A Ratiometric Fluorescent Sensor Based on Chelation-Enhanced Fluorescence of Carbon Dots for Zinc Ion Detection
Abstract
:1. Introduction
2. Results and Discussion
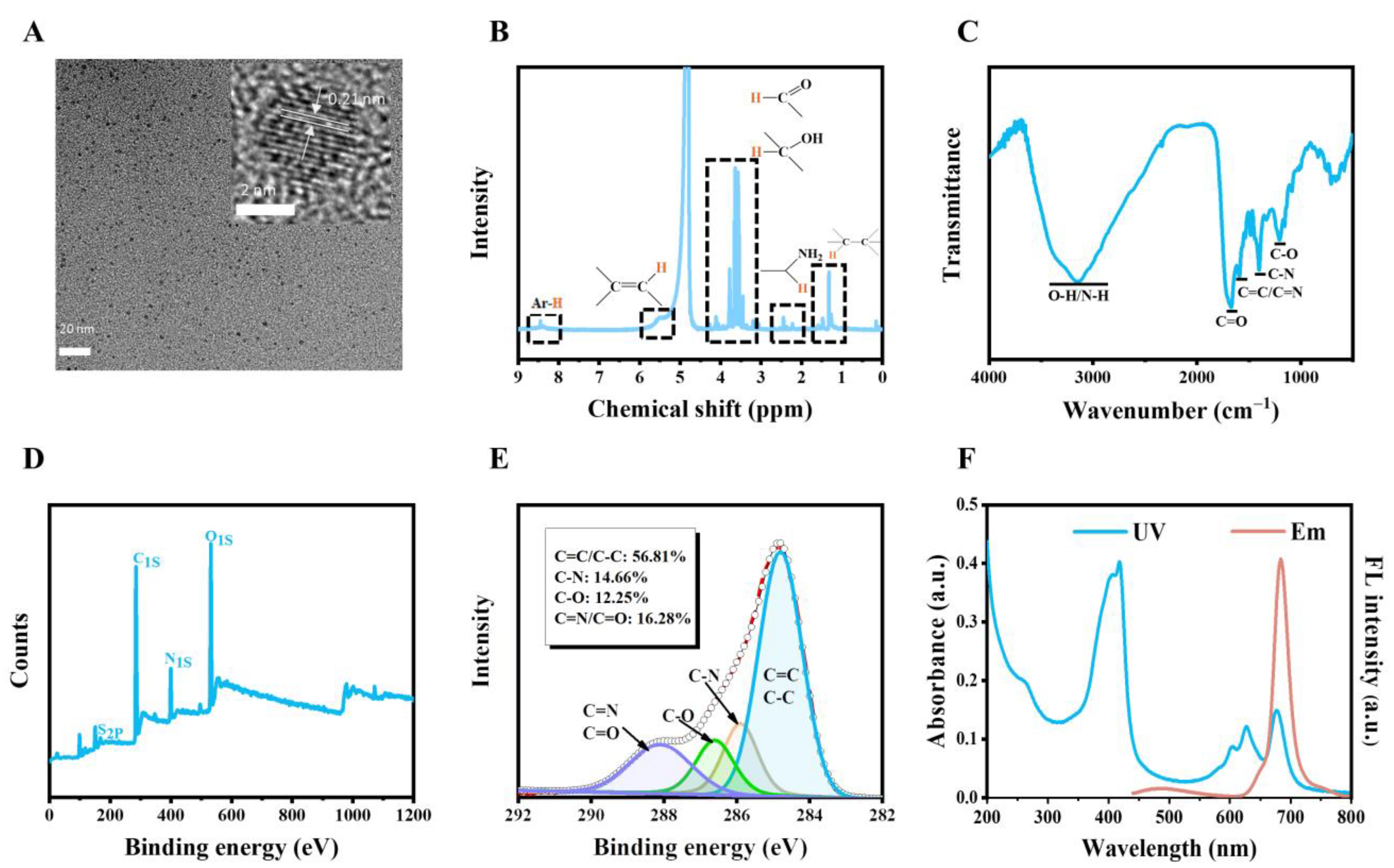
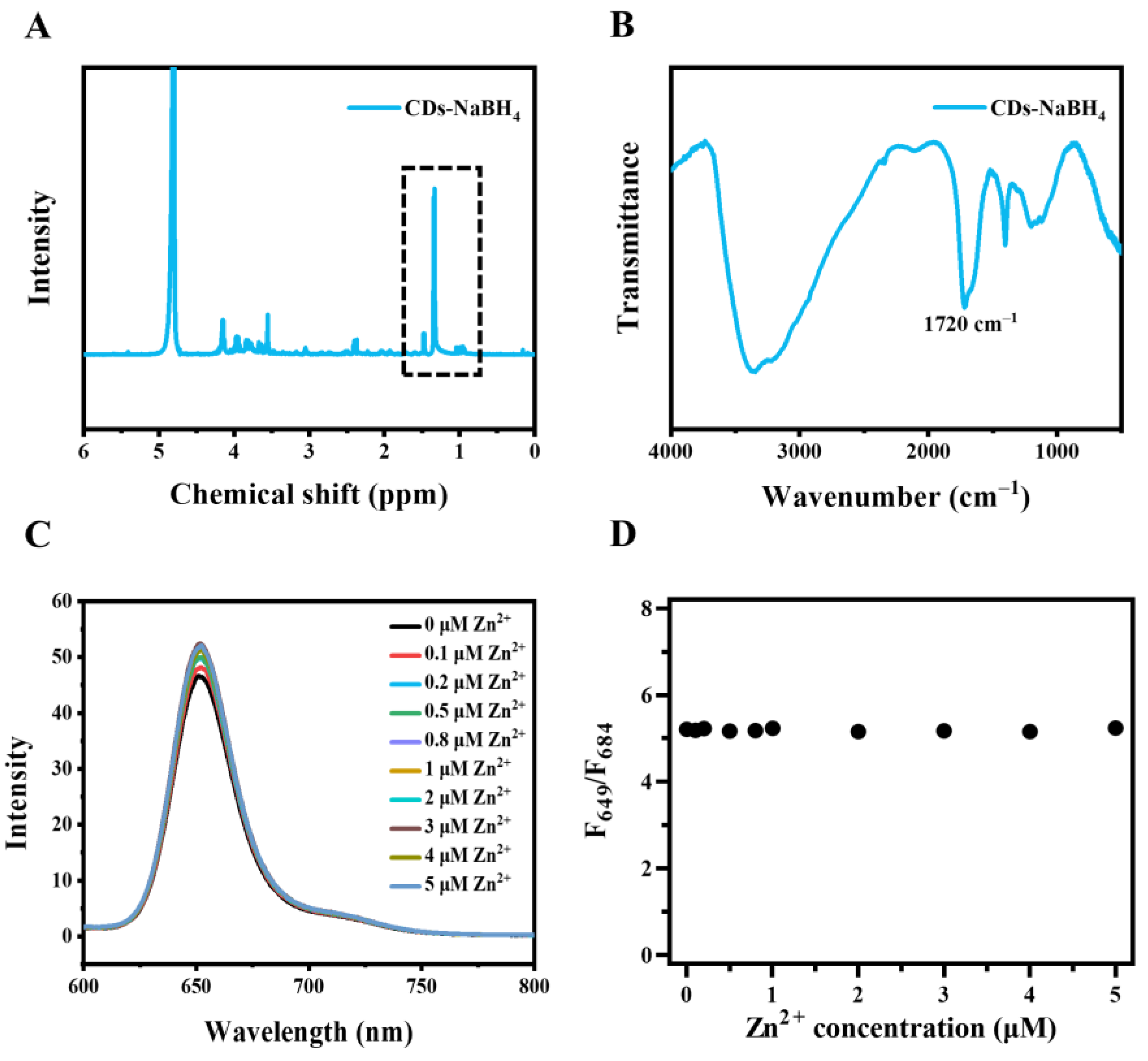
2.1. Characterization of CDs
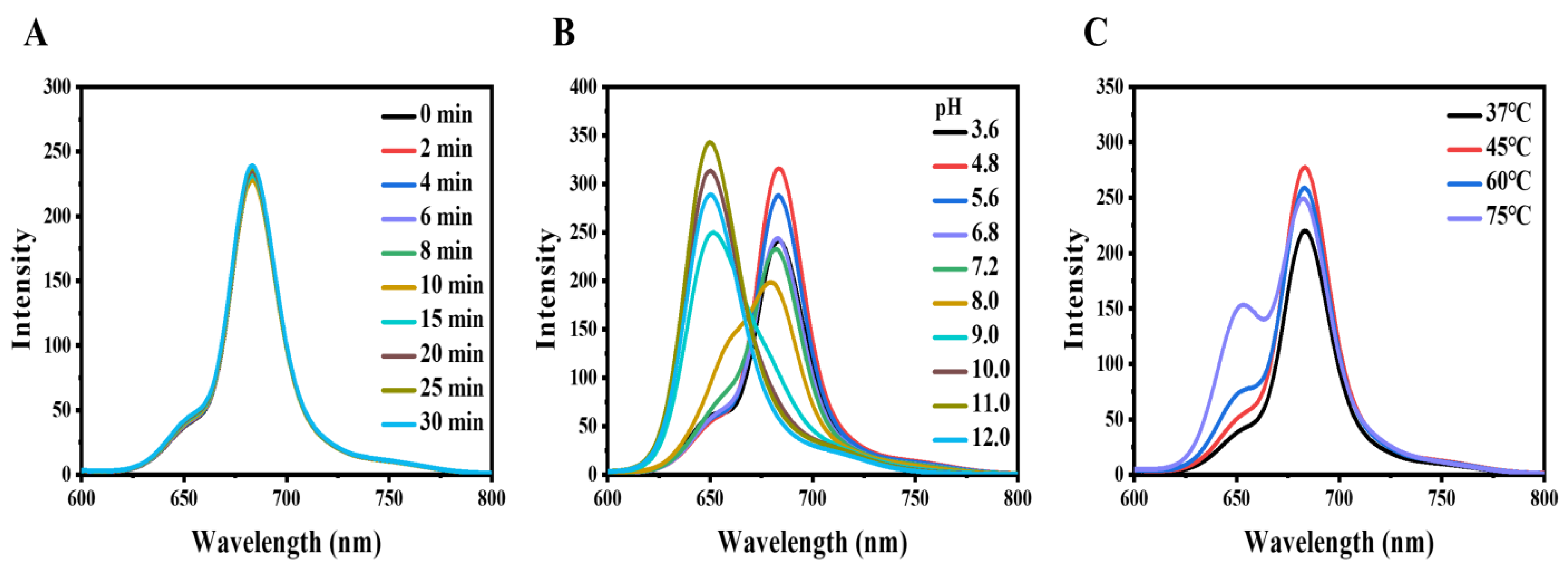
2.2. The Properties of CDs
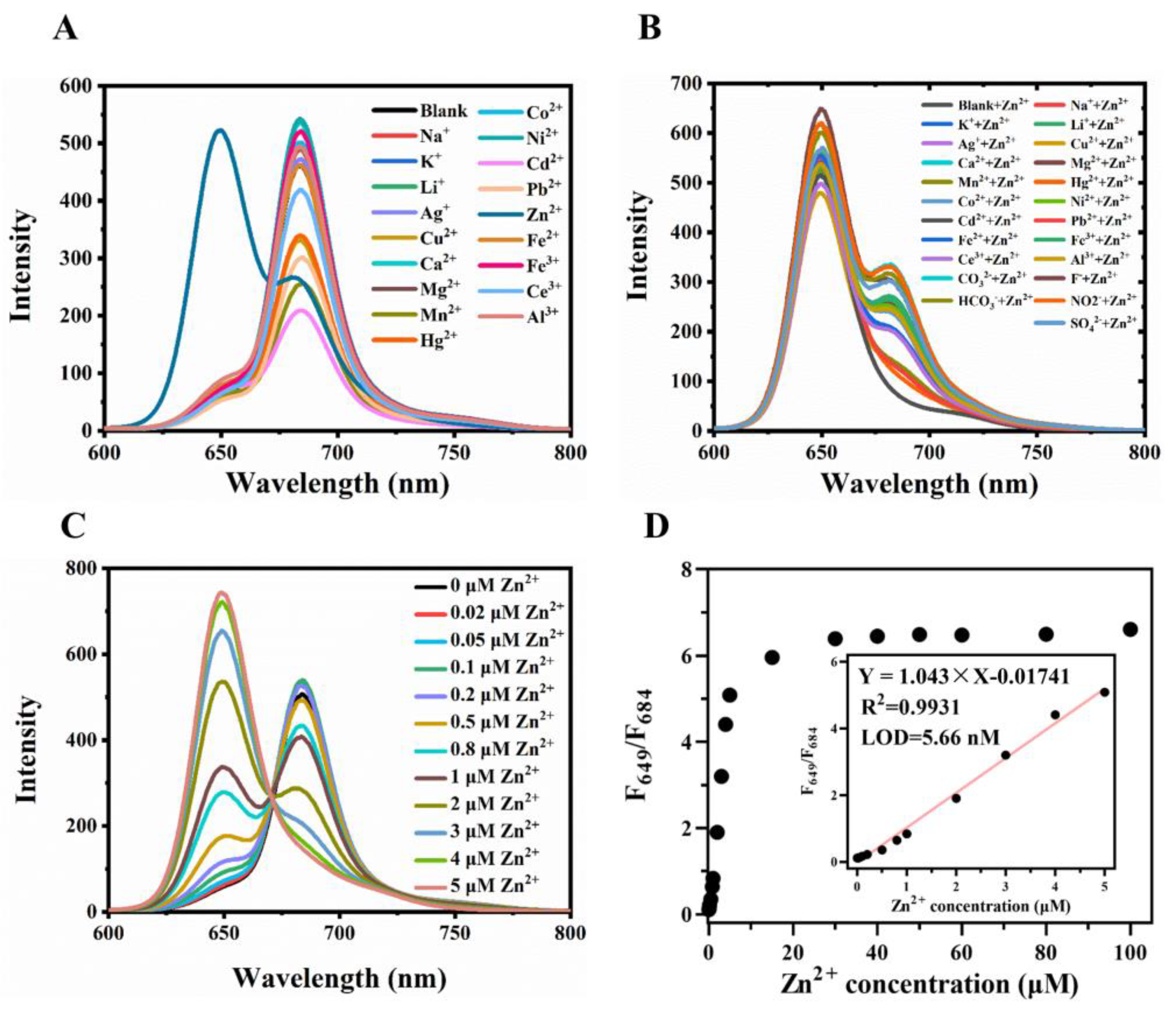
2.3. Colorimetric Fluorescence Detection of Zinc Ion
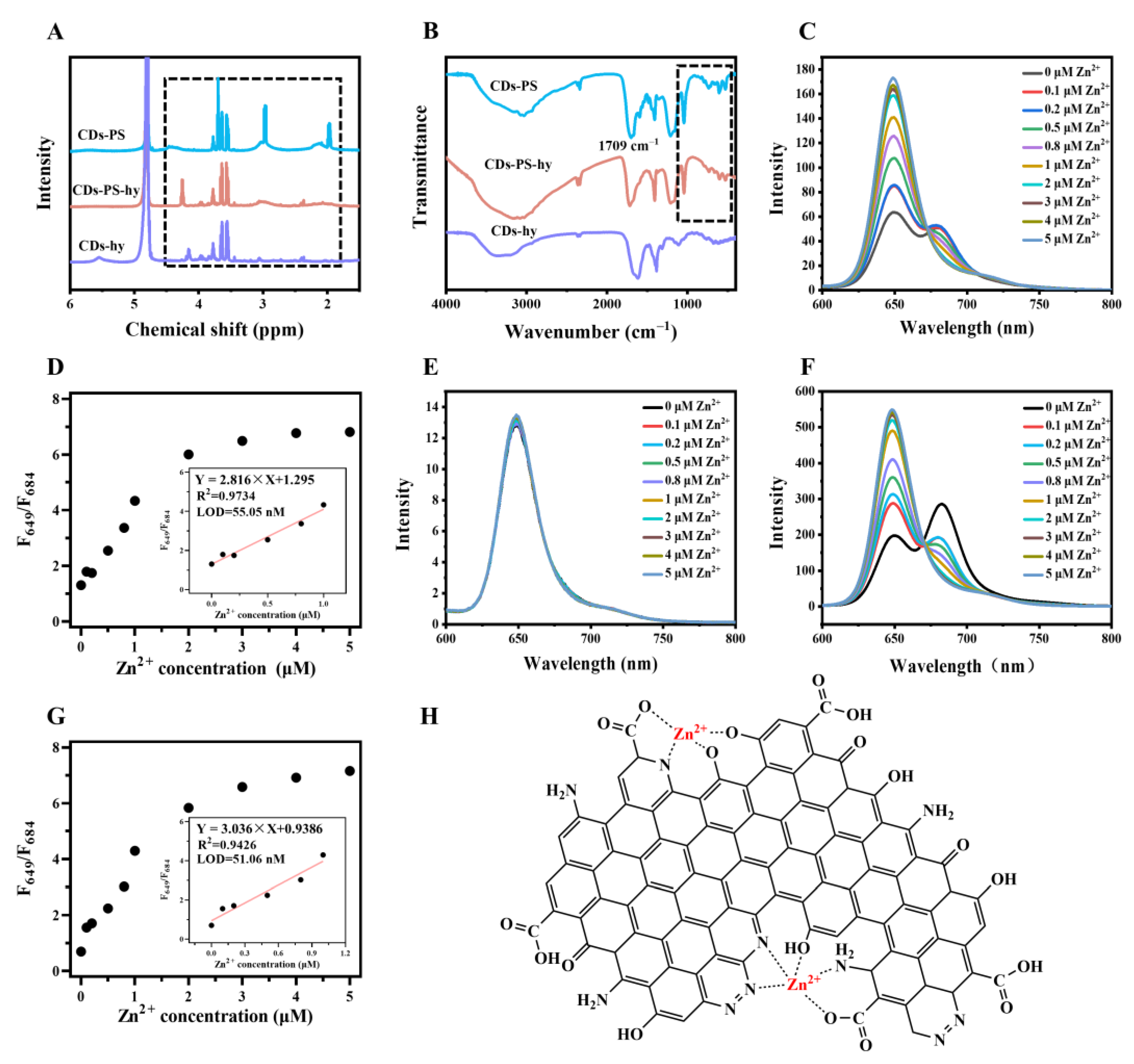
2.4. Effect of Zinc Ions on the Structure of CDs
2.5. The Coordination between CDs and Zinc Ions
2.6. Detection of Zinc Ions in Complex Real Samples
3. Materials and Methods
3.1. Reagents, Materials and Instruments
3.2. Synthesis
3.2.1. Synthesis of CDs
3.2.2. Synthesis of CDs-SPI
3.2.3. Synthesis of CDs-PS
3.2.4. Synthesis of CDs-PS-hy
3.2.5. Synthesis of CDs-hy
3.2.6. Synthesis of CDs-NaBH4
3.3. Effect of Metal Ions on the Fluorescence of CDs
3.4. Fluorescence Stability Test of CDs
3.5. Detection of Zinc Ions by CDs
3.6. Detection of Zn2+ Levels in Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, Y.; Fan, W.; Fan, C.; Li, Y. Ultra-fast zinc ion detection in living cells and zebrafish by a light-up fluorescent probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Shi, Y.G. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef]
