From Oxidized Fatty Acids to Dimeric Species: In Vivo Relevance, Generation and Methods of Analysis
Abstract
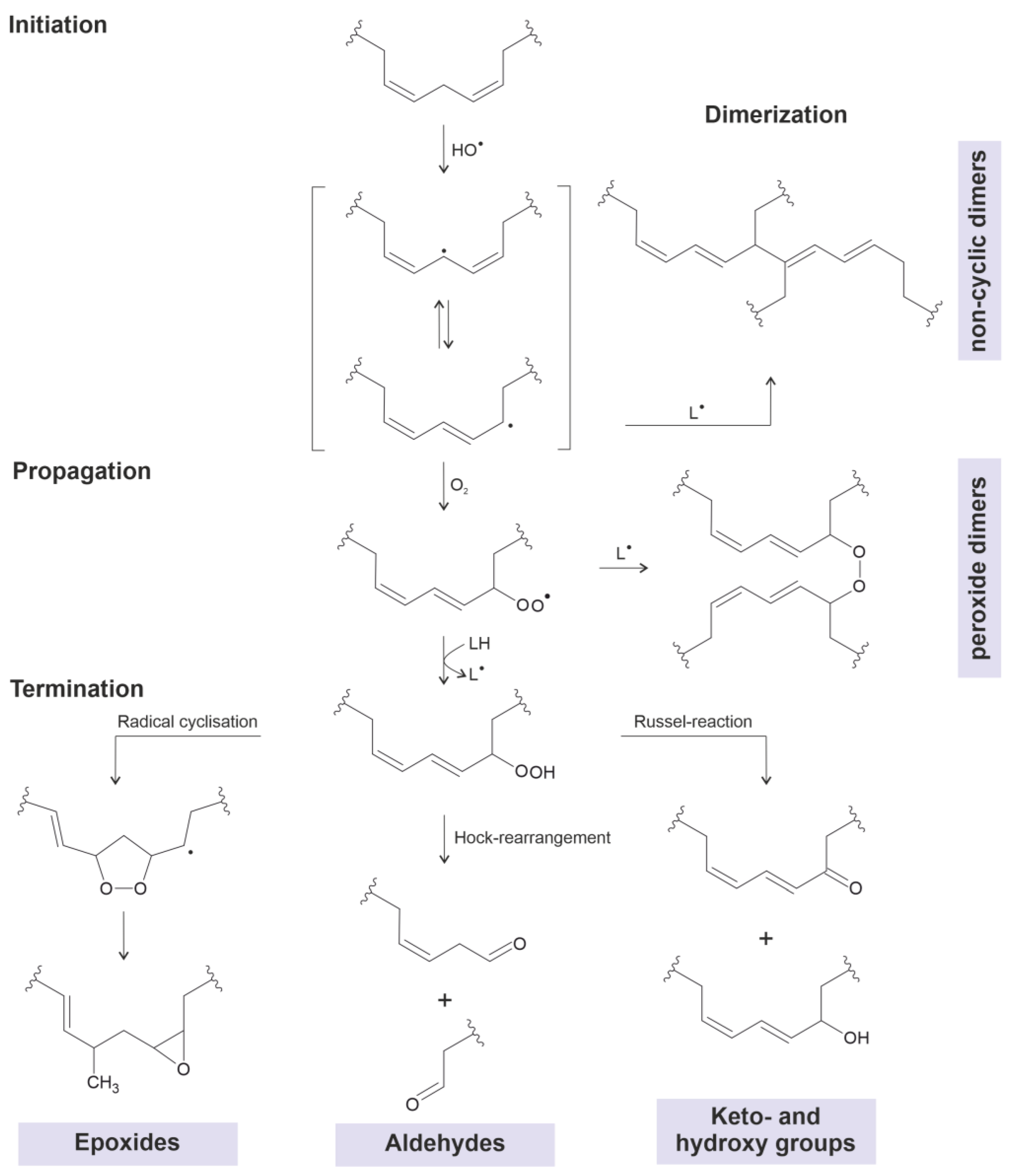
:1. Introduction
2. Industrial Relevance of Fatty Acid Dimers
3. Heat-Induced Dimerization of Vegetable Oils
- The double content of the triacylglycerols (TAG) within the oil;
- The frying temperature;
- The time of frying;
- The (residual) water content of the oil;
- The presence of transition metals which are known to foster the decomposition of initially generated lipid hydroperoxides [24];
- The presence of vitamins and natural antioxidants such as tocopherols [25].
