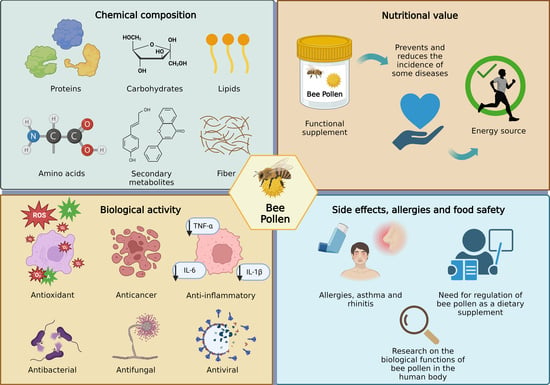
Chemical Properties and Biological Activity of Bee Pollen
Abstract
:1. Introduction
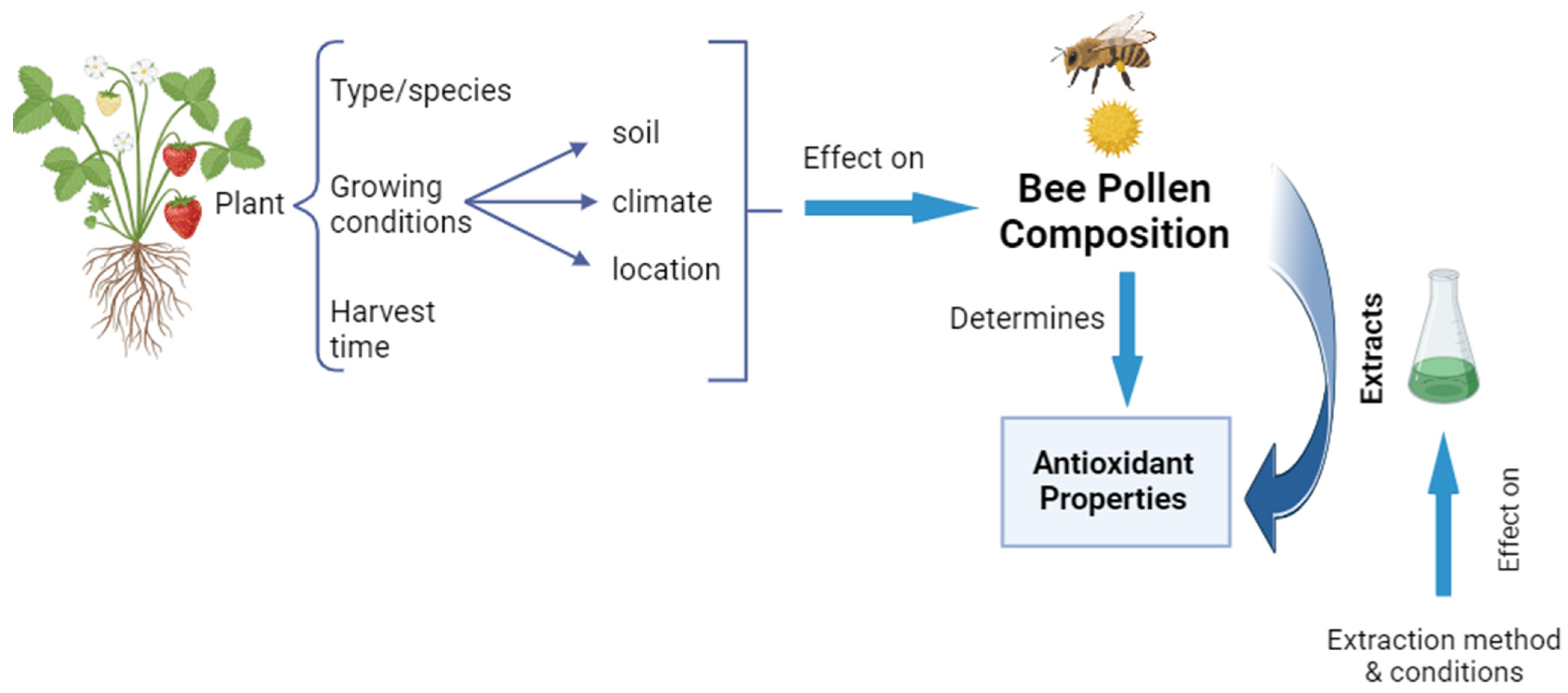
2. Chemical Composition and Nutritional Value
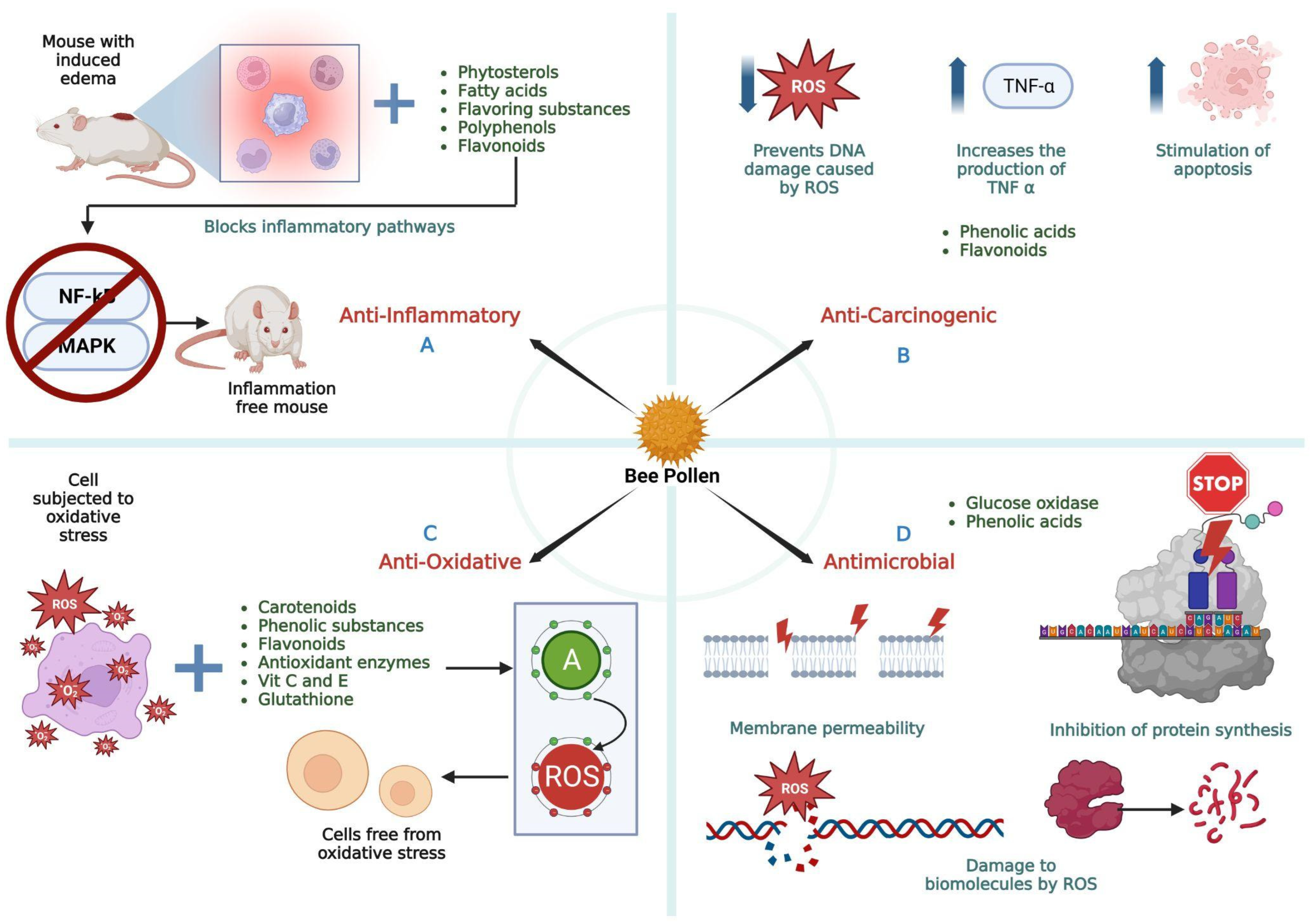
3. Anti-Inflammatory Activity
4. Antioxidant Activity
5. Antimicrobial Activity
5.1. Viruses
5.2. Bacteria
5.3. Fungi
6. Anticarcinogenic Activity
7. Clinical Trials
8. Functional Supplements and Human Health Benefits
9. Side Effects, Allergies, and Food Safety
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Why Bees Are Essential to People and Planet. Available online: https://www.unep.org/news-and-stories/story/why-bees-are-essential-people-and-planet (accessed on 14 June 2022).
