Applications of Prolamin-Based Edible Coatings in Food Preservation: A Review
Abstract
:1. Introduction
2. Preparation of Prolamin-Based Coatings
2.1. Selection of Prolamins
2.2. Extraction of Prolamins
2.2.1. Extraction
2.2.2. Separation and Purification
2.3. Preparation of Coating Solutions
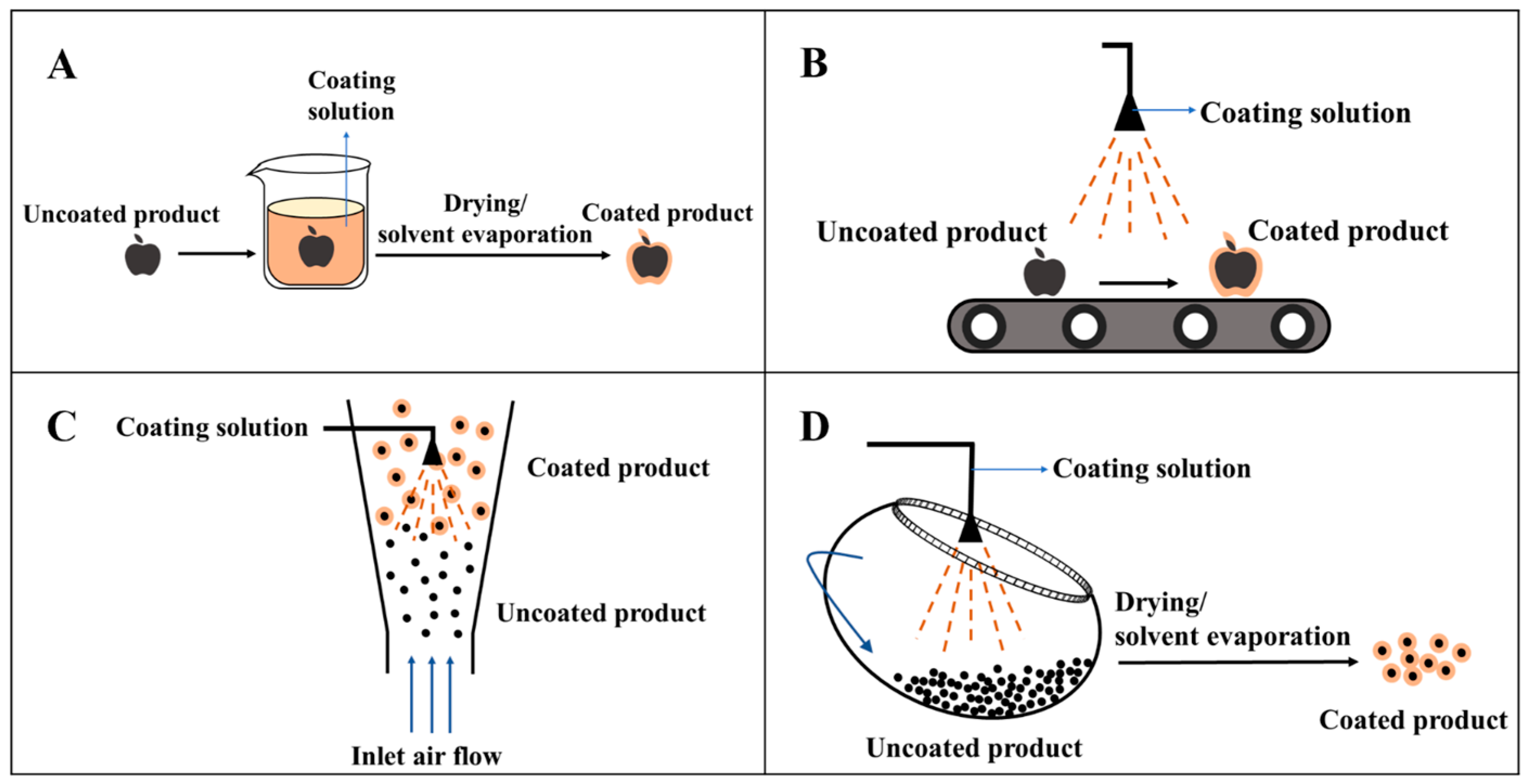
2.4. Coating Processes
2.4.1. Dipping
2.4.2. Spraying
2.4.3. Fluidized-Bed
2.4.4. Panning
3. Modification of Prolamin-Based Coatings
3.1. Mechanical Performance Improvement
3.2. Barrier Performance Improvement
3.3. Water Resistance Improvement
3.4. Other Performance Improvement
| Prolamin | Additive | Apply to | Application | Additional Effect | Ref. |
|---|---|---|---|---|---|
| Zein | Eugenol + Carvacrol + Thymol | Inhibits L. innocua and E. coli | Whole melons | — | [104] |
| Nisin + ethylenediaminetetraacetic acid | Inhibits E. coli, E. aerogenes and C. freundii | Commercial fish balls | — | [105] | |
| gallic acid | Inhibits C. jejuni | — | — | [106] | |
| ethyl-Nα-dodecanoyl-L-arginate hydrochloride (LAE) | Inhibits L. monocytogenes and E. coli | — | LAE addition (Addition amount: 5%, 10%) does not cause substantial changes in morphological, optical, thermal, mechanical and barrier properties. | [107] | |
| Cinnamon essential oil + Chitosan nanoparticles | Inhibits E. coli and S. aureus | — | The tensile strength of the film increases, and the elongation at break decreases. | [108] | |
| Kafirin | Citral | Inhibits bacterial growth | Fresh chicken fillets | The maximum stress and stiffness of the film decrease, while the fracture strain and yield stress increase | [109] |
| Quercetin | Delays lipid oxidation | Fresh chicken fillets | — | ||
| Gliadin | Cinnamaldehyde | Inhibits E. coli and S. aureus | — | The chemical cross-linking of cinnamaldehyde and protein is carried out under alkaline conditions, and the mechanical properties and water stability of the film improve after cross-linking | [110] |
| Cinnamaldehyde | Inhibits P. expansum and A. niger | Bread and cheese spread | — | [111] | |
| Zataria multiflora Boiss essential oil | Inhibits B. subtilis and L. monocytogenes, and has antioxidant properties | Smoked salmon fish fillet | — | [112] |
4. Conclusions and Further Remarks
- (1)
- Except for commercial zein, other prolamins still face low extracting yield, long extracting time, and high cost, which limits their development in actual production and application. The extracting processes using industrial by-products as the raw materials should be optimized and strengthened to improve the comprehensive utilization efficiency of grains. At the same time, attention should be paid to the recovery and reuse of extracting solvents, so as to reduce the pollution and production costs;
- (2)
- Most studies on the extracting processes of prolamins mainly take the extracting yield as the evaluation index. In fact, different extracting methods, extracting solvents, and extracting conditions largely impact the structures and composition of the final prolamin product, ultimately affecting the performance and application effect of the resulting films. Various means should be used to characterize the properties of obtained prolamins, such as the secondary, tertiary, and quaternary structures of prolamins, AA composition, and disulfide bond content, etc., in order to clarify the relationship between the extracting processes and the properties of the resulting films;
- (3)
- The studies on prolamin-based films mainly focus on improving their performance by optimization of the film-forming processes or modifications. However, when prolamin-based films are actually used for food coating, they are very likely to encounter problems such as irregular food shape, poor adhesion between the food surface and the coating solution, and unsatisfactory application environment. Thus, the physical properties of the coating solution (such as rheology, viscosity, density, surface tension, etc.) should be studied and adjusted from the perspective of application, so as to find out the composition and properties of the coating solution suitable for the coating processes;
