Recent Advances and Synergistic Effects of Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts: A Review
Abstract
:1. Introduction
2. Fundamentals of Electrocatalysis Kinetics
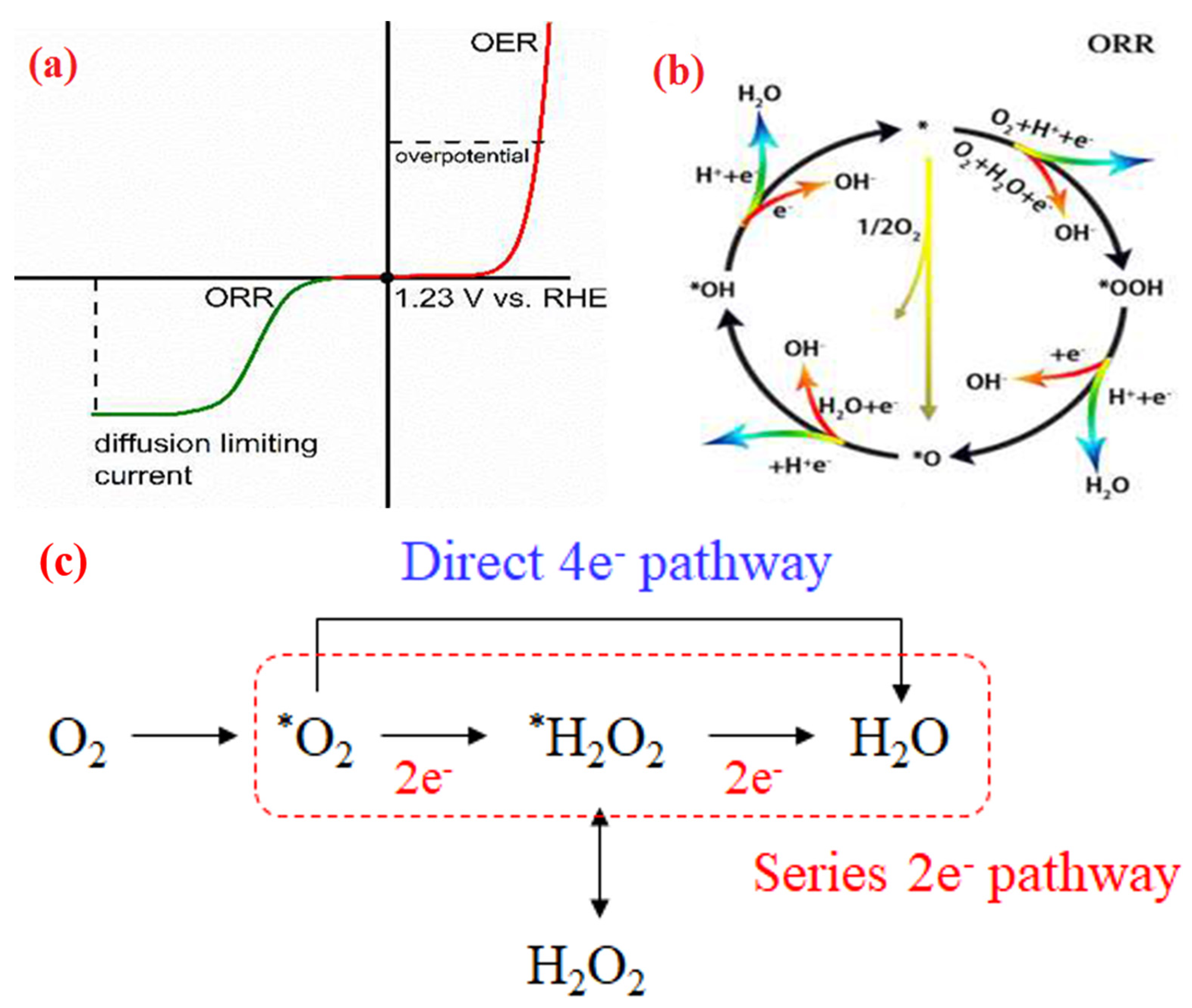
3. General ORR Mechanism
4. Performance Analysis of ORR Electrocatalyst
4.1. RDE and RRDE Method
4.2. Membrane Electrode Assembly (MEA) Test
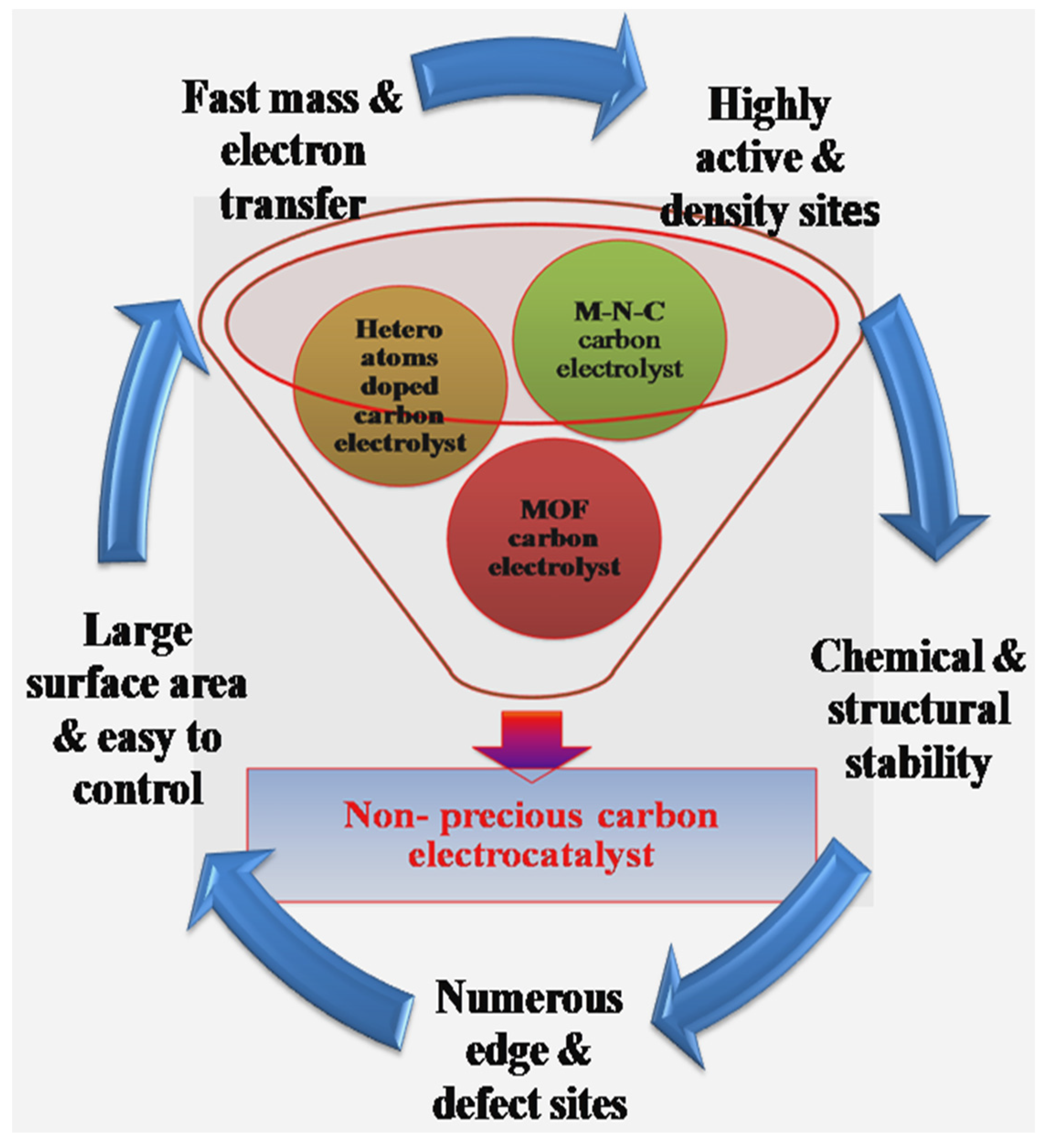
5. Active Sites Engineering
5.1. Active Sites of Hetero Atoms Doped Carbon Electrocatalysts
5.2. Active Sites of M-N-C Carbon Electrocatalysts
5.3. Mechanism of Reaction at ORR Active Sites of Metal-Based Carbon Electrocatalyst
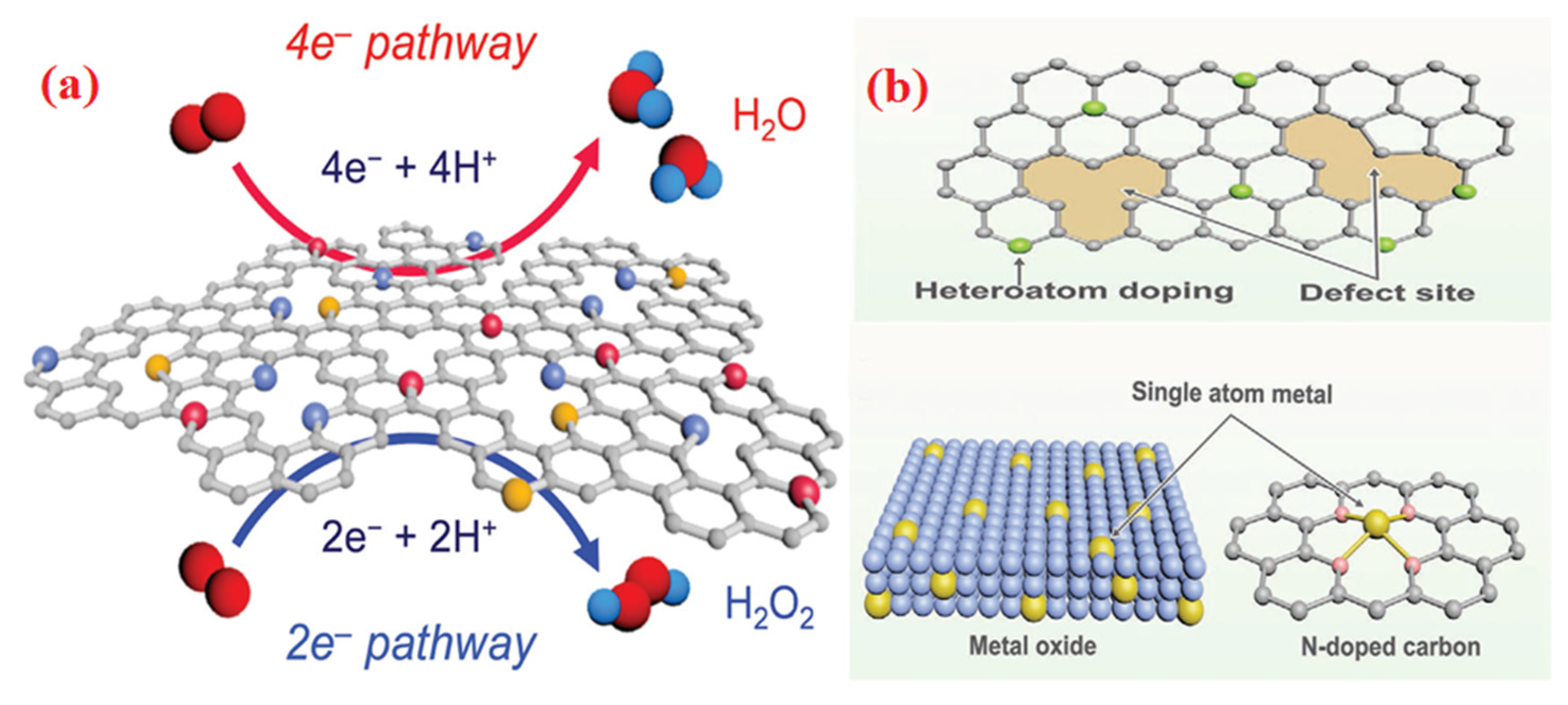
6. Recent Development of Non-Precious Metal Electrocatalyst
6.1. Metal-Free Heteroatom Dopants Electrocatalyst
6.2. M-N-C Carbon Electrocatalyst

| Metal Precursor | Catalysts Name | Metal Content | BET Surface (m2 g−1) | Electrolyte | Eonset (V) | E1/2 (V) | Tafel Slopes (mV dec−1) | Electron Transfer Number | H2O2 Yield | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Fe1/N,S-PC (Fe/N and S-co-doped hierarchical porous carbon) | 2.6 wt.% Fe (ICP-OES) | 998.5 | 0.1 M KOH | - | 0.904 | 84.5 | 3.95 | <9% | [101] |
| Fe | Fe−N−C (P-FeMOF@ZIF-8) | 1.24 wt.% Fe (ICP) | 785.0 | 0.1 M KOH | 1.01 | - | - | - | - | [104] |
| 0.5 M H2SO4 | 0.85 | - | - | - | - | |||||
| Mn | Mn-N-C-HCl-800/1100 | 2.00 wt.% Mn (XPS) | 1511 | 0.5 M H2SO4 | - | 0.815 | - | - | <3% | [107] |
| Fe | Fe/N-PCNs | 3.89 wt.% Fe (XPS) | 864 | 0.1 M KOH | 0.96 | 0.86 | - | ~3.95 | - | [108] |
| 0.1 M HClO4 | 0.88 | 0.79 | - | 3.80 | ||||||
| Mo | Mo/OSG-H | 13.47 wt.% Mo (ICP-OES) | - | 0.1 M KOH | 0.78 | - | 54.7 | 2.1 | - | [109] |
| Fe/Co | CAN-Pc(Fe/Co) | 10.70 wt.% (ICP-OES) | 84.11 | 0.1 M KOH | 1.04 | 0.84 | 54 | 3.94 | <5% | [110] |
| Zn/Co | Zn/CoN-C | 0.33 wt.% Zn 0.14 wt.% Co (ICP-MS) | 1343 | 0.1 M KOH | 1.004 | 0.861 | - | 3.88 | ~5% | [111] |
| 0.1 M HClO4 | 0.97 | 0.796 | - | |||||||
| Zn | Zn–N–C | 9.33 wt% Zn (EDX) 5.64 wt.% Zn (ICP-MS) | 1002 | 0.1 M KOH | - | 0.873 | - | - | <5% | [112] |
| 0.1 M HClO4 | - | 0.746 | - | |||||||
| Co | 10Co-N@DCNF | 0.649 wt.% Co (ICP-MS) | - | 0.1 M KOH | - | 0.83 | 56 | - | <8% | [113] |
| Co | Co-N/S-DSHCN-3.5 (Co, N, and S co-doped hollow carbon nanocages) | - | 429 | 0.1 M KOH | 0.989 | 0.878 | - | 3.9 | <10% | [114] |
| 0.5 M H2SO4 | 0.84 | 0.754 | - | |||||||
| Fe | FeCl1N4/CNS | 1.50 wt.% Fe (ICP-OES) | - | 0.1 M KOH | - | 0.921 | 51 | 3.97–3.99 | <1% | [115] |
| Fe/Co | M/FeCo-SAs-N-C | 5.12 wt.% Fe 4.39 wt.% Co (ICP-MS) 3.32 wt.% Fe 3.33 wt.% Co (XPS) | 1003.7 | 0.1 M HClO4 | 0.981 | 0.851 | - | ~4 | <6% | [116] |
| Mn | 20Mn-NC-second | 3.03 wt.% Mn (ICP-MS) | 715 | 0.5 M H2SO4 | - | 0.8 | 80 | - | <2% | [117] |
| Co | Co-N-C-10 | - | - | 0.1 M HClO4 | 0.92 | 0.79 | 55.8 | - | <2% | [118] |
| Cr | Cr/N/C-950 | 1.90 wt.% Cr (ICP-MS) | 884.9 | 0.1 M HClO4 | - | 0.761 | 37 | - | - | [119] |
| Fe | C-rGO-ZIF-2 | 4.29 wt.% Fe (ICP-OES) | 650 | 0.1 M HClO4 | 0.89 | 0.77 | - | 3.8 | <5% | [120] |
| Ce/Fe | Ce–Fe/NC | 0.83 wt.% Ce 0.88 wt.% Fe (ICP-OES) | 831 | 0.1 M KOH | - | 0.913 | 55.55 | 3.98 | ~3% | [121] |
| 0.1 M HClO4 | - | 0.791 | ||||||||
| Mn | Mn-N-C | - | 1334.3 | 0.1 M KOH | 0.98 | 0.88 | 60.3 | 3.98 | <2% | [122] |
| 0.5 M H2SO4 | - | 0.73 | ||||||||
| Mn/Co | MnCo2O4/N-C | - | 630.5 | 0.1 M KOH | 0.943 | 0.795 | 86 | 3.50–3.83 | <10% | [123] |
6.3. Metal–Organic Framework (MOF) Carbon Electrocatalyst
7. Conclusions and Future Outlook
Future Efficient Carbon-Based Electrocatalyst
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dou, S.; Tao, L.; Huo, J.; Wang, S.; Dai, L. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 1320–1326. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Wei, Q.; Xiong, F.; Tan, S.; Huang, L.; Lan, E.H.; Dunn, B.; Mai, L. Porous One-Dimensional Nanomaterials: Design, Fabrication and Applications in Electrochemical Energy Storage. Adv. Mater. 2017, 29, 1602300. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shen, Y.; He, F.; Li, Y.; Liu, A.; Liu, S.; Zhang, Y. Recent advances of doped carbon as non-precious catalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 15704–15716. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Dou, M.; Ji, J.; Wang, F. Biomass Derived N-Doped Porous Carbon Supported Single Fe Atoms as Superior Electrocatalysts for Oxygen Reduction. Small 2017, 13, 1604290. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Chen, S.; Zhang, Y.; Wang, Y.; Nie, Y.; Ding, W.; Qi, X.; Wei, Z. Controlled synthesis of hollow micro/meso-pore nitrogen-doped carbon with tunable wall thickness and specific surface area as efficient electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 2433–2437. [Google Scholar] [CrossRef]
- Wan, K.; Tan, A.-D.; Yu, Z.-P.; Liang, Z.-X.; Piao, J.-H.; Tsiakaras, P. 2D nitrogen-doped hierarchically porous carbon: Key role of low dimensional structure in favoring electrocatalysis and mass transfer for oxygen reduction reaction. Appl. Catal. B Environ. 2017, 209, 447–454. [Google Scholar] [CrossRef]
- Yu, H.; Fisher, A.; Cheng, D.; Cao, D. Cu,N-codoped Hierarchical Porous Carbons as Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 21431–21439. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, S.; Wang, Y.; Dong, J.; Chen, W.; Li, Z.; Shen, R.; Zheng, L.; Zhuang, Z.; Wang, D.; et al. Isolated Single Iron Atoms Anchored on N-Doped Porous Carbon as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2017, 56, 6937–6941. [Google Scholar] [CrossRef]
- Xia, W.; Zou, R.; An, L.; Xia, D.; Guo, S. A metal–organic framework route to in situ encapsulation of Co@Co3O4@C core@bishell nanoparticles into a highly ordered porous carbon matrix for oxygen reduction. Energy Environ. Sci. 2015, 8, 568–576. [Google Scholar] [CrossRef]