- Krishnan, S.; Wiederkehr Guerra, G.; Bertrand, D.; Wertz-Kanounnikoff, S.; Kettle, C.J. The Pollination Services of Forests—A Review of Forest and Landscape Interventions to Enhance Their Cross-Sectoral Benefits; Forestry Working Paper; No. 15.; FAO & Bioversity International: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Llnskens, H.F.; Jorde, W. Pollen as food and medicine—A review. Econ. Bot. 1997, 51, 78–86. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee products: An emblematic example of underutilized sources of bioactive compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Lesne, P.; Grebenok, R.J.; Rangel, J.; Behmer, S.T. Assessing pollen nutrient content: A unifying approach for the study of bee nutritional ecology. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210510. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Zokaityte, E.; Dauksiene, A.; Jagminas, P.; Klupsaite, D.; Bliznikas, S.; Ruzauskas, M. Variations of the antimicrobial, antioxidant, sensory attributes and biogenic amines content in Lithuania-derived bee products. LWT 2020, 118, 108793. [Google Scholar] [CrossRef]
- Nicolson, S.W. Bee food: The chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 2011, 46, 197–204. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Ares, A.M.; Valverde, S.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Extraction and determination of bioactive compounds from bee pollen. J. Pharm. Biomed. Anal. 2018, 147, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.-F.; Wu, L.-M.; Hu, F.-L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee pollen: Current status and therapeutic potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef] [PubMed]
- Puerto, N.; Prieto, G.; Castro, R. Chemical composition and antioxidant activity of pollen. Review. Chil. J. Agric. Anim. Sci. 2015, 31, 115–126. Available online: https://revistas.udec.cl/index.php/chjaas/article/view/9973 (accessed on 14 June 2022).
- Keskin, M.; Özkök, A. Effects of drying techniques on chemical composition and volatile constituents of bee pollen. Czech J. Food Sci. 2020, 38, 203–208. [Google Scholar] [CrossRef]
- Alimoglu, G.; Guzelmeric, E.; Yuksel, P.I.; Celik, C.; Deniz, I.; Yesilada, E. Monofloral and polyfloral bee pollens: Comparative evaluation of their phenolics and bioactivity profiles. LWT 2021, 142, 110973. [Google Scholar] [CrossRef]
- El Ghouizi, A.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef]
- Kara, Y.; Can, Z.; Kolaylı, S. Applicability of Phenolic Profile Analysis Method Developed with RP-HPLC-PDA to some Bee Product. Braz. Arch. Biol. Technol. 2022, 65, e22210384. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Polak, T.; Pucihar, T.; Lilek, N.; Kandolf Borovšak, A.; Korošec, M. Carbohydrate composition of Slovenian bee pollens. Int. J. Food Sci. Technol. 2018, 53, 1880–1888. [Google Scholar] [CrossRef]
- Spulber, R.; Dogaroglu, M.; Babeanu, N.; Popa, O. Physicochemical characteristics of fresh bee pollen from different botanical origins. Rom. Biotechnol. Lett. 2018, 23, 13357–13365. [Google Scholar]
- singh Bagri, A.; Tiwari, P.; Gloch, E. Botanical origin and chemical composition of bee pollens collected from Apis cerana hives domesticated in the Pauri Garhwal, Western Himalaya, India. Ecol. Quest. 2023, 34, 1–17. [Google Scholar] [CrossRef]
- Dong, J.; Yang, Y.; Wang, X.; Zhang, H. Fatty acid profiles of 20 species of monofloral bee pollen from China. J. Apic. Res. 2015, 54, 503–511. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Escuredo, O.; Seijo, M.C.; Rojo, S.; Vilas-Boas, M.; Falcão, S.I. Phenolic Profile of Castanea Bee Pollen from the Northwest of the Iberian Peninsula. Separations 2023, 10, 270. [Google Scholar] [CrossRef]
- Rojo, S.; Escuredo, O.; Rodríguez-Flores, M.S.; Seijo, M.C. Botanical origin of galician bee pollen (northwest spain) for the characterization of phenolic content and antioxidant activity. Foods 2023, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.S.; Chambó, E.D.; Costa, M.A.P.d.C.; Cavalcante da Silva, S.M.P.; Lopes de Carvalho, C.A.; M. Estevinho, L. Chemical Composition and Biological Activities of Mono- and Heterofloral Bee Pollen of Different Geographical Origins. Int. J. Mol. Sci. 2017, 18, 921. [Google Scholar] [CrossRef] [PubMed]
- Khongkarat, P.; Phuwapraisirisan, P.; Chanchao, C. Phytochemical content, especially spermidine derivatives, presenting antioxidant and antilipoxygenase activities in Thai bee pollens. PeerJ 2022, 10, e13506. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Liu, L.-C.; Wu, M.-C.; Lin, T.-P.; Yang, C.-Y.; Huang, M.-Y. Chemical constituents, antioxidant, and anticancer activities of bee pollen from various floral sources in Taiwan. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12644. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Screening of Indian bee pollen based on antioxidant properties and polyphenolic composition using UHPLC-DAD-MS/MS: A multivariate analysis and ANN based approach. Food Res. Int. 2021, 140, 110041. [Google Scholar] [CrossRef]
- Aylanc, V.; Larbi, S.; Calhelha, R.; Barros, L.; Rezouga, F.; Rodríguez-Flores, M.S.; Seijo, M.C.; El Ghouizi, A.; Lyoussi, B.; Falcão, S.I.; et al. Evaluation of Antioxidant and Anticancer Activity of Mono- and Polyfloral Moroccan Bee Pollen by Characterizing Phenolic and Volatile Compounds. Molecules 2023, 28, 835. [Google Scholar] [CrossRef]
- Gomes, A.N.P.; Camara, C.A.; dos Santos Sousa, A.; de Assis Ribeiro dos Santos, F.; de Santana Filho, P.C.; Dorneles, G.P.; Romão, P.R.T.; Silva, T.M.S. Chemical composition of bee pollen and leishmanicidal activity of rhusflavone. Rev. Bras. Farmacogn. 2021, 31, 176–183. [Google Scholar] [CrossRef]
- Hsu, P.-S.; Wu, T.-H.; Huang, M.-Y.; Wang, D.-Y.; Wu, M.-C. Nutritive Value of 11 Bee Pollen Samples from Major Floral Sources in Taiwan. Foods 2021, 10, 2229. [Google Scholar] [CrossRef]
- Prđun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef]
- Ilie, C.-I.; Oprea, E.; Geana, E.-I.; Spoiala, A.; Buleandra, M.; Gradisteanu Pircalabioru, G.; Badea, I.A.; Ficai, D.; Andronescu, E.; Ficai, A.; et al. Bee pollen extracts: Chemical composition, antioxidant properties, and effect on the growth of selected probiotic and pathogenic bacteria. Antioxidants 2022, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Kaškonas, P.; Maruška, A. Volatile compounds composition and antioxidant activity of bee pollen collected in Lithuania. Chem. Pap. 2015, 69, 291–299. [Google Scholar] [CrossRef]
- Primorac, L.; Bilić Rajs, B.; Gal, K.; Bubalo, D.; Prđun, S.; Flanjak, I. The specificity of monofloral bee pollen fatty acid composition from Croatia and its nutritional value. J. Cent. Eur. Agric. 2023, 24, 104–114. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Assessment of Physico-chemical Properties, Fatty Acid, Amino Acid and Mineral Profile of Bee Pollen from India with a Multivariate Perspective. J. Food Nutr. Res. 2018, 57, 328–340. Available online: https://www.vup.sk/en/download.php?bulID=1997 (accessed on 8 October 2023).
