Investigation of Minerals Extracted during Seawater Desalination Using Two-Dimensional Correlation Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
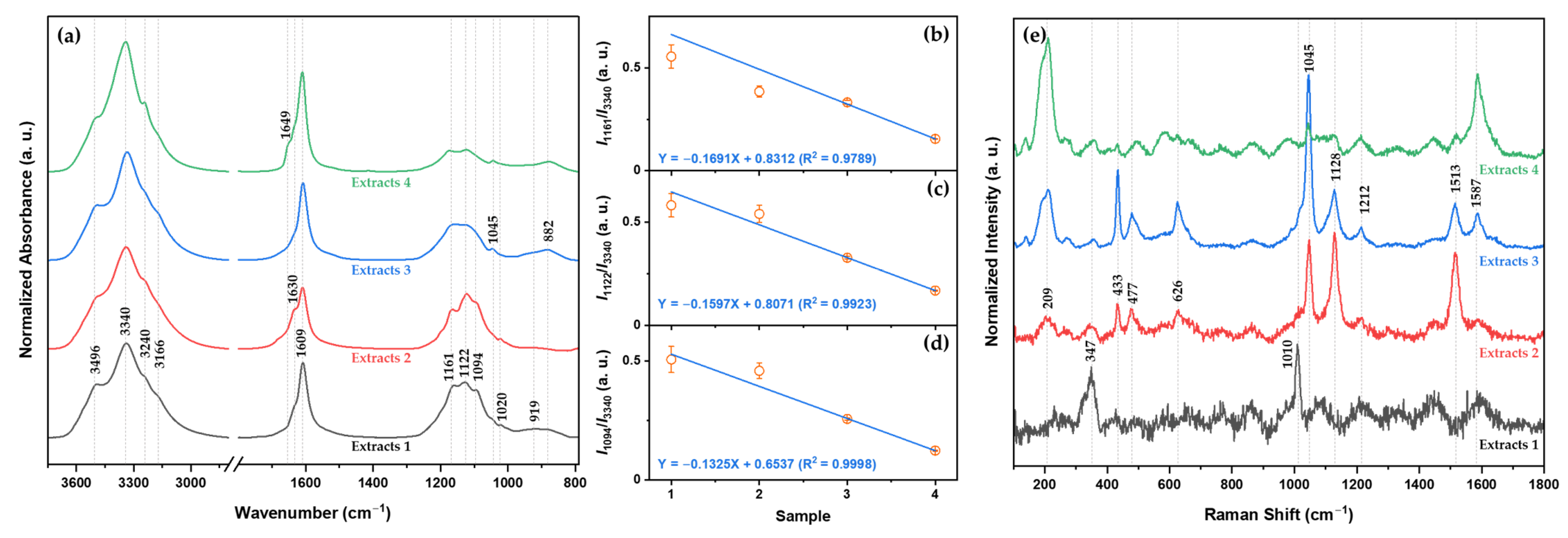
2.1. Identification of the Extracted Mineral Composition during the Desalination Process
Ions and Compounds of Each Extract
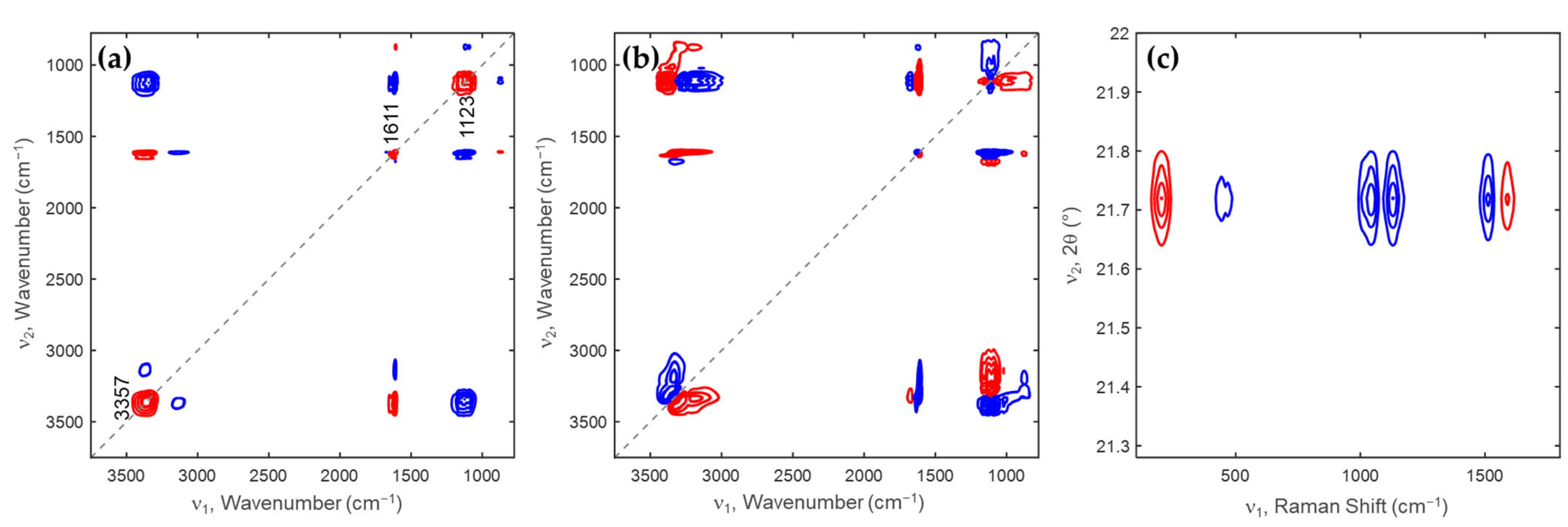
2.2. Two-Dimensional Correlation Spectroscopy Analysis of Minerals Extracted from Each Desalination Step
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.; Fan, N.; Liu, Y.; Liu, X.; Wang, Z.; Xie, X.; Jia, Y. Deep seabed mining: Frontiers in engineering geology and environment. Int. J. Coal Sci. Technol. 2023, 10, 23. [Google Scholar] [CrossRef]
- Vysetti, B. Deep-sea mineral deposits as a future source of critical metals, and environmental issues—A brief review. Miner. Miner. Mater. 2023, 2, 5. [Google Scholar] [CrossRef]
- Martínez-Alvarez, V.; González-Ortega, M.J.; Martin-Gorriz, B.; Soto-García, M.; Maestre-Valero, J.F. 14—Seawater Desalination for Crop Irrigation—Current Status and Perspectives. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 461–492. [Google Scholar]
- Nigiz, F.U.; Hilmioglu, N.D. Chapter 4.6—Vacuum Stripping Membrane Desalination for Marmara Seawater. In Exergetic, Energetic and Environmental Dimensions; Dincer, I., Colpan, C.O., Kizilkan, O., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1017–1027. [Google Scholar]
- Seubert, E.L.; Caron, D.A. Chapter 13—Impact of Algal Blooms and Their Toxins on Reverse Osmosis Desalination Plant Operations. In Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 489–504. [Google Scholar]
- Do Thi, H.T.; Tóth, A.J. Investigation of Carbon Footprints of Three Desalination Technologies: Reverse Osmosis (RO), Multi-Stage Flash Distillation (MSF) and Multi-Effect Distillation (MED). Period. Polytech. Chem. Eng. 2023, 67, 41–48. [Google Scholar] [CrossRef]
- Aladwani, S.H.; Al-Obaidi, M.A.; Mujtaba, I.M. Performance of reverse osmosis based desalination process using spiral wound membrane: Sensitivity study of operating parameters under variable seawater conditions. Clean. Eng. Technol. 2021, 5, 100284. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Bragg-Sitton, S. 13—Hybrid energy systems (HESs) using small modular reactors (SMRs). In Handbook of Small Modular Nuclear Reactors; Carelli, M.D., Ingersoll, D.T., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 319–350. [Google Scholar]