- (4)
- Although prolamins have good water insolubility, prolamin-based films swell under the environment of high-water activity, which makes the barrier performance of the film plummet. It is difficult to provide continuous barrier protection for coated foods. At present, the more effective modification method is to greatly increase the cross-linking degree of prolamin-based films through chemical cross-linking or cross-linking such as irradiation. However, these modification methods are difficult to implement in food coating applications. Moreover, prolamin-based films modified by such methods usually run counter to their original advantages of being edible and easy to degrade. Therefore, seeking non-toxic, green, safe, economical, and easy-to-operate methods should be the research focus to improve the stability of coatings;
- (5)
- Although prolamin-based films do not have a waxy texture like lipid-based films, some prolamins themselves carry a special odor, which may be unpleasant to some users and consumers, and which can be masked or eliminated by some processing means in the subsequent research. And for food coating, it is worth noting how to balance the mechanical properties of the coatings and the resistance of the coatings during chewing.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Shao, L.; Chen, S.; Wang, H.; Zhang, J.; Xu, X.; Wang, H. Advances in understanding the predominance, phenotypes, and mechanisms of bacteria related to meat spoilage. Trends Food Sci. Technol. 2021, 118, 822–832. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Carrión-Granda, X.; Maté, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz Garcia, L.; Qian, J.P.; Yang, X.T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Chen, J.Q.; Liu, X.X.; Wang, X. Research progress of application of polysaccharides, proteins and their composite coatings in preservation of postharvest berries. Storage Process 2022, 22, 97–103. (In Chinese) [Google Scholar]
- Ma, C.F. The Effect of Modified Atmosphere Packaging and Coating Process on the Quality of Frozen Tilapia Fillets. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2017. [Google Scholar]
- Tran, P.H.; Duan, W.; Lee, B.; Tran, T.T. The use of zein in the controlled release of poorly water-soluble drugs. Int. J. Pharm. 2019, 566, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Z.; Lu, R.X.; Zhu, H.Y.; Miao, M. Fresh-keeping effect of pullulan polysaccharide coating treatment on cherry tomato. Food Ferment. Ind. 2023, 49, 86–91. [Google Scholar]
- Zhang, Q.; Yin, L.J.; Chen, F.S. Research progress on polysaccharide-based edible film. Cereals Oils 2020, 33, 1–3. (In Chinese) [Google Scholar]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef]
- Cheng, K.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Cunha, A.P.; Brito, E.S.; Azeredo, H.M.; Gallao, M.I. Mesquite seed gum and palm fruit oil emulsion edible films: Influence of oil content and sonication. Food Hydrocoll. 2016, 56, 227–235. [Google Scholar] [CrossRef]
- Wang, R.L.; Bian, K.; Xu, S.Y. Study on preventing fat deterioration in nuts coated with edible film from vegetable proteins. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2002, 23, 19–22. (In Chinese) [Google Scholar]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R. The major seed storage proteins of spelt wheat, sorghum, millets and pseudocereals. In Pseudocereals and Less Common Cereals; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–24. [Google Scholar]
- Chen, W.Y.; Mu, Y.; Zhu, A.F.; Zhang, B.H. Evaluation of the preparation and preservation effects of zein film. Modern Food Sci. Technol. 2022, 38, 141–147. (In Chinese) [Google Scholar]
