Cobalt Ferrite Synthesized Using a Biogenic Sol–Gel Method for Biomedical Applications
Abstract
:1. Introduction
2. Results
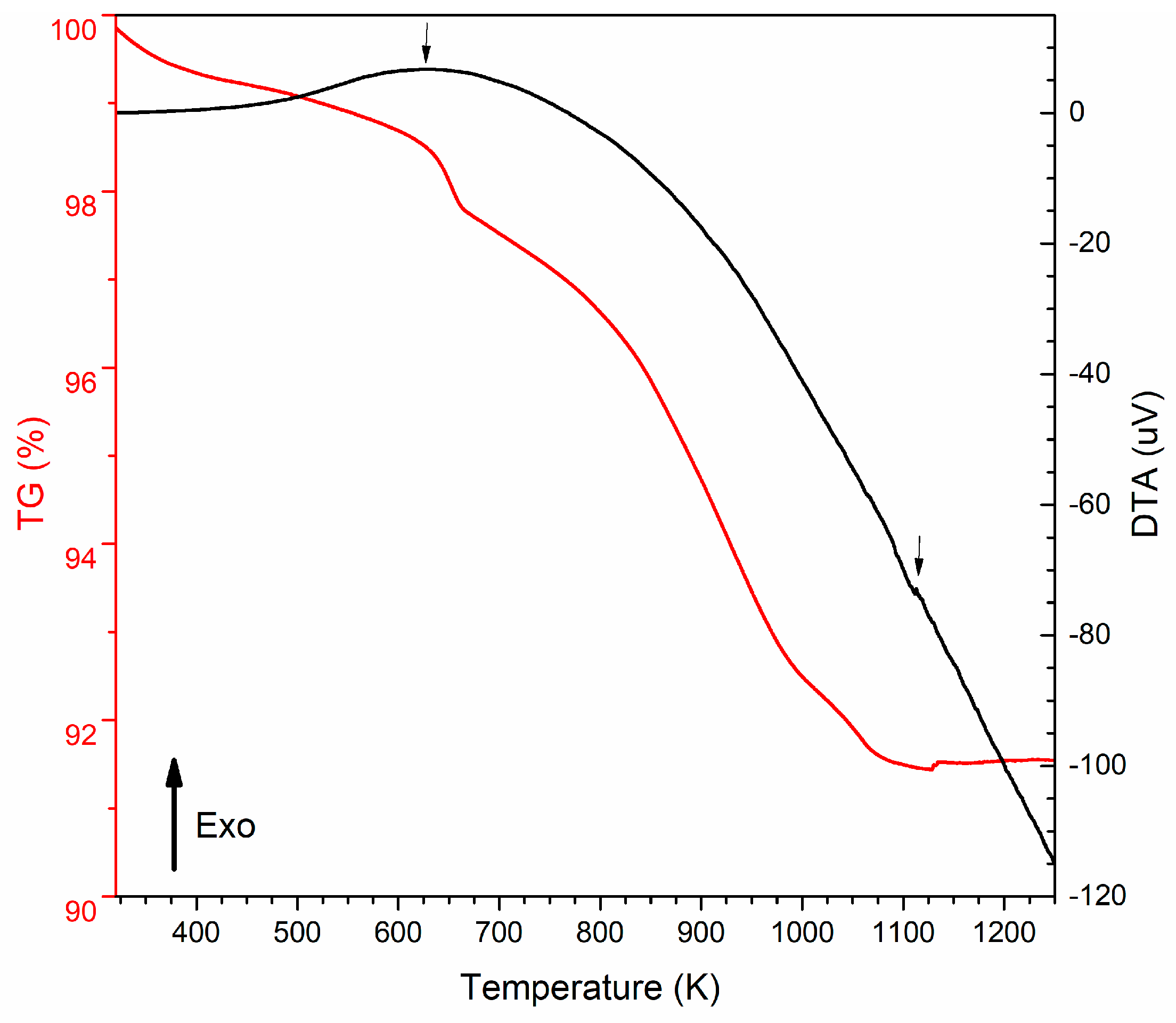
2.1. Thermal Analysis
2.2. Structural Characterization
2.2.1. XRD Diffractograms
