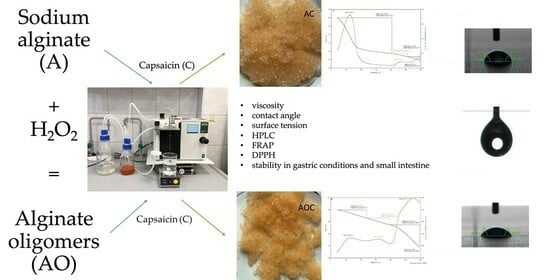
Effect of Chemical Degradation of Sodium Alginate on Capsaicin Encapsulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solutions Characteristics
2.1.1. Viscosity
2.1.2. Contact Angle
2.1.3. Surface Tension
2.2. Capsules Characteristics
2.2.1. Encapsulation Efficiency
2.2.2. Thermo-Gravimetric Analysis (TGA)
2.2.3. Antioxidant Properties
2.2.4. Stability of Capsules and Capsaicin Content under Gastric Conditions
2.2.5. Stability of Capsules and Capsaicin Content under Conditions of the Small Intestine
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.2.1. Preparation of Alginate Oligosaccharides by Oxidation with Hydrogen Peroxide (H2O2)
3.2.2. Preparation of an Oleoresin Solution
3.2.3. Encapsulation of Capsaicin in a Solution of Sodium Alginate Oligomers
3.3. Methods
3.3.1. Viscosity
3.3.2. Contact Angle Measurement
3.3.3. Surface Tension Using the Pendant Drop Method
3.3.4. Determination of Encapsulation Efficiency
3.3.5. Thermal Gravimetric Analysis (TGA)
3.3.6. Antioxidant Properties
Free Radical Scavenging Activity (DPPH)
Ferric Reducing Antioxidant Power (FRAP)
3.3.7. Stability of Capsules under Gastric Conditions
3.3.8. Stability of Capsules in Small Intestine
3.3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bede, P.M.; Prado da Silva, M.H.; Ben-Hur da Silva Figueredo, A.; Finotelli, P.V. Nanostructured magnetic alginate composites for biomedical applications. Polímeros 2017, 27, 267–272. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, T.; Vidart, J.M.M.; Carlos da Silva, M.G.; Gimenes, M.L.; Vieira, M.G.A. Alginate and Sericin: Environmental and Pharmaceutical Applications. In Biological Activities and Application of Marine Polysaccharides, 1st ed.; Shalaby, E.A., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L. Radiation-induced degradation of sodium alginate and its plant growth promotion effect. Arab. J. Chem. 2019, 10, 431–438. [Google Scholar] [CrossRef]
- Piacentini, E. Encapsulation Efficiency. In Encyclopedia of Membranes, 1st ed.; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 706–707. [Google Scholar]
- Labre, F.; Mathieu, S.; Chaud, P.; Morvan, P.Y.; Vallée, R.; Helbert, W.; Fort, S. DMTMM-mediated amidation of alginate oligosaccharides aimed at modulating their interaction with proteins. Carbohydr. Polym. 2018, 184, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Kulig, D.; Król-Kilińska, Ż.; Żarowska, B.; Bobak, Ł.; Jarmoluk, A. Biophysico-Chemical Properties of Alginate Oligomers Obtained by Acid and Oxidation Depolymerization. Polymers 2021, 13, 2258. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Encapsulation of carotenoid-rich oil from Gac peel: Optimisation of the encapsulating process using a spray drier and the storage stability of encapsulated powder. Powder Technol. 2019, 344, 373–379. [Google Scholar] [CrossRef]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Leea MF, X.; Lim, H.P.; Lim, S.L.; Tey, B.T.; Poncelet, D.; Chan, E.S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef]
- Echalier, C.; Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical cross-linking methods for cell encapsulation in hydrogels. Mater. Today Commun. 2019, 20, 100536. [Google Scholar] [CrossRef]
- Segura-Campos, R.M.; Ruiz-Ruiz, J.C.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Capsicum chinense: Composition and Functional Properties. In Functional Properties of Traditional Foods, 1st ed.; Kristbergsson, K., Ötles, S., Eds.; Springer: New York, NY, USA, 2018; Chapter 20; pp. 289–292. [Google Scholar]
- Pelofsky, A.H.J. Surface tension—Viscosity relation for liquids. Chem. Eng. Data 1966, 11, 394–397. [Google Scholar] [CrossRef]
- Chen, L.; Bonaccurso, E. Effects of surface wettability and liquid viscosity on the dynamic wetting of individual drops. Phys. Rev. E 2014, 90, 022401. [Google Scholar] [CrossRef]
- Deng, W.; Zheng, H.; Zhu, Z.; Deng, Y.; Shi, Y.; Wang, D.; Zhong, Y. Effect of Surfactant Formula on the Film Forming Capacity, Wettability, and Preservation Properties of Electrically Sprayed Sodium Alginate Coats. Foods 2023, 12, 2197. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Komes, D.; Karlović, S.; Djaković, S.; Špoljarič, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2011, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Bannikova, A.; Evteev, A.; Pankin, K.; Evdokimov, I.; Kasapis, S. Microencapsulation of fish oil with alginate: In-vitro evaluation and controlled release. LWT 2018, 90, 310–315. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.; Poncelet, D.; Renard, D. Characterization of Core-Shell Alginate Capsules. Food Biophys. 