4. Fatty Acid Dimers with In Vivo Relevance
4.1. Branched-Chain FAHFA
4.2. ω-FAHFA
4.3. Ornithine-FAHFA
4.4. Wax Esters
5. Dimeric Fatty Acids Generated by HOCl
6. Summary and Outlook
- The first compounds (industrial dimeric fatty acids) are generated in the absence of oxygen when fatty acids are heated in the presence of a suitable catalyst. They do not contain oxygen bridges but are linked via C-C linkages. They have useful mechanical properties and are widely used in industry. According to the current knowledge, these compounds are considered non-toxic since they are scarcely soluble in polar solvents. However, the question of whether they may accumulate in adipose tissue is still open.
- The second compound class arises when vegetable oils are heated in the presence of oxygen. The evaluation of the formation mechanism is difficult because the majority of investigations have been performed using complex oil mixtures. It is not yet clear whether these are harmful compounds, but it is commonly accepted that simultaneously generated aldehydes (through scission at the double-bond position) are much more harmful [34]. This particularly applies if reaction products with amino groups (fried food) are considered.
- The third class of FAHFAs is generated if a hydroxyl fatty acid reacts with a “normal” fatty acid. Their generation requires enzyme catalysis. The effects of FAHFAs as potential drugs are currently under intensive investigation. Several studies have provided convincing evidence that some FAHFAs possess antidiabetic and anti-inflammatory effects—but there may also be severe side effects. For instance, some selected FAHFAs induced hepatic steatosis and fibrosis in mice [64].
- The last compound class has only been loosely investigated so far. These fatty acid oligomers are generated as the consequence of the oxidation/chlorination of unsaturated fatty acids such as oleic acid by HOCl. In contrast to all other compounds mentioned here, neither elevated temperatures nor enzymes nor any catalysts are necessary in vitro to generate oligomers from fatty acid chlorohydrins. This makes them interesting for many reasons. These compounds may be useful to monitor the in vivo generation of HOCl because they undergo a slower metabolic turnover in comparison to the native lipids [91].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emerit, I. Reactive oxygen species, chromosome mutation, and cancer: Possible role of clastogenic factors in carcinogenesis. Free Radic. Biol. Med. 1994, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ma, M.; Tan, Z.; Zheng, H.; Liu, X. Neutrophil: A new player in metastatic cancers. Front. Immunol. 2020, 11, 565165. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Fuchs, B.; Arnhold, J.; Arnold, K. Contribution of reactive oxygen species to cartilage degradation in rheumatic diseases: Molecular pathways, diagnosis and potential therapeutic strategies. Curr. Med. Chem. 2003, 10, 2123–2145. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, G. Linoleic acid peroxidation—the dominant lipid peroxidation process in low density lipoprotein—and its relationship to chronic diseases. Chem. Phys. Lipids 1998, 95, 105–162. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Biomarkers of lipid peroxidation in clinical material. Biochim. Biophys. Acta 2014, 1840, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Tirosh, O.; Cohen, G.; Sasson, S.; Zarkovic, N. Reactive aldehydes—second messengers of free radicals in diabetes mellitus. Free Radic. Res. 2013, 47 (Suppl. 1), 39–48. [Google Scholar] [CrossRef] [PubMed]
- Sakaino, M.; Sano, T.; Kato, S.; Shimizu, N.; Ito, J.; Rahmania, H.; Imagi, J.; Nakagawa, K. Carboxylic acids derived from triacylglycerols that contribute to the increase in acid value during the thermal oxidation of oils. Sci. Rep. 2022, 12, 12460. [Google Scholar] [CrossRef]
- Browne, R.W.; Armstrong, D. HPLC analysis of lipid-derived polyunsaturated fatty acid peroxidation products in oxidatively modified human plasma. Clin. Chem. 2000, 46, 829–836. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Major, I.; Ladole, M.R.; Doke, R.B.; Patil, N.R.; Kulkarni, R.D. Dimer fatty acid—A renewable building block for high-performance polymeric materials. Ind. Crops Prod. 2023, 200, 116817. [Google Scholar] [CrossRef]