- Shi, P.-C.; Yi, J.-D.; Liu, T.-T.; Li, L.; Zhang, L.-J.; Sun, C.-F.; Wang, Y.-B.; Huang, Y.-B.; Cao, R. Hierarchically porous nitrogen-doped carbon nanotubes derived from core–shell ZnO@zeolitic imidazolate framework nanorods for highly efficient oxygen reduction reactions. J. Mater. Chem. A 2017, 5, 12322–12329. [Google Scholar] [CrossRef]
- Dou, S.; Shen, A.; Tao, L.; Wang, S. Molecular doping of graphene as metal-free electrocatalyst for oxygen reduction reaction. Chem. Commun. 2014, 50, 10672–10675. [Google Scholar] [CrossRef]
- Song, H.; Li, H.; Wang, H.; Key, J.; Ji, S.; Mao, X.; Wang, R. Chicken bone-derived N-doped porous carbon materials as an oxygen reduction electrocatalyst. Electrochim. Acta 2014, 147, 520–526. [Google Scholar] [CrossRef]
- Ma, Z.; Dou, S.; Shen, A.; Tao, L.; Dai, L.; Wang, S. Sulfur-Doped Graphene Derived from Cycled Lithium-Sulfur Batteries as a Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2015, 54, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, A.; Wei, X.; Zhou, K.; Chen, W.; Chen, F.; Xu, J.; Wang, S.; Dai, L. Ultrathin Wrinkled N-Doped Carbon Nanotubes for Noble-Metal Loading and Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 20507–20512. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.; Dai, L. BCN Graphene as Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2012, 51, 4209–4212. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Wang, D.; Dou, S.; Ma, Z.; Wu, J.; Tao, L.; Shen, A.; Ouyang, C.; Liu, Q.; et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842. [Google Scholar] [CrossRef] [PubMed]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef]
- Bayatsarmadi, B.; Zheng, Y.; Vasileff, A.; Qiao, S. Recent Advances in Atomic Metal Doping of Carbon-based Nanomaterials for Energy Conversion. Small 2017, 13, 1700191. [Google Scholar] [CrossRef]
- Zhang, H.; Osgood, H.; Xie, X.; Shao, Y.; Wu, G. Engineering nanostructures of PGM-free oxygen-reduction catalysts using metal-organic frameworks. Nano Energy 2017, 31, 331–350. [Google Scholar] [CrossRef]
- Barkholtz, H.M.; Liu, D.-J. Advancements in rationally designed PGM-free fuel cell catalysts derived from metal–organic frameworks. Mater. Horiz. 2017, 4, 20–37. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Tan, Q.; Lu, L.; Wang, Z.; Wu, G. Metal–Organic Frameworks and Their Derived Materials as Electrocatalysts and Photocatalysts for CO2 Reduction: Progress, Challenges, and Perspectives. Chem.—A Eur. J. 2018, 24, 18137–18157. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA; Weinheim, Germany, 2001. [Google Scholar]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Griffith, J.S.; Roughton, F.J.W. On the magnetic properties of some haemoglobin complexes, Proceedings of the Royal Society of London. Ser. A Math. Phys. Sci. 1997, 235, 23–36. [Google Scholar] [CrossRef]
- Weiss, J.J. Nature of the Iron–Oxygen Bond in Oxyhæmoglobin. Nature 1964, 203, 183. [Google Scholar] [CrossRef]
- Yeager, E. Recent Advances in the Science of Electrocatalysis. J. Electrochem. Soc. 1981, 128, 160C. [Google Scholar] [CrossRef]
- Adžić, R.R.; Wang, J.X. Configuration and Site of O2 Adsorption on the Pt(111) Electrode Surface. J. Phys. Chem. B 1998, 102, 8988–8993. [Google Scholar] [CrossRef]
- Du, C.; Sun, Y.; Shen, T.; Yin, G.; Zhang, J. Applications of RDE and RRDE Methods in Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 231–277. [Google Scholar] [CrossRef]
- Biddinger, E.J.; von Deak, D.; Singh, D.; Marsh, H.; Tan, B.; Knapke, D.S.; Ozkan, U.S. Examination of Catalyst Loading Effects on the Selectivity of CNx and Pt/VC ORR Catalysts Using RRDE. J. Electrochem. Soc. 2011, 158, B402. [Google Scholar] [CrossRef]
- Nagai, T.; Jahn, C.; Jia, H. Improved Accelerated Stress Tests for ORR Catalysts Using a Rotating Disk Electrode. J. Electrochem. Soc. 2019, 166, F3111–F3115. [Google Scholar] [CrossRef]
- Kocha, S.S.; Shinozaki, K.; Zack, J.W.; Myers, D.J.; Kariuki, N.N.; Nowicki, T.; Stamenkovic, V.; Kang, Y.; Li, D.; Papageorgopoulos, D. Best Practices and Testing Protocols for Benchmarking ORR Activities of Fuel Cell Electrocatalysts Using Rotating Disk Electrode. Electrocatalysis 2017, 8, 366–374. [Google Scholar] [CrossRef]
- Mills, A.; O’Rourke, C. Wireless rotating disk electrode (wRDE) for assessing heterogeneous water oxidation catalysts (WOCs). Chem. Commun. 2016, 52, 7727–7730. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Asen, L.; Ercolano, G.; Dionigi, F.; Zalitis, C.; Hawkins, A.; Bonastre, A.M.; Seidl, L.; Knoll, A.C.; Sharman, J.; et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources 2018, 392, 274–284. [Google Scholar] [CrossRef]
- Bernt, M.; Hartig-Weiß, A.; Tovini, M.F.; El-Sayed, H.A.; Schramm, C.; Schröter, J.; Gebauer, C.; Gasteiger, H.A. Current Challenges in Catalyst Development for PEM Water Electrolyzers. Chem. Ing. Technol. 2020, 92, 31–39. [Google Scholar] [CrossRef]
- Zhong, G.; Xu, S.; Liu, L.; Zheng, C.Z.; Dou, J.; Wang, F.; Fu, X.; Liao, W.; Wang, H. Effect of Experimental Operations on the Limiting Current Density of Oxygen Reduction Reaction Evaluated by Rotating-Disk Electrode. ChemElectroChem 2020, 7, 1107–1114. [Google Scholar] [CrossRef]
- Ratera, I.; Veciana, J. Playing with organic radicals as building blocks for functional molecular materials, Chem. Soc. Rev. 2012, 41, 303–349. [Google Scholar] [CrossRef]
- Kang, S.-F.; Chang, H.-M. Coagulation of textile secondary effluents with fenton’s reagent. Water Sci. Technol. 1997, 36, 215–222. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Neyerlin, K.C.; Gu, W.; Jorne, J.; Gasteiger, H.A. Determination of Catalyst Unique Parameters for the Oxygen Reduction Reaction in a PEMFC. J. Electrochem. Soc. 2006, 153, A1955. [Google Scholar] [CrossRef]