- Wang, Y.; Lao, S.; Ding, W.; Zhang, Z.; Liu, S. A novel ratiometric fluorescent probe for detection of iron ions and zinc ions based on dual-emission carbon dots. Sens. Actuators B Chem. 2019, 284, 186–192. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, C.W.; Weiss, J.H. Zinc: New clues to diverse roles in brain ischemia. Trends Pharmacol. Sci. 2011, 32, 480–486. [Google Scholar] [CrossRef]
- Yan, L.Q.; Yang, H.; Li, J.; Zhou, C.P.; Li, L.; Wu, X.Z. A near infrared fluorescent probe for detection and bioimaging of zinc ions and hypochloric acid. Anal. Chim. Acta 2022, 1206, 339750. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Choi, S.; Cui, C.; Luo, Y.; Kim, S.H.; Ko, J.K.; Huo, X.F.; Ma, J.J.; Fu, L.W.; Souza, R.F.; Korichneva, I.; et al. Selective inhibitory effects of zinc on cell proliferation in esophageal squamous cell carcinoma through Orai1. FASEB J. 2018, 32, 404–416. [Google Scholar] [CrossRef]
- Waheed, A.; Abdel-Azeim, S.; Ullah, N.; Oladepo, S.A. Design and synthesis of two new terbium and europium complex-based luminescent probes for the selective detection of zinc ions. Luminescence 2020, 35, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Karmegam, M.V.; Karuppannan, S.; Christopher Leslee, D.B.; Subramanian, S.; Gandhi, S. Phenothiazine-rhodamine-based colorimetric and fluorogenic ‘turn-on’ sensor for Zn2+ and bioimaging studies in live cells. Luminescence 2020, 35, 90–97. [Google Scholar] [CrossRef]
- Kara, D.; Fisher, A.; Hill, S.J. Preconcentration and determination of trace elements with 2,6-diacetylpyridine functionalized amberlite XAD-4 by flow injection and atomic spectroscopy. Analyst 2005, 130, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Liu, S.; Liang, W.; Li, D.; Wang, L.L.; He, Y.Q. A regenerable fluorescent quantum dot based nanoprobe for zinc(II), and the design of a molecular logic gate. Microchim. Acta 2015, 182, 2009–2017. [Google Scholar] [CrossRef]
- Khan, S.A.; Alam, M.Z.; Mohasin, M.; Ahmad, S.; Salma, U.; Parveen, H.; Mukhtar, S.; Al-Anazi, M.; Alotaibi, F.A.; Abdelaziz, M.A. Ultrasound-assisted synthesis of chalcone: A highly sensitive and selective fluorescent chemosensor for the detection of Fe3+ in aqueous media. J. Fluoresc. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Jiang, W.; Fu, Q.; Fan, H.; Wang, W. An NBD fluorophore-based sensitive and selective fluorescent probe for zinc ion. Chem. Commun. 2008, 2, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Qiu, S.; Lu, R.; Pu, S. A multi-functional fluorescent sensor for Zn2+ and HSO4− based on a new diarylethene derivative. Tetrahedron Lett. 2018, 59, 3365–3372. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Feng, Q.; Li, N.; Li, K.; Hou, H.; Zhang, B. ‘Turn-on’ fluorescent chemosensors based on naphthaldehyde-2-pyridinehydrazone compounds for the detection of zinc ion in water at neutral pH. Luminescence 2018, 33, 29–33. [Google Scholar] [CrossRef]
- Menéndez, G.O.; López, C.S.; Jares-Erijman, E.A.; Spagnuolo, C.C. A versatile near-infrared asymmetric tricarbocyanine for zinc ion sensing in water. Photochem. Photobiol. 2013, 89, 1354–1361. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Suo, F.; Yu, C.; Zhang, D.; Chen, J.; Wu, Q.; Jing, S.; Li, L.; Huang, W. A novel naphthofluorescein-based probe for ultrasensitive point-of-care testing of zinc(II) ions and its bioimaging in living cells and zebrafishes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117949. [Google Scholar] [CrossRef] [PubMed]
- Jonaghani, M.Z.; Zali-Boeini, H.; Moradi, H. A coumarin based highly sensitive fluorescent chemosensor for selective detection of zinc ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Oh, E.T.; Park, H.J.; Lee, K.H. Ratiometric fluorescent probe based on symmetric peptidyl receptor with picomolar affinity for Zn2+ in aqueous solution. Sens. Actuators B Chem. 2017, 245, 996–1003. [Google Scholar] [CrossRef]
- Czaplinska, B.; Spaczynska, E.; Musiol, R. Quinoline fluorescent probes for zinc—From diagnostic to therapeutic molecules in treating neurodegenerative diseases. Med. Chem. 2018, 14, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Makhal, S.C.; Guchhait, N. Evaluating the merit of a diethylamino coumarinderived thiosemicarbazone as an intramolecular charge transfer probe: Efficient Zn(II) mediated emission swing from green to yellow. Photochem. Photobiol. Sci. 2020, 18, 2031–2041. [Google Scholar] [CrossRef]
- Tian, X.T.; Yin, X.B. Carbon dots, unconventional preparation strategies, and applications beyond photoluminescence. Small 2019, 15, e1901803. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Lee, S.T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Recent advances of carbon dots in imaging-guided theranostics. TrAC Trends Anal. Chem. 2021, 134, 116116. [Google Scholar] [CrossRef]