- Gercek, Y.C.; Celik, S.; Bayram, S. Screening of Plant Pollen Sources, Polyphenolic Compounds, Fatty Acids and Antioxidant/Antimicrobial Activity from Bee Pollen. Molecules 2021, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, S.; Zhang, S.; Peng, S.; Cao, W.; Ho, C.-T.; Bai, N. Bioactive Constituents of F. esculentum Bee Pollen and Quantitative Analysis of Samples Collected from Seven Areas by HPLC. Molecules 2019, 24, 2705. [Google Scholar] [CrossRef]
- Spulber, R.; Colța, T.; Băbeanu, N. Chemical Diversity of Polyphenols From bee Pollen and Propolis. AgroLife Sci. J. 2017, 6, 183–194. [Google Scholar]
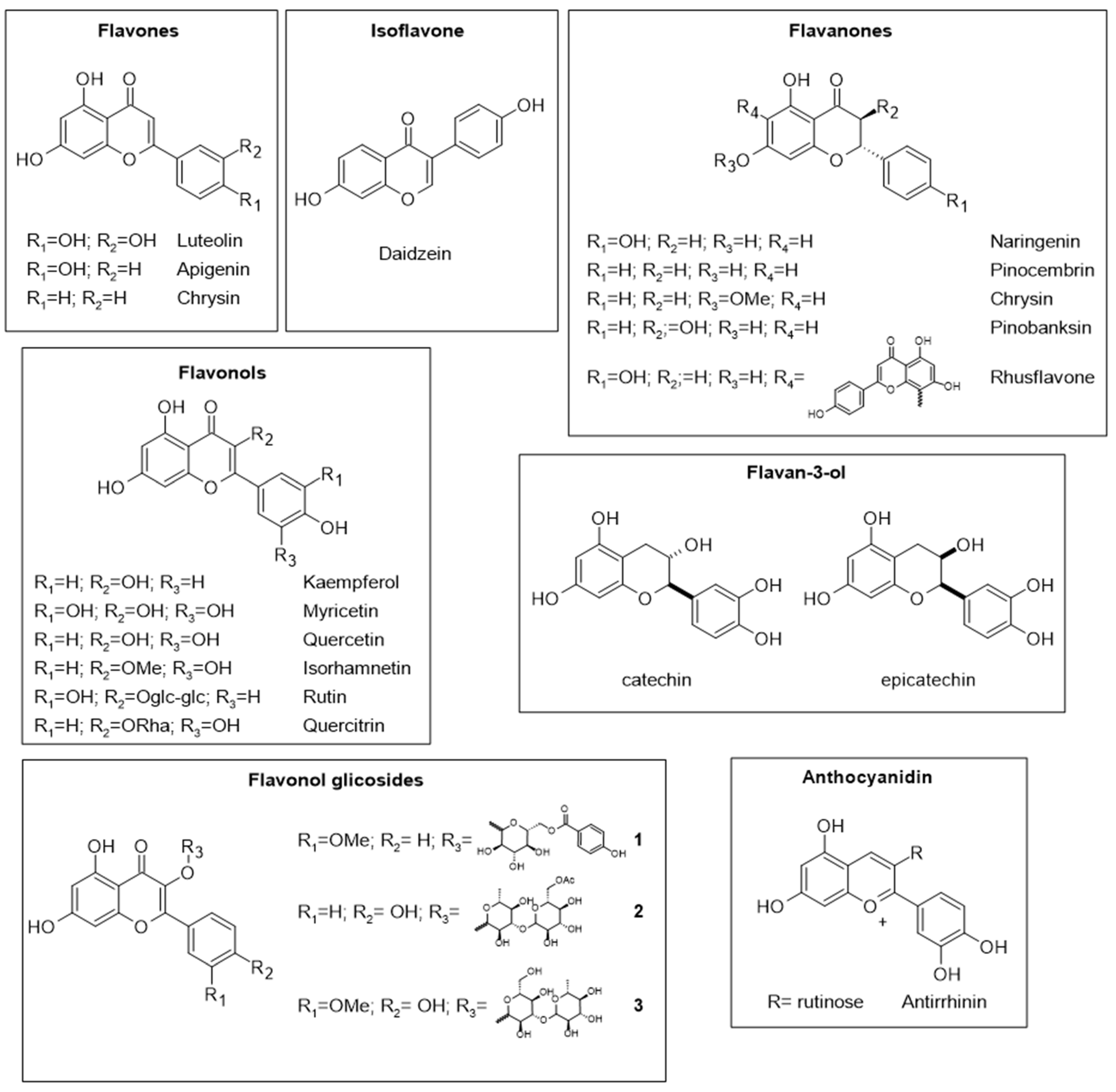
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, M.; Nagpal, A.K.; Katnoria, J. Kaur Polyphenolic Characterization of Pollen Grains of Some Medicinal Plant Species using Ultra High Performance Liquid Chromatography (UHPLC). J. Appl. Nat. Sci. 2021, 13, 840–845. [Google Scholar] [CrossRef]
- Tutun, H.; Kaya, M.M.; Usluer, M.S.; Kahraman, H.A. Bee pollen: Its antioxidant activity. Uludağ Arıcılık Derg. 2021, 21, 119–131. [Google Scholar] [CrossRef]
- Perveen, S.; Orfali, R.; Al-Taweel, A.M.; Khan, A.; Alghanem, B.; Shaibah, H. Simultaneous identification of phenolic and flavonoid contents in bee pollen by HPLC-ESI-MS data. Biomed. Res. 2019, 30, 770–774. [Google Scholar] [CrossRef]
- Mutlu, C.; Erbas, M. Turkish bee pollen: Composition, regional discrimination and polyphenol bioaccessibility. Food Biosci. 2023, 53, 102805. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhou, Q.; Wang, L.; Huang, W.; Wang, R. Effect of ultrasonic and ball-milling treatment on cell wall, nutrients, and antioxidant capacity of rose bee pollen, and identification of bioactive components. J. Sci. Food Agric. 2019, 99, 5350–5357. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Del Bo’, C.; Quicazán, M.C.; Corrrea, A.R.; Simonetti, P. Nutrients, phytochemicals and botanical origin of commercial bee pollen from different geographical areas. J. Food Compos. Anal. 2018, 73, 29–38. [Google Scholar] [CrossRef]
- Ghouizi, A.E.; Menyiy, N.E.; Falcão, S.I.; Vilas-Boas, M.; Lyoussi, B. Chemical composition, antioxidant activity, and diuretic effect of Moroccan fresh bee pollen in rats. Vet. World 2020, 13, 1251–1261. [Google Scholar] [CrossRef]