- Jaouen, F.; Goellner, V.; Lefèvre, M.; Herranz, J.; Proietti, E.; Dodelet, J. Oxygen reduction activities compared in rotating-disk electrode and proton exchange membrane fuel cells for highly active FeNC catalysts. Electrochim. Acta 2013, 87, 619–628. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Jung, E.; Shin, H.; Antink, W.H.; Sung, Y.-E.; Hyeon, T. Recent Advances in Electrochemical Oxygen Reduction to H2O2: Catalyst and Cell Design. ACS Energy Lett. 2020, 5, 1881–1892. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Medina, J.; Wang, Z.; Cruz-Silva, R.; Morelos-Gomez, A.; Wang, F.; Yao, X.; Terrones, M.; Endo, M. Defect Engineering and Surface Functionalization of Nanocarbons for Metal-Free Catalysis. Adv. Mater. 2019, 31, e1805717. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.; Chen, X.; Li, B.; Hou, T.; Zhang, B.; Zhang, Q.; Titirici, M.; Wei, F. Topological Defects in Metal-Free Nanocarbon for Oxygen Electrocatalysis. Adv. Mater. 2016, 28, 6845–6851. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Zhuang, L.; Liu, H.; Yan, X.; Wang, X.; Liu, J.; Wang, J.; Zheng, Y.; Xiao, Z.; et al. Identification of active sites for acidic oxygen reduction on carbon catalysts with and without nitrogen doping. Nat. Catal. 2019, 2, 688–695. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect Graphene as a Trifunctional Catalyst for Electrochemical Reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Zhao, Z.L.; Wang, M.; Xiang, B.; Li, J.; Feng, Z.; Xu, H.; Gu, M. Ultrahigh-Loading of Ir Single Atoms on NiO Matrix to Dramatically Enhance Oxygen Evolution Reaction. J. Am. Chem. Soc. 2020, 142, 7425–7433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Yang, Y.; Wang, L.; Mu, Z.; Zhu, H.; Zhu, X.; Xing, H.; Xia, H.; Huang, B.; et al. A General Method for Transition Metal Single Atoms Anchored on Honeycomb-Like Nitrogen-Doped Carbon Nanosheets. Adv. Mater. 2020, 32, e1906905. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Peng, H.; Liu, F.; Liu, X.; Liao, S.; You, C.; Tian, X.; Nan, H.; Luo, F.; Song, H.; Fu, Z.; et al. Effect of Transition Metals on the Structure and Performance of the Doped Carbon Catalysts Derived From Polyaniline and Melamine for ORR Application. ACS Catal. 2014, 4, 3797–3805. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, C.; Zhang, D.; Gong, L.; Zhu, Y.; Zhao, Z.; Xu, Q.; Li, H.; Xia, Z. Guiding Principles for Designing Highly Efficient Metal-Free Carbon Catalysts. Adv. Mater. 2019, 31, e1805252. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the Electrocatalytic Oxygen Reduction Activity of Graphene-Based Catalysts: A Roadmap to Achieve the Best Performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Vojvodic, A.; Nørskov, J.K. New design paradigm for heterogeneous catalysts. Natl. Sci. Rev. 2015, 2, 140–143. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Wang, C.; Tao, W.; Huang, M.; Zuo, M.; Yang, Y.; Yang, K.; Zhang, L.; Chen, S.; et al. Turning main-group element magnesium into a highly active electrocatalyst for oxygen reduction reaction. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Chen, H.; Du, X.; Zhang, B.; Hong, Z.; Sun, S.; Wang, W. Progress and Challenges Toward the Rational Design of Oxygen Electrocatalysts Based on a Descriptor Approach. Adv. Sci. 2019, 7, 1901614. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Song, J.; Dou, S.; Li, X.; Wang, J.; Wang, X. Strategies to Break the Scaling Relation toward Enhanced Oxygen Electrocatalysis. Matter 2019, 1, 1494–1518. [Google Scholar] [CrossRef]
- Woo, J.; Lim, J.S.; Kim, J.H.; Joo, S.H. Heteroatom-doped carbon-based oxygen reduction electrocatalysts with tailored four-electron and two-electron selectivity. Chem. Commun. 2021, 57, 7350–7361. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, X.; Liu, Y.; Ai, X.; Asefa, T.; Zou, X. Active Site Engineering in Porous Electrocatalysts. Adv. Mater. 2020, 32, e2002435. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Z.; Kuang, Y.; Xu, C.; Yang, L.; Zhou, M.; He, B.; Jing, M.; Li, Z.; Li, F.; et al. Edge-Rich Quasi-Mesoporous Nitrogen-Doped Carbon Framework Derived from Palm Tree Bark Hair for Electrochemical Applications. ACS Appl. Mater. Interfaces 2018, 10, 27047–27055. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Qiao, Y.; Shi, Z.; Tang, W.; Li, C.M. Hierarchically Porous N-Doped Carbon Nanotubes/Reduced Graphene Oxide Composite for Promoting Flavin-Based Interfacial Electron Transfer in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2018, 10, 11671–11677. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, R.; Liu, H.; Zhou, L.; Wang, Y.; Zhang, Y.; Fan, G. Flexible Active-Site Engineering of Monometallic Co-Layered Double Hydroxides for Achieving High-Performance Bifunctional Electrocatalyst toward Oxygen Evolution and H2O2 Reduction. ACS Appl. Mater. Interfaces 2020, 12, 12919–12929. [Google Scholar] [CrossRef]
- Zhao, J.; Dumont, J.H.; Martinez, U.; Macossay, J.; Artyushkova, K.; Atanassov, P.; Gupta, G. Graphite Intercalation Compounds Derived by Green Chemistry as Oxygen Reduction Reaction Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 42678–42685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, T.; Kou, Z.; Shen, L.; Zhao, Y.; Wang, Z.; Wang, X.; Yang, Z.; Du, J.; Xu, J.; et al. Negative Pressure Pyrolysis Induced Highly Accessible Single Sites Dispersed on 3D Graphene Frameworks for Enhanced Oxygen Reduction. Angew. Chem. Int. Ed. 2020, 59, 20465–20469. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Liu, J.; Liu, M.; Wang, F. Hexagonal Fe2N Coupled with N-Doped Carbon: Crystal-Plane-Dependent Electrocatalytic Activity for Oxygen Reduction. ACS Catal. 2020, 10, 2443–2451. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Jin, Y.; Tong, Y.; Lu, Q.; Gao, F. Quantum Effects Allow the Construction of Two-Dimensional Co3O4-Embedded Nitrogen-Doped Porous Carbon Nanosheet Arrays from Bimetallic MOFs as Bifunctional Oxygen Electrocatalysts. Chem.—A Eur. J. 2018, 24, 14522–14530. [Google Scholar] [CrossRef]