- Wu, H.; Pang, L.F.; Fu, M.J.; Guo, X.F.; Wang, H. Boron and nitrogen codoped carbon dots as fluorescence sensor for Fe3+ with improved selectivity. J. Pharm. Biomed. Anal. 2020, 180, 113052. [Google Scholar] [CrossRef]
- Bu, D.; Song, H.; Li, Z.; Wei, L.; Zhang, H.; Yu, M. Carbon-dot-based ratiometric fluorescent probe of intracellular zinc ion and persulfate ion with low dark toxicity. Luminescence 2020, 35, 1319–1327. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S. CDs-MnO2-TPPS ternary system for ratiometric fluorescence detection of ascorbic acid and alkaline phosphatase. ACS Omega 2021, 6, 16565–16572. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.; Zhang, L.; Jiang, K.; Lin, H. Near-infrared emissive carbon dots for two-photon fluorescence bioimaging. Nanoscale 2016, 8, 17350–17356. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, M.; Hu, J.; Bao, L.; Tang, B.; Wei, X.; Zhao, J.; Jin, Z.; Luo, Q.; Pang, D. Quantitatively switchable pH-sensitive photoluminescence of carbon nanodots. J. Phys. Chem. Lett. 2021, 12, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Li, S.P.; Liu, X.F.; Shi, B.F.; Huang, Y.J.; Zhao, S.L. Nitrogen and sulfur co-doped carbon dot-based ratiometric fluorescent probe for Zn2+ sensing and imaging in living cells. Microchim. Acta 2022, 189, 107. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Z.; Li, Y.; Ma, S.; Hu, P.; Zhong, X. A quantum dot-based “off-on” fluorescent probe for biological detection of zinc ions. Analyst 2013, 138, 2181–2191. [Google Scholar] [CrossRef]