2.2.2. Raman Spectroscopy
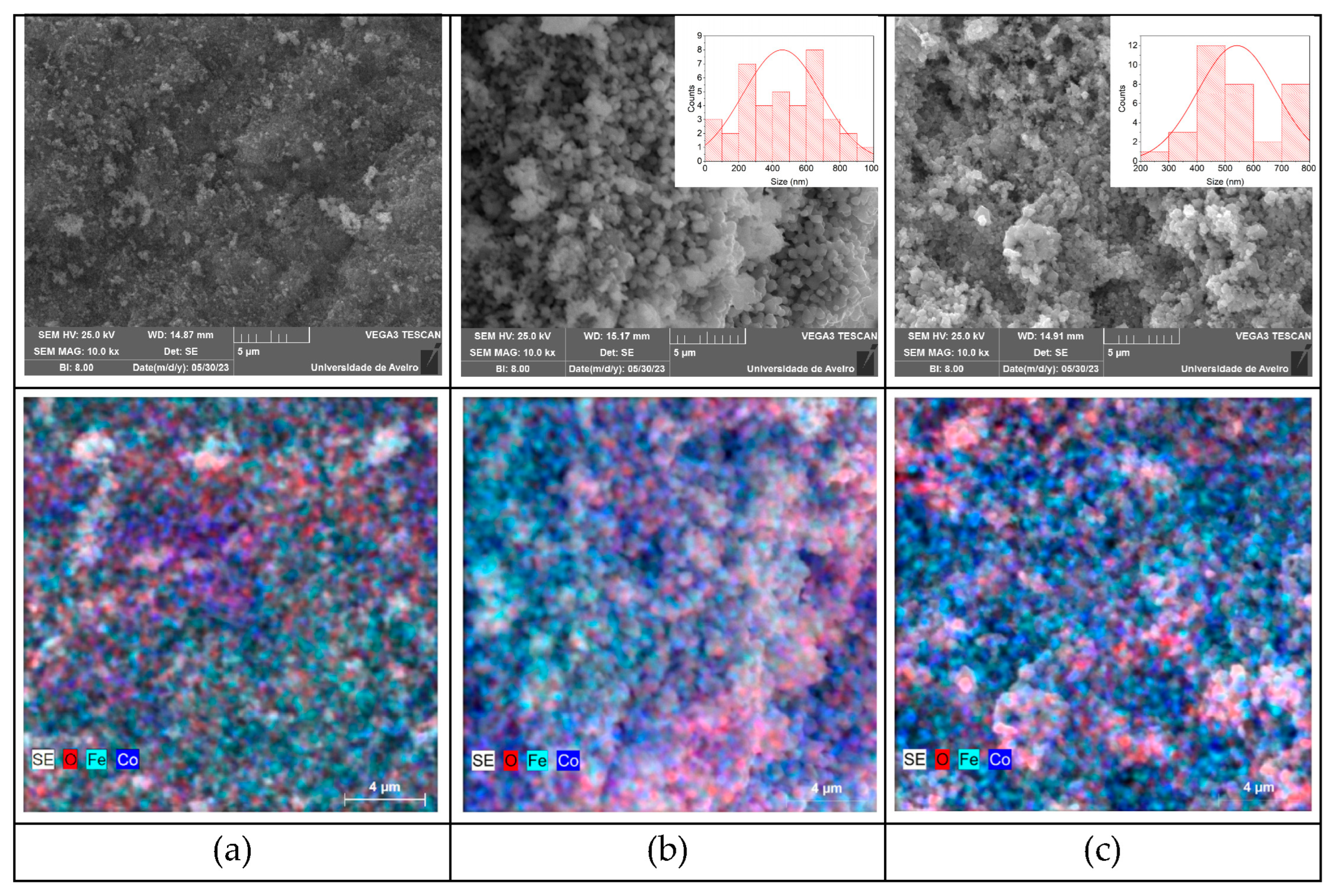
2.3. Morphological Characterization
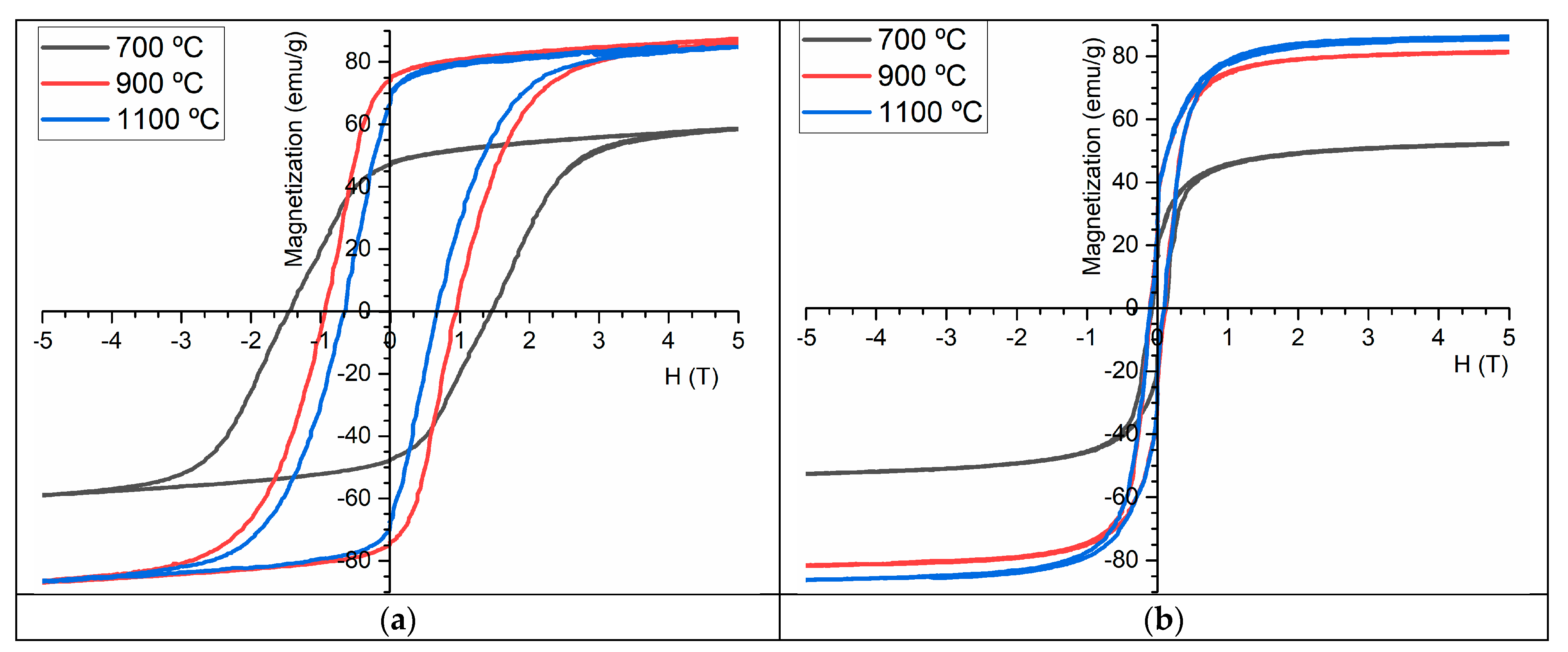
2.4. Magnetic Characterization
2.5. Specific Absorption Rate
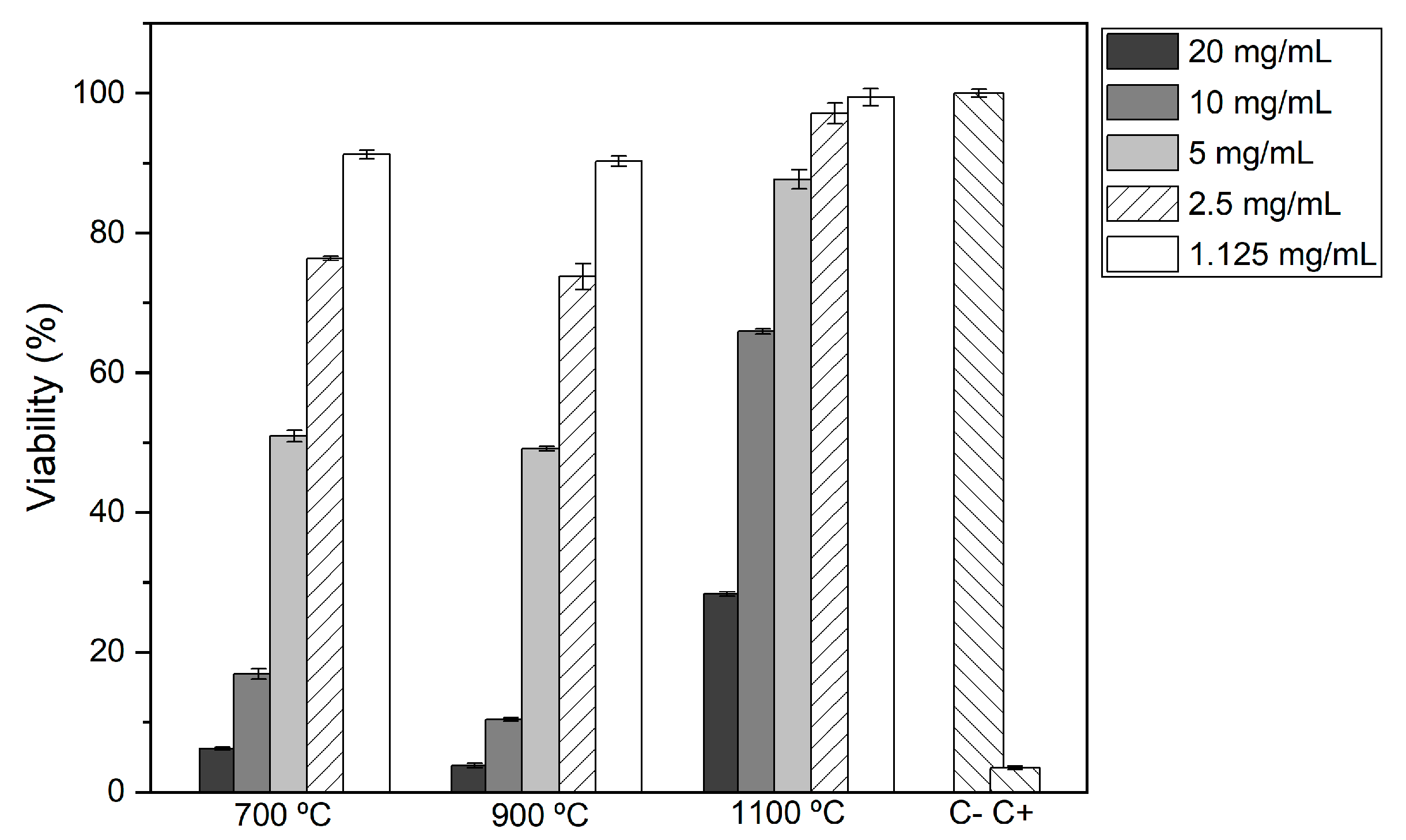
2.6. Cytotoxicity
3. Materials and Methods
3.1. Cobalt Ferrite Powder Preparation
3.2. Structural and Morphological Characterization
3.3. Magnetic Characterization
3.4. Biological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.N.C. Coating of Magnetite Nanoparticles with Chitosan for Magnetic Hyperthermia. Master’s Thesis, Universidade de Aveiro, Aveiro, Portugal, 2016. Available online: https://ria.ua.pt/handle/10773/21895 (accessed on 30 September 2023).