- Hager, J.V.; Rawles, S.D.; Xiong, Y.L.; Newman, M.C.; Webster, C.D. Edible corn-zein-based coating incorporated with nisin or lemongrass essential oil inhibits listeria monocytogenes on cultured hybrid striped bass, morone chrysops× morone saxatilis, fillets during refrigerated and frozen storage. J. World Aquacult. Soc. 2019, 50, 204–218. [Google Scholar] [CrossRef]
- Zhang, C.H.; Chang, N.; Gao, H.N. Preparation of edible composite film with wheat gluten and zein and its application in hazelnut kernels preservation. China Oils Fats 2010, 35, 21–24. [Google Scholar]
- Lu, L.L. Preparation and Functional Study of Novel Zein Drug Carriers. Master’s Thesis, South China University of Technology, Guangzhou, China, 2020. [Google Scholar]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. Starch-Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Zang, M.; Shan, C.Y.; Ma, Y.F.; Nei, X.; Ma, S.H. Research progress of cereal gliadin. Chin. Wild Plant Resour. 2017, 36, 46–49. (In Chinese) [Google Scholar]
- Derose, R.T.; Ma, D.; Kwon, I.; Hasnain, S.E.; Klassy, R.C.; Hall, T.C. Characterization of the kafirin gene family from sorghum reveals extensive homology with zein from maize. Plant Mol. Biol. 1989, 12, 245–256. [Google Scholar] [CrossRef]
- Cao, W.; Li, F.; Huang, Q.R. The extraction of hordein and properties of hordein. Grain Process. 2015, 40, 40–43. [Google Scholar]
- Xiao, J.; Li, Y.; Li, J.; Gonzalez, A.P.; Xia, Q.; Huang, Q. Structure, morphology, and assembly behavior of kafirin. J. Agric. Food. Chem. 2015, 63, 216–224. [Google Scholar] [CrossRef]
- Taylor, J.; Taylor, J.; Dutton, M.F.; De Kock, S. Identification of kafirin film casting solvents. Food Chem. 2005, 90, 401–408. [Google Scholar] [CrossRef]
- Qazanfarzadeh, Z.; Kadivar, M.; Shekarchizadeh, H.; Porta, R. Rye secalin characterisation and use to improve zein-based film performance. Int. J. Food Sci. Technol. 2021, 56, 742–752. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y. Wheat gluten–based coatings and films: Preparation, properties, and applications. J. Food Sci. 2023, 88, 582–594. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Lagarón, J.M.; López-Rubio, A.; Gavara, R. Gliadins polymerized with cysteine: Effects on the physical and water barrier properties of derived films. Biomacromolecules 2004, 5, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Muñoz, P.; Kanavouras, A.; Ng, P.K.; Gavara, R. Development and characterization of biodegradable films made from wheat gluten protein fractions. J. Agric. Food. Chem. 2003, 51, 7647–7654. [Google Scholar] [CrossRef]
- Oliviero, M.; Di Maio, E.; Iannace, S. Effect of molecular structure on film blowing ability of thermoplastic zein. J. Appl. Polym. Sci. 2010, 115, 277–287. [Google Scholar] [CrossRef]
- Belton, P.S.; Delgadillo, I.; Halford, N.G.; Shewry, P.R. Kafirin structure and functionality. J. Cereal Sci. 2006, 44, 272–286. [Google Scholar] [CrossRef]
- Dong, S.; Xu, H.; Tan, J.; Xie, M.; Yu, G. The structure and amphipathy characteristics of modified γ-zeins by sds or alkali in conjunction with heating treatment. Food Chem. 2017, 233, 361–368. [Google Scholar] [CrossRef]
- Holding, D.R. Recent advances in the study of prolamin storage protein organization and function. Front. Plant Sci. 2014, 5, 276. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crop. Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Hou, M.Y.; Fan, W.; Xu, Y. Extraction and characterization comparison of prolamin from wet and dried distiller’s grains of baijiu. Food Ferment. Ind. 2020, 46, 99–103. [Google Scholar]
- Wang, Y.; Tilley, M.; Bean, S.; Sun, X.S.; Wang, D. Comparison of methods for extracting kafirin proteins from sorghum distillers dried grains with solubles. J. Agric. Food. Chem. 2009, 57, 8366–8372. [Google Scholar] [CrossRef]
- Selling, G.W.; Woods, K.K. Improved isolation of zein from corn gluten meal using acetic acid and isolate characterization as solvent. Cereal Chem. 2008, 85, 202–206. [Google Scholar] [CrossRef]
- Parris, N.; Dickey, L.C. Extraction and solubility characteristics of zein proteins from dry-milled corn. J. Agric. Food. Chem. 2001, 49, 3757–3760. [Google Scholar] [CrossRef]