- Thabit, Q.; Nassour, A.; Nelles, M. Water Desalination Using the Once-through Multi-Stage Flash Concept: Design and Modeling. Materials 2022, 15, 6131. [Google Scholar] [CrossRef]
- Alhimali, H.; Jafary, T.; Al-Mamun, A.; Baawain, M.S.; Vakili-Nezhaad, G.R. New insights into the application of microbial desalination cells for desalination and bioelectricity generation. Biofuel Res. J. 2019, 6, 1090–1099. [Google Scholar] [CrossRef]
- Minas, K.; Karunakaran, E.; Bond, T.; Gandy, C.; Honsbein, A.; Madsen, M.; Amezaga, J.; Amtmann, A.; Templeton, M.R.; Biggs, C.A.; et al. Biodesalination: An emerging technology for targeted removal of Na+ and Cl− from seawater by cyanobacteria. Desalin. Water Treat. 2014, 55, 2647–2668. [Google Scholar] [CrossRef]
- Mourad, A.A.H.; Mohammad, A.F.; Al-Marzouqi, A.H.; Altarawneh, M.; Al-Marzouqi, M.H.; El-Naas, M.H. A process for CO2 capture and brine salinity reduction through reaction with potassium hydroxide: A multi-stage evaluation. J. Nat. Gas Sci. Eng. 2022, 106, 104756. [Google Scholar] [CrossRef]
- Truong-Lam, H.S.; Seo, S.D.; Jeon, C.; Lee, G.-p.; Lee, J.D. A gas hydrate process for high-salinity water and wastewater purification. Desalination 2022, 529, 115651. [Google Scholar] [CrossRef]
- Kress, N.; Gertner, Y.; Shoham-Frider, E. Seawater quality at the brine discharge site from two mega size seawater reverse osmosis desalination plants in Israel (Eastern Mediterranean). Water Res. 2020, 171, 115402. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Cheng, G.; Wu, Y.; Hou, X.; Wang, W.; Yin, J. Mineral particles fouling analysis and cleaning in seawater reverse osmosis desalination. Desalin. Water Treat. 2020, 198, 71–79. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef]
- Bang, J.-H.; Yoo, Y.; Lee, S.-W.; Song, K.; Chae, S. CO2 Mineralization Using Brine Discharged from a Seawater Desalination Plant. Minerals 2017, 7, 207. [Google Scholar] [CrossRef]
- Mirrielees, J.A.; Kirpes, R.M.; Haas, S.M.; Rauschenberg, C.D.; Matrai, P.A.; Remenapp, A.; Boschi, V.L.; Grannas, A.M.; Pratt, K.A.; Ault, A.P. Probing Individual Particles Generated at the Freshwater-Seawater Interface through Combined Raman, Photothermal Infrared, and X-ray Spectroscopic Characterization. ACS Meas. Sci. Au 2022, 2, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Wang, B.; Guo, X.-J.; Zou, C.-W.; Tan, X.-D. Investigating adsorption performance of heavy metals onto humic acid from sludge using Fourier-transform infrared combined with two-dimensional correlation spectroscopy. Environ. Sci. Pollut. Res. 2019, 26, 9842–9850. [Google Scholar] [CrossRef]