- Burg, D.A.; Kleiman, R. Preparation of meadowfoam dimer acids and dimer esters, and their use as lubricants. J. Americ. Oil Chem. Soc. 1991, 68, 600–603. [Google Scholar] [CrossRef]
- Vendamme, R.; Olaerts, K.; Gomes, M.; Degens, M.; Shigematsu, T.; Eevers, W. Interplay between viscoelastic and chemical tunings in fatty-acid-based polyester adhesives: Engineering biomass toward functionalized step-growth polymers and soft networks. Biomacromolecules 2012, 13, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- den Otter, I.M.J.A.M. The dimerization of oleic acid with a montmorillonite catalyst I: Important process parameters; some main reactions. Fette Seifen Anstrichm. 1970, 72, 667–673. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, X.; Liang, X.; Ji, J. Improving the stability and efficiency of dimeric fatty acids production by increasing the brønsted acidity and basal spacing of montmorillonite. Eur. J. Lipid Sci. Technol. 2020, 122, 1900342. [Google Scholar] [CrossRef]
- Zeman, I.; Ranný, M.; Winterová, L. Chromatographic analysis of fatty acid dimers. J. Chromatogr. A 1986, 354, 283–292. [Google Scholar] [CrossRef]
- Rao, T.; Kale, V.; Vijayalakshmi, P.; Gangadhar, A.; Subbarao, R.; Lakshminarayana, G. Chromatographic methods for the determination of monomer, dimer and trimer fractions in dimer fatty acids. J. Chromatogr. A 1989, 466, 403–406. [Google Scholar] [CrossRef]
- Kolar, M.J.; Nelson, A.T.; Chang, T.; Ertunc, M.E.; Christy, M.P.; Ohlsson, L.; Härröd, M.; Kahn, B.B.; Siegel, D.; Saghatelian, A. Faster protocol for endogenous fatty acid esters of hydroxy fatty acid (FAHFA) measurements. Anal. Chem. 2018, 90, 5358–5365. [Google Scholar] [CrossRef]
- Nelson, A.B.; Chow, L.S.; Hughey, C.C.; Crawford, P.A.; Puchalska, P. Artifactual FA dimers mimic FAHFA signals in untargeted metabolomics pipelines. J. Lipid Res. 2022, 63, 100201. [Google Scholar] [CrossRef]
- Le Gresley, A.; Ampem, G.; de Mars, S.; Grootveld, M.; Naughton, D.P. “Real-world” evaluation of lipid oxidation products and trace metals in french fries from two chain fast-food restaurants. Front. Nutr. 2021, 8, 620952. [Google Scholar] [CrossRef]
- Schiller, J.; Süß, R.; Petković, M.; Hanke, G.; Vogel, A.; Arnold, K. Effects of thermal stressing on saturated vegetable oils and isolated triacylglycerols—product analysis by MALDI-TOF mass spectrometry, NMR and IR spectroscopy. Eur. J. Lipid Sci. Technol. 2002, 104, 496–505. [Google Scholar] [CrossRef]
- Koelmel, J.P.; Aristizabal-Henao, J.J.; Ni, Z.; Fedorova, M.; Kato, S.; Otoki, Y.; Nakagawa, K.; Lin, E.Z.; Godri Pollitt, K.J.; Vasiliou, V.; et al. A novel technique for redox lipidomics using mass spectrometry: Application on vegetable oils used to fry potatoes. J. Am. Soc. Mass Spectrom. 2021, 32, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77-86. [Google Scholar] [CrossRef] [PubMed]
- Warner, K. Impact of high-temperature food processing on fats and oils. Adv. Exp. Med. Biol. 1999, 459, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Ren, F.-R.; Shan, G.-Q.; Qin, H.; Mao, L.; Zhu, B.-Z. Molecular mechanism of metal-independent decomposition of organic hydroperoxides by halogenated quinoid carcinogens and the potential biological implications. Chem. Res. Toxicol. 2015, 28, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hirooka, N.; Kajimoto, G. Microwave heating effects on relative stabilities of tocopherols in oils. J. Food Sci. 1991, 56, 1042–1046. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Tasioula-Margari, M.; Dobarganes, M.C. Quantitation and distribution of altered fatty acids in frying fats. J. Americ. Oil Chem. Soc. 1995, 72, 1171–1176. [Google Scholar] [CrossRef]
- Dobarganes, M.C. Formation of Dimers and Oligomers. Available online: https://lipidlibrary.aocs.org/chemistry/physics/frying-oils/formation-of-dimers-and-oligomers (accessed on 30 October 2023).