- Zhang, Y.; Knibbe, R.; Sunarso, J.; Zhong, Y.; Zhou, W.; Shao, Z.; Zhu, Z. Recent Progress on Advanced Materials for Solid-Oxide Fuel Cells Operating Below 500 °C. Adv. Mater. 2017, 29, 17703452017. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Wang, L.; Li, Y.; Yakobson, B.I.; Tour, J.M. Oxidized Laser-Induced Graphene for Efficient Oxygen Electrocatalysis. Adv. Mater. 2018, 30, e1707319. [Google Scholar] [CrossRef]
- Du, R.; Zhang, N.; Zhu, J.; Wang, Y.; Xu, C.; Hu, Y.; Mao, N.; Xu, H.; Duan, W.; Zhuang, L.; et al. Nitrogen-Doped Carbon Nanotube Aerogels for High-Performance ORR Catalysts. Small 2015, 11, 3903–3908. [Google Scholar] [CrossRef]
- Wu, R.; Wan, X.; Deng, J.; Huang, X.; Chen, S.; Ding, W.; Li, L.; Liao, Q.; Wei, Z. NaCl protected synthesis of 3D hierarchical metal-free porous nitrogen-doped carbon catalysts for the oxygen reduction reaction in acidic electrolyte. Chem. Commun. 2019, 55, 9023–9026. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, R.; Liao, H.; Hou, Y. Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2013, 2, 88–97. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Ming, J.; Wang, Q.; Wang, H.; Cao, Y.; Peng, F.; Yang, Y.; Yu, H. Electronic synergism of pyridinic- and graphitic-nitrogen on N-doped carbons for the oxygen reduction reaction. Chem. Sci. 2018, 10, 1589–1596. [Google Scholar] [CrossRef]
- Tian, K.; Wang, J.; Cao, L.; Yang, W.; Guo, W.; Liu, S.; Li, W.; Wang, F.; Li, X.; Xu, Z.; et al. Single-site pyrrolic-nitrogen-doped sp2-hybridized carbon materials and their pseudocapacitance. Nat. Commun. 2020, 11, 3884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of Phosphorus-Doped Graphene and its Multifunctional Applications for Oxygen Reduction Reaction and Lithium Ion Batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Hu, M.; Iqbal, Z.; Wang, X. N8− Polynitrogen Stabilized on Boron-Doped Graphene as Metal-Free Electrocatalysts for Oxygen Reduction Reaction. ACS Catal. 2020, 10, 160–167. [Google Scholar] [CrossRef]
- Li, R.; Liu, F.; Zhang, Y.; Guo, M.; Liu, D. Nitrogen, Sulfur Co-Doped Hierarchically Porous Carbon as a Metal-Free Electrocatalyst for Oxygen Reduction and Carbon Dioxide Reduction Reaction. ACS Appl. Mater. Interfaces 2020, 12, 44578–44587. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-S.; Li, S.-L.; Tang, Y.-J.; Li, K.; Zhou, L.; Kong, N.; Lan, Y.-Q.; Bao, J.-C.; Dai, Z.-H. Heteroatoms ternary-doped porous carbons derived from MOFs as metal-free electrocatalysts for oxygen reduction reaction. Sci. Rep. 2014, 4, 5130. [Google Scholar] [CrossRef]
- Kahan, R.J.; Hirunpinyopas, W.; Cid, J.; Ingleson, M.J.; Dryfe, R.A.W. Well-Defined Boron/Nitrogen-Doped Polycyclic Aromatic Hydrocarbons Are Active Electrocatalysts for the Oxygen Reduction Reaction. Chem. Mater. 2019, 31, 1891–1898. [Google Scholar] [CrossRef]
- Ai, W.; Luo, Z.; Jiang, J.; Zhu, J.; Du, Z.; Fan, Z.; Xie, L.; Zhang, H.; Huang, W.; Yu, T. Nitrogen and Sulfur Codoped Graphene: Multifunctional Electrode Materials for High-Performance Li-Ion Batteries and Oxygen Reduction Reaction. Adv. Mater. 2014, 26, 6186–6192. [Google Scholar] [CrossRef]
- Chang, Y.; Hong, F.; He, C.; Zhang, Q.; Liu, J. Nitrogen and Sulfur Dual-Doped Non-Noble Catalyst Using Fluidic Acrylonitrile Telomer as Precursor for Efficient Oxygen Reduction. Adv. Mater. 2013, 25, 4794–4799. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J.; Zhou, H.; Fu, C.; Wang, G.; Yang, L.; Xu, C.; Chen, Z.; Yang, W.; Kuang, Y. Hydrothermal preparation of nitrogen, boron co-doped curved graphene nanoribbons with high dopant amounts for high-performance lithium sulfur battery cathodes. J. Mater. Chem. A 2017, 5, 7403–7415. [Google Scholar] [CrossRef]
- Meng, Y.; Voiry, D.; Goswami, A.; Zou, X.; Huang, X.; Chhowalla, M.; Liu, Z.; Asefa, T. N-, O-, and S-Tridoped Nanoporous Carbons as Selective Catalysts for Oxygen Reduction and Alcohol Oxidation Reactions. J. Am. Chem. Soc. 2014, 136, 13554–13557. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Voiry, D.; Chhowalla, M.; Asefa, T. Efficient Metal-Free Electrocatalysts for Oxygen Reduction: Polyaniline-Derived N- and O-Doped Mesoporous Carbons. J. Am. Chem. Soc. 2013, 135, 7823–7826. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jia, Y.; Chang, G.; Chen, J.; Liu, H.; Wang, J.; Hu, Y.; Xia, Y.; Yang, D.; Yao, X. A Defect-Driven Metal-free Electrocatalyst for Oxygen Reduction in Acidic Electrolyte. Chem 2018, 4, 2345–2356. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active Sites and Mechanism of Oxygen Reduction Reaction Electrocatalysis on Nitrogen-Doped Carbon Materials. Adv. Mater. 2018, 31, e1804297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, Q.; Chen, Z.; Jose, V.; Jiang, X.; Fu, G.; Lee, J.-M.; Huang, S. B, N-doped ultrathin carbon nanosheet superstructure for high-performance oxygen reduction reaction in rechargeable zinc-air battery. Carbon 2020, 164, 398–406. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Tian, X.; Luo, L.; Fang, J.; Gao, H.; Jiang, Z.-J. Hydrothermal Synthesis of Boron and Nitrogen Codoped Hollow Graphene Microspheres with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 19398–19407. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef]
- Ai, K.; Liu, Y.; Ruan, C.; Lu, L.; Lu, G. Sp2C-Dominant N-Doped Carbon Sub-micrometer Spheres with a Tunable Size: A Versatile Platform for Highly Efficient Oxygen-Reduction Catalysts. Adv. Mater. 2013, 25, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Sakaushi, K.; Eckardt, M.; Lyalin, A.; Taketsugu, T.; Behm, R.J.; Uosaki, K. Microscopic Electrode Processes in the Four-Electron Oxygen Reduction on Highly Active Carbon-Based Electrocatalysts. ACS Catal. 2018, 8, 8162–8176. [Google Scholar] [CrossRef]