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; Zhang, S.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.S.; Graça, M.P.F.; Lucas, J.; Valente, M.A.; Soares, P.I.P.; Lança, M.C.; Vieira, T.; Silva, J.C.; Borges, J.P.; Jinga, L.-I.; et al. Nanostructured LiFe5O8 by a Biogenic Method for Applications from Electronics to Medicine. Nanomaterials 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Romão, J.; Matos, R.; Silva, J.C.; Borges, J.P. Design and engineering of magneto-responsive devices for cancer theranostics: Nano to macro perspective. Prog. Mater. Sci. 2021, 116, 100742. [Google Scholar] [CrossRef]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2018, 174, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Kandala, S.K.; Wabler, M.; Zhou, H.; Cornejo, C.; Armour, M.; Hedayati, M.; Zhang, Y.; DeWeese, T.L.; Herman, C.; et al. Magnetic nanoparticle hyperthermia enhances radiation therapy: A study in mouse models of human prostate cancer. Int. J. Hyperth. 2015, 31, 359–374. [Google Scholar] [CrossRef]
- Kafrouni, L.; Savadogo, O. Recent progress on magnetic nanoparticles for magnetic hyperthermia. Prog. Biomater. 2016, 5, 147–160. [Google Scholar] [CrossRef]
- Salih, S.J.; Mahmood, W.M. Review on magnetic spinel ferrite (MFe2O4) nanoparticles: From synthesis to application. Heliyon 2023, 9, e16601. [Google Scholar] [CrossRef]
- Gavilán, H.; Rizzo, G.M.R.; Silvestri, N.; Mai, B.T.; Pellegrino, T. Scale-up approach for the preparation of magnetic ferrite nanocubes and other shapes with benchmark performance for magnetic hyperthermia applications. Nat. Protoc. 2023, 18, 783–809. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Lv, Y.; Herng, T.S.; Xu, X.; Xia, W.; Zhang, T.; Fang, J.; Xiao, W.; Ding, J. Orientation Mediated Enhancement on Magnetic Hyperthermia of Fe3O4 Nanodisc. Adv. Funct. Mater. 2014, 25, 812–820. [Google Scholar] [CrossRef]
- Bao, J.; Guo, S.; Zu, X.; Zhuang, Y.; Fan, D.; Zhang, Y.; Shi, Y.; Pang, X.; Ji, Z.; Cheng, J. Magnetic vortex nanoring coated with gadolinium oxide for highly enhanced T1-T2 dual-modality magnetic resonance imaging-guided magnetic hyperthermia cancer ablation. Biomed. Pharmacother. 2022, 150, 112926. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Christiansen, M.G.; Anikeeva, P. Maximizing hysteretic losses in magnetic ferrite nanoparticles via model-driven synthesis and materials optimization. ACS Nano 2013, 7, 8990–9000. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, M.; Qian, J.; Gao, D.; Liang, X. Tunable Fe3O4 Nanorods for Enhanced Magnetic Hyperthermia Performance. Sci. Rep. 2020, 10, 8331. [Google Scholar] [CrossRef] [PubMed]