- Jiang, Q.-H.; Zhang, C.-B.; Yang, J.; Zhao, Y.; Xu, Y.-Z.; Wang, D.-J. Investigation on structural changes of isotactic polypropylene mesophase in the heating process by using two-dimensional infrared correlation spectroscopy. Chin. Chem. Lett. 2015, 26, 197–199. [Google Scholar] [CrossRef]
- Dong, L.; Shan, C.; Liu, Y.; Sun, H.; Yao, B.; Gong, G.; Jin, X.; Wang, S. Characterization and Mechanistic Study of Heavy Metal Adsorption by Facile Synthesized Magnetic Xanthate-Modified Chitosan/Polyacrylic Acid Hydrogels. Int. J. Environ. Res. Public Health 2022, 19, 11123. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Yang, A.; Zhu, Q.; Sun, H.; Sun, P.; Yao, B.; Zang, Y.; Du, X.; Dong, L. Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water. Polymers 2022, 14, 1107. [Google Scholar] [CrossRef]
- Ryabchikov, B.E.; Panteleev, A.A.; Gladush, M.G. Performance testing of seawater desalination by nanofiltration. Pet. Chem. 2012, 52, 465–474. [Google Scholar] [CrossRef]
- Shawky, H.A.; Abdel Fatah, A.A.; Abo ElFadl, M.M.S.; El-Aassar, A.H.M. Design of a small mobile PV-driven RO water desalination plant to be deployed at the northwest coast of Egypt. Desalin. Water Treat. 2015, 55, 3755–3766. [Google Scholar] [CrossRef]
- Noda, I. Generalized two-dimensional correlation method applicable to infrared, Raman, and other types of spectroscopy. Appl. Spectrosc. 1993, 47, 1329–1336. [Google Scholar] [CrossRef]
- Noda, I.; Ozaki, Y. Two-Dimensional Correlation Spectroscopy: Applications in Vibrational and Optical Spectroscopy; John Wiley & Sons Ltd.: Chichester, UK, 2005. [Google Scholar]
- Sharkh, B.A.; Al-Amoudi, A.A.; Farooque, M.; Fellows, C.M.; Ihm, S.; Lee, S.; Li, S.; Voutchkov, N. Seawater desalination concentrate—A new frontier for sustainable mining of valuable minerals. NPJ Clean Water 2022, 5, 9. [Google Scholar] [CrossRef]
- McNally, J.S.; Foo, Z.H.; Deshmukh, A.; Orme, C.J.; Lienhard, J.H.; Wilson, A.D. Solute displacement in the aqueous phase of water-NaCl-organic ternary mixtures relevant to solvent-driven water treatment. RSC Adv. 2020, 10, 29516–29527. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Ye, P.; Cheng, W.; Cheng, H.; Du, Z.; Cheng, F. Selective adsorption of octadecylamine hydrochloride on the surface of KCl crystal: Adsorption energy based on density functional theory. Adsorption 2019, 25, 1553–1558. [Google Scholar] [CrossRef]