- Wu, K.; Chen, X.; Liu, S.; Pan, Y.; Cheong, W.-C.; Zhu, W.; Cao, X.; Shen, R.; Chen, W.; Luo, J.; et al. Porphyrin-like Fe-N4 sites with sulfur adjustment on hierarchical porous carbon for different rate-determining steps in oxygen reduction reaction. Nano Res. 2018, 11, 6260–6269. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Role of Nitrogen Moieties in N-Doped 3D-Graphene Nanosheets for Oxygen Electroreduction in Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 11623–11632. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, C.; Liu, F.; Hou, Y. Hybrid of Iron Nitride and Nitrogen-Doped Graphene Aerogel as Synergistic Catalyst for Oxygen Reduction Reaction. Adv. Funct. Mater. 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wei, D.; Li, M. A “MOF-Protective-Pyrolysis” Strategy for the Preparation of Fe–N–C Catalysts and the Role of Fe, N, and C in the Oxygen Reduction Reaction in Acidic Medium. ACS Appl. Mater. Interfaces 2019, 11, 35755–35763. [Google Scholar] [CrossRef]
- Zhao, C.; Li, B.; Liu, J.; Zhang, Q. Intrinsic Electrocatalytic Activity Regulation of M–N–C Single-Atom Catalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 4448–4463. [Google Scholar] [CrossRef]
- Yang, S.; Xue, X.; Liu, X.; Liu, W.; Bao, J.; Huang, Y.; Su, H.; Yuan, S.; Li, H.-M. Scalable Synthesis of Micromesoporous Iron-Nitrogen-Doped Carbon as Highly Active and Stable Oxygen Reduction Electrocatalyst. ACS Appl. Mater. Interfaces 2019, 11, 39263–39273. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Yang, F.; Li, B.; Stracensky, T.; Karakalos, S.; Mukerjee, S.; Jia, Q.; Su, D.; Wang, G.; et al. Atomically Dispersed MnN4 Catalysts via Environmentally Benign Aqueous Synthesis for Oxygen Reduction: Mechanistic Understanding of Activity and Stability Improvements. ACS Catal. 2020, 10, 10523–10534. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, S.; Lu, X.; Fan, W.; Chi, B.; Ye, Y.; Shi, X.; Zeng, J.; Li, X.; Liao, S. Two-Dimensional Bimetallic Zn/Fe-Metal-Organic Framework (MOF)-Derived Porous Carbon Nanosheets with a High Density of Single/Paired Fe Atoms as High-Performance Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 13878–13887. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Jiao, Y.; Shi, B.; Liu, J.; Xie, Z.; Chen, X.; Zhang, Q.; Qiao, S. Coordination Tunes Selectivity: Two-Electron Oxygen Reduction on High-Loading Molybdenum Single-Atom Catalysts. Angew. Chem. Int. Ed. 2020, 59, 9171–9176. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Dou, M.; Zhang, Z.; Dai, L.; Wang, F. Two-Dimensional Conjugated Aromatic Networks as High-Site-Density and Single-Atom Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2019, 58, 14724–14730. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, B.; Hu, Y.; Liu, W.; Zhao, Y.; Yang, R.; Li, Z.; Luo, J.; Chi, B.; Jiang, Z.; et al. An Isolated Zinc–Cobalt Atomic Pair for Highly Active and Durable Oxygen Reduction. Angew. Chem. Int. Ed. 2019, 58, 2622–2626. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Yang, N.; Deng, M.; Ibraheem, S.; Deng, J.; Li, J.; Li, L.; Wei, Z. Ultrahigh-Loading Zinc Single-Atom Catalyst for Highly Efficient Oxygen Reduction in Both Acidic and Alkaline Media. Angew. Chem. Int. Ed. 2019, 58, 7035–7039. [Google Scholar] [CrossRef]
- Yang, Q.; Jia, Y.; Wei, F.; Zhuang, L.; Yang, D.; Liu, J.; Wang, X.; Lin, S.; Yuan, P.; Yao, X. Understanding the Activity of Co-N4−xCx in Atomic Metal Catalysts for Oxygen Reduction Catalysis. Angew. Chem. Int. Ed. 2020, 59, 6122–6127. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Liu, Y.; Yang, M.; Li, X.; He, Y.; Li, H.; Chen, H.; Lin, Z. Dual-Shelled Multidoped Hollow Carbon Nanocages with Hierarchical Porosity for High-Performance Oxygen Reduction Reaction in Both Alkaline and Acidic Media. Nano Lett. 2020, 20, 5639–5645. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Xu, R.; Chen, W.; Zheng, L.; Han, A.; Zhu, Y.; Zhang, J.; Zhang, H.; Luo, J.; et al. Electronic structure engineering to boost oxygen reduction activity by controlling the coordination of the central metal. Energy Environ. Sci. 2018, 11, 2348–2352. [Google Scholar] [CrossRef]
- Yin, S.; Yang, J.; Han, Y.; Li, G.; Wan, L.; Chen, Y.; Chen, C.; Qu, X.; Jiang, Y.; Sun, S. Construction of Highly Active Metal-Containing Nanoparticles and FeCo-N4 Composite Sites for the Acidic Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 21976–21979. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, H.; Chen, Y.; Zhu, J.; Gao, L.; Jin, Z.; Ge, J.; Jiang, Z.; Chen, S.; Liu, C.; et al. Identification of binuclear Co2N5 active sites for oxygen reduction reaction with more than one magnitude higher activity than single atom CoN4 site. Nano Energy 2018, 46, 396–403. [Google Scholar] [CrossRef]
- Luo, E.; Zhang, H.; Wang, X.; Gao, L.; Gong, L.; Zhao, T.; Jin, Z.; Ge, J.; Jiang, Z.; Liu, C.; et al. Single-Atom Cr−N4 Sites Designed for Durable Oxygen Reduction Catalysis in Acid Media. Angew. Chem. Int. Ed. 2019, 58, 12469–12475. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shao, Z. Tunable and convenient synthesis of highly dispersed Fe–Nx catalysts from graphene-supported Zn–Fe-ZIF for efficient oxygen reduction in acidic media. RSC Adv. 2019, 9, 42236–42244. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Hou, Y.; Liu, P.; Hao, X.; Liu, Y.; Xu, B.; Guo, J. One-step synthesis of CeFeO3 nanoparticles on porous nanocarbon frameworks derived from ZIF-8 for a boosted oxygen reduction reaction in pH universal electrolytes. J. Mater. Chem. A 2022, 10, 13013–13020. [Google Scholar] [CrossRef]
- Fu, W.; Wang, X.-L.; Yang, X.-X.; He, X.-Q. MnCo2O4 Anchored on Nitrogen-Doped Carbon Nanomaterials as an Efficient Electrocatalyst for Oxygen Reduction. ChemistrySelect 2018, 3, 4228–4236. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Cullen, D.A.; Uddin, M.A.; Langhorst, L.; Li, B.; Karakalos, S.; Kropf, A.J.; Wegener, E.C.; Sokolowski, J.; et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2019, 12, 250–260. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Wang, M.; Lin, C.; Yang, W.; Meng, J.; Xu, Y.; Owusu, K.A.; Jiang, B.; Chen, C.; et al. Boosting oxygen reduction activity with low-temperature derived high-loading atomic cobalt on nitrogen-doped graphene for efficient Zn–air batteries. Chem. Commun. 2019, 55, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, D.; Cao, D.; Zeng, X.C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 2018, 1, 339–348. [Google Scholar] [CrossRef]