- Schober, T.J.; Bean, S.R.; Tilley, M.; Smith, B.M.; Ioerger, B.P. Impact of different isolation procedures on the functionality of zein and kafirin. J. Cereal Sci. 2011, 54, 241–249. [Google Scholar] [CrossRef]
- Anderson, T.J.; Lamsal, B.P. Development of new method for extraction of α-zein from corn gluten meal using different solvents. Cereal Chem. 2011, 88, 356–362. [Google Scholar] [CrossRef]
- Lai, C.J.; Wu, L.Y.; Hu, L.F.; Tu, J.; Dong, W.H. Aggregation state and structural properties of zein in different solvents. Mod. Food Sci. Technol. 2021, 6, 115–123. [Google Scholar]
- Dianda, N.; Rouf, T.B.; Bonilla, J.C. Effect of solvent polarity on the secondary structure, surface and mechanical properties of biodegradable kafirin films. J. Cereal Sci. 2019, 90, 102856. [Google Scholar] [CrossRef]
- Pena-Serna, C.; Lopes-Filho, J.F. Influence of ethanol and glycerol concentration over functional and structural properties of zein–oleic acid films. Mater. Chem. Phys. 2013, 142, 580–585. [Google Scholar] [CrossRef]
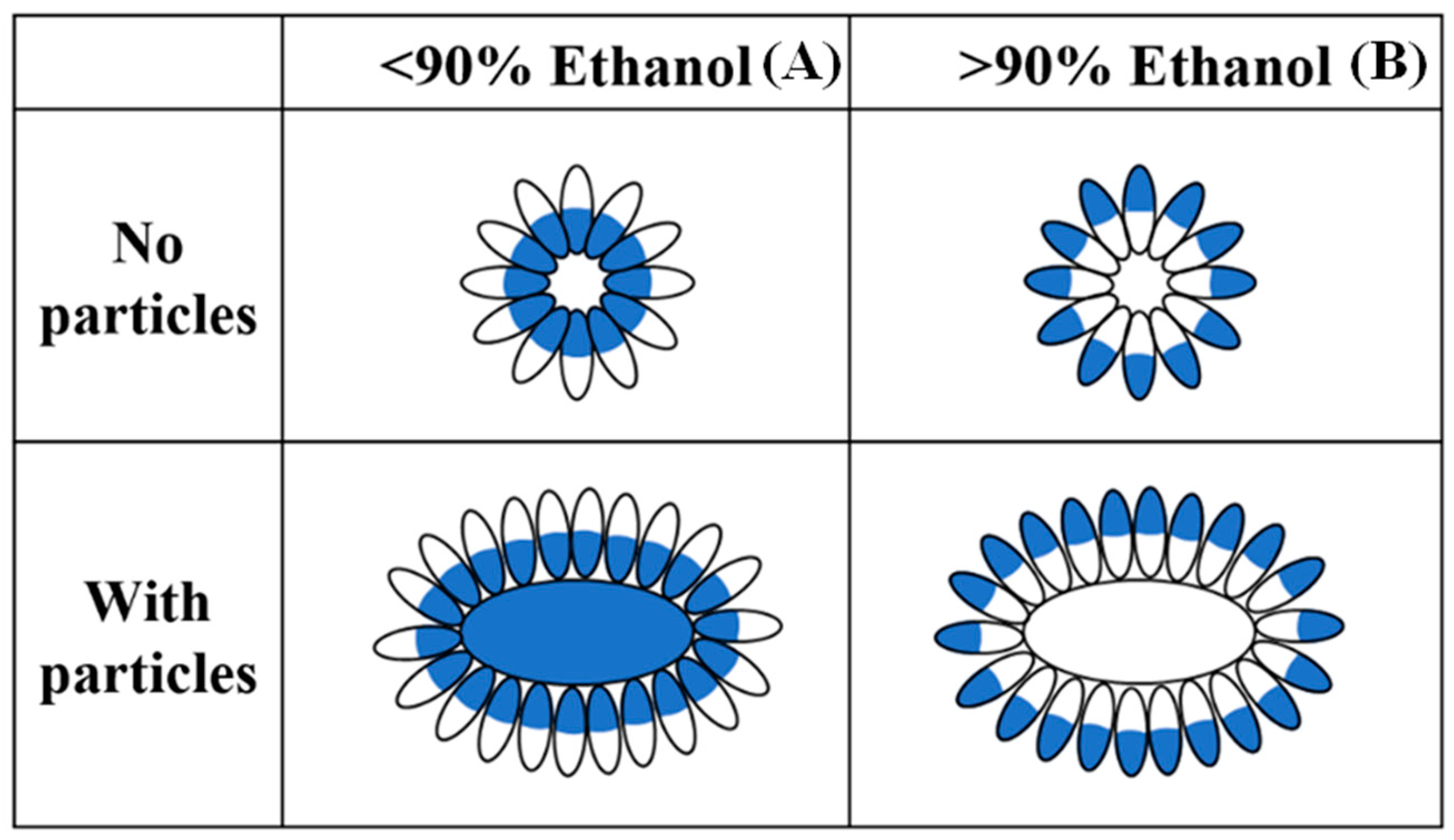
- Kim, S.; Xu, J. Aggregate formation of zein and its structural inversion in aqueous ethanol. J. Cereal Sci. 2008, 47, 1–5. [Google Scholar] [CrossRef]
- Yin, Y.; Yin, S.; Yang, X.; Tang, C.; Wen, S.; Chen, Z.; Xiao, B.; Wu, L. Surface modification of sodium caseinate films by zein coatings. Food Hydrocoll. 2014, 36, 1–8. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, N.; Danno, G.; Takezawa, H.; Izumi, Y. Three-dimensional structure of maize α-zein proteins studied by small-angle X-ray scattering. Biochim. Et Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1997, 1339, 14–22. [Google Scholar] [CrossRef]
- Shi, K.; Kokini, J.L.; Huang, Q. Engineering zein films with controlled surface morphology and hydrophilicity. J. Agric. Food. Chem. 2009, 57, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing spray systems for application of edible coatings. Compr. Rev. Food. Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Boddula, R.; Ahamed, M.I.; Asiri, A.M. Polymers Coatings: Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Luciano, C.G.; Caicedo Chacon, W.D.; Valencia, G.A. Starch-based coatings for food preservation: A review. Starch-Stärke 2022, 74, 2100279. [Google Scholar] [CrossRef]
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Influence of coating application methods on the postharvest quality of cassava. Int. J. Food Sci. 2019, 2019, 2148914. [Google Scholar] [CrossRef]
- Cisneros Zevallos, L.; Krochta, J.M. Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Lin, S.Y.; Krochta, J.M. Fluidized-bed system for whey protein film coating of peanuts. J. Food Process Eng. 2006, 29, 532–546. [Google Scholar] [CrossRef]
- Patel, M.K. Technological improvements in electrostatic spraying and its impact to agriculture during the last decade and future research perspectives—A review. Eng. Agric. Environ. Food 2016, 9, 92–100. [Google Scholar] [CrossRef]
- Sasaki, R.S.; Teixeira, M.M.; Fernandes, H.C.; Monteiro, P.M.D.B.; Rodrigues, D.E.; Alvarenga, C.B.D. Parameters of electrostatic spraying and its influence on the application efficiency. Rev. Ceres 2013, 60, 474–479. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.; Avena-Bustillos, R.J.; De Berrios, J.; Sambo, P.; Mchugh, T.H. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Solís-Morales, D.; Sáenz-Hernández, C.M.; Ortega-Rivas, E. Attrition reduction and quality improvement of coated puffed wheat by fluidised bed technology. J. Food Eng. 2009, 93, 236–241. [Google Scholar] [CrossRef]
- Nascimento, R.F.; Ávila, M.F.; Taranto, O.P.; Kurozawa, L.E. Agglomeration in fluidized bed: Bibliometric analysis, a review, and future perspectives. Powder Technol. 2022, 406, 117597. [Google Scholar] [CrossRef]
- Prata, A.S.; Maudhuit, A.; Boillereaux, L.; Poncelet, D. Development of a control system to anticipate agglomeration in fluidised bed coating. Powder Technol. 2012, 224, 168–174. [Google Scholar] [CrossRef]