- Zeb, A. Chemistry and liquid chromatography methods for the analyses of primary oxidation products of triacylglycerols. Free Radic. Res. 2015, 49, 549–564. [Google Scholar] [CrossRef]
- Fullana, A.; Carbonell-Barrachina, A.A.; Sidhu, S. Comparison of volatile aldehydes present in the cooking fumes of extra virgin olive, olive, and canola oils. J. Agric. Food Chem. 2004, 52, 5207–5214. [Google Scholar] [CrossRef]
- Gibson, M.; Percival, B.C.; Edgar, M.; Grootveld, M. Low-field benchtop NMR spectroscopy for quantification of aldehydic lipid oxidation products in culinary oils during shallow frying episodes. Foods 2023, 12, 1254. [Google Scholar] [CrossRef]
- Skinner, J.; Arora, P.; McMath, N.; Penumetcha, M. Determination of oxidized lipids in commonly consumed foods and a preliminary analysis of their binding affinity to PPARγ. Foods 2021, 10, 1702. [Google Scholar] [CrossRef]
- Thompson, L.U.; Aust, R. Lipid changes in french fries and heated oils during commercial deep frying and their nutritional and toxicological implications. Can. Inst. Food Technol. J. 1983, 16, 246–253. [Google Scholar] [CrossRef]
- Cherif, A.; Slama, A. Stability and change in fatty acids composition of soybean, corn, and sunflower oils during the heating process. J. Food Qual. 2022, 2022, 6761029. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Márquez-Ruiz, G. Formation and analysis of oxidized monomeric, dimeric, and higher oligomeric triglycerides. In Deep Frying; Academic Press: Cambridge, MA, USA, 2007; pp. 87–110. ISBN 9781893997929. [Google Scholar]
- Takahashi, H.; Kato, S.; Shimizu, N.; Otoki, Y.; Ito, J.; Sakaino, M.; Sano, T.; Imagi, J.; Nakagawa, K. Elucidation of olive oil oxidation mechanisms by analysis of triacylglycerol hydroperoxide isomers using LC-MS/MS. Molecules 2022, 27, 5282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qin, W.; Li, M.; Shen, Q.; Saleh, A.S. Application of chromatographic techniques in the detection and identification of constituents formed during food frying: A review. Comp. Rev. Food Sci. Food Safe 2015, 14, 601–633. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G. Separation and Quantification of Oxidized Monomeric, Dimeric and Oligomeric Fatty Acids. Available online: https://lipidlibrary.aocs.org/chemistry/physics/frying-oils/separation-and-quantification-of-oxidized-monomeric-dimeric-and-oligomeric-fatty-acids (accessed on 30 October 2023).