- Haseli, P.; Majewski, P.; Christo, F.; Raven, M.; Klose, S.; Bruno, F. Experimental Kinetic Analysis of Potassium Extraction from Ultrapotassic Syenite Using NaCl-CaCl2 Salt Mixture. ACS Omega 2020, 5, 16421–16429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Li, J.; Sun, X. Processing, microstructure and mechanical properties of biomedical magnesium with a specific two-layer structure. Prog. Nat. Sci. Mater. 2013, 23, 183–189. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Dashti, A.; Nasiri, S.; Monshi, A.; Vilkauskas, A.; Janusas, G. Measurement Modulus of Elasticity Related to the Atomic Density of Planes in Unit Cell of Crystal Lattices. Materials 2020, 13, 4380. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Linares-Fernandez, L.; Doehne, E.; Sebastian, E. Effects of ferrocyanide ions on NaCl crystallization in porous stone. J. Cryst. Growth 2002, 243, 503–516. [Google Scholar] [CrossRef]
- Abdou, S.M.; Moharam, H. Characterization of table salt samples from different origins and ESR detection of the induced effects due to gamma irradiation. J. Phys. Conf. Ser. 2019, 1253, 012036. [Google Scholar] [CrossRef]
- Ferchaud, C.J.; Zondag, H.A.; Veldhuis, J.B.J.; Boer, R.D. Study of the reversible water vapour sorption process of MgSO4.7H2O and MgCl2.6H2O under the conditions of seasonal solar heat storage. J. Phys. Conf. Ser. 2012, 395, 012069. [Google Scholar] [CrossRef]
- Nalajala, V.S.; Kothamasu, N.J.; Mandapati, R.N.; Chaganti, R.V.S.N.; Subbaiah, T. Preparation of Magnesium Chloride from Sea Water Bitterns using Techniques of Spray and Thermal Drying. J. Mater. Eng. Perform. 2023, 32, 2537–2542. [Google Scholar] [CrossRef]
- Shaddel, S.; Grini, T.; Ucar, S.; Azrague, K.; Andreassen, J.P.; Osterhus, S.W. Struvite crystallization by using raw seawater: Improving economics and environmental footprint while maintaining phosphorus recovery and product quality. Water Res. 2020, 173, 115572. [Google Scholar] [CrossRef] [PubMed]
- Kelch, S.E.; Youngman, R.E.; Ferrage, E.; Basinski, J.J.; Wang, J.; Aristilde, L. Quantitative Spectroscopic Analysis of Water Populations in the Hydrated Nanopore Environments of a Natural Montmorillonite. J. Phys. Chem. C 2021, 125, 26552–26565. [Google Scholar] [CrossRef]
- Bales, C.; Gantenbein, P.; Jaenig, D.; Kerskes, H.; Summer, K.; Van Essen, M.; Weber, R. Laboratory Tests of Chemical Reactions and Prototype Sorption Storage Units: Report B4 of Subtask B. 2008. Available online: https://api.semanticscholar.org/CorpusID:96465242 (accessed on 1 October 2023).