2019, 14, 467–478. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Zhang, J.; Wu, W.; Wang, M.; Fan, L. Thermal Degradation of Sodium Alginate-Incorporated Soy Protein Isolate/Glycerol Composite Membranes. In Proceedings of the 17th IAPRI World Conference on Packaging, Tianjin, China, 12–15 October 2010; pp. 402–405. [Google Scholar]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclet. Quim. 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Amna, T.; Gharsan, F.N.; Shang, K.; Hassan, M.S.; Khil, M.S.; Hwang, I. Electrospun Twin Fibers Encumbered with Intrinsic Antioxidant Activity as Prospective Bandage. Macromol. Res. 2019, 27, 663–669. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Chatzitaki, A.T.; Karavasili, C.; Katsamenis, O.L.; Tzetzis, D.; Mystiridou, E.; Bouropoulos, N.; Fatouros, D.G. Controlled Release of 5-Fluorouracil from Alginate Beads Encapsulated in 3D Printed pH-Responsive Solid Dosage Forms. AAPS Pharm. Sci. Tech. 2018, 19, 3362–3375. [Google Scholar] [CrossRef]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Development of surfactant-coated alginate capsules containing Lactobacillus plantarum. Food Hydrocoll. 2018, 82, 490–499. [Google Scholar] [CrossRef]
- Hudita, A.; Galateanu, B.; Costache, M.; Negrei, C.; Ion, R.M.; Iancu, L.; Ginghina, O. In Vitro Cytotoxic Protective Effect of Alginate-Encapsulated Capsaicin Might Improve Skin Side Effects Associated with the Topical Application of Capsaicin. Molecules 2021, 26, 1455. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Pereira, S.; Pohl, L.; Ketelhut, S.; Kemper, B.; Gorzelanny, C.; Galla, H.J.; Moerschbacher, B.M.; Goycoolea, F.M. Chitosan encapsulation modulates the effect of capsaicin on the tight junctions of MDCK cells. Sci. Rep. 2015, 5, 10048. [Google Scholar] [CrossRef] [PubMed]
- Kulig, D.; Król-Kilińska, Ż.; Bobak, Ł.; Żarowska, B.; Jarmoluk, A.; Zimoch-Korzycka, A. Functional Properties of Chitosan Oligomers Obtained by Enzymatic Hydrolysis. Polymers 2023, 15, 3801. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Yeh, J.Y.; Chen, P.C.; Hsu, C.K. Phenolic content and DPPH radical scavening activity of yam-containing surimi gels influenced by salt and heating. Asian J. Health Inf. Sci. 2007, 2, 1–11. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Analytic. Biochem. 1996, 293, 70–76. [Google Scholar] [CrossRef]
- Arenales-Sierra, I.M.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Rodríguez-Hernández, L.; Alvarez-Ramírez, J. Calcium alginate beads loaded with Mg(OH)2 improve L. casei viability under simulated gastric condition. LWT 2019, 112, 108220. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Na Bhuket, P.R.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef]
- Gorbunova, N.; Bannikova, A.; Evteev, A.; Evdokimov, I.; Kasapis, S. Alginate-based encapsulation of extracts from beta Vulgaris cv. beet greens: Stability and controlled release under simulated gastrointestinal conditions. LWT 2018, 93, 442–449. [Google Scholar] [CrossRef]




| Hydrolysis Time [h] | Viscosity [mPas] |
|---|---|
| 0 | 249.590 a ± 2.115 |
| 2 | 17.985 b ± 0.412 |
| 3 | 17.141 b ± 0.253 |
| 4 | 17.065 b ± 0.191 |
| 24 | 16.327 c ± 0.291 |
| Hydrolysis Time [h] | SFT [mN/m] |
|---|---|
| 0 | 78.297 a ± 0.176 |
| 2 | 74.680 b ± 0.278 |
| 3 | 71.187 c ± 0.240 |
| 4 | 70.510 d ± 0.392 |
| 24 | 69.133 e ± 0.450 |
| Variants | Stability Time [min] | Capsaicin Content [µg/g] |
|---|---|---|
| AO | 1080.00 b ± 2.00 | - |
| A | 95.00 a ± 1.00 | - |
| AC | 92.00 a ± 5.00 | 0.10–0.32 * |
| AOC | 1075.00 b ± 5.00 | 0.10–0.32 ** |
| Coding | Alginate [A] Concentration [%] | Alginate Oligomers [AO] Concentration [%] | Capsaicin [C] Content [%] |
|---|---|---|---|
| AO | - | 1.5 | - |
| A | 1.5 | - | - |
| AC | 1.5 | - | 0.245 |
| AOC | - | 1.5 | 0.245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulig, D.; Bobak, Ł.; Jarmoluk, A.; Szmaja, A.; Król-Kilińska, Ż.; Zimoch-Korzycka, A. Effect of Chemical Degradation of Sodium Alginate on Capsaicin Encapsulation. Molecules 2023, 28, 7844. https://doi.org/10.3390/molecules28237844
Kulig D, Bobak Ł, Jarmoluk A, Szmaja A, Król-Kilińska Ż, Zimoch-Korzycka A. Effect of Chemical Degradation of Sodium Alginate on Capsaicin Encapsulation. Molecules. 2023; 28(23):7844. https://doi.org/10.3390/molecules28237844
Chicago/Turabian StyleKulig, Dominika, Łukasz Bobak, Andrzej Jarmoluk, Aleksandra Szmaja, Żaneta Król-Kilińska, and Anna Zimoch-Korzycka. 2023. "Effect of Chemical Degradation of Sodium Alginate on Capsaicin Encapsulation" Molecules 28, no. 23: 7844. https://doi.org/10.3390/molecules28237844