- Reda, M.; Hansen, H.A.; Vegge, T. DFT Study of the Oxygen Reduction Reaction on Carbon-Coated Iron and Iron Carbide. ACS Catal. 2018, 8, 10521–10529. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Shen, H.; Li, W.; Shen, T.; Ali, Z.; Tang, T.; Guo, S.; Sun, Q.; Hou, Y. N-Doped Carbon Nanosheet Networks with Favorable Active Sites Triggered by Metal Nanoparticles as Bifunctional Oxygen Electrocatalysts. ACS Energy Lett. 2018, 3, 2914–2920. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.-Z. Determination of the Electron Transfer Number for the Oxygen Reduction Reaction: From Theory to Experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Li, L.; Tang, C.; Zheng, Y.; Xia, B.; Zhou, X.; Xu, H.; Qiao, S.-Z. Tailoring Selectivity of Electrochemical Hydrogen Peroxide Generation by Tunable Pyrrolic-Nitrogen-Carbon. Adv. Energy Mater. 2020, 10, 2000789. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zeng, Y.; Chen, J.; Qiu, L.; Zhou, H.; Sun, C.; Yu, Y.; Zhu, C.; Zhu, Z. Single Fe Atom on Hierarchically Porous S, N-Codoped Nanocarbon Derived from Porphyra Enable Boosted Oxygen Catalysis for Rechargeable Zn-Air Batteries. Small 2019, 15, 1900307. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Liu, Q.; Sarnello, E.; Tang, C.; Chng, M.; Shui, J.; Li, T.; Pennycook, S.J.; Han, M.; Zhao, D. MOF-Derived Carbon Networks with Atomically Dispersed Fe–Nx Sites for Oxygen Reduction Reaction Catalysis in Acidic Media. ACS Mater. Lett. 2019, 1, 37–43. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, D.; Zhao, M.; Chen, H.; Fan, X.; Gao, B.; Gu, L.; Guo, Y.; Qin, J.; Wei, J.; et al. Cross-Linked Polyphosphazene Hollow Nanosphere-Derived N/P-Doped Porous Carbon with Single Nonprecious Metal Atoms for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 14639–14646. [Google Scholar] [CrossRef]
- She, W.; Xie, Q.; Huang, Y.; Xie, C.; Zhang, X.; Sun, M.; Wang, F.; Xiao, J. Microstructure Engineering of Fe/Fe3C-Decorated Metal–Nitrogen–Carbon Mesoporous Nanospheres via a Self-Template Method for Enhancing Oxygen Reduction Activity. ACS Appl. Mater. Interfaces 2020, 12, 28065–28074. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal–organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331. [Google Scholar] [CrossRef]
- Li, S.-L.; Xu, Q. Metal–organic frameworks as platforms for clean energy. Energy Environ. Sci. 2013, 6, 1656–1683. [Google Scholar] [CrossRef]
- Liao, P.-Q.; Shen, J.-Q.; Zhang, J.-P. Metal–organic frameworks for electrocatalysis. Coord. Chem. Rev. 2018, 373, 22–48. [Google Scholar] [CrossRef]
- Song, G.; Wang, Z.; Wang, L.; Li, G.; Huang, M.; Yin, F. Preparation of MOF(Fe) and its catalytic activity for oxygen reduction reaction in an alkaline electrolyte. Chin. J. Catal. 2014, 35, 185–195. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.; Kiani, M.; Chen, Y.; Chen, J.; Wang, G.; Chan, S.H.; Wang, R. Synthesis of MOF-Derived Nonprecious Catalyst with High Electrocatalytic Activity for Oxygen Reduction Reaction. Ind. Eng. Chem. Res. 2018, 57, 12087–12095. [Google Scholar] [CrossRef]
- Bai, F.; Huang, H.; Hou, C.; Zhang, P. Porous carbon-coated cobalt sulfide nanocomposites derived from metal organic frameworks (MOFs) as an advanced oxygen reduction electrocatalyst. New J. Chem. 2016, 40, 1679–1684. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Li, Y.; Zhu, R.; Pang, H. Applications of Metal–Organic-Framework-Derived Carbon Materials. Adv. Mater. 2019, 31, 1804740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ge, J.; Liu, C.; Xing, W. Atomic-level dispersed catalysts for PEMFCs: Progress and future prospects. EnergyChem 2019, 1, 100018. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Hou, C.-C.; Wei, Y.-S.; Xu, Q. Multiple catalytic sites in MOF-based hybrid catalysts for organic reactions. Org. Biomol. Chem. 2020, 18, 8508–8525. [Google Scholar] [CrossRef] [PubMed]
- Windberg, T.; Ebert, T.; Uhlig, D.; Schulze, S.; Spange, S. Hierarchically structured carbon/carbon nanocomposites with adjustable porosity fabricated by twin polymerization. Microporous Mesoporous Mater. 2017, 246, 62–71. [Google Scholar] [CrossRef]
- Ma, W.; Yu, P.; Ohsaka, T.; Mao, L. An efficient electrocatalyst for oxygen reduction reaction derived from a Co-porphyrin-based covalent organic framework. Electrochem. Commun. 2015, 52, 53–57. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Song, J.; Du, D.; Lin, Y. Metal-Organic Framework-Derived Non-Precious Metal Nanocatalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2017, 7, 1700363. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent Progress in Metal-Organic Frameworks for Applications in Electrocatalytic and Photocatalytic Water Splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Xing, L.; Zhang, G.; Sun, S. Metal-organic framework derived carbon materials for electrocatalytic oxygen reactions: Recent progress and future perspectives. Carbon 2020, 156, 77–92. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.; He, C.; Duan, C. Metal–Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Solomon, M.B.; Church, T.L.; D’Alessandro, D.M. Perspectives on metal–organic frameworks with intrinsic electrocatalytic activity. CrystEngComm 2017, 19, 4049–4065. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhou, S.; Yu, F.; Yu, M.; Chiang, C.-Y.; Zhou, W.; Zhao, J.; Qiu, J. Metal–Organic-Framework-Derived Hybrid Carbon Nanocages as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution. Adv. Mater. 2017, 29, 1700874. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Wang, R.; Wang, S.; Lv, Y.-R.; Xu, H.; Zang, S.-Q. Metal–organic framework-derived Co9S8 embedded in N, O and S-tridoped carbon nanomaterials as an efficient oxygen bifunctional electrocatalyst. J. Mater. Chem. A 2019, 7, 7389–7395. [Google Scholar] [CrossRef]
