Coatings on Lithium Battery Separators: A Strategy to Inhibit Lithium Dendrites Growth
Abstract
:1. Introduction
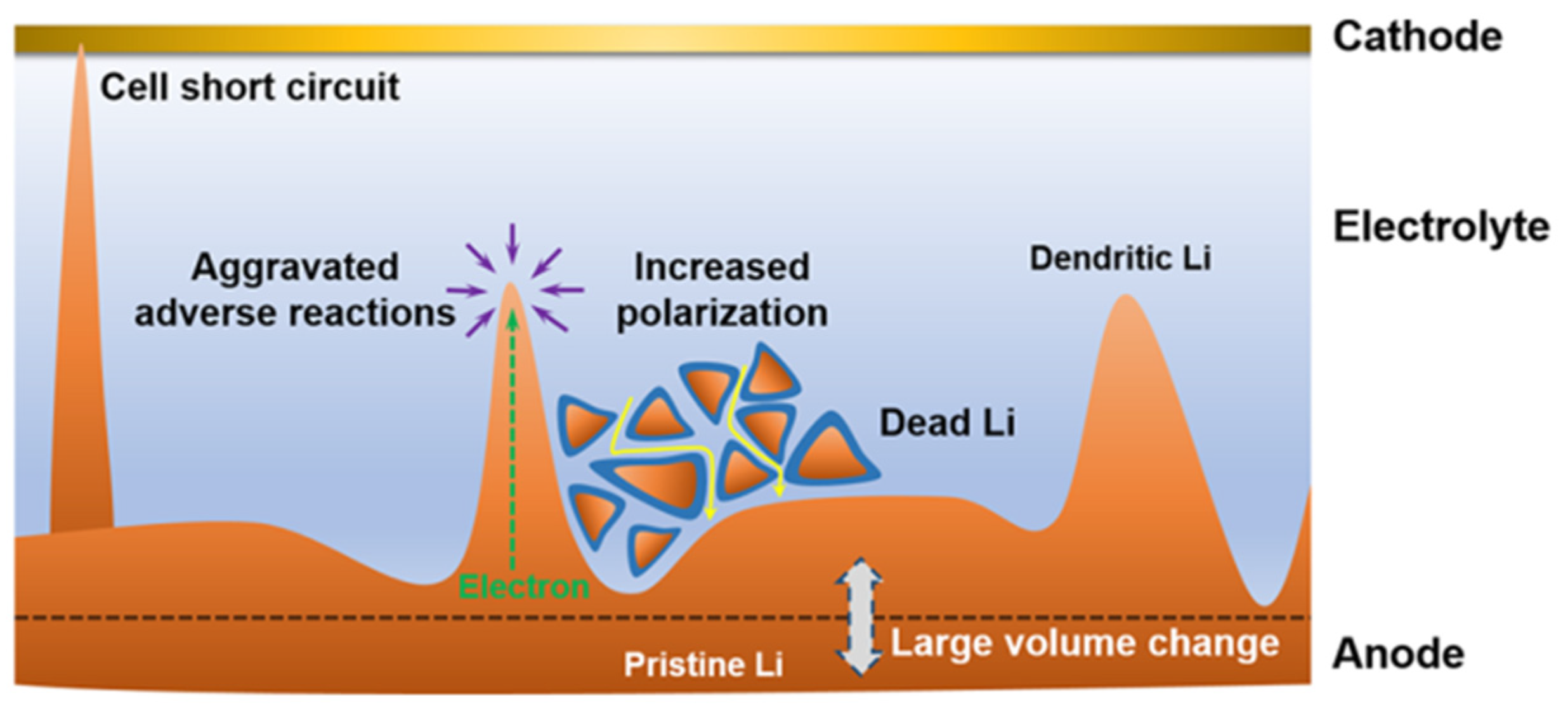
2. Formation of Lithium Dendrite and Inhibition Principle
3. Modification by Metal Coatings
4. Modification by Metal Oxides Coatings
5. Modification by Nitride Coatings
6. Modification by Other Coatings
7. Conclusions and Outlook
- (1)
- The different coating thicknesses will affect the energy density of the battery, as reported. This phenomenon needs further study, and the mechanism needs to be explored.
- (2)
- Currently, partially modified coatings can be used as nucleation sites or lithiophilic sites to homogenize lithium deposition. If these nucleation sites and lithiophilic sites are covered by lithium deposition, does that mean that the modified coating loses its effect? Is there any way to improve this? More attention should be paid to the effective time constancy of the modified layer.