- Ruedas-Rama, M.J.; Hall, E.A.H. Azamacrocycle activated quantum dot for zinc ion detection. Anal. Chem. 2008, 80, 8260–8268. [Google Scholar] [CrossRef]
- Enbanathan, S.; Munusamy, S.; Jothi, D.; Manojkumar, S.; Manickam, S.; Iyer, S.K. Zinc ion detection using a benzothiazole-based highly selective fluorescence “turn-on” chemosensor and its real-time application. RSC Adv. 2022, 12, 27839–27845. [Google Scholar] [CrossRef]
- Hosseini, M.; Vaezi, Z.; Ganjali, M.R.; Faridbod, F.; Abkenar, S.D.; Alizadeh, K.; Salavati-Niasari, M. Fluorescence “turn-on” chemosensor for the selective detection of zinc ion based on Schiff-base derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 978–982. [Google Scholar] [CrossRef]
- Ghorai, P.; Pal, K.; Karmakar, P.; Saha, A. The development of two fluorescent chemosensors for the selective detection of Zn2+ and Al3+ ions in a quinoline platform by tuning the substituents in the receptor part: Elucidation of the structures of the metal-bound chemosensors and biological studies. Dalton Trans. 2020, 49, 4758–4773. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, W.B.; Cheng, W.X.; Gu, Y.P.; Chen, Y.M.; Zhou, W.H.; Yu, X.F.; Chen, M.M.; Zhu, M.R.; Fan, K.L.; et al. Red emissive carbon dot superoxide dismutase nanozyme for bioimaging and ameliorating acute lung injury. Adv. Funct. Mater. 2023, 33, 2213856. [Google Scholar] [CrossRef]