- Kyselka, J.; Bleha, R.; Dragoun, M.; Bialasová, K.; Horáčková, Š.; Schätz, M.; Sluková, M.; Filip, V.; Synytsya, A. Antifungal Polyamides of Hydroxycinnamic Acids from Sunflower Bee Pollen. J. Agric. Food Chem. 2018, 66, 11018–11026. [Google Scholar] [CrossRef] [PubMed]
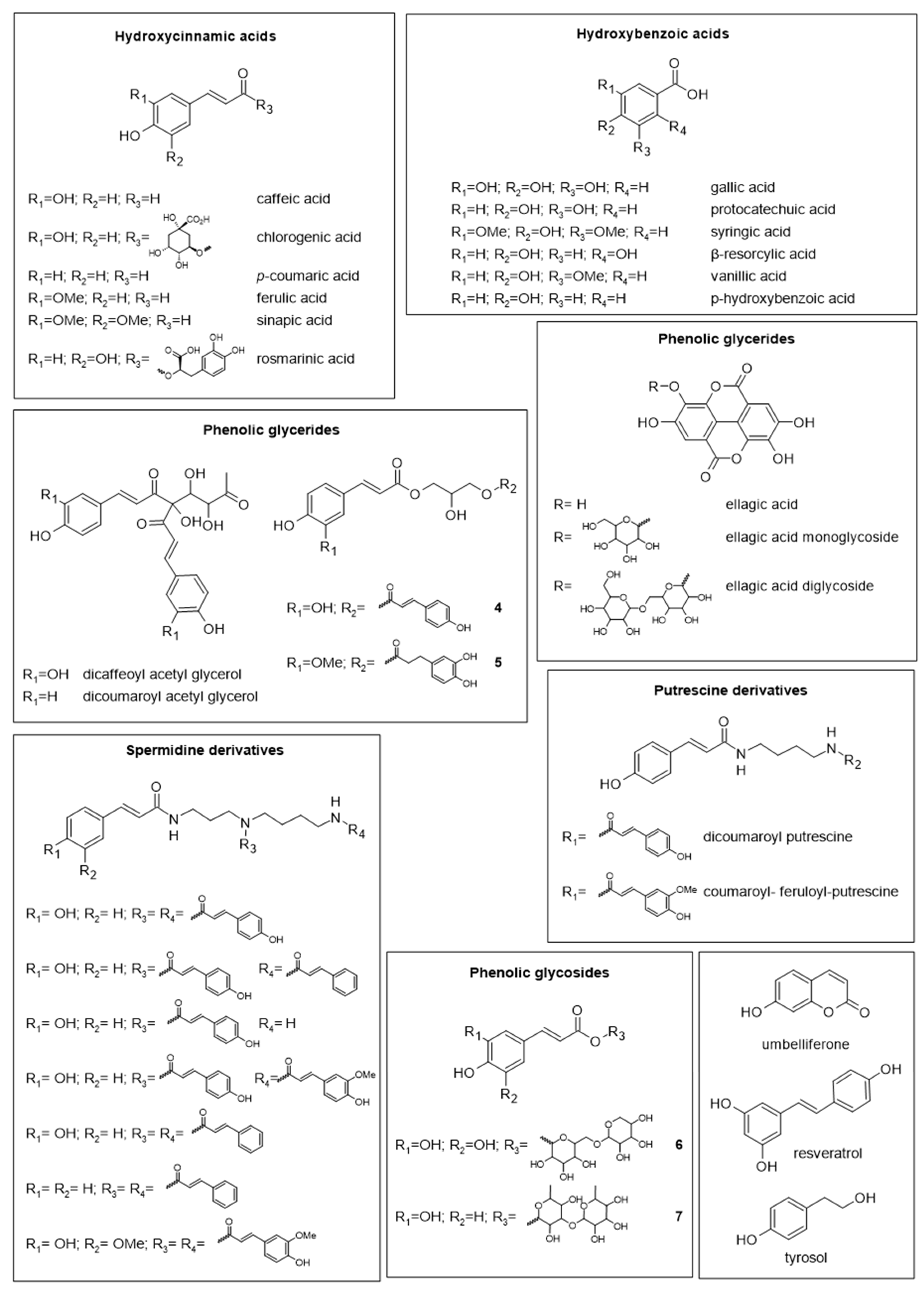
- Qiao, J.; Feng, Z.; Zhang, Y.; Xiao, X.; Dong, J.; Haubruge, E.; Zhang, H. Phenolamide and flavonoid glycoside profiles of 20 types of monofloral bee pollen. Food Chem. 2023, 405, 134800. [Google Scholar] [CrossRef]
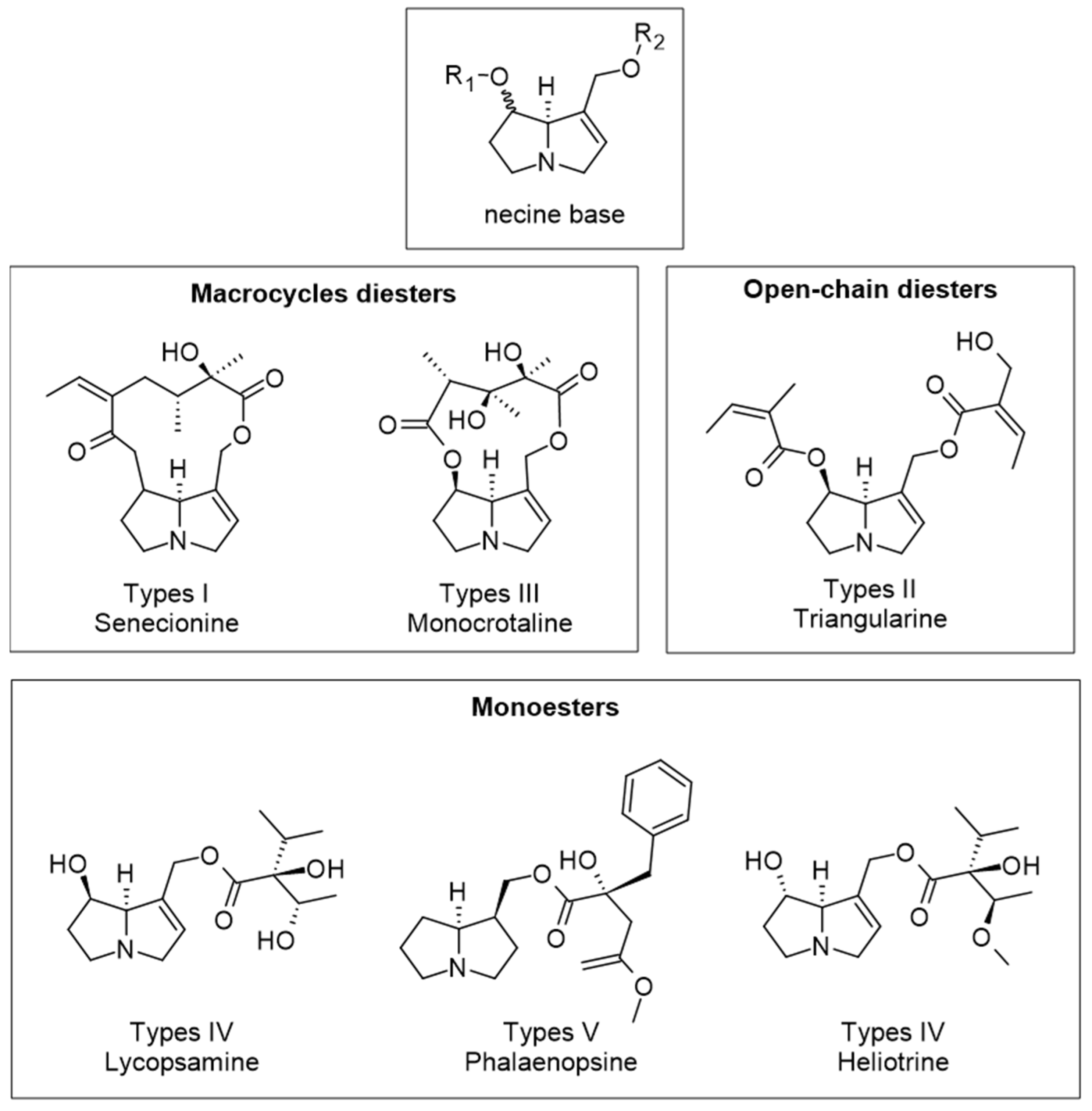
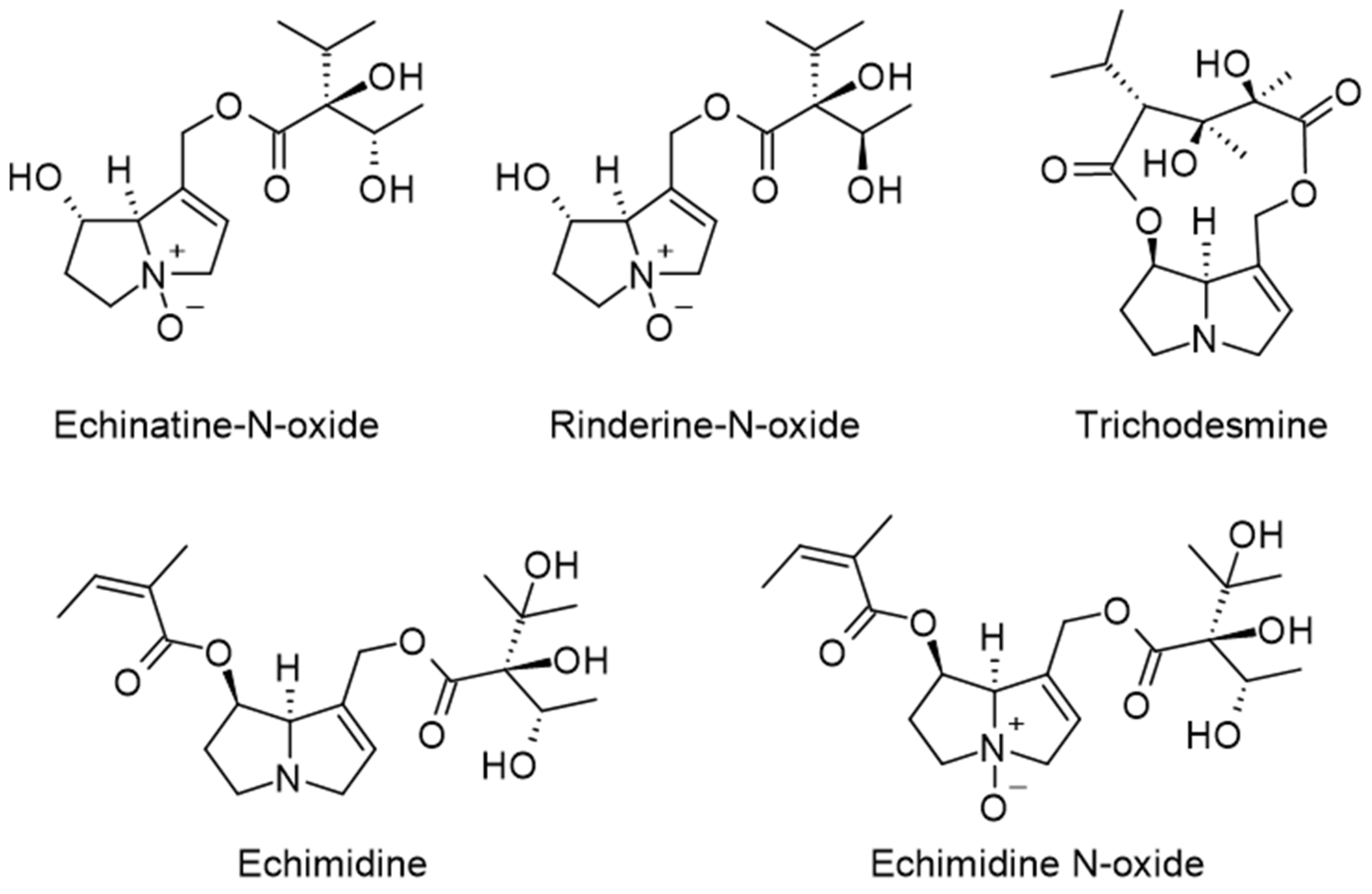
- Brugnerotto, P.; Seraglio, S.K.T.; Schulz, M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Pyrrolizidine alkaloids and beehive products: A review. Food Chem. 2021, 342, 128384. [Google Scholar] [CrossRef]
- Kwon, Y.; Gu, Y.; Jeong, Y. Evaluation of pyrrolizidine alkaloids in Korean commercial honeys and bee pollens. Food Sci. Technol. Res. 2022, 28, 123–132. [Google Scholar] [CrossRef]
- Inacio, L.D.; Merlanti, R.; Lucatello, L.; Bisutti, V.; Contiero, B. Pyrrolizidine alkaloids in bee pollen identified by LC-MS/MS analysis and colour parameters using multivariate class modeling. Heliyon 2020, 6, e03593. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- da Silva, B.R.; Orsso, C.E.; Gonzalez, M.C.; Sicchieri, J.M.F.; Mialich, M.S.; Jordao, A.A.; Prado, C.M. Phase angle and cellular health: Inflammation and oxidative damage. Rev. Endocr. Metab. Disord. 2023, 24, 543–562. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Huang, Q.; Zhang, L.; Liu, X.; Liu, R.; Lu, Q. Antioxidant and anti-inflammatory activities of rape bee pollen after fermentation and their correlation with chemical components by ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry-based untargeted metabolomics. Food Chem. 2023, 409, 135342. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Uddin, G.; Rauf, A.; Siddiqui, B.S.; Muhammad, N.; Khan, A.; Shah, S.U.A. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine 2014, 21, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, M.; Wan, Z.; Liang, J.; Betti, M.; Hrynets, Y.; Xue, X.; Wu, L.; Wang, K. Bee Pollen Extracts Modulate Serum Metabolism in Lipopolysaccharide-Induced Acute Lung Injury Mice with Anti-Inflammatory Effects. J. Agric. Food Chem. 2019, 67, 7855–7868. [Google Scholar] [CrossRef]