- (3)
- At present, flexible wearable electronic devices are developing rapidly, and lithium metal batteries can be an ideal energy supply choice. So, flexible separator modifications and coatings need to be developed and investigated.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Xu, C.; Behrens, P.; Gasper, P.; Smith, K.; Hu, M.; Tukker, A.; Steubing, B. Electric vehicle batteries alone could satisfy short-term grid storage demand by as early as 2030. Nat. Commun. 2023, 14, 119. [Google Scholar] [CrossRef]
- Lyzwinski, L.; Elgendi, M.; Shokurov, A.V.; Cuthbert, T.J.; Ahmadizadeh, C.; Menon, C. Opportunities and challenges for sweat-based monitoring of metabolic syndrome via wearable technologies. Commun. Eng. 2023, 2, 48. [Google Scholar] [CrossRef]
- Khunte, A.; Sangha, V.; Oikonomou, E.K.; Dhingra, L.S.; Aminorroaya, A.; Mortazavi, B.J.; Coppi, A.; Brandt, C.A.; Krumholz, H.M.; Khera, R. Detection of left ventricular systolic dysfunction from single-lead electrocardiography adapted for portable and wearable devices. NPJ Digit. Med. 2023, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Cham, Switzerland, 2016; pp. 1–619. [Google Scholar]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 ≥ x ≥ −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, P.J.; Depicciotto, L.A.; Bruce, P.G.; Goodenough, J.B. Electrochemical extraction of lithium from LiMn2O4. Mater. Res. Bull. 1984, 19, 179–187. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Ravet, N.; Gauthier, M.; Zaghib, K.; Mauger, A.; Goodenough, J.; Gendron, F.; Julien, C.M. Mechanism of the Fe2+ reduction at low temperature, for LiFePO4 synthesis from a polymer additive. Chem. Mater. 1970, 19, 2595–2602. [Google Scholar] [CrossRef]
- Song, L.; Du, J.; Xiao, Z.; Jiang, P.; Cao, Z.; Zhu, H. Research progress on the surface of high-nickel nickel–cobalt–manganese ternary cathode materials: A Mini Review. Front. Chem. 2020, 8, 761. [Google Scholar] [CrossRef]
- Han, X.; Gong, Y.; Fu, K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G.; et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2016, 16, 572–579. [Google Scholar] [CrossRef]
- Lee, J.K.; Oh, C.; Kim, N.; Hwang, J.Y.; Sun, Y.K. Rational design of silicon-based composites for high-energy storage devices. J. Mater. Chem. A 2016, 4, 5366–5384. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Pan, D. Solutions for the problems of silicon–carbon anode materials for lithium-ion batteries. R. Soc. Open Sci. 2018, 5, 172370. [Google Scholar] [CrossRef]
- Liu, S.; Ren, Z.; Fakudze, S.; Shang, Q.; Chen, J.; Liu, C.; Han, J.; Tian, Z. Structural evolution of graphitic carbon derived from ionic liquids-dissolved cellulose and its application as lithium-ion battery anodes. Langmuir 2022, 38, 320–331. [Google Scholar] [CrossRef]
- Xin, F.; Whittingham, M.S. Challenges and development of tin-based anode with high volumetric capacity for Li-ion batteries. Electrochem. Energy Rev. 2020, 3, 643–655. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Cao, G. Promises and challenges of tin-based compounds as anode materials for lithium-ion batteries. Int. Mater. Rev. 2015, 60, 330–352. [Google Scholar] [CrossRef]