- Gao, W.H.; He, J.Y.; Chen, L.; Meng, X.Q.; Ma, Y.N.; Cheng, L.L.; Tu, K.S.; Gao, X.F.; Liu, C.; Zhang, M.Z.; et al. Deciphering the catalytic mechanism of superoxide dismutase activity of carbon dot nanozyme. Nat. Commun. 2023, 14, 160. [Google Scholar] [CrossRef] [PubMed]



| Probe | Linear Range | Response Time | LOD | Ref. |
|---|---|---|---|---|
| DPA-P-DTC-QDs | 0.9–16 μM | 9 min | 0.7 μM | [37] |
| CdSe-ZnS QD | 5–500 μM | 3 min | 2.4 μM | [38] |
| CDs | 250–600 μM | Immediate detection | 0.36 mM | [32] |
| N-S CDs | 0.01–1 μM | 40 min | 5.0 nM | [36] |
| DCDs | 2.5–50 μM | 5 min | 1.2 μM | [4] |
| BIPP | 0.1–1 μM | Not mentioned | 23.6 nM | [39] |
| Probe | 0–10 μM | Less than 20 s | 15.3 nM | [9] |
| ZN-2 | 0–10 μM | 20 min | 0.74 μM | [21] |
| L | 0.16–10 μM | Not mentioned | 0.15 μM | [40] |
| HL1 | Not mentioned | Not mentioned | 0.14 μM | [41] |
| CDs | 0.02–5 μM | Immediate detection | 5.66 nM | This study |
| CDs-SPI | Ultrapure water | Immediate detection | 2.48 nM | This study |
| τ1 (ns) | τ2 (ns) | τ3 (ns) | τave (ns) | |
|---|---|---|---|---|
| CDs + 0 μM Zn2+-650 | 1.7899 (4.85%) | 4.5394 (90.40%) | 13.0837 (4.75%) | 4.8119 |
| CDs + 0.5 μM Zn2+-650 | 1.9599 (14.10%) | 4.3687 (80.84%) | 12.4657 (5.06%) | 4.4388 |
| CDs + 1 μM Zn2+-650 | 1.9775 (23.41%) | 4.1601 (71.93%) | 12.9282 (4.66%) | 4.0577 |
| CDs + 2 μM Zn2+-650 | 1.8694 (28.03%) | 3.5503 (67.42%) | 12.9373 (4.55%) | 3.5063 |