- Phadatare, M.R.; Meshram, J.V.; Gurav, K.V.; Kim, J.H.; Pawar, S.H. Enhancement of specific absorption rate by exchange coupling of the core–shell structure of magnetic nanoparticles for magnetic hyperthermia. J. Phys. D Appl. Phys. 2016, 49, 095004. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, P.; Pathak, S.; Jain, K.; Garg, P.; Pant, M.; Mahapatro, A.K.; Rath, D.; Wang, L.; Kim, S.-K.; et al. A threefold increase in SAR performance for magnetic hyperthermia by compositional tuning in zinc-substituted iron oxide superparamagnetic nanoparticles with superior biocompatibility. J. Alloys Compd. 2023, 968, 171868. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Ahangar, H.A.; Shaari, A.H.; Hashim, M. Simple Synthesis and Characterization of Cobalt Ferrite Nanoparticles by a Thermal Treatment Method. J. Nanomater. 2010, 2010, e907686. [Google Scholar] [CrossRef]
- Amiri, S.; Shokrollahi, H. The role of cobalt ferrite magnetic nanoparticles in medical science. Mater. Sci. Eng. C 2013, 33, 1–8. [Google Scholar] [CrossRef]
- Lucas, J.M.F.; Teixeira, S.S.; Gavinho, S.R.; Prezas, P.R.; Silva, C.C.; Sales, A.J.M.; Valente, M.A.; Almeida, A.F.; Freire, F.N.; Salgueiro, C.C.M.; et al. Niobium oxide prepared by sol–gel using powder coconut water. J. Mater. Sci. Mater. Electron. 2019, 30, 11346–11353. [Google Scholar] [CrossRef]
- Fortes, S.S.; Duque, J.G.S.; Macêdo, M.A. Nanocrystals of BaFe12O19 obtained by the proteic sol–gel process. Phys. B Condens. Matter 2006, 384, 88–90. [Google Scholar] [CrossRef]
- Brito, P.C.A.; Gomes, R.F.; Duque, J.G.S.; Macêdo, M.A. SrFe12O19 prepared by the proteic sol–gel process. Phys. B Condens. Matter 2006, 384, 91–93. [Google Scholar] [CrossRef]
- Carvalho, J.P.F.; Vieira, T.; Silva, J.C.; Soares, P.I.P.; Ferreira, N.M.; Amorim, C.O.; Teixeira, S.S.; Graça, M.P.F. Potassium Ferrite for Biomedical Applications. Materials 2023, 16, 3880. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.V.S.; Montes, P.J.R.; Soares, F.M.S.; Santos, C.; Valerio, M.E.G. Influence of co-dopant in the europium reduction in SrAl2O4 host. J. Synchrotron Radiat. 2014, 21, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Pereira, L.; Ribeiro, C.; Tofanello, A.; Costa, J.; De Moura, C.; Garcia, M.; De Moura, E. Gold Supported on Strontium Surface-Enriched CoFe2O4 Nanoparticles: A Strategy for the Selective Oxidation of Benzyl Alcohol. J. Braz. Chem. Soc. 2019, 30, 1317–1325. [Google Scholar] [CrossRef]