- Chen, J.; Adit, G.; Li, L.; Zhang, Y.; Chua, D.H.C.; Lee, P.S. Optimization strategies toward functional sodium-ion batteries. Energy Environ. Mater. 2023, 6, e12633. [Google Scholar] [CrossRef]
- Wang, J.; Yin, H.; Wang, Z.; Gao, J.; Jiang, Q.; Xu, Y.; Chen, Z. High-performance Sn-based anode with robust lignin-derived hard carbon support for sodium-ion batteries. Asia-Pac. J. Chem. Eng. 2022, 17, e2768. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Yan, B.; Zhao, W.; Zhang, Q.; Kong, Q.; Chen, G.; Zhang, C.; Han, J.; Jiang, S.; He, S. One stone for four birds: A “chemical blowing” strategy to synthesis wood-derived carbon monoliths for high-mass loading capacitive energy storage in low temperature. J. Colloid. Interface Sci. 2023, 653, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, L.; Liu, Z.; Jiang, L.; Lan, T.; Zhang, C.; Liu, K.; He, S. High rate performance supercapacitors based on N, O Co-doped hierarchical porous carbon foams synthesized via chemical blowing and dual templates. Molecules 2023, 28, 6994. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium–sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Peng, H.J.; Huang, J.Q.; Cheng, X.B.; Zhang, Q. Review on high-loading and high-energy lithium–sulfur batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Lu, Y.C.; Gallant, B.M.; Kwabi, D.G.; Harding, J.R.; Mitchell, R.R.; Whittingham, M.S.; Shao-Horn, Y. Lithium–oxygen batteries: Bridging mechanistic understanding and battery performance. Energy Environ. Sci. 2013, 6, 750–768. [Google Scholar] [CrossRef]
- Shen, X.; Liu, H.; Cheng, X.B.; Yan, C.; Huang, J.Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Stor. Mater. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Eftekhari, A. The rise of lithium–selenium batteries. Sustain. Energy Fuels 2017, 1, 14–29. [Google Scholar] [CrossRef]
- Deng, W.N.; Li, Y.H.; Xu, D.F.; Zhou, W.; Xiang, K.X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- He, D.; Lu, J.; He, G.; Chen, H. Recent advances in solid-electrolyte interphase for Li metal anode. Front. Chem. 2022, 10, 916132. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From materials to cell: State-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem. Rev. 2021, 122, 903–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Yin, Y.X.; Zuo, T.T.; Dong, W.; Li, J.Y.; Shi, J.L.; Zhang, C.H.; Li, N.W.; Li, C.J.; Guo, Y.G. Stable Li metal anodes via regulating lithium plating/stripping in vertically aligned microchannels. Adv. Mater. 2017, 29, 1703729. [Google Scholar] [CrossRef]
- Nan, Y.; Li, S.; Li, B.; Yang, S. An artificial TiO2/lithium n-butoxide hybrid SEI layer with facilitated lithium-ion transportation ability for stable lithium anodes. Nanoscale 2019, 11, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhao, N.; Zhang, Z.; Wan, H.; Lin, H.; Liu, M.; Shen, C.; He, H.; Guo, X.; Zhang, J.G.; et al. Stabilizing Li/electrolyte interface with a transplantable protective layer based on nanoscale LiF domains. Nano Energy 2017, 39, 662–672. [Google Scholar] [CrossRef]