| CDs + 0 μM Zn2+-680 | 4.7963 (96.09%) | 15.5196 (3.91%) | 5.2156 | |
| CDs + 0.5 μM Zn2+-680 | 4.7806 (95.75%) | 14.0733 (4.25%) | 5.1755 | |
| CDs + 1 μM Zn2+-680 | 4.7104 (94.37%) | 12.2045 (5.63%) | 5.1323 | |
| CDs + 2 μM Zn2+-680 | 1.5277 (1.77%) | 4.7273 (93.66) | 14.4138 (4.57%) | 5.1133 |
| Sample | Original (mg/mL) | Measured (mg/mL) | RSD% (n = 3) |
|---|---|---|---|
| 1 | 0.5 | 0.49 | 4.1 |
| 2 | 0.5 | 0.47 | 4.6 |
| 3 | 0.5 | 0.51 | 5.3 |
| 4 | 1.561 × 10−3 (ICP-MS) | 1.57 × 10−3 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, G.; Jia, Z.; Yu, M.; Zhang, M.; Xu, C. A Ratiometric Fluorescent Sensor Based on Chelation-Enhanced Fluorescence of Carbon Dots for Zinc Ion Detection. Molecules 2023, 28, 7818. https://doi.org/10.3390/molecules28237818
Lu G, Jia Z, Yu M, Zhang M, Xu C. A Ratiometric Fluorescent Sensor Based on Chelation-Enhanced Fluorescence of Carbon Dots for Zinc Ion Detection. Molecules. 2023; 28(23):7818. https://doi.org/10.3390/molecules28237818
Chicago/Turabian StyleLu, Guangrong, Zhenzhen Jia, Mengdi Yu, Mingzhen Zhang, and Changlong Xu. 2023. "A Ratiometric Fluorescent Sensor Based on Chelation-Enhanced Fluorescence of Carbon Dots for Zinc Ion Detection" Molecules 28, no. 23: 7818. https://doi.org/10.3390/molecules28237818
APA StyleLu, G., Jia, Z., Yu, M., Zhang, M., & Xu, C. (2023). A Ratiometric Fluorescent Sensor Based on Chelation-Enhanced Fluorescence of Carbon Dots for Zinc Ion Detection. Molecules, 28(23), 7818. https://doi.org/10.3390/molecules28237818