- Benali, A.; Saher, L.; Bejar, M.; Dhahri, E.; Graca, M.F.P.; Valente, M.A.; Sanguino, P.; Helguero, L.A.; Bachari, K.; Silva, A.M.S.; et al. Synthesis and physico-chemical characterization of Bi-doped Cobalt ferrite nanoparticles: Cytotoxic effects against breast and prostate cancer cell lines. Eur. Phys. J. Plus 2022, 137, 559. [Google Scholar] [CrossRef]
- Babay, S.; Mhiri, T.; Toumi, M. Synthesis, structural and spectroscopic characterizations of maghemite γ-Fe2O3 prepared by one-step coprecipitation route. J. Mol. Struct. 2015, 1085, 286–293. [Google Scholar] [CrossRef]
- Kharat, P.B.; Somvanshi, S.B.; Kounsalye, J.S.; Deshmukh, S.S.; Khirade, P.P.; Jadhav, K.M. Temperature dependent viscosity of cobalt ferrite/ethylene glycol ferrofluids. AIP Conf. Proc. 2018, 1942, 050044. [Google Scholar] [CrossRef]
- Zhang, W.; Stolojan, V.; Silva, S.R.P.; Wu, C.W. Raman, EELS and XPS studies of maghemite decorated multi-walled carbon nanotubes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 715–718. [Google Scholar] [CrossRef]
- Kirik, N.; Krylov, A.; Boronin, A.; Koshcheev, S.; Solovyov, L.; Rabchevskii, E.; Shishkina, N.; Anshits, A. The Relationship between the Structural Characteristics of α-Fe2O3 Catalysts and Their Lattice Oxygen Reactivity Regarding Hydrogen. Materials 2023, 16, 4466. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Hou, J.; Zhang, X.; Gao, Y.; Wang, K. Facet-Dependent SERS Activity of Co3O4. Int. J. Mol. Sci. 2022, 23, 15930. [Google Scholar] [CrossRef] [PubMed]
- Hadjiev, V.G.; Iliev, M.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199. [Google Scholar] [CrossRef]
- Hennous, M.; Ramana, E.V.; Tobaldi, D.M.; Costa, B.F.O.; Valente, M.A.; Labrincha, J.; Karmaoui, M. Synthesis, structure and magnetic properties of multipod-shaped cobalt ferrite nanocrystals. N. J. Chem. 2019, 43, 10259–10269. [Google Scholar] [CrossRef]
- Ravindra, A.V.; Ju, S. Mesoporous CoFe2O4 nanocrystals: Rapid microwave-hydrothermal synthesis and effect of synthesis temperature on properties. Mater. Chem. Phys. 2023, 303, 127818. [Google Scholar] [CrossRef]
- Cao, D.; Li, H.; Pan, L.; Li, J.; Wang, X.; Jing, P.; Cheng, X.; Wang, W.; Wang, J.; Liu, Q. High saturation magnetization of γ-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci. Rep. 2016, 6, 32360. [Google Scholar] [CrossRef]
- Ardeshirfard, H.; Elhamifar, D. An efficient method for the preparation of magnetic Co3O4 nanoparticles and the study of their catalytic application. Front. Catal. 2023, 3, 1194977. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Costa, L.C.; Graça, M.P.F. Biocompatibility, Bioactivity, and Antibacterial Behaviour of Cerium-Containing Bioglass®. Nanomaterials 2022, 12, 4479. [Google Scholar] [CrossRef]