- Tan, J.; Matz, J.; Dong, P.; Shen, J.; Ye, M. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 2021, 11, 2100046. [Google Scholar] [CrossRef]
- Chen, R.; Qu, W.; Guo, X.; Li, L.; Wu, F. The pursuit of solid-state electrolytes for lithium batteries: From comprehensive insight to emerging horizons. Mater. Horiz. 2016, 3, 487–516. [Google Scholar] [CrossRef]
- Rosero Navarro, N.C.; Kajiura, R.; Miura, A.; Tadanaga, K. Organic-inorganic hybrid materials for interface design in all-solid-state batteries with a garnet-type solid electrolyte. ACS Appl. Energy Mater. 2020, 3, 11260–11268. [Google Scholar] [CrossRef]
- Liu, B.; Gong, Y.; Fu, K.; Han, X.; Yao, Y.; Pastel, G.; Yang, C.; Xie, H.; Wachsman, E.D.; Hu, L. Garnet solid electrolyte protected Li-metal batteries. ACS Appl. Mater. Interfaces 2017, 9, 18809–18815. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wu, H.B.; Liu, F.; Shen, J.; Mo, R.; Chen, G.; Tan, G.; Chen, J.; Kong, X.; Lu, X.; et al. Particulate anion sorbents as electrolyte additives for lithium batteries. Adv. Funct. Mater. 2020, 30, 2003055. [Google Scholar] [CrossRef]
- Ho, V.C.; Ngo, D.T.; Le, H.T.T.; Verma, R.; Kim, H.-S.; Park, C.N.; Park, C.J. Effect of an organic additive in the electrolyte on suppressing the growth of Li dendrites in Li metal-based batteries. Electrochim. Acta 2018, 279, 213–223. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, L.P.; Li, Z.; Liang, J.L.; Zhou, M.Y.; Zhao, C.Z.; Zeng, X.; Li, B.Q.; Chen, A.; Zhang, X.Q.; et al. Highly soluble organic nitrate additives for practical lithium metal batteries. Carbon Energy 2022, 5, e283. [Google Scholar] [CrossRef]
- Ouyang, Y.; Cui, C.; Guo, Y.; Wei, Y.; Zhai, T.; Li, H. In situ formed LiZn alloy skeleton for stable lithium anodes. ACS Appl. Mater. Interfaces 2020, 12, 25818–25825. [Google Scholar] [CrossRef]
- Yue, X.Y.; Wang, W.W.; Wang, Q.C.; Meng, J.K.; Zhang, Z.Q.; Wu, X.J.; Yang, X.Q.; Zhou, Y.N. CoO nanofiber decorated nickel foams as lithium dendrite suppressing host skeletons for high energy lithium metal batteries. Energy Stor. Mater. 2018, 14, 335–344. [Google Scholar] [CrossRef]
- Qing, P.; Wu, Z.; Wang, A.; Huang, S.; Long, K.; Naren, T.; Chen, D.; He, P.; Huang, H.; Chen, Y.; et al. Highly reversible lithium metal anode enabled by 3D lithiophilic-lithiophobic dual-skeletons. Adv. Mater. 2023, 35, 2211203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.W.; Chen, J.L.; Tian, T.; Shen, B.; Peng, Y.D.; Song, Y.H.; Jiang, B.; Lu, L.L.; Yao, H.B.; Yu, S.H. Sustainable separators for high-performance lithium ion batteries enabled by chemical modifications. Adv. Funct. Mater. 2019, 29, 1902023. [Google Scholar] [CrossRef]
- Lin, C.E.; Zhang, H.; Song, Y.Z.; Zhang, Y.; Yuan, J.J.; Zhu, B.K. Carboxylated polyimide separator with excellent lithium ion transport properties for a high-power density lithium-ion battery. J. Mater. Chem. A 2018, 6, 991–998. [Google Scholar] [CrossRef]