- Bisharat, L.; Barker, S.A.; Narbad, A.; Craig, D.Q. In vitro drug release from acetylated high amylose starch-zein films for oral colon-specific drug delivery. Int. J. Pharm. 2019, 556, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, H.J.; Guo, H.; Song, J.Y.; He, X.H.; Xu, X.H.; Zao, H.; Xin, D.H. Performance of zein film modified by glycosylation and in vitro release analysis of hard capsule. Trans. Chin. Soc. Agric. Eng. 2021, 37, 302–309. [Google Scholar]
- Wang, Q.; Chen, W.; Ma, C.; Chen, S.; Liu, X.; Liu, F. Enzymatic synthesis of sodium caseinate-egcg-carboxymethyl chitosan ternary film: Structure, physical properties, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2022, 222, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Byaruhanga, Y.B.; Erasmus, C.; Taylor, J.R. Effect of microwave heating of kafirin on the functional properties of kafirin films. Cereal Chem. 2005, 82, 565–573. [Google Scholar] [CrossRef]
- Byaruhanga, Y.B.; Emmambux, M.N.; Belton, P.S.; Wellner, N.; Ng, K.G.; Taylor, J. Alteration of kafirin and kafirin film structure by heating with microwave energy and tannin complexation. J. Agric. Food. Chem. 2006, 54, 4198–4207. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100, 105443. [Google Scholar] [CrossRef]
- Emmambux, M.N.; Stading, M.; Taylor, J. Sorghum kafirin film property modification with hydrolysable and condensed tannins. J. Cereal Sci. 2004, 40, 127–135. [Google Scholar] [CrossRef]
- Girard, A.L.; Teferra, T.; Awika, J.M. Effects of condensed vs. hydrolysable tannins on gluten film strength and stability. Food Hydrocoll. 2019, 89, 36–43. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Krochta, J.M. Protein-based films and coatings. In Edible Coatings and Films to Improve Food Quality; CRC Press: Boca Raton, FL, USA, 2011; pp. 13–78. [Google Scholar]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- álvarez-Castillo, E.; Ramos, M.; Bengoechea, C.; Martínez, I.; Romero, A. Effect of blend mixing and formulation on thermophysical properties of gluten-based plastics. J. Cereal Sci. 2020, 96, 103090. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Oromiehie, A.R.; Musavi, M.; D-Jomeh, Z.E.; Rad, E.R.; Milani, J. Effect of plasticizing sugars on rheological and thermal properties of zein resins and mechanical properties of zein films. Food Res. Int. 2006, 39, 882–890. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Q.; Wang, Y.Y.; Yan, X.; Guo, X.F. Selection and optimization of plasticizers and their effects on moisture barrier property of zein film. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2018, 39, 20–25. [Google Scholar]
- Pommet, M.; Redl, A.; Guilbert, S.; Morel, M. Intrinsic influence of various plasticizers on functional properties and reactivity of wheat gluten thermoplastic materials. J. Cereal Sci. 2005, 42, 81–91. [Google Scholar] [CrossRef]
- Wu, L.Y. Study on Modification, Surface Properties and Film Forming Properties of Zein. Ph.D. Dissertation, South China University of Technology, Guangzhou, China, 2010. [Google Scholar]
- Skurtys, O.; Acevedo, C. Food Hydrocolloid Edible Films and Coatings; Nova Sciences Publishers: New York, NY, USA, 2010. [Google Scholar]
- Han, J.H. Edible films and coatings: A review. In Innovations in Food Packaging, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; Chapter 9; pp. 213–255. [Google Scholar]
- Lu, Y.N.; Cui, H.P.; Ren, H.W.; Guo, X.F. Effect of plasticizers on the barrier property of zein film. Cereals Oils 2012, 25, 25–27. [Google Scholar]
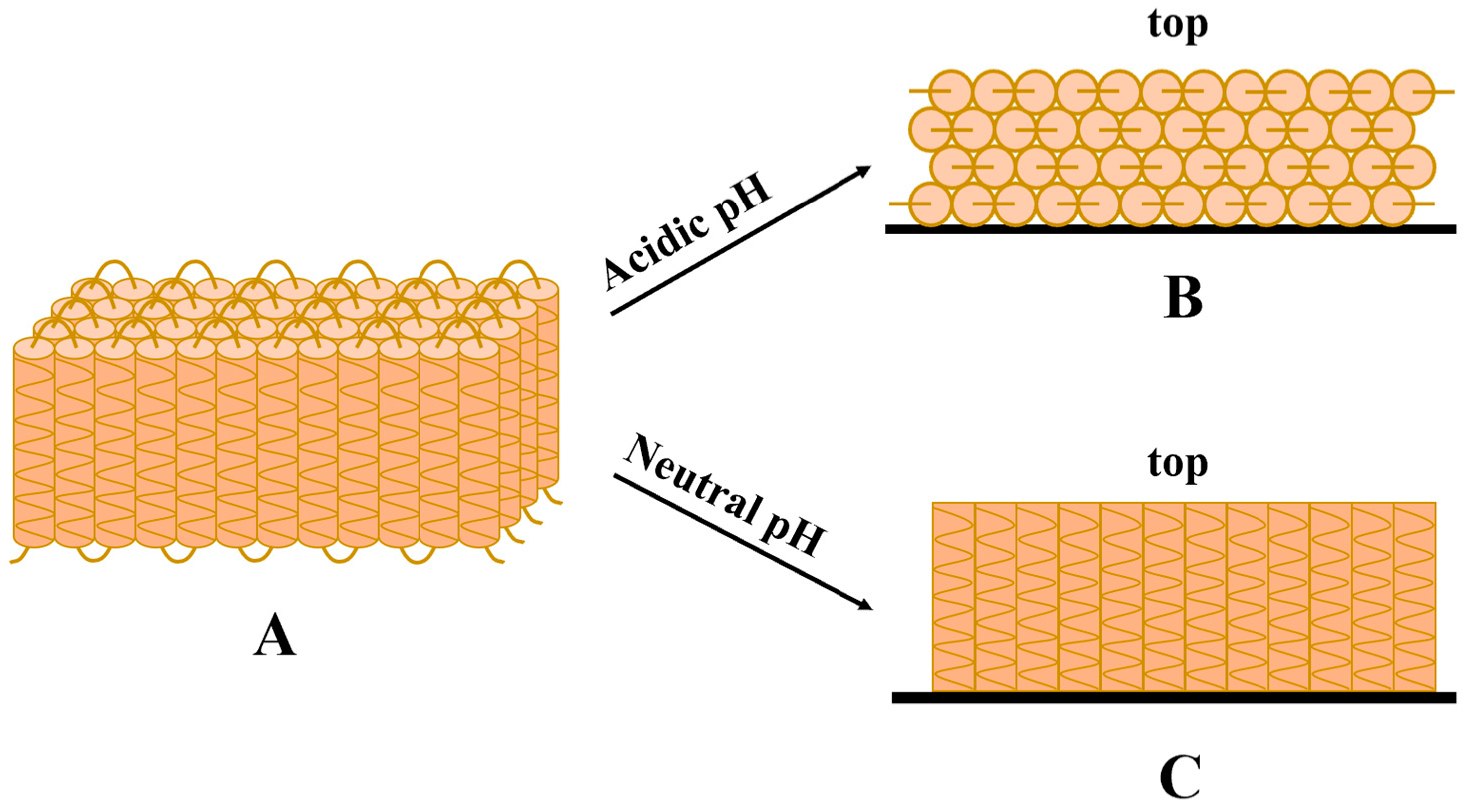
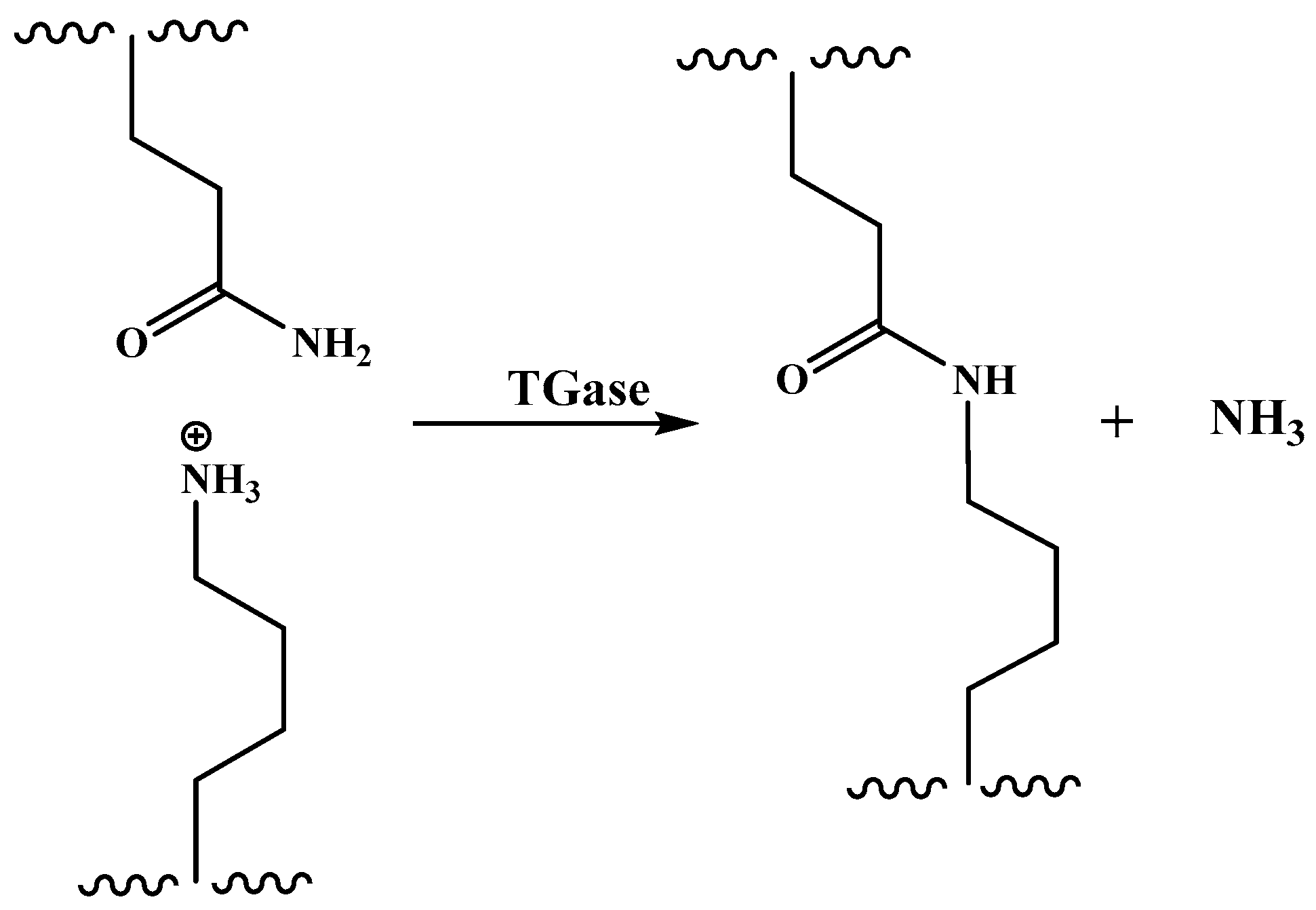
- Masamba, K.; Li, Y.; Hategekimana, J.; Ma, J.; Zhong, F. Effect of drying temperature and ph alteration on mechanical and water barrier properties of transglutaminase cross linked zein–oleic acid composite films. LWT-Food Sci. Technol. 2016, 65, 518–531. [Google Scholar] [CrossRef]
