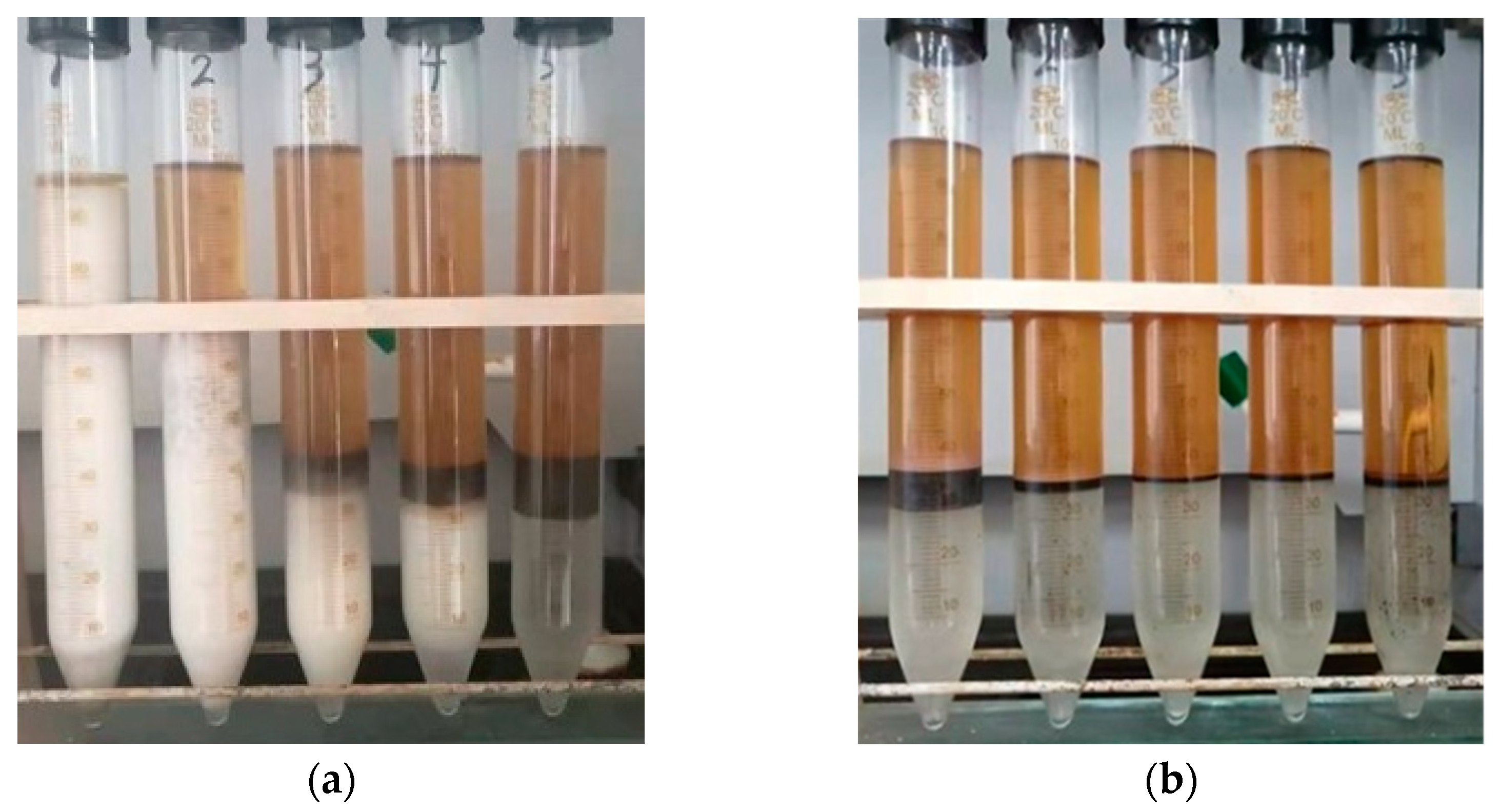

| Concentration /wt.% | Top/mL | Middle/mL | Bottle/mL | |||
|---|---|---|---|---|---|---|
| GSD | HND | GSD | HND | GSD | HND | |
| 0.1 | 2 | 62 | 96 | 11 | 2 | 27 |
| 0.5 | 28 | 63 | 69 | 3 | 3 | 34 |
| 1.0 | 57 | 63 | 8 | 1 | 35 | 36 |
| 1.5 | 58 | 63 | 7 | 1 | 35 | 36 |
| 2.0 | 58 | 64 | 12 | 2 | 30 | 34 |
| Concentration /wt.% | Zeta Potential /mV |
|---|---|
| 0 | −52.59 |
| 0.5 | 52.35 |
| 1.0 | −49.91 |
| 2.0 | −43.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Su, C.; Xiong, Y.; Wei, L.; Gu, C.; Ye, H.; Xiao, Q.; Luo, X. Synthesis, Performance, Mechanism: A Hyperbranched Phase Reverse Nano-Demulsifier for Condensate Emulsion. Molecules 2023, 28, 7692. https://doi.org/10.3390/molecules28237692
Liang L, Su C, Xiong Y, Wei L, Gu C, Ye H, Xiao Q, Luo X. Synthesis, Performance, Mechanism: A Hyperbranched Phase Reverse Nano-Demulsifier for Condensate Emulsion. Molecules. 2023; 28(23):7692. https://doi.org/10.3390/molecules28237692
Chicago/Turabian StyleLiang, Lei, Chao Su, Yujia Xiong, Lei Wei, Congyue Gu, Haifeng Ye, Qinghua Xiao, and Xingyu Luo. 2023. "Synthesis, Performance, Mechanism: A Hyperbranched Phase Reverse Nano-Demulsifier for Condensate Emulsion" Molecules 28, no. 23: 7692. https://doi.org/10.3390/molecules28237692
APA StyleLiang, L., Su, C., Xiong, Y., Wei, L., Gu, C., Ye, H., Xiao, Q., & Luo, X. (2023). Synthesis, Performance, Mechanism: A Hyperbranched Phase Reverse Nano-Demulsifier for Condensate Emulsion. Molecules, 28(23), 7692. https://doi.org/10.3390/molecules28237692