- Yao, S.; Yang, Y.; Liang, Z.; Chen, J.; Ding, J.; Li, F.; Liu, J.; Xi, L.; Zhu, M.; Liu, J. A dual−functional cationic covalent organic frameworks modified separator for high energy lithium metal batteries. Adv. Funct. Mater. 2023, 33, 2212466. [Google Scholar] [CrossRef]
- Jang, E.K.; Ahn, J.; Yoon, S.; Cho, K.Y. High Dielectric, Robust composite protective layer for dendrite-free and LiPF6 degradation-free lithium metal anode. Adv. Funct. Mater. 2019, 29, 1905078. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, X.Q.; Cheng, X.B.; Peng, H.J.; Zhao, C.Z.; Yan, C.; Huang, J.Q. Artificial soft-rigid protective layer for dendrite-free lithium metal anode. Adv. Funct. Mater. 2018, 28, 1705838. [Google Scholar] [CrossRef]
- Hou, G.; Ci, C.; Guo, H.; Zhang, X.; Sun, Q.; Cheng, J.; Salpekar, D.; Ai, Q.; Chen, L.; Puthirath, A.B.; et al. Facile construction of a hybrid artificial protective layer for stable lithium metal anode. Chem. Eng. J. 2020, 391, 123542. [Google Scholar] [CrossRef]
- Kaskhedikar, N.A.; Maier, J. Lithium storage in carbon nanostructures. Adv. Mater. 2009, 21, 2664–2680. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Yann Liaw, B.; Metzler, V.; Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wang, H.; Hu, C.; Jin, Y.; Liu, J.; Yan, H. Recent progresses in the suppression method based on the growth mechanism of lithium dendrite. J. Energy Chem. 2018, 27, 513–527. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ren, X.; Niu, C.; Yu, L.; Engelhard, M.H.; Cho, I.; Ryou, M.H.; Jin, H.S.; Kim, H.T.; Liu, J.; et al. Suppressing lithium dendrite growth by metallic coating on a separator. Adv. Funct. Mater. 2017, 27, 1704391. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, F.; Chen, N.; Ma, Y.; Yang, C.; Shang, Y.; Liu, H.; Li, L.; Chen, R. Reversing the dendrite growth direction and eliminating the concentration polarization via an internal electric field for stable lithium metal anodes. Chem. Sci. 2022, 13, 9277–9284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiong, S.; Wang, J.; Jiao, X.; Li, S.; Zhang, C.; Song, Z.; Song, J. Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Stor. Mater. 2019, 19, 24–30. [Google Scholar] [CrossRef]
- Lin, L.; Liu, F.; Yan, X.; Chen, Q.; Zhuang, Y.; Zheng, H.; Lin, J.; Wang, L.; Han, L.; Wei, Q.; et al. Dendrite-free reverse lithium deposition induced by ion rectification layer toward superior lithium metal batteries. Adv. Funct. Mater. 2021, 31, 2104081. [Google Scholar] [CrossRef]
- Yue, C.; Sun, S.; Jang, M.; Park, E.; Son, B.; Son, H.; Liu, Z.; Wang, D.H.; Paik, U.; Song, T. A robust solid electrolyte interphase layer coated on polyethylene separator surface induced by Ge interlayer for stable Li-metal batteries. Electrochim. Acta 2021, 370, 137703. [Google Scholar] [CrossRef]
- Din, M.M.U.; Murugan, R. Metal coated polypropylene separator with enhanced surface wettability for high capacity lithium metal batteries. Sci. Rep. 2019, 9, 16795. [Google Scholar] [CrossRef]
- Huang, Z.J.; Han, Z.Y.; Jiang, B.Z.; Zhang, Y.B.; Gu, S.C.; Zhang, C.; Pan, Z.Z.; Nishihara, H.; Yang, Q.H.; Lv, W. Regulating Li-ion flux through a dense yet highly ionic conductive interlayer for stable Li deposition. Adv. Mater. Interfaces 2022, 9, 2200457. [Google Scholar] [CrossRef]