- Li, J.-S.; Li, S.-L.; Tang, Y.-J.; Han, M.; Dai, Z.-H.; Bao, J.-C.; Lan, Y.-Q. Nitrogen-doped Fe/Fe3C@graphitic layer/carbon nanotube hybrids derived from MOFs: Efficient bifunctional electrocatalysts for ORR and OER. Chem. Commun. 2015, 51, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal–Organic Framework Derived Hybrid Co3O4-Carbon Porous Nanowire Arrays as Reversible Oxygen Evolution Electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Barman, B.K.; Nanda, K.K. CoFe Nanoalloys Encapsulated in N-Doped Graphene Layers as a Pt-Free Multifunctional Robust Catalyst: Elucidating the Role of Co-Alloying and N-Doping. ACS Sustain. Chem. Eng. 2018, 6, 12736–12745. [Google Scholar] [CrossRef]
- Yan, W.; Cao, X.; Wang, R.; Sha, Y.; Cui, P.; Cui, S. N co-doped rod-like porous carbon derived from S, N organic ligand assembled Ni-MOF as an efficient electrocatalyst for oxygen reduction reaction. J. Solid State Chem. 2019, 275, 167–173. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, S.; Xu, L.; Wang, D.; Li, Y. An efficient multifunctional hybrid electrocatalyst: Ni2P nanoparticles on MOF-derived Co,N-doped porous carbon polyhedrons for oxygen reduction and water splitting. Chem. Commun. 2018, 54, 12101–12104. [Google Scholar] [CrossRef]
- Li, D.; Xu, H.-Q.; Jiao, L.; Jiang, H.-L. Metal-organic frameworks for catalysis: State of the art, challenges, and opportunities. EnergyChem 2019, 1, 100005. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wang, R.; Hong, M. Structural Evolution from Metal–Organic Framework to Hybrids of Nitrogen-Doped Porous Carbon and Carbon Nanotubes for Enhanced Oxygen Reduction Activity. Chem. Mater. 2015, 27, 7610–7618. [Google Scholar] [CrossRef]
- Ge, L.; Yang, Y.; Wang, L.; Zhou, W.; De Marco, R.; Chen, Z.; Zou, J.; Zhu, Z. High activity electrocatalysts from metal–organic framework-carbon nanotube templates for the oxygen reduction reaction. Carbon 2015, 82, 417–424. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Y.; Liang, Y.; Kong, B.; Zhang, J.; Song, J.; Bao, Q.; Simon, G.P.; Jiang, S.P.; Wang, H. Nitrogen-Doped Nanoporous Carbon/Graphene Nano-Sandwiches: Synthesis and Application for Efficient Oxygen Reduction. Adv. Funct. Mater. 2015, 25, 5768–5777. [Google Scholar] [CrossRef]
- Cichocka, M.O.; Liang, Z.; Feng, D.; Back, S.; Siahrostami, S.; Wang, X.; Samperisi, L.; Sun, Y.; Xu, H.; Hedin, N.; et al. A Porphyrinic Zirconium Metal–Organic Framework for Oxygen Reduction Reaction: Tailoring the Spacing between Active-Sites through Chain-Based Inorganic Building Units. J. Am. Chem. Soc. 2020, 142, 15386–15395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-W.; Li, Y.-A.; Wang, X.-R.; Chen, G.-J.; Liu, Q.-K.; Ma, J.-P.; Dong, Y.-B. Fabrication of Cd(ii)-MOF-based ternary photocatalytic composite materials for H2 production via a gel-to-crystal approach. Chem. Commun. 2015, 51, 15906–15909. [Google Scholar] [CrossRef]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Liberman, I.; Shimoni, R.; Ifraemov, R.; Rozenberg, I.; Singh, C.; Hod, I. Active-Site Modulation in an Fe-Porphyrin-Based Metal–Organic Framework through Ligand Axial Coordination: Accelerating Electrocatalysis and Charge-Transport Kinetics. J. Am. Chem. Soc. 2020, 142, 1933–1940. [Google Scholar] [CrossRef]
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Gao, M.-R.; Liu, C.-H.; Li, S.-L.; Jiang, H.-L.; Lan, Y.-Q.; Han, M.; Yu, S.-H. Porous Molybdenum-Based Hybrid Catalysts for Highly Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2015, 54, 12928–12932. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zeng, J.; Peng, C.; Wang, R. MoO2/C hollow nanospheres synthesized by solvothermal method as anode material for lithium-ion batteries. Ionics 2019, 25, 437–445. [Google Scholar] [CrossRef]
- Jing, Y.; Cheng, Y.; Wang, L.; Liu, Y.; Yu, B.; Yang, C. MOF-derived Co, Fe, and Ni co-doped N-enriched hollow carbon as efficient electrocatalyst for oxygen reduction reaction. Chem. Eng. J. 2020, 397, 125539. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, J.; Zhang, Y.; Xu, W.; Xing, W.; Xu, D.; Zhang, Y.; Zhang, X. ZIF-8 Derived Graphene-Based Nitrogen-Doped Porous Carbon Sheets as Highly Efficient and Durable Oxygen Reduction Electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 14235–14239. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, J.-C.; Ding, S.; Lyu, Z.; Feng, S.; Tian, H.; Huyan, C.; Xu, M.; Li, T.; Du, D.; et al. 2D Single-Atom Catalyst with Optimized Iron Sites Produced by Thermal Melting of Metal–Organic Frameworks for Oxygen Reduction Reaction. Small Methods 2020, 4, 1900827. [Google Scholar] [CrossRef]
- Hou, C.-C.; Zou, L.; Sun, L.; Zhang, K.; Liu, Z.; Li, Y.; Li, C.; Zou, R.; Yu, J.; Xu, Q. Single-Atom Iron Catalysts on Overhang-Eave Carbon Cages for High-Performance Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 7384–7389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Chen, M.; Yu, B.; Wang, B.; Wang, X.; Zhang, W.; Yang, D. MOF derived multi-metal oxides anchored N, P-doped carbon matrix as efficient and durable electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2021, 581, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Huang, T.; Wen, Z.; Mao, S.; Cui, S.; Chen, J. Metal−Organic Framework-Derived Nitrogen-Doped Core-Shell-Structured Porous Fe/Fe3C@C Nanoboxes Supported on Graphene Sheets for Efficient Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 1400337. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, H.; Du, L.; He, L.; Zhao, K.; Chang, L.; Yin, G.; Zhao, H.; Liu, S.; Tang, Z. Carbonized Nanoscale Metal–Organic Frameworks as High Performance Electrocatalyst for Oxygen Reduction Reaction. ACS Nano 2014, 8, 12660–12668. [Google Scholar] [CrossRef]