- Masamba, K.; Li, Y.; Hategekimana, J.; Liu, F.; Ma, J.; Zhong, F. Effect of type of plasticizers on mechanical and water barrier properties of transglutaminase cross-linked zein–oleic acid composite films. Int. J. Food Eng. 2016, 12, 365–376. [Google Scholar] [CrossRef]
- Erdogan, I.; Demir, M.; Bayraktar, O. Olive leaf extract as a crosslinking agent for the preparation of electrospun zein fibers. J. Appl. Polym. Sci. 2015, 132, 41388. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, Y. Water-stable electrospun zein fibers for potential drug delivery. J. Biomater. Sci. Polym. Ed. 2011, 22, 1393–1408. [Google Scholar] [CrossRef]
- Xu, H.; Liu, P.; Mi, X.; Xu, L.; Yang, Y. Potent and regularizable crosslinking of ultrafine fibrous protein scaffolds for tissue engineering using a cytocompatible disaccharide derivative. J. Mat. Chem. B 2015, 3, 3609–3616. [Google Scholar] [CrossRef]
- Xu, J.L. Improving Strategies and Related Mechanisms for Mechanical Properties of Collagen Fiber Edible Film. Ph.D. Dissertation, Jiangnan University, Wuxi, China, 2021. [Google Scholar]
- Reddy, N.; Tan, Y.; Li, Y.; Yang, Y. Effect of glutaraldehyde crosslinking conditions on the strength and water stability of wheat gluten fibers. Macromol. Mater. Eng. 2008, 293, 614–620. [Google Scholar] [CrossRef]
- Prata, A.S.; Zanin, M.H.; Ré, M.I.; Grosso, C.R. Release properties of chemical and enzymatic crosslinked gelatin-gum arabic microparticles containing a fluorescent probe plus vetiver essential oil. Colloids Surf. B Biointerfaces 2008, 67, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sessa, D.J.; Mohamed, A.; Byars, J.A.; Hamaker, S.; Selling, G.W. Properties of films from corn zein reacted with glutaraldehyde. J. Appl. Polym. Ence 2010, 105, 2877–2883. [Google Scholar] [CrossRef]
- Anyango, J.O.; Taylor, J.; Taylor, J.R. Improvement in water stability and other related functional properties of thin cast kafirin protein films. J. Agric. Food. Chem. 2011, 59, 12674–12682. [Google Scholar] [CrossRef]
- Casali, D.M.; Yost, M.J.; Matthews, M.A. Eliminating glutaraldehyde from crosslinked collagen films using supercritical CO2. J. Biomed. Mater. Res. Part A 2018, 106, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Developing water stable gliadin films without using crosslinking agents. J. Polym. Environ. 2010, 18, 277–283. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Tong, Y.; Li, Y.; Zhang, J.; Chen, C.; Ren, F.; Hou, C.; Wang, P. Composite films with properties improved by increasing the compatibility of sodium caseinate and zein in a heated 60% ethanol solvent. Food Hydrocoll. 2023, 134, 108017. [Google Scholar] [CrossRef]
- Xu, H.; Shen, L.; Xu, L.; Yang, Y. Low-temperature crosslinking of proteins using non-toxic citric acid in neutral aqueous medium: Mechanism and kinetic study. Ind. Crop. Prod. 2015, 74, 234–240. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Q.; Li, G.; Qiu, Y.; Wei, Q. Electrospun water-stable zein/ethyl cellulose composite nanofiber and its drug release properties. Mater. Sci. Eng. C 2017, 74, 86–93. [Google Scholar] [CrossRef]
- Arancibia, M.Y.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Release of volatile compounds and biodegradability of active soy protein lignin blend films with added citronella essential oil. Food Control 2014, 44, 7–15. [Google Scholar] [CrossRef]
- Mouzakitis, C.; Sereti, V.; Matsakidou, A.; Kotsiou, K.; Biliaderis, C.G.; Lazaridou, A. Physicochemical properties of zein-based edible films and coatings for extending wheat bread shelf life. Food Hydrocoll. 2022, 132, 107856. [Google Scholar] [CrossRef]
- Garcia, F.; Lin, W.; Mellano, V.; Davidov-Pardo, G. Effect of biopolymer coatings made of zein nanoparticles and ε-polylysine as postharvest treatments on the shelf-life of avocados (persea americana mill. Cv. Hass). J. Agric. Food Res. 2022, 7, 100260. [Google Scholar] [CrossRef]
- Huang, T.; Lin, J.; Fang, Z.; Yu, W.; Li, Z.; Xu, D. Preparation and characterization of irradiated kafirin-quercetin film for packaging cod (gadus morhua) during cold storage at 4 °C. Food Bioproc. Technol. 2020, 13, 522–532. [Google Scholar] [CrossRef]