- Criscuolo, A.; Nepachalovich, P.; Garcia-Del Rio, D.F.; Lange, M.; Ni, Z.; Baroni, M.; Cruciani, G.; Goracci, L.; Blüher, M.; Fedorova, M. Analytical and computational workflow for in-depth analysis of oxidized complex lipids in blood plasma. Nat. Commun. 2022, 13, 6547. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Prabutzki, P.; Leopold, J.; Nimptsch, A.; Lemmnitzer, K.; Vos, D.R.N.; Hopf, C.; Schiller, J. A new update of MALDI-TOF mass spectrometry in lipid research. Prog. Lipid Res. 2022, 86, 101145. [Google Scholar] [CrossRef]
- Engel, K.M.; Schiller, J. A comparison of PC oxidation products as detected by MALDI-TOF and ESI-IT mass spectrometry. Chem. Phys. Lipids 2017, 203, 33–45. [Google Scholar] [CrossRef]
- Tietel, Z.; Hammann, S.; Meckelmann, S.W.; Ziv, C.; Pauling, J.K.; Wölk, M.; Würf, V.; Alves, E.; Neves, B.; Domingues, M.R. An overview of food lipids toward food lipidomics. Comp. Rev. Food Sci. Food Safe. 2023, 22, 4302–4354. [Google Scholar] [CrossRef]
- Schiller, J.; Süß, R.; Petković, M.; Arnold, K. Thermal stressing of unsaturated vegetable oils: Effects analysed by MALDI-TOF mass spectrometry, 1H and 31P NMR spectroscopy. Eur. Food Res. Technol. 2002, 215, 282–286. [Google Scholar] [CrossRef]
- Patrikios, I.S.; Mavromoustakos, T.M. Monounsaturated fatty acid ether oligomers formed during heating of virgin olive oil show agglutination activity against human red blood cells. J. Agric. Food Chem. 2014, 62, 867–874. [Google Scholar] [CrossRef]
- Park, K.J.; Kim, M.; Seok, S.; Kim, Y.-W.; Kim, D.H. Quantitative analysis of cyclic dimer fatty acid content in the dimerization product by proton NMR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 149, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Spyros, A.; Dais, P. Application of (31)P NMR spectroscopy in food analysis. 1. quantitative determination of the mono- and diglyceride composition of olive oils. J. Agric. Food Chem. 2000, 48, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Dais, P.; Spyros, A. 31P NMR spectroscopy in the quality control and authentication of extra-virgin olive oil: A review of recent progress. Magn. Reson. Chem. 2007, 45, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Riecan, M.; Paluchova, V.; Lopes, M.; Brejchova, K.; Kuda, O. Branched and linear fatty acid esters of hydroxy fatty acids (FAHFA) relevant to human health. Pharmacol. Ther. 2022, 231, 107972. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. Fatty acyl esters of hydroxy fatty acid (FAHFA) lipid families. Metabolites 2020, 10, 512. [Google Scholar] [CrossRef]
- Brejchova, K.; Balas, L.; Paluchova, V.; Brezinova, M.; Durand, T.; Kuda, O. Understanding FAHFAs: From structure to metabolic regulation. Prog. Lipid Res. 2020, 79, 101053. [Google Scholar] [CrossRef]
- Ebrecht, A.C.; Mofokeng, T.M.; Hollmann, F.; Smit, M.S.; Opperman, D.J. Lactones from unspecific peroxygenase-catalyzed in-chain hydroxylation of saturated fatty acids. Org. Lett. 2023, 25, 4990–4995. [Google Scholar] [CrossRef]
- Zhu, Q.-F.; Yan, J.-W.; Ni, J.; Feng, Y.-Q. FAHFA footprint in the visceral fat of mice across their lifespan. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158639. [Google Scholar] [CrossRef]
- Slivniak, R.; Domb, A.J. Macrolactones and polyesters from ricinoleic acid. Biomacromolecules 2005, 6, 1679–1688. [Google Scholar] [CrossRef]
- Balas, L.; Feillet-Coudray, C.; Durand, T. Branched fatty acyl esters of hydroxyl fatty acids (FAHFAs), appealing beneficial endogenous fat against obesity and type-2 diabetes. Chemistry 2018, 24, 9463–9476. [Google Scholar] [CrossRef] [PubMed]