- Mokhtari, M.B.; El Ouar, I.; Zeghina, I.; Tartouga, M.A.; Ghorab, A.; Bahri, L.; Bensouici, C. Palynological analysis, phenolic components and anti-inflammatory activity of some bee pollens collected from the northeast region of algeria. Uludağ Arıcılık Derg. 2022, 22, 45–58. [Google Scholar] [CrossRef]
- Kosedag, M.; Gulaboglu, M. Pollen and bee bread expressed highest anti-inflammatory activities among bee products in chronic inflammation: An experimental study with cotton pellet granuloma in rats. Inflammopharmacology. 2023, 31, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.F.d.S.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.M.; Rocha, C.Q.d.; Lima, S.T.d.J.R.M.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef]
- Tobwor, P.; Deenarn, P.; Pruksatrakul, T.; Jiemsup, S.; Yongkiettrakul, S.; Vichai, V.; Phromson, M.; Chaiyapechara, S.; Jangsutthivorawat, W.; Yotbuntueng, P.; et al. Biochemical characterization of the cyclooxygenase enzyme in penaeid shrimp. PLoS ONE 2021, 16, e0250276. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid. Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Shafiee-Khamneh, A.; Heshmati-Afshar, F.; Delazar, A. Anti-inflammatory and anti-angiogenesis effect of bee pollen methanolic extract using air pouch model of inflammation. Res. Pharm. Sci. 2020, 15, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Martín-Muñoz, M.F.; Bartolome, B.; Caminoa, M.; Bobolea, I.; Ara, M.C.G.; Quirce, S. Bee pollen: A dangerous food for allergic children. Identification of responsible allergens. Allergol. Immunopathol. 2010, 38, 263–265. [Google Scholar] [CrossRef]
- Feás, X.; Vázquez-Tato, M.P.; Estevinho, L.; Seijas, J.A.; Iglesias, A. Organic bee pollen: Botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules 2012, 17, 8359–8377. [Google Scholar] [CrossRef]
- Saraiva, L.C.; Cunha, F.V.; Léllis, D.R.; Nunes, L.C. Composición, actividad biológica y toxicidad del polen apícola: Estado-del-arte. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2018, 17, 426–440. Available online: https://blacpma.ms-editions.cl/index.php/blacpma/article/view/130 (accessed on 8 October 2023).