- Yan, J.; Liu, F.Q.; Gao, J.; Zhou, W.; Huo, H.; Zhou, J.J.; Li, L. Low-cost regulating lithium deposition behaviors by transition metal oxide coating on separator. Adv. Funct. Mater. 2021, 31, 2007255. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Fu, S.; Luo, R.; Qu, W.; Hu, X.; Chen, R.; Wu, F.; Li, L. In situ formation of a Li-Sn alloy protected layer for inducing lateral growth of dendrites. J. Mater. Chem. A 2020, 8, 23574–23579. [Google Scholar] [CrossRef]
- Chen, L.; Lin, X.; Dang, W.; Huang, H.; Liu, G.; Yang, Z. Tantalum oxide nanosheets/polypropylene composite separator constructing lithium-ion channels for stable lithium metal batteries. Adv. Compos. Hybrid. Mater. 2022, 6, 12. [Google Scholar] [CrossRef]
- An, Q.; Liu, Q.; Wang, S.; Liu, L.; Wang, H.; Sun, Y.; Duan, L.; Zhao, G.; Guo, H. Oxygen vacancies with localized electrons direct a functionalized separator toward dendrite-free and high loading LiFePO4 for lithium metal batteries. J. Energy Chem. 2022, 75, 38–45. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, Q.; Liu, L.; Li, G.; Yang, L.; Wang, X.; Ma, Y.; Hu, Z. Thermally conductive AlN-network shield for separators to achieve dendrite-free plating and fast Li-ion transport toward durable and high-rate lithium-metal anodes. Adv. Sci. 2022, 9, 2200411. [Google Scholar] [CrossRef]
- Yan, M.; Wang, C.Y.; Fan, M.; Zhang, Y.; Xin, S.; Yue, J.; Zeng, X.X.; Liang, J.Y.; Song, Y.X.; Yin, Y.X.; et al. In situ derived mixed ion/electron conducting layer on top of a functional separator for high-performance, dendrite-free rechargeable lithium-metal batteries. Adv. Funct. Mater. 2023, 2301638. [Google Scholar] [CrossRef]
- Ma, Y.; Qu, W.; Hu, X.; Qian, J.; Li, Y.; Li, L.; Lu, H.; Du, H.; Wu, F.; Chen, R. Induction/inhibition effect on lithium dendrite growth by a binary modification layer on a separator. ACS Appl. Mater. Interfaces 2022, 14, 44338–44344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, F.; Srinivas, K.; Yu, B.; Chen, X.; Wang, B.; Wang, X.; Liu, D.; Zhang, Z.; He, J.; et al. Fe3N@N-doped graphene as a lithiophilic interlayer for highly stable lithium metal batteries. Energy Stor. Mater. 2022, 45, 656–666. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, X.; Li, S.; Zheng, Q.; Jiang, S.; Xu, Y.; He, B.; Ma, L.; Luo, Y.; Wang, Y.; et al. Boosting thermal and mechanical properties: Achieving high-safety separator chemically bonded with nano TiN particles for high performance lithium-ion batteries. Small 2023, 19, 202300378. [Google Scholar] [CrossRef]
- Liao, C.; Wang, W.; Wang, J.; Han, L.; Qiu, S.; Song, L.; Gui, Z.; Kan, Y.; Hu, Y. Magnetron sputtering deposition of silicon nitride on polyimide separator for high-temperature lithium-ion batteries. J. Energy Chem. 2021, 56, 1–10. [Google Scholar] [CrossRef]