- Liberati-Čizmek, A.-M.; Biluš, M.; Brkić, A.L.; Barić, I.C.; Bakula, M.; Hozić, A.; Cindrić, M. Analysis of fatty acid esters of hydroxyl fatty acid in selected plant food. Plant Foods Hum. Nutr. 2019, 74, 235–240. [Google Scholar] [CrossRef]
- Kuda, O.; Brezinova, M.; Silhavy, J.; Landa, V.; Zidek, V.; Dodia, C.; Kreuchwig, F.; Vrbacky, M.; Balas, L.; Durand, T.; et al. Nrf2-mediated antioxidant defense and peroxiredoxin 6 are linked to biosynthesis of palmitic acid ester of 9-hydroxystearic acid. Diabetes 2018, 67, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Planey, S.L.; Zacharias, D.A. Palmitoyl acyltransferases, their substrates, and novel assays to connect them. Mol. Membr. Biol. 2009, 26, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.J.; Kamat, S.S.; Parsons, W.H.; Homan, E.A.; Maher, T.; Peroni, O.D.; Syed, I.; Fjeld, K.; Molven, A.; Kahn, B.B.; et al. Branched fatty acid esters of hydroxy fatty acids are preferred substrates of the MODY8 protein carboxyl ester lipase. Biochemistry 2016, 55, 4636–4641. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Ertunc, M.E.; Konduri, S.; Zhang, J.; Pinto, A.M.; Chu, Q.; Kahn, B.B.; Siegel, D.; Saghatelian, A. Discovery of FAHFA-containing triacylglycerols and their metabolic regulation. J. Am. Chem. Soc. 2019, 141, 8798–8806. [Google Scholar] [CrossRef]
- Cudlman, L.; Machara, A.; Vrkoslav, V.; Polášek, M.; Bosáková, Z.; Blanksby, S.J.; Cvačka, J. Characterization of triacylglycerol estolide isomers using high-resolution tandem mass spectrometry with nanoelectrospray Ionization. Biomolecules 2023, 13, 475. [Google Scholar] [CrossRef]
- Wang, J.; Liang, G.; Zhao, T.-J. Adipose triglyceride lipase: The first transacylase for FAHFAs. Life Metab. 2023, 2, loac016. [Google Scholar] [CrossRef]
- Benlebna, M.; Balas, L.; Gaillet, S.; Durand, T.; Coudray, C.; Casas, F.; Feillet-Coudray, C. Potential physio-pathological effects of branched fatty acid esters of hydroxy fatty acids. Biochimie 2021, 182, 13–22. [Google Scholar] [CrossRef]
- Tuteja, S. Good fats: Lipidomics approach identify novel regulators of glucose homeostasis. Circ. Cardiovasc. Genet. 2014, 7, 965–966. [Google Scholar] [CrossRef]
- Benlebna, M.; Balas, L.; Bonafos, B.; Pessemesse, L.; Vigor, C.; Grober, J.; Bernex, F.; Fouret, G.; Paluchova, V.; Gaillet, S.; et al. Long-term high intake of 9-PAHPA or 9-OAHPA increases basal metabolism and insulin sensitivity but disrupts liver homeostasis in healthy mice. J. Nutr. Biochem. 2020, 79, 108361. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef]
- Moyo, K.M.; Choi, J.; Chang, J.; Soedono, S.; Nguyet, D.V.H.; Song, Y.-R.; Park, S.J.; Go, G.-W.; Lee, D.Y.; Cho, K.W. 12-OAHSA is a component of olive oil and mitigates obesity-induced inflammation. J. Nutr. Biochem. 2022, 110, 109127. [Google Scholar] [CrossRef] [PubMed]
- Takumi, H.; Kato, K.; Ohto-N, T.; Nakanishi, H.; Kamasaka, H.; Kuriki, T. Analysis of fatty acid esters of hydroxyl fatty acid in nut oils and other plant oils. J. Oleo Sci. 2021, 70, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and glycolipids in plants and algae. Subcell. Biochem. 2016, 86, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Ito, J.; Shimizu, N.; Takahashi, T.; Kato, C.; Parida, I.S.; Jutanom, M.; Ishihara, K.; Nakagawa, K. Structural analysis and anti-inflammatory effect of a digalactosyldiacylglycerol-monoestolide, a characteristic glycolipid in oats. Nutrients 2022, 14, 4153. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.; Lee, J.; Moraes-Vieira, P.M.; Donaldson, C.J.; Sontheimer, A.; Aryal, P.; Wellenstein, K.; Kolar, M.J.; Nelson, A.T.; Siegel, D.; et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 2018, 27, 419–427.e4. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Guijas, C.; Astudillo, A.M.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Sequestration of 9-hydroxystearic acid in FAHFA (Fatty Acid Esters of Hydroxy Fatty Acids) as a protective mechanism for colon carcinoma cells to avoid apoptotic cell death. Cancers 2019, 11, 524. [Google Scholar] [CrossRef]
- Kokotou, M.G. Analytical methods for the determination of fatty acid esters of hydroxy fatty acids (FAHFAs) in biological samples, plants and foods. Biomolecules 2020, 10, 1092. [Google Scholar] [CrossRef]
- Iturrospe, E.; Robeyns, R.; Da Silva, K.M.; van de Lavoir, M.; Boeckmans, J.; Vanhaecke, T.; van Nuijs, A.L.N.; Covaci, A. Metabolic signature of HepaRG cells exposed to ethanol and tumor necrosis factor alpha to study alcoholic steatohepatitis by LC-MS-based untargeted metabolomics. Arch. Toxicol. 2023, 97, 1335–1353. [Google Scholar] [CrossRef]
- Ma, Y.; Kind, T.; Vaniya, A.; Gennity, I.; Fahrmann, J.F.; Fiehn, O. An in silico MS/MS library for automatic annotation of novel FAHFA lipids. J. Cheminform. 2015, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Tong, L.; Duan, X.; Petznick, A.; Wenk, M.R.; Shui, G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid Res. 2014, 55, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Ball, B.A.; Scoggin, K.; Troedsson, M.H.; Squires, E.L. Lipidomics of equine amniotic fluid: Identification of amphiphilic (O-acyl)-ω-hydroxy-fatty acids. Theriogenology 2018, 105, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Scoggin, K.; Ball, B.A.; Troedsson, M.H.; Squires, E.L. Lipidomics of equine sperm and seminal plasma: Identification of amphiphilic (O-acyl)-ω-hydroxy-fatty acids. Theriogenology 2016, 86, 1212–1221. [Google Scholar] [CrossRef]
- Kim, S.-K.; Park, Y.-C. Biosynthesis of ω-hydroxy fatty acids and related chemicals from natural fatty acids by recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2019, 103, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.E.; Ailuri, R.; Marshall, D.L.; Brown, S.H.J.; Saville, J.T.; Narreddula, V.R.; Boase, N.R.; Poad, B.L.J.; Trevitt, A.J.; Willcox, M.D.P.; et al. Mass spectrometry-directed structure elucidation and total synthesis of ultra-long chain (O-acyl)-ω-hydroxy fatty acids. J. Lipid Res. 2018, 59, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Kalužíková, A.; Vrkoslav, V.; Harazim, E.; Hoskovec, M.; Plavka, R.; Buděšínský, M.; Bosáková, Z.; Cvačka, J. Cholesteryl esters of ω-(O-acyl)-hydroxy fatty acids in vernix caseosa. J. Lipid Res. 2017, 58, 1579–1590. [Google Scholar] [CrossRef]
- Geiger, O.; González-Silva, N.; López-Lara, I.M.; Sohlenkamp, C. Amino acid-containing membrane lipids in bacteria. Prog. Lipid Res. 2010, 49, 46–60. [Google Scholar] [CrossRef]
- Zhang, X.; Ferguson-Miller, S.M.; Reid, G.E. Characterization of ornithine and glutamine lipids extracted from cell membranes of Rhodobacter sphaeroides. J. Am. Soc. Mass Spectrom. 2009, 20, 198–212. [Google Scholar] [CrossRef]
- Wood, P.L.; Erol, E. Construction of a bacterial lipidomics analytical platform: Pilot validation with bovine paratuberculosis serum. Metabolites 2023, 13, 809. [Google Scholar] [CrossRef]