- Leja, M.; Mareczek, A.; Wyżgolik, G.; Klepacz-Baniak, J.; Czekońska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Aličić, D.; Šubarić, D.; Jašić, M.; Pašalić, H.; Ačkar, Đ. Antioxidant properties of pollen. Hrana Zdr. Boles. Znan. Stručni Časopis Za Nutr. Dijetetiku 2014, 3, 6–12. [Google Scholar]
- Fatrcová-Šramková, K.; Nôžková, J.; Kačániová, M.; Máriássyová, M.; Rovná, K.; Stričík, M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health Part B 2013, 48, 133–138. [Google Scholar] [CrossRef]
- Carpes, S.T.; Begnini, R.; de Alencar, S.M.; Masson, M.L. Study of preparations of bee pollen extracts, antioxidant and antibacterial activity. Ciência E Agrotecnologia 2007, 31, 1818–1825. [Google Scholar] [CrossRef]
- Kim, S.B.; Jo, Y.H.; Liu, Q.; Ahn, J.H.; Hong, I.P.; Han, S.M.; Hwang, B.Y.; Lee, M.K. Optimization of Extraction Condition of Bee Pollen Using Response Surface Methodology: Correlation between Anti-Melanogenesis, Antioxidant Activity, and Phenolic Content. Molecules 2015, 20, 19764–19774. [Google Scholar] [CrossRef]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The profile of polyphenolic compounds, contents of total phenolics and flavonoids, and antioxidant and antimicrobial properties of bee products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Lu, Q. Separation and Characterization of Phenolamines and Flavonoids from Rape Bee Pollen, and Comparison of Their Antioxidant Activities and Protective Effects Against Oxidative Stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef]
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef]
- Corona, M.; Branchiccela, B.; Alburaki, M.; Palmer-Young, E.C.; Madella, S.; Chen, Y.; Evans, J.D. Decoupling the effects of nutrition, age, and behavioral caste on honey bee physiology, immunity, and colony health. Front. Physiol. 2023, 14, 1149840. [Google Scholar] [CrossRef]
- Asoutis Didaras, N.; Dimitriou, T.; Daskou, M.; Karatasou, K.; Mossialos, D. In vitro assessment of the antiviral activity of greek bee bread and bee collected pollen against enterovirus d68. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e4859. [Google Scholar] [CrossRef]
- Futui, W.; Thongwai, N. Antimicrobial and antioxidant activities, total phenolic and flavonoid contents of bee pollen crude extracts. Int. J. Biosci. Biochem. Bioinform. 2020, 10, 42–48. [Google Scholar] [CrossRef]
- Sadeq, O.; Mechchate, H.; Es-safi, I.; Bouhrim, M.; Jawhari, F.Z.; Ouassou, H.; Kharchoufa, L.; N. AlZain, M.; M. Alzamel, N.; Mohamed Al kamaly, O.; et al. Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria fruticosa, Achillea fragrantissima, and Phoenix dactylifera. Plants 2021, 10, 676. [Google Scholar] [CrossRef]
- Grubbs, K.J.; May, D.S.; Sardina, J.A.; Dermenjian, R.K.; Wyche, T.P.; Pinto-Tomás, A.A.; Clardy, J.; Currie, C.R. Pollen Streptomyces Produce Antibiotic That Inhibits the Honey Bee Pathogen Paenibacillus larvae. Front. Microbiol. 2021, 12, 632637. [Google Scholar] [CrossRef] [PubMed]
- Nader, R.A.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Beehive products as antibacterial agents: A review. Antibiotics 2021, 10, 717. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; Oumokhtar, B.; Lyoussi, B. Antioxidant and Antibacterial Effects of Pollen Extracts on Human Multidrug-Resistant Pathogenic Bacteria. J. Food Qual. 2021, 2021, 5560182. [Google Scholar] [CrossRef]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef]
- Alnaqdy, A.; Al-Jabri, A.; Al Mahrooqi, Z.; Nzeako, B.; Nsanze, H. Inhibition effect of honey on the adherence of Salmonella to intestinal epithelial cells in vitro. Int. J. Food Microbiol. 2005, 103, 347–351. [Google Scholar] [CrossRef]
- Soares de Arruda, V.A.; Vieria dos Santos, A.; Figueiredo Sampaio, D.; da Silva Araújo, E.; de Castro Peixoto, A.L.; Estevinho, L.M.; de Almeida-Muradian, L.B. Brazilian bee pollen: Phenolic content, antioxidant properties and antimicrobial activity. J. Apic. Res. 2021, 60, 775–783. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee products in dermatology and skin care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Plutino, M.; Lucini, L.; Aromolo, R.; Martinelli, E.; Souto, E.B.; Santini, A.; Pignatti, G. Bee products: A representation of biodiversity, sustainability, and health. Life 2021, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Xiao, G.; Liu, G.; Dong, H.; Liu, R.; Lu, Q. Phenolamide extract of apricot bee pollen alleviates glucolipid metabolic disorders and modulates the gut microbiota and metabolites in high-fat diet-induced obese mice. Food Funct. 2023, 14, 4662–4680. [Google Scholar] [CrossRef]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 1 August 2022).
- Premratanachai, P.; Chanchao, C. Review of the anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef]
- Münstedt, K.; Männle, H. Bee products and their role in cancer prevention and treatment. Complement. Ther. Med. 2020, 51, 102390. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Puthong, S.; Arung, E.T.; Chanchao, C. In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pac. J. Trop. Biomed. 2014, 4, 549–556. [Google Scholar] [CrossRef]
- Portokalakis, I.; Yusof, H.; Ghanotakis, D.; Nigam, P.; Owusu-Apenten, R. Manuka Honey-induced Cytotoxicity against MCF7 Breast Cancer Cells is Correlated to Total Phenol Content and Antioxidant Power. J. Adv. Biol. Biotechnol. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Amalia, E.; Diantini, A.; Subarnas, A. Water-soluble propolis and bee pollen of Trigona spp. from South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF-7 cell line. Oncol. Lett. 2020, 20, 274. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, J.; Huang, W.; Zhu, L.; Shao, M.; Dordoe, C.; Ahn, Y.-J.; Wang, D.; Zhao, Y.; Xiong, Y.; et al. The botanical origin and antioxidant, anti-BACE1 and antiproliferative properties of bee pollen from different regions of South Korea. BMC Complement. Med. Ther. 2020, 20, 236. [Google Scholar] [CrossRef]
- Arung, E.T.; Ramadhan, R.; Khairunnisa, B.; Amen, Y.; Matsumoto, M.; Nagata, M.; Kusuma, I.W.; Paramita, S.; Tandirogang, N.; Takemoto, N.; et al. Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. Saudi J. Biol. Sci. 2021, 28, 7182–7189. [Google Scholar] [CrossRef]
- Radev, Z. Variety in Protein Content of Pollen from 50 Plants from Bulgaria. Bee World 2018, 95, 81–83. [Google Scholar] [CrossRef]
- Rozman, A.S.; Hashim, N.; Maringgal, B.; Abdan, K. A comprehensive review of stingless bee products: Phytochemical composition and beneficial properties of honey, propolis, and pollen. Appl. Sci. 2022, 12, 6370. [Google Scholar] [CrossRef]
- Saisavoey, T.; Sangtanoo, P.; Srimongkol, P.; Reamtong, O.; Karnchanatat, A. Hydrolysates from bee pollen could induced apoptosis in human bronchogenic carcinoma cells (ChaGo-K-1). J. Food Sci. Technol. 2021, 58, 752–763. [Google Scholar] [CrossRef]
- Wan Omar, W.A.; Azhar, N.A.; Harif Fadzilah, N.; Nik Mohamed Kamal, N.N.S. Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 265–269. [Google Scholar] [CrossRef]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2020, 33, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical prospects of bee products: Special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, A.A.; Abdella, E.M.; Ahmed, R.R.; Ahmed, Y.K. Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of Egyptian bee pollen and propolis extracts. Cytotechnology 2014, 66, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-D.; Lou, Y.-J. A steroid fraction of chloroform extract from bee pollen of Brassica campestris induces apoptosis in human prostate cancer PC-3 cells. Phytother. Res. 2007, 21, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Zăhan, M.; Mărghitaş, L.A.; Dezmirean, D.S.; Erler, S.; Bobiş, O. Antiproliferative activity and apoptotic effects of Filipendula ulmaria pollen against C26 mice colon tumour cells. J. Apic. Sci. 2016, 60, 135–144. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Amina, M.; Alqahtani, A.S.; Alqahtani, M.S.; Malik, A.; Hatshan, M.R.; Siddiqui, M.R.H.; Khan, M.; Shaik, M.R.; Ola, M.S.; et al. Pollen Bee Aqueous Extract-Based Synthesis of Silver Nanoparticles and Evaluation of Their Anti-Cancer and Anti-Bacterial Activities. Processes 2020, 8, 524. [Google Scholar] [CrossRef]
- Di Chiacchio, I.M.; Gómez-Abenza, E.; Paiva, I.M.; de Abreu, D.J.M.; Rodríguez-Vidal, J.F.; Carvalho, E.E.N.; Carvalho, S.M.; Solis-Murgas, L.D.; Mulero, V. Bee pollen in zebrafish diet affects intestinal microbiota composition and skin cutaneous melanoma development. Sci. Rep. 2022, 12, 9998. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M.; et al. Bee pollen: Clinical trials and patent applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.; Teixeira, E.W.; Alves, M.L.; Moreti, A.C.; Otsuk, I.P.; Borguini, R.G.; Salatino, A. Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from Southeast Brazil. J. Agric. Food Chem. 2011, 59, 5516–5522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Firenzuoli, F. Therapeutic efficacy of orally administered pollen for nonallergic diseases: An umbrella review. Phytother. Res. 2019, 11, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Conquer, J.; Giese, N.; Khalsa, K.P.S.; Sklar, J.; Weissner, W.; Woods, J. An evidence-based systematic review of bee pollen by the Natural Standard Research Collaboration. J. Diet. Suppl. 2009, 6, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.; Karabagias, V.; Gatzias, I.; Riganakos, K. Bio-Functional Properties of Bee Pollen: The Case of “Bee Pollen Yoghurt”. Coatings 2018, 8, 423. [Google Scholar] [CrossRef]
- Conte, P.; Del Caro, A.; Balestra, F.; Piga, A.; Fadda, C. Bee pollen as a functional ingredient in gluten-free bread: A physical-chemical, technological and sensory approach. LWT 2018, 90, 1–7. [Google Scholar] [CrossRef]
- CC Pollen CO What to Look For in Bee Pollen. Available online: https://www.beepollen.com/what-to-look-for-in-bee-pollen/ (accessed on 8 October 2023).