- Lal, S.S.S.T. Tempo-oxidized cellulose nanofiber/kafirin protein thin film crosslinked by maill. Cellulose 2019, 26, 6099–6118. [Google Scholar] [CrossRef]
- Jia, F.; Huang, Y.; Zhao, J.; Luo, S.; Hou, Y.; Hu, S.-Q. Physicochemical and functional properties of dialdehyde polysaccharides crosslinked gliadin film cooperated by citric acid. J. Cereal Sci. 2021, 102, 103349. [Google Scholar] [CrossRef]
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Muriel-Gallet, V.; Gavara, R.; Hernández-Muñoz, P. Zein films and coatings as carriers and release systems of zataria multiflora boiss. Essential oil for antimicrobial food packaging. Food Hydrocoll. 2017, 70, 260–268. [Google Scholar] [CrossRef]
- Ge, X.; Huang, X.; Zhou, L.; Wang, Y. Essential oil-loaded antimicrobial and antioxidant zein/poly (lactic acid) film as active food packaging. Food Packag. Shelf Life 2022, 34, 100977. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef] [PubMed]
- Becaro, A.A.; Siqueira, M.C.; Puti, F.C.; de Moura, M.R.; Correa, D.S.; Marconcini, J.M.; Mattoso, L.H.; Ferreira, M.D. Cytotoxic and genotoxic effects of silver nanoparticle/carboxymethyl cellulose on allium cepa. Environ. Monit. Assess. 2017, 189, 352. [Google Scholar] [CrossRef]
- Boyacı, D.; Iorio, G.; Sozbilen, G.S.; Alkan, D.; Trabattoni, S.; Pucillo, F.; Farris, S.; Yemenicioğlu, A. Development of flexible antimicrobial zein coatings with essential oils for the inhibition of critical pathogens on the surface of whole fruits: Test of coatings on inoculated melons. Food Packag. Shelf Life 2019, 20, 100316. [Google Scholar] [CrossRef]
- Lin, L.S.; Wang, B.J.; Weng, Y.M. Quality preservation of commercial fish balls with antimicrobial zein coatings. J. Food Qual. 2011, 34, 81–87. [Google Scholar] [CrossRef]
- Alkan, D.; Aydemir, L.Y.; Arcan, I.; Yavuzdurmaz, H.; Atabay, H.I.; Ceylan, C.; Yemenicioglu, A. Development of flexible antimicrobial packaging materials against campylobacter jejuni by incorporation of gallic acid into zein-based films. J. Agric. Food. Chem. 2011, 59, 11003–11010. [Google Scholar] [CrossRef]
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R. Novel antimicrobial zein film for controlled release of lauroyl arginate (lae). Food Hydrocoll. 2016, 61, 547–554. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Giteru, S.G.; Oey, I.; Ali, M.A.; Johnson, S.K.; Fang, Z. Effect of kafirin-based films incorporating citral and quercetin on storage of fresh chicken fillets. Food Control 2017, 80, 37–44. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.J.; Huang, Y.; Zhao, J.; Hou, Y.; Hu, S. Development and characterization of gliadin-based bioplastic films enforced by cinnamaldehyde. J. Cereal Sci. 2021, 99, 103208. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Antifungal properties of gliadin films incorporating cinnamaldehyde and application in active food packaging of bread and cheese spread foodstuffs. Int. J. Food Microbiol. 2013, 166, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, Z.; Pedram Nia, A.; Saeidi-Asl, M.; Armin, M.; Heydari-Majd, M. Evaluation of antimicrobial properties of gliadin nanofibers containing zataria multiflora boiss essential oil and its effect on shelf-life extension of smoked salmon fish fillet. Res. Innov. Food Sci. Technol. 2022, 11, 141–154. [Google Scholar]

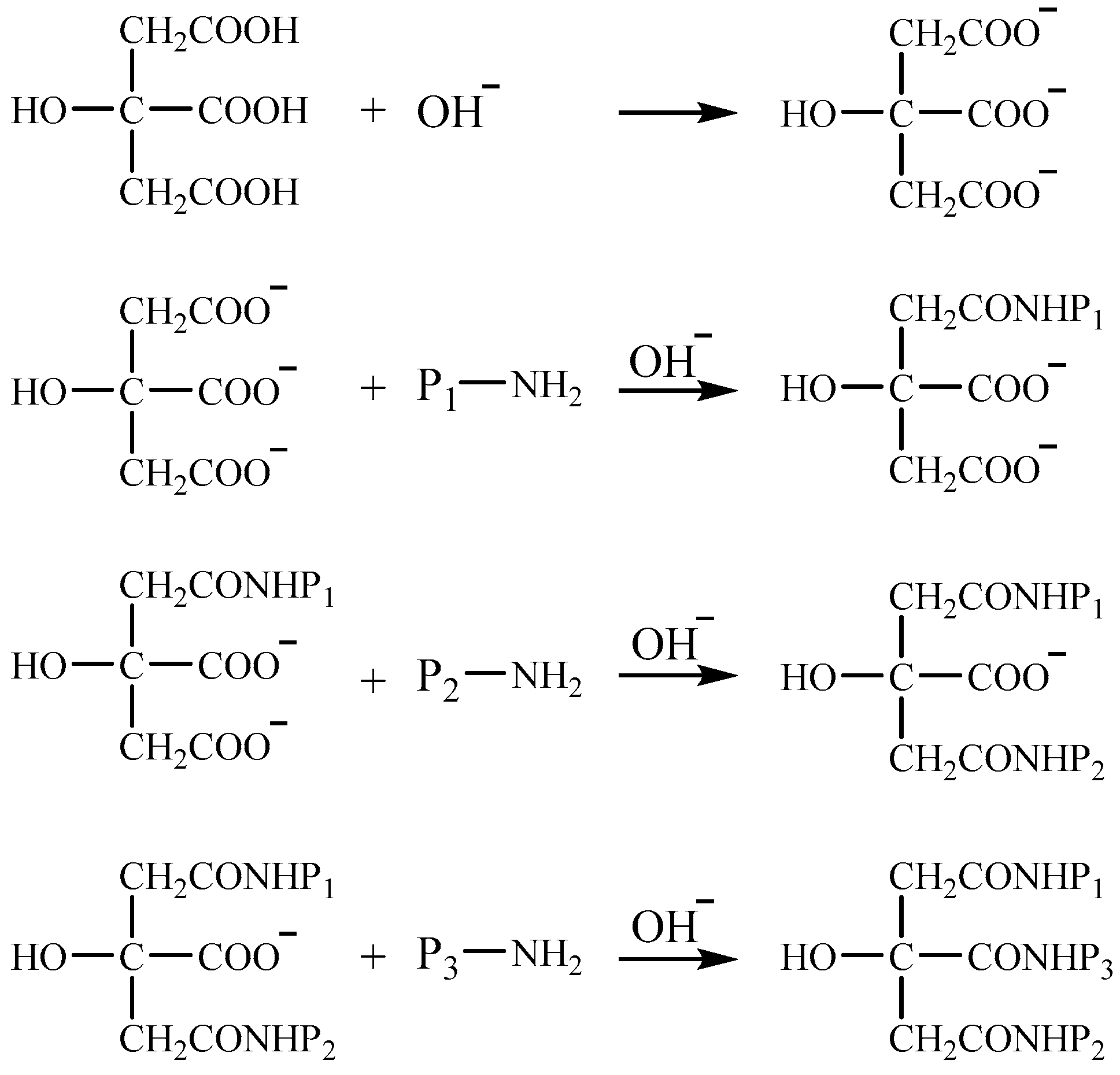
| Amino Acid | Zein | Kafirin | Gliadin | Hordein |
|---|---|---|---|---|
| Ala | 11.3 | 12.4 | 2.3 | 1.6 |
| Arg | 1.1 | 1.0 | 2.4 | 1.6 |
| Asp | 3.9 | 6.5 | 1.3 | 0.7 |
| Cys | 1.9 | 0.4 | 2.7 | 0.5 |
| Glu | 20.7 | 30.0 | 38.4 | 42.0 |
| Gly | 3.8 | 1.1 | 3.3 | 1.7 |
| His | 2.1 | 0.9 | 1.6 | 0.2 |
| Ile | 3.7 | 4.8 | 3.7 | 5.8 |
| Leu | 18.7 | 19.2 | 7.0 | 6.0 |
| Lys | 0.2 | 0.1 | 0.5 | 5.4 |
| Met | 1.9 | 1.0 | 0.6 | 0.8 |
| Phe | 5.3 | 6.4 | 4.7 | 2.3 |
| Pro | 8.7 | 10.0 | 15.0 | 22.7 |
| Ser | 5.9 | 4.1 | 7.7 | 2.1 |
| Thr | 3.1 | 2.6 | 2.8 | 2.6 |