- Wältermann, M.; Stöveken, T.; Steinbüchel, A. Key enzymes for biosynthesis of neutral lipid storage compounds in prokaryotes: Properties, function and occurrence of wax ester synthases/acyl-CoA: Diacylglycerol acyltransferases. Biochimie 2007, 89, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Ludovici, M.; Galante, M.; Sinagra, J.-L.; Picardo, M. Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry. J. Lipid Res. 2010, 51, 3377–3388. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Murphy, R.C. Electrospray mass spectrometry of human hair wax esters. J. Lipid Res. 2007, 48, 1231–1246. [Google Scholar] [CrossRef]
- Brasser, A.J.; Barwacz, C.A.; Dawson, D.V.; Brogden, K.A.; Drake, D.R.; Wertz, P.W. Presence of wax esters and squalene in human saliva. Arch. Oral Biol. 2011, 56, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.A.; Ndi, C.; Semple, S.J.; Buirchell, B.; Møller, B.L.; Staerk, D. PTP1B-inhibiting branched-chain fatty acid dimers from eremophila oppositifolia subsp. angustifolia Identified by high-resolution PTP1B inhibition profiling and HPLC-PDA-HRMS-SPE-NMR analysis. J. Nat. Prod. 2020, 83, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Schröter, J.; Griesinger, H.; Reuß, E.; Schulz, M.; Riemer, T.; Süß, R.; Schiller, J.; Fuchs, B. Unexpected products of the hypochlorous acid-induced oxidation of oleic acid: A study using high performance thin-layer chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2016, 1439, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Schröter, J.; Schiller, J. Chlorinated phospholipids and fatty acids: (Patho)physiological relevance, potential toxicity, and analysis of lipid chlorohydrins. Oxid. Med. Cell. Longev. 2016, 2016, 8386362. [Google Scholar] [CrossRef] [PubMed]
- Schröter, J.; Süß, R.; Schiller, J. MALDI-TOF MS to monitor the kinetics of phospholipase A2-digestion of oxidized phospholipids. Methods 2016, 104, 41–47. [Google Scholar] [CrossRef]
- De-Madaria, E.; Molero, X.; Bonjoch, L.; Casas, J.; Cárdenas-Jaén, K.; Montenegro, A.; Closa, D. Oleic acid chlorohydrin, a new early biomarker for the prediction of acute pancreatitis severity in humans. Ann. Intensive Care 2018, 8, 1. [Google Scholar] [CrossRef]
- Franco-Pons, N.; Casas, J.; Fabriàs, G.; Gea-Sorlí, S.; de-Madaria, E.; Gelpí, E.; Closa, D. Fat necrosis generates proinflammatory halogenated lipids during acute pancreatitis. Ann. Surg. 2013, 257, 943–951. [Google Scholar] [CrossRef]
- Brennan, E.; Kantharidis, P.; Cooper, M.E.; Godson, C. Pro-resolving lipid mediators: Regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 2021, 17, 725–739. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leopold, J.; Prabutzki, P.; Engel, K.M.; Schiller, J. From Oxidized Fatty Acids to Dimeric Species: In Vivo Relevance, Generation and Methods of Analysis. Molecules 2023, 28, 7850. https://doi.org/10.3390/molecules28237850
Leopold J, Prabutzki P, Engel KM, Schiller J. From Oxidized Fatty Acids to Dimeric Species: In Vivo Relevance, Generation and Methods of Analysis. Molecules. 2023; 28(23):7850. https://doi.org/10.3390/molecules28237850
Chicago/Turabian StyleLeopold, Jenny, Patricia Prabutzki, Kathrin M. Engel, and Jürgen Schiller. 2023. "From Oxidized Fatty Acids to Dimeric Species: In Vivo Relevance, Generation and Methods of Analysis" Molecules 28, no. 23: 7850. https://doi.org/10.3390/molecules28237850
APA StyleLeopold, J., Prabutzki, P., Engel, K. M., & Schiller, J. (2023). From Oxidized Fatty Acids to Dimeric Species: In Vivo Relevance, Generation and Methods of Analysis. Molecules, 28(23), 7850. https://doi.org/10.3390/molecules28237850