- Okhrimenko, L.; Favergeon, L.; Johannes, K.; Kuznik, F. New kinetic model of the dehydration reaction of magnesium sulfate hexahydrate: Application for heat storage. Thermochim. Acta 2020, 687, 178569. [Google Scholar] [CrossRef]
- Seeger, M.; Otto, W.; Flick, W.; Bickelhaupt, F.; Akkerman, O.S. Magnesium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Bardi, U. Extracting Minerals from Seawater: An Energy Analysis. Sustainability 2010, 2, 1–13. [Google Scholar] [CrossRef]
- Mudkudkar, N. Design and Fabrication of Solar Desalination Unit. Int. J. Res. Appl. Sci. Eng. Technol. 2019, 7, 2509–2516. [Google Scholar] [CrossRef]
- Zhao, L.J.; Zhang, Y.H.; Wei, Z.F.; Cheng, H.; Li, X.H. Magnesium sulfate aerosols studied by FTIR spectroscopy: Hygroscopic properties, supersaturated structures, and implications for seawater aerosols. J. Phys. Chem. A 2006, 110, 951–958. [Google Scholar] [CrossRef]
- Smith, D.H.; Seshadri, K.S. Infrared spectra of Mg2Ca(SO4)3, MgSO4, hexagonal CaSO4, and orthorhombic CaSO4. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1999, 55, 795–805. [Google Scholar] [CrossRef]
- Lesiecki, M.L.; Nibler, J.W. Infrared and Raman spectra and structures of matrix isolated magnesium dihalides: MgF2, MgCl2, MgBr2, and MgI2. J. Chem. Phys. 2008, 64, 871–884. [Google Scholar] [CrossRef]
- Cziczo, D.J.; Abbatt, J.P.D. Infrared Observations of the Response of NaCl, MgCl2, NH4HSO4, and NH4NO3 Aerosols to Changes in Relative Humidity from 298 to 238 K. J. Phys. Chem. A 2000, 104, 2038–2047. [Google Scholar] [CrossRef]