- Liu, K.; Zhuo, D.; Lee, H.-W.; Liu, W.; Lin, D.; Lu, Y.; Cui, Y. Extending the life of lithium-based rechargeable batteries by reaction of lithium dendrites with a novel silica nanoparticle sandwiched separator. Adv. Mater. 2017, 29, 1603987. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Zhao, R.; Qi, X.; Li, K.; Sun, Q.; Ma, M.; Qie, L.; Huang, Y. A “dendrite-eating” separator for high-areal-capacity lithium-metal batteries. Energy Stor. Mater. 2020, 31, 181–186. [Google Scholar] [CrossRef]
- Wang, L.; Fu, S.; Zhao, T.; Qian, J.; Chen, N.; Li, L.; Wu, F.; Chen, R. In situ formation of a LiF and Li-Al alloy anode protected layer on a Li metal anode with enhanced cycle life. J. Mater. Chem. A 2020, 8, 1247–1253. [Google Scholar] [CrossRef]
- Tan, L.; Wei, C.; Zhang, Y.; An, Y.; Xiong, S.; Feng, J. LiF-rich and self-repairing interface induced by MgF2 engineered separator enables dendrite-free lithium metal batteries. Chem. Eng. J. 2022, 442, 136243. [Google Scholar] [CrossRef]
- Han, D.; Wang, X.; Zhou, Y.N.; Zhang, J.; Liu, Z.; Xiao, Z.; Zhou, J.; Wang, Z.; Zheng, J.; Jia, Z.; et al. A graphene-coated thermal conductive separator to eliminate the dendrite-induced local hotspots for stable lithium cycling. Adv. Energy Mater. 2022, 12, 2201190. [Google Scholar] [CrossRef]

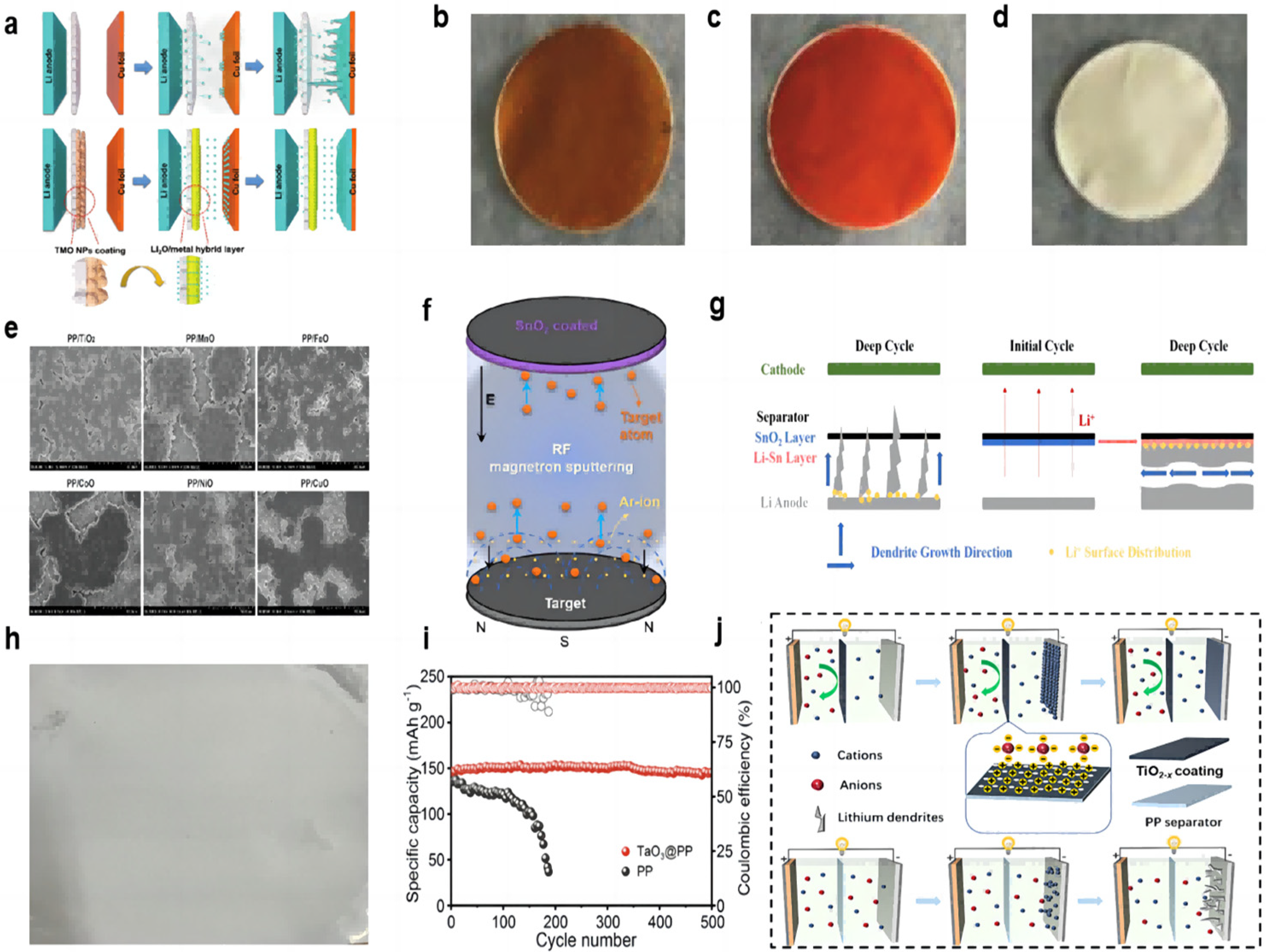


| Material | Method | Separator | Electrolyte | Cathode | Loading Amount | Performance |
|---|---|---|---|---|---|---|
| Cu [54] | DC magnetron sputtering | PE separator (ND420) | 1 M LiPF6 in EC/ DEC (1:1 v/v) with 1 wt% VC and 10 wt% FEC additives | LCO | 0.5 mAh cm−2 | 1 C 280 cycles 95% |
| Au [55] | DC magnetron sputtering | Celgard 2325 | Ether electrolyte: 1 M LiTFSI in DOL/DME (1:1 v/v) Ester electrolyte: 1.0 M LiPF6 in EC/DEC/EMC (1:1:1 v/v/v) | LFP NCM | 2.4 mg cm−2 2.8 mg cm−2 | 1 C 350 cycles 97.8% 1 C 300 cycles 75.1% |
| Mg [56] | DC magnetron sputtering | Celgard 2325 | 1 M LiPF6 in EC/DEC (1:1 v/v) | LCO | 8.0 mg cm−2 | 1 C 400 cycles 80% 2 C 500 cycles 70.6% |
| Zn [57] | DC magnetron sputtering | Celgard 2500 | 1 M LiTFSI in DOL/DME (1:1 v/v) with 2 wt% of LiNO3 additive | LFP | 4.0 mg cm−2 11 mg cm−2 19.2 mg cm−2 | 5 C 300 cycles 121 mAhg−1 1 C 200 cycles 135 mAh g−1 0.33 C 120 cycles 144 mAh g−1 |
| Ge [58] | Thermal evaporation | PE separator | 1.3 M LiPF6 in EC/DEC (1:1 v/v) with 5 wt% FEC additive | LCO | 3 mg cm−2 | 100 mA g−1 400 cycles 92% |
| Nb [59] | RF magnetron sputtering | Celgard 2325 | 1 M LiTFSI in DME/DOL (1:1 v/v) | LNMC | 2.5 mg cm−2 | 0.2 C 120 cycles 130 mAh g−1 |
| Material | Method | Separator | Electrolyte | Cathode | Loading Amount | Performance |
|---|---|---|---|---|---|---|
| Fe2O3/Fe3O4 [60] | vacuum filtration | PP separator | 1 M LiTFSI in DOL/DME (1:1 v/v) with 1 wt% LiNO3 additive | LFP | 1 mg cm–2 | 0.5 C 250 cycles 94.7% (Fe3O4) 0.5 C 250 cycles 98.3% (Fe2O3) |
| MnO [61] | coating | PP separator | 1 M LiTFSI in DOL/DME (1:1 v/v) with 1 wt% LiNO3 additive | LFP | / | 1 C 600 cycles (with LiNO3 additive) |
| SnO2 [62] | RF magnetron sputtering | commercial Celgard separator | 1 M LiTFSI and 0.2 M LiNO3 in DOL/DME (1:1 v/v) (Li||Li cell) 1 M LiPF6 in EC/DMC/DEC (1:1:1 v/v/v) (full cell) | LFP | / | 1 C 300 cycles 126.8 mAh g−1 |