- Gomes, H.C.; Teixeira, S.S.; Graça, M.P.F. Synthesis of calcium ferrite for energy storage applications. J. Alloys Compd. 2022, 921, 166026. [Google Scholar] [CrossRef]
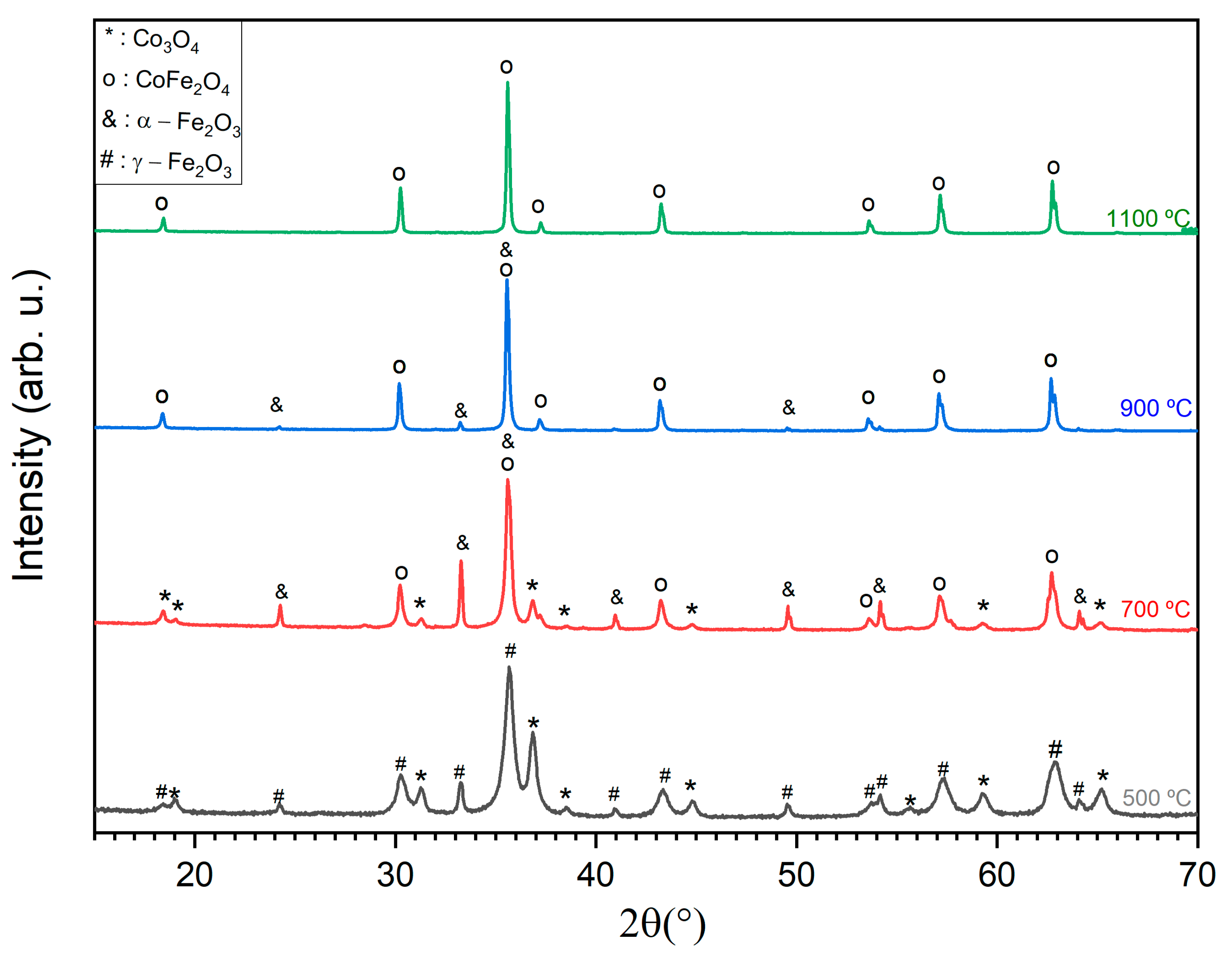


| HT Temperature (°C) | Crystalline Phase and Composition (wt%) | R Expected (Rexp) | Weighted R Profile (Rwp) | Goodness-of-Fit (GF) |
|---|---|---|---|---|
| 500 | (22.7) γ-(77.3) | 1.82 | 2.84 | 1.56 |
| 700 | (63.8) α- (23.6) (7.6) | 1.69 | 6.13 | 3.63 |
| 900 | (94.7) α- (5.3) | 1.68 | 3.82 | 2.27 |
| 1000 | (97.6) α- (2.4) | 1.66 | 3.64 | 2.18 |
| 1100 | (100) | 1.77 | 2.93 | 1.65 |
| 500 °C | 700 °C | 900 °C | 1000 °C | 1100 °C | Attribution | |
|---|---|---|---|---|---|---|
| Vibrational Bands (cm−1) | 300.16 | |||||
| 675.55 | ||||||
| 1356.40 | ||||||
| 285.14 | ||||||
| 401.93 | 290.15 | 290.15 | ||||
| 603.81 | 605.47 | 605.47 | ||||
| 1304.51 | ||||||
| 181.70 | 171.66 | |||||
| 477.00 | 220.00 | 692.23 | 692.23 | |||
| 512.18 | 687.23 | |||||
| 205.06 | ||||||
| 206.73 | 206.73 | 296.82 | ||||
| 465.33 | 466.34 | 465.33 | 468.33 | |||
| 569.44 | 569.44 | 563.56 | ||||
| 685.56 |
| Sample | SAR (W/g) | |
|---|---|---|
| 700 °C | ||
| 900 °C | ||
| 1100 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, P.; Costa, B.; Carvalho, J.P.F.; Soares, P.I.P.; Vieira, T.; Henriques, C.; Valente, M.A.; Teixeira, S.S. Cobalt Ferrite Synthesized Using a Biogenic Sol–Gel Method for Biomedical Applications. Molecules 2023, 28, 7737. https://doi.org/10.3390/molecules28237737
Gomes P, Costa B, Carvalho JPF, Soares PIP, Vieira T, Henriques C, Valente MA, Teixeira SS. Cobalt Ferrite Synthesized Using a Biogenic Sol–Gel Method for Biomedical Applications. Molecules. 2023; 28(23):7737. https://doi.org/10.3390/molecules28237737
Chicago/Turabian StyleGomes, Patrícia, Bárbara Costa, João P. F. Carvalho, Paula I. P. Soares, Tânia Vieira, Célia Henriques, Manuel Almeida Valente, and Sílvia Soreto Teixeira. 2023. "Cobalt Ferrite Synthesized Using a Biogenic Sol–Gel Method for Biomedical Applications" Molecules 28, no. 23: 7737. https://doi.org/10.3390/molecules28237737
APA StyleGomes, P., Costa, B., Carvalho, J. P. F., Soares, P. I. P., Vieira, T., Henriques, C., Valente, M. A., & Teixeira, S. S. (2023). Cobalt Ferrite Synthesized Using a Biogenic Sol–Gel Method for Biomedical Applications. Molecules, 28(23), 7737. https://doi.org/10.3390/molecules28237737