- Niu, G.; Ruditskiy, A.; Vara, M.; Xia, Y. Toward continuous and scalable production of colloidal nanocrystals by switching from batch to droplet reactors. Chem. Soc. Rev. 2015, 44, 5806–5820. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, G.; Lu, N.; Wang, J.; Tong, L.; Wang, L.; Kim, M.J.; Xia, Y. Continuous and Scalable Production of Well-Controlled Noble-Metal Nanocrystals in Milliliter-Sized Droplet Reactors. Nano Lett. 2014, 14, 6626–6631. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiao, L.; Yang, Y.; DiSalvo, F.J.; Abruña, H.D. High-Loading Intermetallic Pt3Co/C Core–Shell Nanoparticles as Enhanced Activity Electrocatalysts toward the Oxygen Reduction Reaction (ORR). Chem. Mater. 2018, 30, 1532–1539. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; Guadarrama-Pérez, O.; Silva-Martínez, S.; Cuevas-Arteaga, C.; Guadarrama-Pérez, V.H. Oxygen reduction reaction (ORR) electrocatalysts in constructed wetland-microbial fuel cells: Effect of different carbon-based catalyst biocathode during bioelectricity production. Electrochim. Acta 2021, 370, 137745. [Google Scholar] [CrossRef]

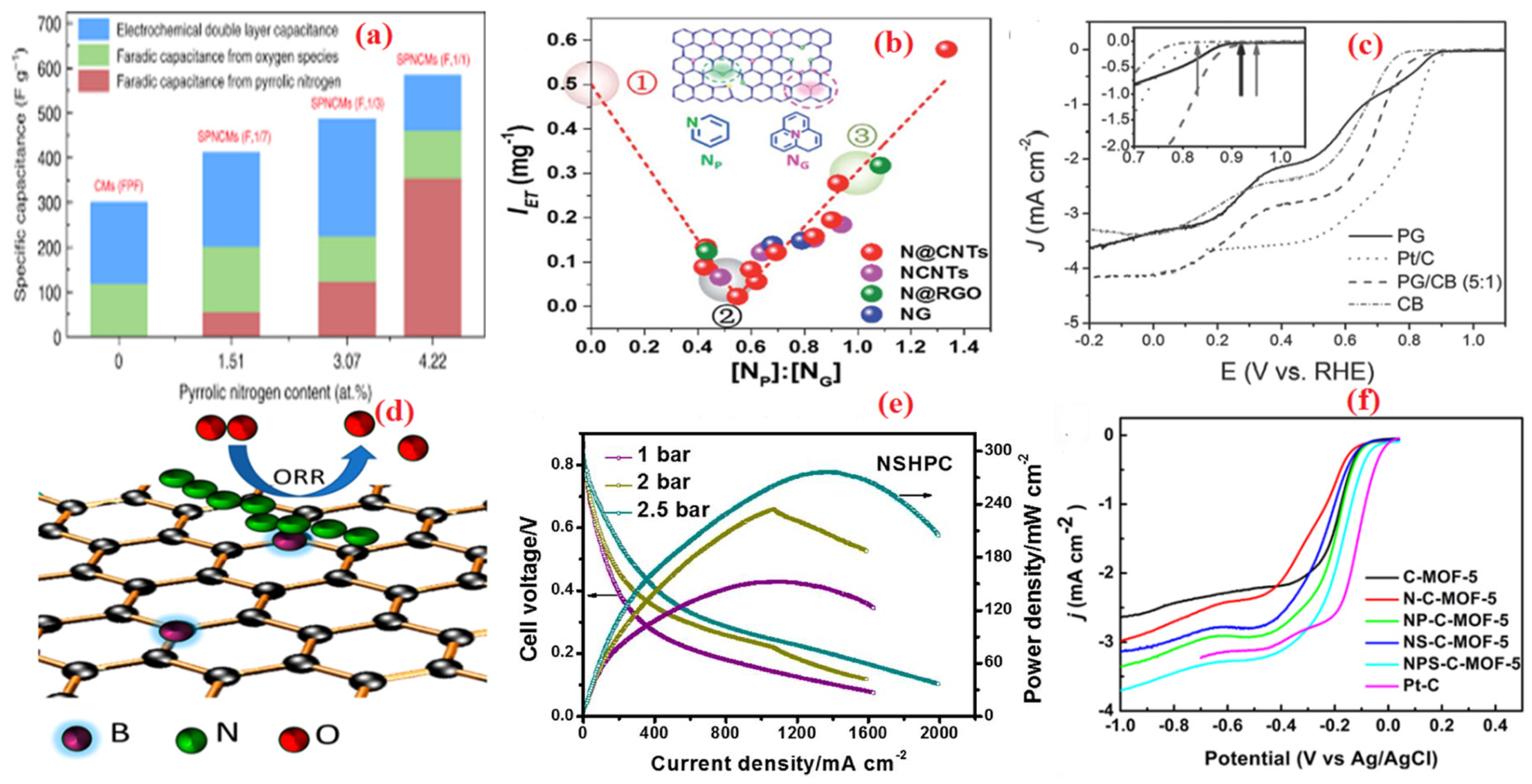
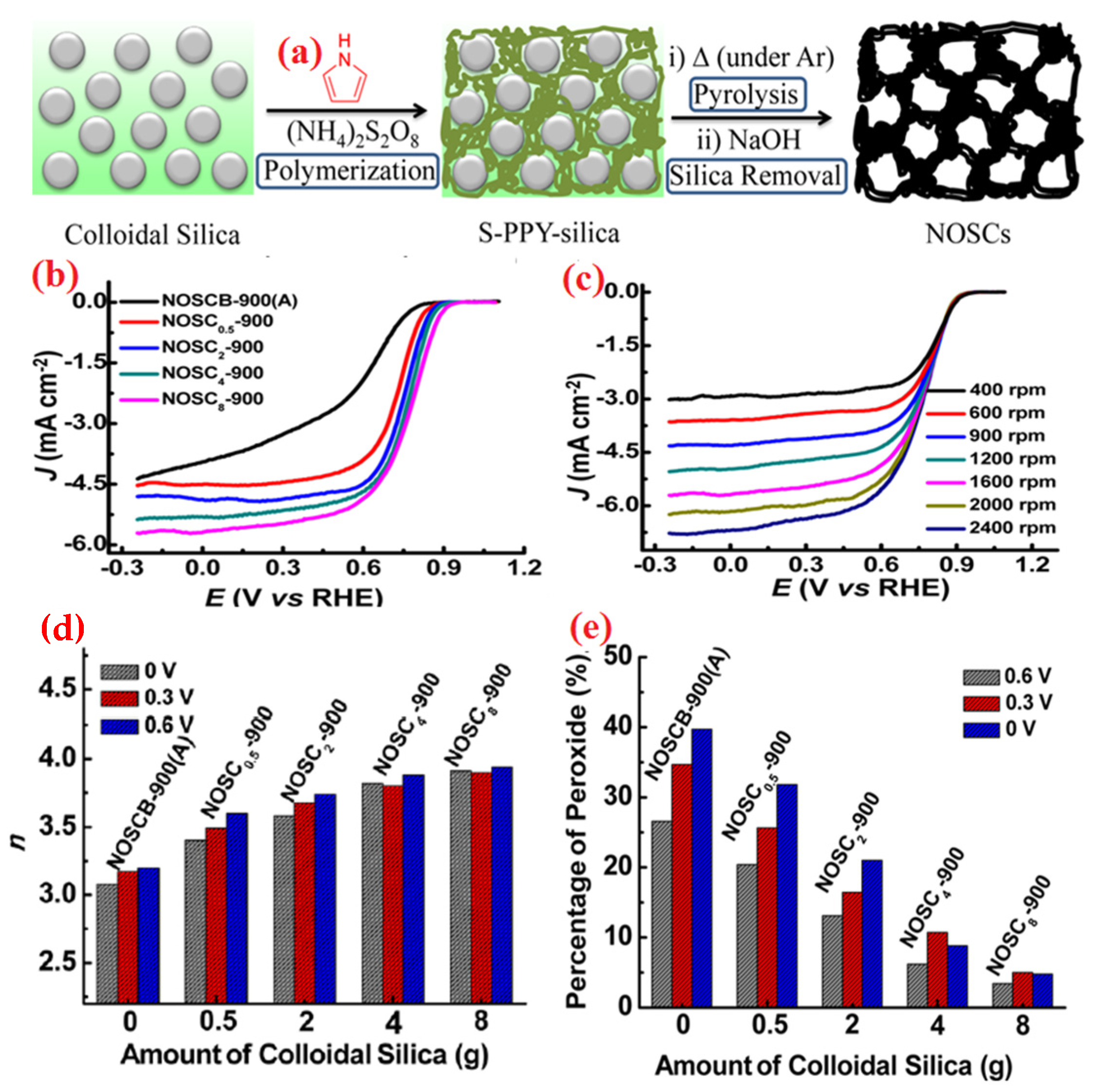


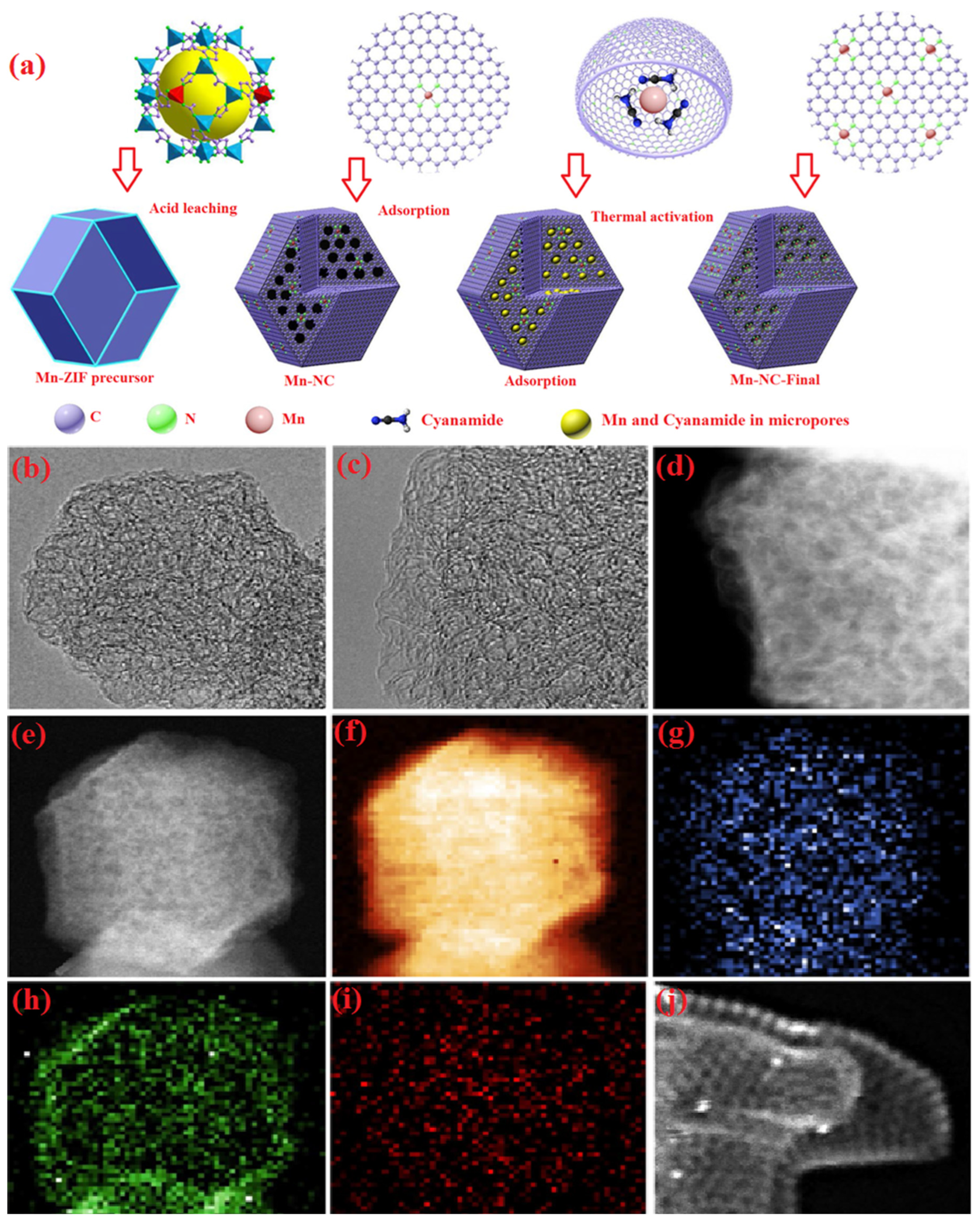
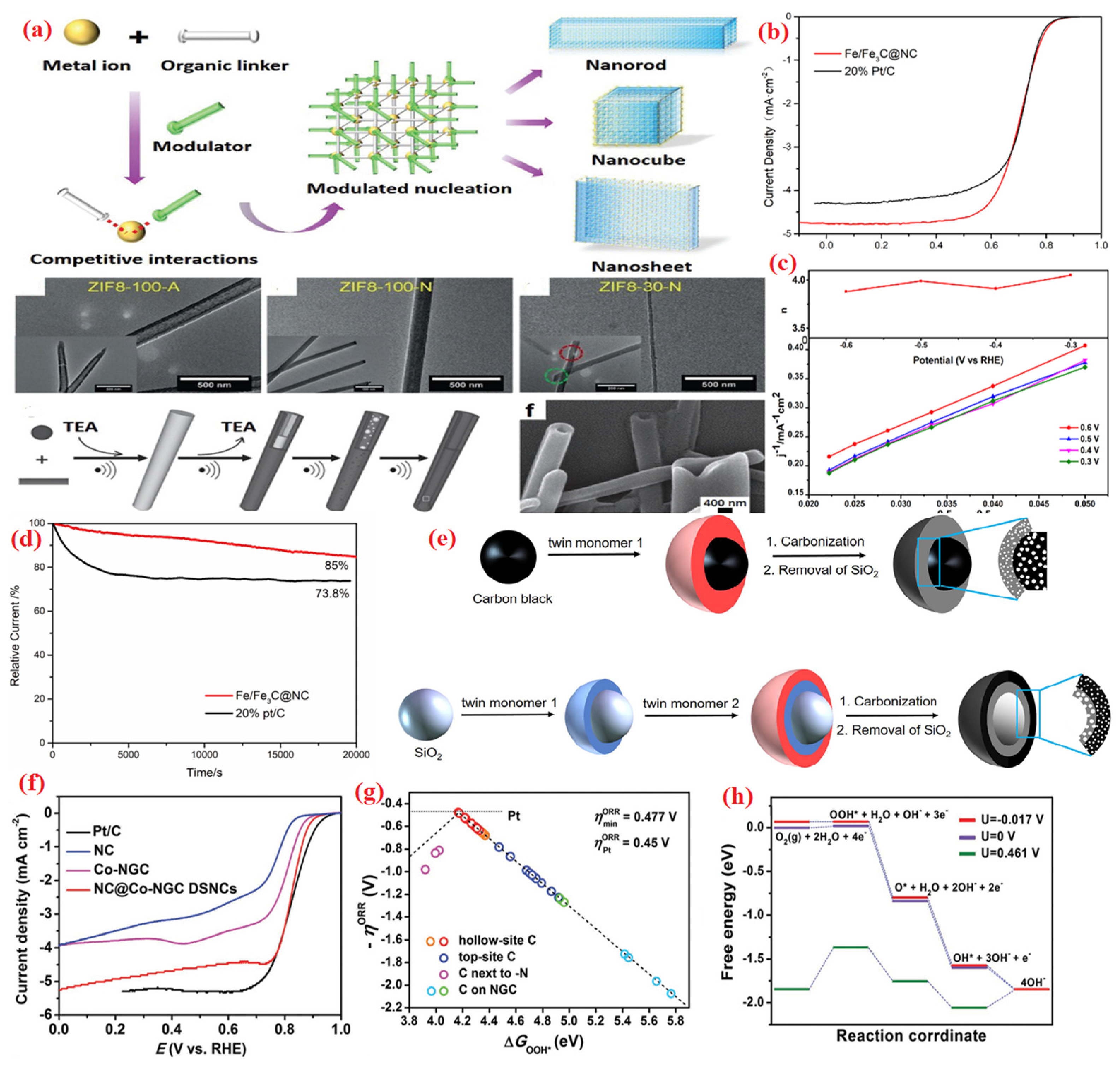
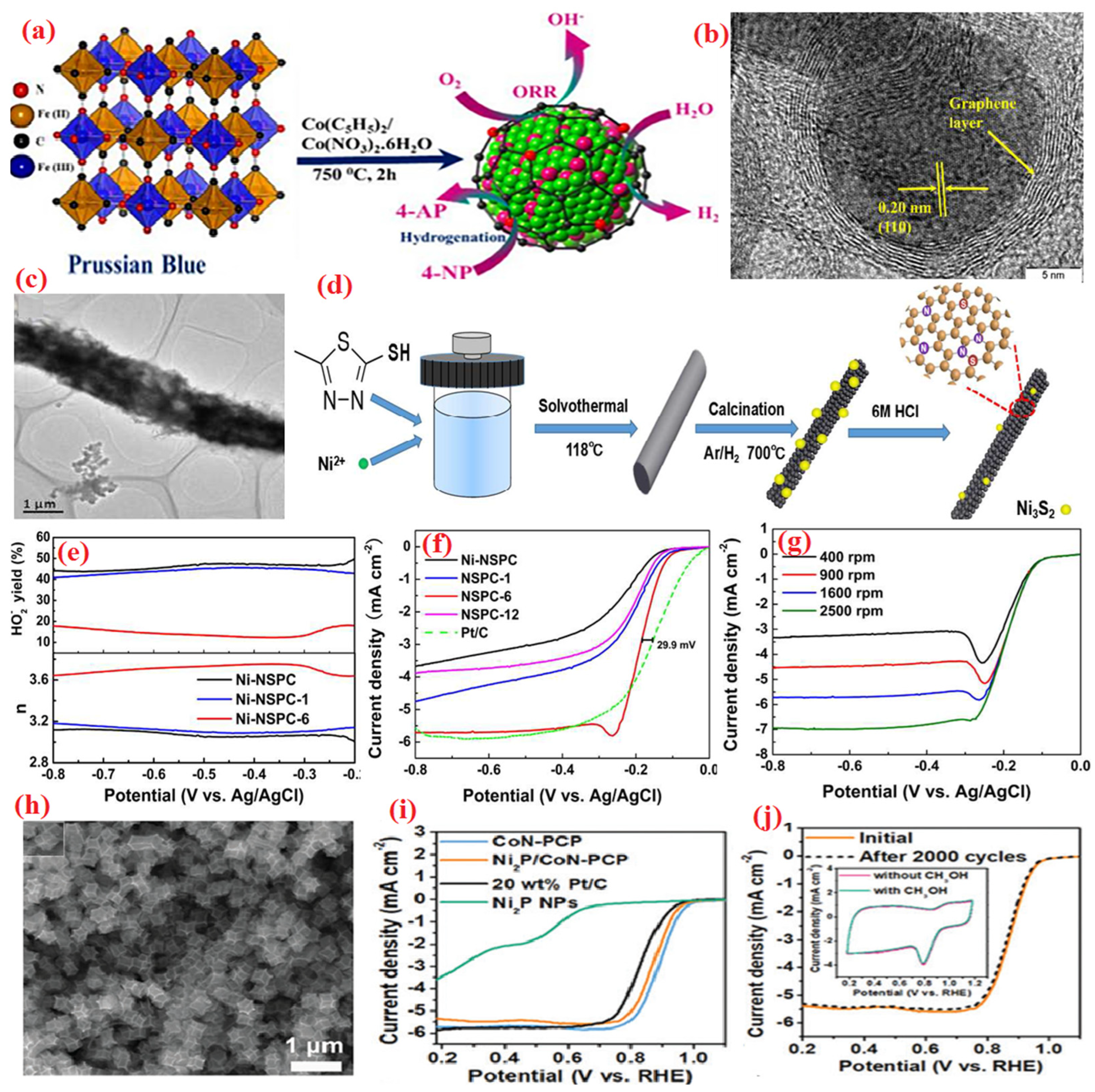

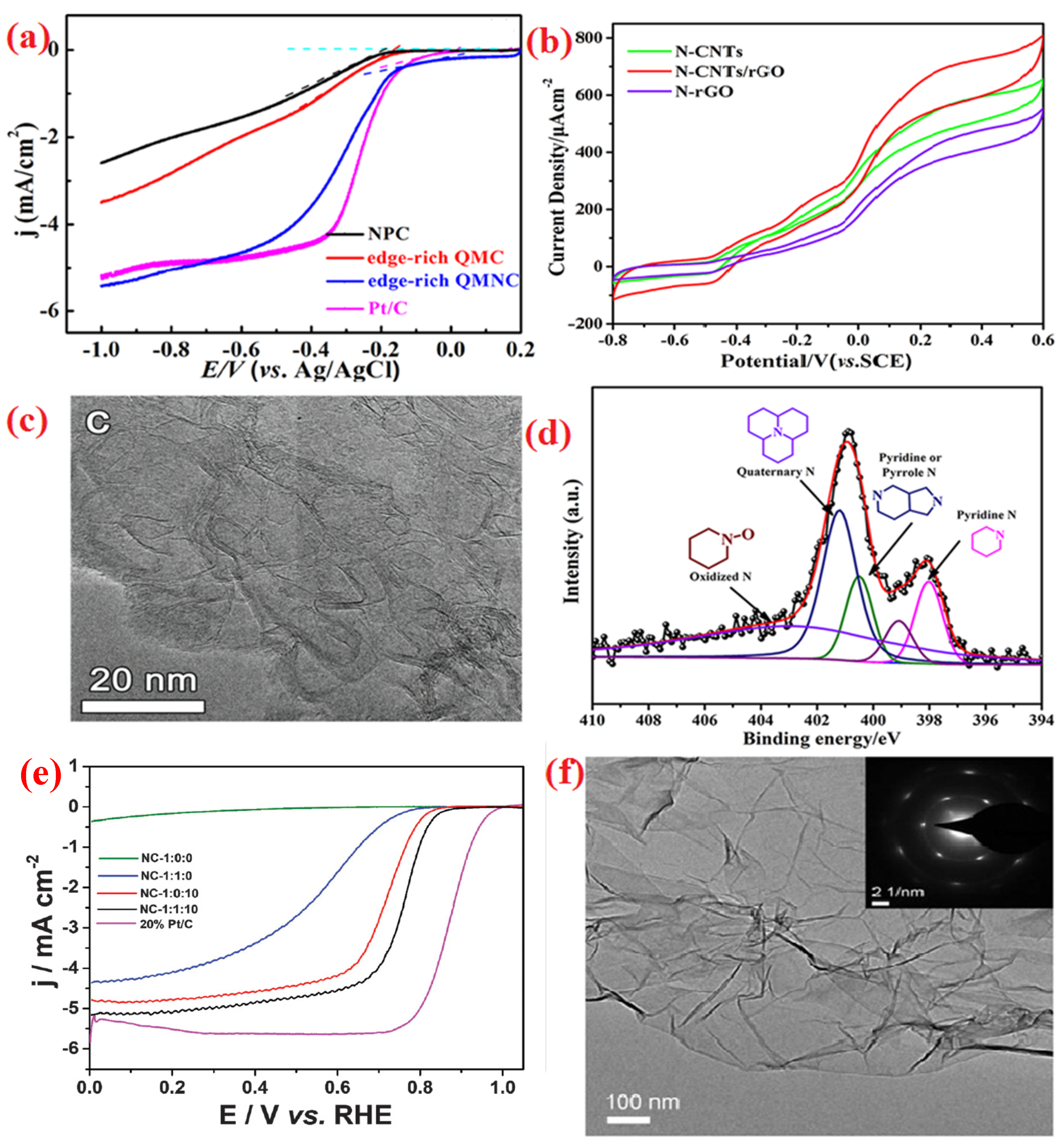
| Heteroatom Doped | Metal Precursor | Catalyst Name | Metal Content | BET Surface (m2 g−1) | Electrolyte | Eonset (V) | E1/2 (V) | Tafel Slopes (mV dec−1) | Electron Transfer Number | H2O2 Yield | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitrogen | - | Edge-rich QMNC (edge-rich quasi-mesoporous nitrogen-doped carbon) | - | 1970 | 0.1 M KOH | 1.05 | - | - | 3.9 | - | [70] |
| Nitrogen | - | N-CNTs/rGO1:1 | - | 424 | - | - | - | - | - | - | [71] |
| Nitrogen | Fe | m-GIC-HT(N-Fe) | - | - | 0.1 M NaOH | 0.82 | 0.72 | - | 3 | - | [73] |
| 0.5 M H2SO4 | 0.8 | - | - | - | - | ||||||
| Nitrogen | Co | Co SAs/3D GFs | 1.16 ± 0.03% Co (XPS) 1.38 ± 0.05 wt% Co (ICP) | 915.47 | 0.1 M KOH | 1.032 | 0.901 | 71 | 3.99 | - | [74] |
| Nitrogen | Zn, Co | 2D-MCo3O4-NCNAs | - | 161.6 | 0.1 M KOH | 1.54 | 0.74 | 74 | ~ 3.9 | 3% | [76] |
| Nitrogen | - | LIG-O (oxidized laser-induced graphene) | - | 246.8 | 0.1 M KOH | 1.52 | - | 56 | 4.0 at 0.6 V | - | [78] |
| Nitrogen | Fe, Mn, Co, Ni | N-CNT-1030 | 198.1 ppm Fe 1452.4 ppm Mn 11.3 ppm Co 4.7 ppm Ni (ICP-AES) | Up to 869 | 0.1 M KOH | −0.055 | −0.263 | - | 3.70–3.85 | - | [79] |
| Nitrogen | - | NC-1:1:10 | - | 888.15 | 0.1 M HClO4 | 0.901 | 0.755 | - | 4.19–4.31 | - | [80] |
| Nitrogen | - | N@CNTs | - | 43–81 | 0.1 M KOH | - | - | 70–105 | 2.2–4.2 | - | [82] |
| Nitrogen, Sulfur | - | NSHPC (N, S co-doped hierarchically porous carbon) | - | 1043.3 | 0.1 M KOH | 1.04 | 0.89 | 62.5 | 3.8–4.0 | <8.5% | [86] |
| 0.1 M HClO4 | 0.84 | 0.74 | 59.7 | ~4.0 | - | ||||||
| Boron | - | PN-B1G-GCE | - | 108.3 | 0.1 M KOH | - | - | - | 3.8–4.0 | - | [85] |
| Boron, Nitrogen | - | BDD2 (boron-doped diamond) | - | - | 0.1 M KOH | −0.634 | - | - | 2.65 | - | |
| Nitrogen, Sulfur | - | NSCA-700-1000 (NSCA: N modified S-defect carbon aerogel) | - | 1307.2 | 0.5 M H2SO4 | 0.885 | 0.76 | - | 4 | - | [88] |
| 0.1 M HClO4 | 0.885 | 0.76 | - | 4 | <3% | ||||||
| 0.1 M KOH | - | 0.85 | - | 4 | - | ||||||
| Boron, Nitrogen | - | NB/C (B, N-doped Ultrathin Carbon Nanosheet) | - | 1085 | 0.1 M KOH | - | 0.8 | 69 | 3.89 | 18–22% | [94] |
| Boron, Nitrogen | - | NBGHSs (Boron and nitrogen co-doped hollow graphene microspheres) | - | 512 | 0.1 M KOH | - | - | 66 | - | - | [96] |
| Nitrogen | - | N-doped HsGDY 900 °C (Hydrogen-substituted graphdiyne) | - | 1754 | 0.1 M KOH | 1.02 | 0.85 | 64.4 | 3.92 | <4% | [97] |
| 0.1 M HClO4 | 0.86 | 0.64 | 76.7 | 3.88–3.95 | <6% | [98] | |||||
| Nitrogen | - | N-rich PDA-based carbon SMSs | - | 422.4 | 0.1 M KOH | −0.1 | −0.1 | - | - | - | [99] |
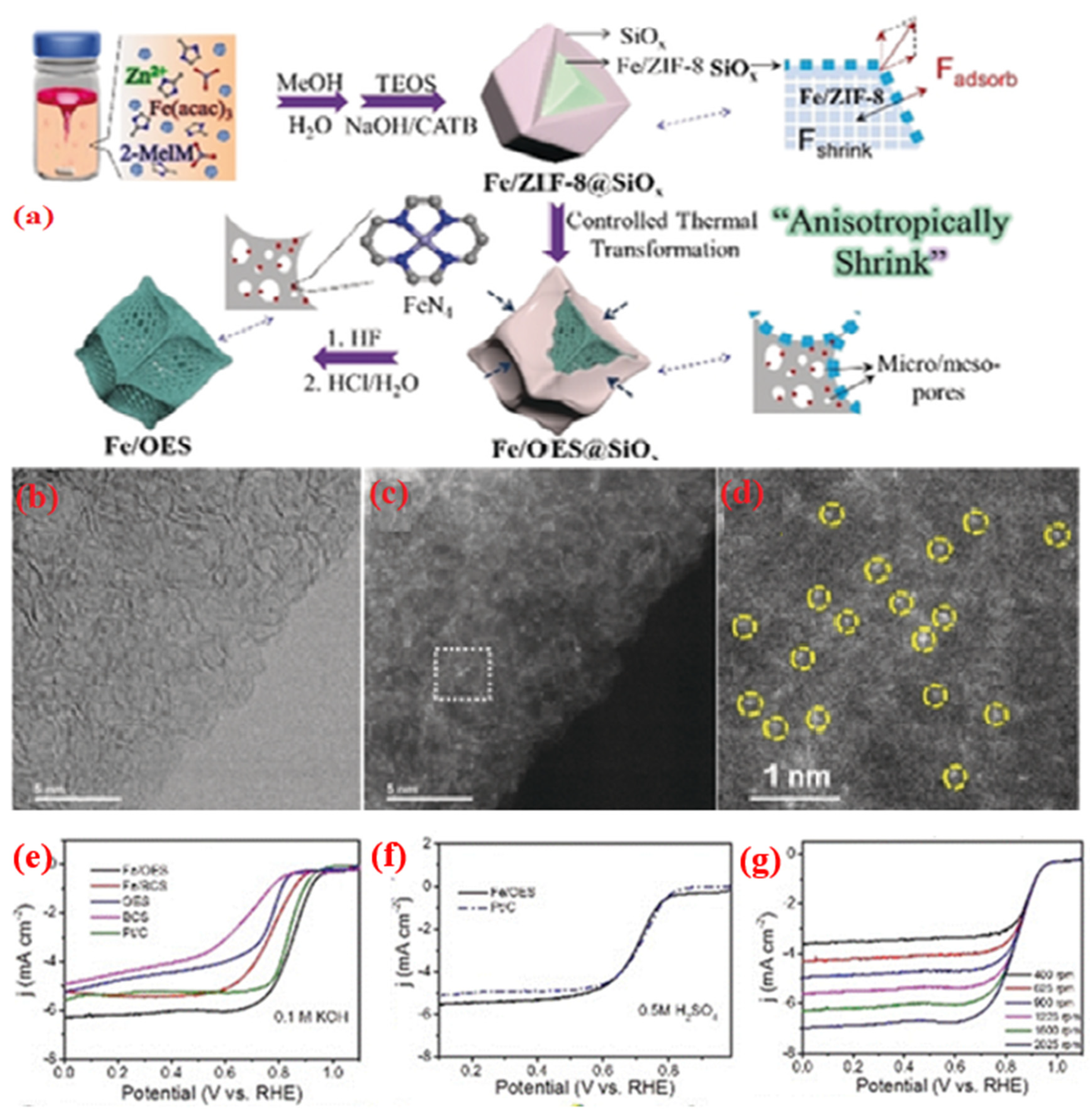
| Metal Precursor | Catalysts Name | Metal Content | BET Surface (m2 g−1) | Electrolyte | Eonset (V) | E1/2 (V) | Tafel Slope (mV dec−1) | Electron Transfer Number | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Co-Fe | MCF/NPCCNT-40 hybrid catalyst | - | 74 | 1.0 M KOH | - | - | 45.9 | - | [176] |
| Fe | MOF(Fe) | - | 1600 ± 300 | 1.0 M KOH | - | - | 137 | - | [138] |
| Fe | Fe/Fe3C@NGL-NCNT | - | - | 1.0 M KOH | - | - | - | 3.6 | [155] |
| Fe | Fe/Fe3C@NC | - | 107 | 1.0 M KOH | 0.85 | 0.7 | - | 3.88–4.05 | [139] |
| Fe | N-doped Fe/Fe3C@C/RGO | - | 34 | 1.0 M KOH | 0.95 | - | 72 | 3.08–3.52 | [177] |
| Co | PC-CoS1.097 NCs | - | 172 | 1.0 M KOH | - | - | - | 3.8–4.0 | [140] |
| Fe | CNPs | - | 326 | 1.0 M KOH | 1.03 | 0.92 | - | 3.97 | [178] |
| Co | Co-N-C @F127 | 1.38 wt.% Co (EELS) 4.4 wt.% Co (XPS) 6.2 wt.% Co (ICP-AES) | 825 | 0.5 M H2SO4 | 0.93 | 0.84 | - | - | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payattikul, L.; Chen, C.-Y.; Chen, Y.-S.; Raja Pugalenthi, M.; Punyawudho, K. Recent Advances and Synergistic Effects of Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts: A Review. Molecules 2023, 28, 7751. https://doi.org/10.3390/molecules28237751
Payattikul L, Chen C-Y, Chen Y-S, Raja Pugalenthi M, Punyawudho K. Recent Advances and Synergistic Effects of Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts: A Review. Molecules. 2023; 28(23):7751. https://doi.org/10.3390/molecules28237751
Chicago/Turabian StylePayattikul, Laksamee, Chen-Yu Chen, Yong-Song Chen, Mariyappan Raja Pugalenthi, and Konlayutt Punyawudho. 2023. "Recent Advances and Synergistic Effects of Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts: A Review" Molecules 28, no. 23: 7751. https://doi.org/10.3390/molecules28237751
APA StylePayattikul, L., Chen, C.-Y., Chen, Y.-S., Raja Pugalenthi, M., & Punyawudho, K. (2023). Recent Advances and Synergistic Effects of Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts: A Review. Molecules, 28(23), 7751. https://doi.org/10.3390/molecules28237751