| TaO3 [63] | vacuum filtration | commercial PP separator | 1 M LiTFSI in DOL /DME (1:1 v/v) with 1 wt% LiNO3 additive | LFP | 12 mg cm−2 | 0.5 C 500 cycles 145 mAh g−1 |
| TiO2−x [64] | coating | PP separator | 1 M LiTFSI in DOL/DME (1:1 v/v) with 5 wt% LiNO3 additive | LFP | 2.4 mg cm−2 (1 C/4 C) 9.24 mg cm−2 (0.5 C) | 1 C 400 cycles 97.4% 4 C 350 cycles 93.9% 0.5 C 900 cycles 113.8 mAh g−1 |
| Material | Method | Separator | Electrolyte | Cathode | Loading Amount | Performance |
|---|---|---|---|---|---|---|
| AlN [65] | vacuum filtration | Celgard 2400 | 1 M LiTFSI in DOL/DME (1:1 v/v) with 2 wt% LiNO3 additive 1 M LiPF6 in EC/DMC (1:1 v/v) | LFP | 2.0 mg cm−2 | 1 C 400 cycles 94.8% |
| Mg3N2 [66] | coating | Celgard separator | 1 M LiPF6 in EC/DMC/DEC (1:1:1 v/v/v) | NCM622 | 3.0 mg cm−2 | 0.5 C 600 cycles 75.9% |
| InN [67] | DC magnetron sputtering | Celgard separator | 1 M LiTFSI in DOL/DME (1:1 v/v) with 0.2 M LiNO3 additive 1 M LiPF6 in EC/DEC/DMC (1:1:1 v/v/v) with 5% FEC additive | LFP | / | 1 C 300 cycles 92.1% |
| Fe3N@NG [68] | electro- static adsorption ammonization process | Celgard 2500 | 1 M LiTFSI in DOL/DME(1:1 v/v) with 1.0 wt% LiNO3 additive (Li||Cu,Li||Li) 1 M LiPF6 in EC/DEC (1:1 v/v) (full cell) | LFP | / | 2 C decay rate of 0.08% |
| Material | Method | Separator | Electrolyte | Cathode | Loading Amount | Performance |
|---|---|---|---|---|---|---|
| SiO2 [71] | sol–gel method | PE separator | 1 M LiPF6 in EC/DEC (1:1 v/v) | / | / | / |
| Si [72] | coating | Celgard 2325 | 1 M LiPF6 in EC and DEC (1:1 wt/wt) with 10 wt% FEC and 1 wt% VC additive | LFP | 20.0 mg cm−2 | 0.2/0.5 mA cm−2 100 cycles 2.30 mAh cm−2 |
| AlF3 [73] | phase inversion method | Celgard separator | 1.0 M LiTFSI in DOL/DME (1:1 v/v) with 0.2 M LiNO3 additive 1.0 M LiPF6 in EC/DC/DEC (1:1:1 v/v/v) (full cell) | LFP | / | 3 C 300 cycles 78.3% |
| MgF2 [74] | coating | PE separator | 1 M LiPF6 in EC/DEC/DMC (1:1:1 v/v/v) | NCM811 | 0.82 mg cm−2 | 2 C 400 cycles 84.5% |
| Graphene [75] | vacuum filtration | Celgard 2500 separator | 1 M LiTFSI in DOL/DME (1:1 v/v) with 2% LiNO3 additive (Li||Cu/Li cell) 1 M LiPF6 in EC/DEC/DMC (1:1:1 v/v/v) | NCM811 | 30.06 mg cm−2 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Tan, R.; Li, J.; Huang, J.; Song, W. Coatings on Lithium Battery Separators: A Strategy to Inhibit Lithium Dendrites Growth. Molecules 2023, 28, 7788. https://doi.org/10.3390/molecules28237788
Cheng H, Tan R, Li J, Huang J, Song W. Coatings on Lithium Battery Separators: A Strategy to Inhibit Lithium Dendrites Growth. Molecules. 2023; 28(23):7788. https://doi.org/10.3390/molecules28237788
Chicago/Turabian StyleCheng, Huchao, Ruiqin Tan, Jia Li, Jinhua Huang, and Weijie Song. 2023. "Coatings on Lithium Battery Separators: A Strategy to Inhibit Lithium Dendrites Growth" Molecules 28, no. 23: 7788. https://doi.org/10.3390/molecules28237788
APA StyleCheng, H., Tan, R., Li, J., Huang, J., & Song, W. (2023). Coatings on Lithium Battery Separators: A Strategy to Inhibit Lithium Dendrites Growth. Molecules, 28(23), 7788. https://doi.org/10.3390/molecules28237788