- Rzepecka-Stojko, A.; Kabała-Dzik, A.; Kubina, R.; Jasik, K.; Kajor, M.; Wrześniok, D.; Stojko, J. Protective Effect of Polyphenol-Rich Extract from Bee Pollen in a High-Fat Diet. Molecules 2018, 23, 805. [Google Scholar] [CrossRef]
- Farag, S.A.; El-Rayes, T.K. Effect of Bee-pollen Supplementation on Performance, Carcass Traits and Blood Parameters of Broiler Chickens. Asian J. Anim. Vet. Adv. 2016, 11, 168–177. [Google Scholar] [CrossRef]
- Senyuk, B.; Boreyko, L.D.; Yurnyuk, S. Correction of Clinical And Biochemical Parameters Using Bee Pollen in Patients with Diabetes. Int. Sci. Prof. Period. J. “Unity Sci.” 2016, 138–140. Available online: https://core.ac.uk/reader/144958607 (accessed on 8 October 2023).
- Yan, S.; Wang, K.; Wang, X.; Ou, A.; Wang, F.; Wu, L.; Xue, X. Effect of fermented bee pollen on metabolic syndrome in high-fat diet-induced mice. Food Sci. Hum. Wellness 2021, 10, 345–355. [Google Scholar] [CrossRef]
- Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of schisandrachinensis bee pollen on nonalcoholic fatty liver disease and gut microbiota in highfat diet induced obese mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Jasik, K.; Buszman, E. Anti-Atherogenic Activity of Polyphenol-Rich Extract from Bee Pollen. Nutrients 2017, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Daudu, O.M. Bee Pollen Extracts as Potential Antioxidants and Inhibitors of α-Amylase and α-Glucosidase Enzymes In Vitro Assessment. J. Apic. Sci. 2019, 63, 315–325. [Google Scholar] [CrossRef]
- Rahayu, A.N.; Wirjatmadi, B.; Adriani, M.; Soenarnatalina, M.; Winarni, D.; Hartiningsih, S. Bee Pollen Effect on Blood Glucose Levels in Alloxan-induced Male Wistar Rats. Health Notions 2018, 2, 10–13. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced type 2 diabetes in rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Ahmed, O.M.; Hozayen, W.G.; Ahmed, M.A. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-Induced diabetic wistar rats. Biomed. Pharmacother. 2018, 97, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Su, G.; Wang, L.; Xia, Q.; Liu, R.; Lu, Q.; Zhang, J. Identification and mechanism of effective components from rape (Brassica napus L.) bee pollen on serum uric acid level and xanthine oxidase activity. J. Funct. Foods 2018, 47, 241–251. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, X.; Zhao, H.; Yang, E.; Wang, Y.; Cheng, N.; Cao, W. Impact of Camellia japonica Bee Pollen Polyphenols on Hyperuricemia and Gut Microbiota in Potassium Oxonate-Induced Mice. Nutrients 2021, 13, 2665. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Geng, Q.; Huang, H.; Yao, H.; Du, T.; Chen, L.; Wu, Z.; Miao, X.; Shi, P. Antioxidative and Cardioprotective Effects of Schisandra chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats. Molecules 2019, 24, 1090. [Google Scholar] [CrossRef] [PubMed]
- Khalil, F.A.; El-Sheikh, N.M. The effects of dietary Egyptian propolis and bee pollen supplementation against toxicity of sodium fluoride in rats. J. Am. Sci. 2010, 6, 310–316. Available online: https://typeset.io/papers/the-effects-of-dietary-egyptian-propolis-and-bee-pollen-1kexg2sm5a (accessed on 8 October 2023).