| Tyr | 3.2 | 5.5 | 1.5 | 2.3 |
| Val | 4.5 | 5.0 | 4.7 | 1.9 |
| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Dipping | The uncoated product is dipped into the film-forming liquid; the solvent in the coating-forming liquid on the surface of the product volatilizes to form a coating | Simple operation and low cost; can be applied to irregular surfaces | Uneven coating thickness; cannot be serialized |
| Spraying | The uncoated product is conveyed by a conveyor belt and passes under a fixed coating solution nozzle, where the coating solution is sprayed on the product and then cured into a coating | Uniform coating, less cross-contamination, and controllable coating thickness; can be used for coated foods with large surface area | Requires the coating solution to have high fluidity and is generally used for low-viscosity coating solutions |
| Fluidized-bed | The particles to be coated are risen from the bottom under the action of airflow, followed by using a fixed nozzle to spray the coating solution onto the surface of fluidized powder/granular products | Suitable for batch production; can be used for dry particles with low density and small size | Uncontrolled agglomeration of the coated particles |
| Panning | The uncoated products are placed into a rotating pan; as the pan rotates, the coating solution is sprayed out and adheres onto the surface of the product | Can produce products in large quantities at the same time | Discontinuous operation, long operation time, difficult cleaning, high cost, and high requirements for the scale and shape of the products to be coated |
| Prolamin | Modification | Modification Effect | Application | Application Effects | Ref. |
|---|---|---|---|---|---|
| Zein | Zein + sunflower oil complex | Large decrease in tensile Young’s modulus and strength with increasing level of sunflower oil in zein films | Wheat bread | Coated breads exhibited retardation in moisture migration from crumb to crust compared to uncoated counterparts | [94] |
| Zein | Zein + ε-polylysine nanoparticles complex | — | Avocado | By day 36 in ambient storage in this study, coated avocados retained enough of their initial physical appearance and texture | [95] |
| Kafirin | Electron beam irradiate kafirin-quercetin film | Irradiation significantly increased mechanical and thermal properties of KQ films, while decreasing water vapor permeability, water solubility, and transparency | Cod fillets during cold storage at 4 °C. | Shelf life of cod fillets wrapped in irradiated prolamin film increased from 4 to 7 days compared to fillets prepared with polyethylene coating | [96] |
| Kafirin | TEMPO-oxidized cellulose nanofiber + kafirin cross-linked by Maillard reaction | Young’s Modulus shows significant increases at 0.5% of TO-CNF; with a gradual decrease at 3% of TO-CNF | — | — | [97] |
| Gliadin | Dialdehyde polysaccharides + citric acid cross-linked | The mechanical properties, water-resistant properties, thermal stability, antibacterial properties of the gliadin films were all advanced | — | — | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Kuang, Y.; Xu, P.; Chen, X.; Bi, Y.; Peng, D.; Li, J. Applications of Prolamin-Based Edible Coatings in Food Preservation: A Review. Molecules 2023, 28, 7800. https://doi.org/10.3390/molecules28237800
Zhang S, Kuang Y, Xu P, Chen X, Bi Y, Peng D, Li J. Applications of Prolamin-Based Edible Coatings in Food Preservation: A Review. Molecules. 2023; 28(23):7800. https://doi.org/10.3390/molecules28237800
Chicago/Turabian StyleZhang, Shuning, Yongyan Kuang, Panpan Xu, Xiaowei Chen, Yanlan Bi, Dan Peng, and Jun Li. 2023. "Applications of Prolamin-Based Edible Coatings in Food Preservation: A Review" Molecules 28, no. 23: 7800. https://doi.org/10.3390/molecules28237800
APA StyleZhang, S., Kuang, Y., Xu, P., Chen, X., Bi, Y., Peng, D., & Li, J. (2023). Applications of Prolamin-Based Edible Coatings in Food Preservation: A Review. Molecules, 28(23), 7800. https://doi.org/10.3390/molecules28237800