- Yamaguchi, M.; Hamamoto, R.; Uchiyama, S.; Ishiyama, K.; Hashimoto, K. Preventive Effects of Bee Pollen Cistus ladaniferus Extract on Bone Loss in Streptozotocin-Diabetic Rats In Vivo. J. Health Sci. 2007, 53, 190–195. [Google Scholar] [CrossRef]
- Hamamoto, R.; Ishiyama, K.; Hashimoto, K.; Yamaguchi, M. Characterization of the Active Component in Bee Pollen Cistus ladaniferus Extract in Stimulating Bone Calcification and in Inhibiting Bone Resorption in Vitro. J. Health Sci. 2006, 52, 607–612. [Google Scholar] [CrossRef]
- Kolesarova, A.; Bakova, Z.; Capcarova, M.; Galik, B.; Juracek, M.; Simko, M.; Toman, R.; Sirotkin, A.V. Consumption of bee pollen affects rat ovarian functions. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, A.; Capcarova, M.; Bakova, Z.; Galik, B.; Juracek, M.; Simko, M.; Sirotkin, A.V. The effect of bee pollen on secretion activity, markers of proliferation and apoptosis of porcine ovarian granulosa cells in vitro. J. Environ. Sci. Health B 2011, 46, 207–212. [Google Scholar] [CrossRef]
- Naseri, L.; Khazaei, M.R.; Khazaei, M. Synergic effect of bee pollen and metformin on proliferation and apoptosis of granulosa cells: Rat model of polycystic ovary syndrome. J. Food Biochem. 2022, 46, e13635. [Google Scholar] [CrossRef]
- Shaldoum, F.; El-Kott, A.F.; Ouda, M.M.A.; Abd-Ella, E.M. Immunomodulatory effects of bee pollen on doxorubicin-induced bone marrow/spleen immunosuppression in rat. J. Food Biochem. 2021, 45, e13747. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Tokura, T.; Nakano, N.; Hara, M.; Niyonsaba, F.; Ushio, H.; Yamamoto, Y.; Tadokoro, T.; Okumura, K.; Ogawa, H. Inhibitory effect of honeybee-collected pollen on mast cell degranulation in vivo and in vitro. J. Med. Food 2008, 11, 14–20. [Google Scholar] [CrossRef]
- Medeiros, K.C.P.; Figueiredo, C.A.V.; Figueredo, T.B.; Freire, K.R.L.; Santos, F.A.R.; Alcantara-Neves, N.M.; Silva, T.M.S.; Piuvezam, M.R. Anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J. Ethnopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Bae, H.J.; Zhang, J.; Kwon, Y.; Koo, B.; Jung, I.H.; Kim, H.M.; Park, J.H.; Lew, J.H.; Ryu, J.H. The Ameliorating Effects of Bee Pollen on Scopolamine-Induced Cognitive Impairment in Mice. Biol. Pharm. Bull. 2019, 42, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, Y.; Zhang, Y.; Zhuang, Y. Antioxidant and Anti-tyrosinase Activities of Phenolic Extracts from Rape Bee Pollen and Inhibitory Melanogenesis by cAMP/MITF/TYR Pathway in B16 Mouse Melanoma Cells. Front. Pharmacol. 2017, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Shahali, Y. Allergy after ingestion of bee-gathered pollen: Influence of botanical origins. Ann. Allergy Asthma Immunol. 2015, 114, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Végh, R.; Csóka, M.; Sörös, C.; Sipos, L. Food safety hazards of bee pollen—A review. Trends Food Sci. Technol. 2021, 114, 490–509. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient-The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Pitsios, C.; Chliva, C.; Mikos, N.; Kompoti, E.; Nowak-Wegrzyn, A.; Kontou-Fili, K. Bee pollen sensitivity in airborne pollen allergic individuals. Ann. Allergy Asthma Immunol. 2006, 97, 703–706. [Google Scholar] [CrossRef]
- Matuszewska, E.; Plewa, S.; Pietkiewicz, D.; Kossakowski, K.; Matysiak, J.; Rosiński, G.; Matysiak, J. Mass Spectrometry-Based Identification of Bioactive Bee Pollen Proteins: Evaluation of Allergy Risk after Bee Pollen Supplementation. Molecules 2022, 27, 7733. [Google Scholar] [CrossRef]
- Chang, F.; Eng, L.; Chang, C. Food allergy labeling laws: International guidelines for residents and travelers. Clin. Rev. Allergy Immunol. 2023, 65, 148–165. [Google Scholar] [CrossRef]
- Beggs, P.J.; Clot, B.; Sofiev, M.; Johnston, F.H. Climate change, airborne allergens, and three translational mitigation approaches. eBioMedicine 2023, 93, 104478. [Google Scholar] [CrossRef]

| Component | Content | Ref |
|---|---|---|
| Proteins | 2–60% | [6] |
| 10–40% | [13] | |
| 14–30% | [14] | |
| 22.7% | [15] | |
| Carbohydrates | 4–69% | [6] |
| 15–60% | [13] | |
| 40–85% | [14] | |
| 30.8% | [15] | |
| Lipids | 1–20% | [13] |
| 1–10% | [14] | |
| 4–7% | [6] | |
| 5.1% | [15] | |
| Amino acids | 10.4% | [15] |
| Phenolic compound | 1.6% | [15] |
| Fiber | 0.3–20% | [6] |
| Component | Measuring Component | Estimation Method | Ref |
|---|---|---|---|
| Proteins | Nitrogen content | Kjeldahl digestion | [8] |
| Dumas combustion | |||
| Basic amino acid residues content | Bradford colorimetric assay | ||
| Peptide bonds | Lowry colorimetric assay | ||
| Bicinchoninic acid (BCA) colorimetric assay | |||
| Carbohydrates | Soluble sugars, starch, and fructosan | Phenol-sulfuric acid colorimetric assay | [8] |
| Lipids | Lipids content | Folch gravimetric assay | [8] |
| Bligh and Dyer gravimetric assay | |||
| Loveridge gravimetric assay | |||
| Vanillin colorimetric assay | |||
| Phenolic compound | Phenolic profiles | High-performance liquid chromatography (HPLC) | [20] |
| Fiber | Soluble and insoluble dietary fiber | Enzymatic gravimetric technique | [21] |
| Potential Usage | Desired Effect | Results * | Bee Pollen/Extract Type | Ref. |
|---|---|---|---|---|
| Hypercholesterolemia | Reduction of cholesterol levels |
| polyphenol-rich bee pollen ethanol extract | [124] |
| basal diet supplemented with bee-pollen | [125] | ||
| aqueous suspension | [126] | ||
| Metabolic syndrome | Prevention of metabolic syndrome |
| yeast-fermented wall-broken bee pollen | [127] |
| Obesity | Body weight loss, reduction of lipid accumulation in serum and liver |
| ethanol extract | [128] |
| Atherosclerosis | Reduction or prevention of chronic inflammation in the aorta and medium-sized arteries |
| ethanol extract | [129] |
| Hyperglycemia | Reduction of blood glucose levels |
| water extracts | [130] |
| bee pollen | [131] | ||
| bee pollen | [132] | ||
| bee pollen suspension | [133] | ||
| aqueous suspension | [126] | ||
| Diabetic testicular-pituitary system dysfunction | Protection against diabetes-induced dysfunction |
| suspension | [133] |
| Hyperuricemia | Reduction of uric acid levels |
| n-butanol extract | [134] |
| ethyl acetate extract | [135] | ||
| Myocardial dysfunction and damage | Cardioprotective agent |
| ethanol extract | [136] |
| Fluorosis | Reduction of the fluoride toxic effects |
| bee pollen | [137] |
| Malnourishment | Source of essential amino acids, fatty acids, and micronutrients |
| bee pollen | [1] |
| Bone metabolism | Preventive effects on bone loss |
| water extract | [138] |
| Increase in calcium content in metaphyseal and diaphyseal tissues. Inhibition of osteoclastic bone resorption in rats. | [139] | |||
| Ovary health | Regulation of the ovarian functions |
| rape seed bee pollen | [140] |
| rape seed bee pollen | [141] | ||
| Prevention of polycystic ovary syndrome |
| suspension | [142] | |
| Immunostimulant |
|
| water extract | [143] |
| Anti-allergic agent | Prevention of allergy |
| phenolic extract | [144] |
| phenolic extract | [145] | ||
| Cognitive dysfunction | Improvement of cognitive impairment |
| ethanol extract | [146] |
| Skin conditions | Against abnormal melanogenesis, hyperpigmentation |
| phenolic extracts | [78,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pólit, C.; Gonzalez-Pastor, R.; Heredia-Moya, J.; Carrera-Pacheco, S.E.; Castillo-Solis, F.; Vallejo-Imbaquingo, R.; Barba-Ostria, C.; Guamán, L.P. Chemical Properties and Biological Activity of Bee Pollen. Molecules 2023, 28, 7768. https://doi.org/10.3390/molecules28237768
Rodríguez-Pólit C, Gonzalez-Pastor R, Heredia-Moya J, Carrera-Pacheco SE, Castillo-Solis F, Vallejo-Imbaquingo R, Barba-Ostria C, Guamán LP. Chemical Properties and Biological Activity of Bee Pollen. Molecules. 2023; 28(23):7768. https://doi.org/10.3390/molecules28237768
Chicago/Turabian StyleRodríguez-Pólit, Cristina, Rebeca Gonzalez-Pastor, Jorge Heredia-Moya, Saskya E. Carrera-Pacheco, Fabián Castillo-Solis, Roberto Vallejo-Imbaquingo, Carlos Barba-Ostria, and Linda P. Guamán. 2023. "Chemical Properties and Biological Activity of Bee Pollen" Molecules 28, no. 23: 7768. https://doi.org/10.3390/molecules28237768
APA StyleRodríguez-Pólit, C., Gonzalez-Pastor, R., Heredia-Moya, J., Carrera-Pacheco, S. E., Castillo-Solis, F., Vallejo-Imbaquingo, R., Barba-Ostria, C., & Guamán, L. P. (2023). Chemical Properties and Biological Activity of Bee Pollen. Molecules, 28(23), 7768. https://doi.org/10.3390/molecules28237768