- Eom, H.-J.; Gupta, D.; Cho, H.-R.; Hwang, H.J.; Hur, S.D.; Gim, Y.; Ro, C.-U. Single-particle investigation of summertime and wintertime Antarctic sea spray aerosols using low-Z particle EPMA, Raman microspectrometry, and ATR-FTIR imaging techniques. Atmos. Chem. Phys. 2016, 16, 13823–13836. [Google Scholar] [CrossRef]
- Prieto-Taboada, N.; Gomez-Laserna, O.; Martinez-Arkarazo, I.; Olazabal, M.A.; Madariaga, J.M. Raman spectra of the different phases in the CaSO4-H2O system. Anal. Chem. 2014, 86, 10131–10137. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.M.; Gomer, N.R.; Sharma, S.K.; McKay, C. Remote Raman Spectroscopy for Planetary Exploration: A Review. Appl. Spectrosc. 2012, 66, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Freeman, J.J.; Jolliff, B.L.; Chou, I.M. Sulfates on Mars: A systematic Raman spectroscopic study of hydration states of magnesium sulfates. Geochim. Cosmochim. Acta 2006, 70, 6118–6135. [Google Scholar] [CrossRef]
- Shi, E.; Wang, A.; Ling, Z. MIR, VNIR, NIR, and Raman spectra of magnesium chlorides with six hydration degrees: Implication for Mars and Europa. J. Raman Spectrosc. 2019, 51, 1589–1602. [Google Scholar] [CrossRef]
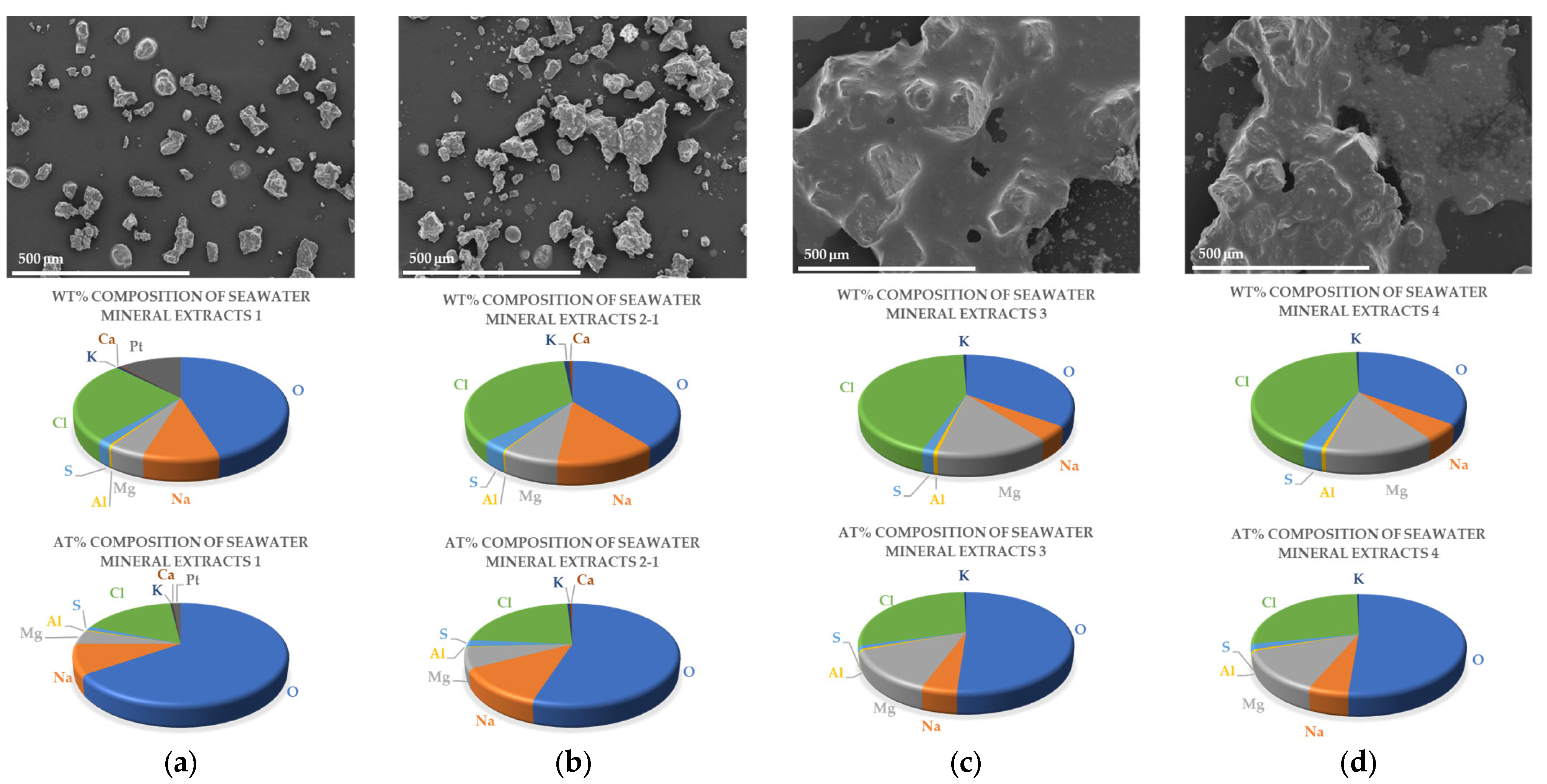

| Sample | Extract 1 | Extract 2 | Extract 3 | Extract 4 | |
|---|---|---|---|---|---|
| Atoms | |||||
| Oxygen (O) | Weight (%) | 45.16 | 39.22 | 35 | 35.26 |
| Atomic (%) | 65.38 | 54.97 | 51.12 | 51.29 | |
| Sodium (Na) | Weight (%) | 9.61 | 12.69 | 4.43 | 5.02 |
| Atomic (%) | 9.68 | 12.38 | 4.5 | 5.09 | |
| Magnesium (Mg) | Weight (%) | 4.68 | 7.18 | 14.16 | 13.87 |
| Atomic (%) | 4.46 | 6.62 | 13.61 | 13.28 | |
| Aluminium (Al) | Weight (%) | 0.37 | 0.23 | 0.53 | 0.53 |
| Atomic (%) | 0.32 | 0.19 | 0.46 | 0.46 | |
| Sulfur (S) | Weight (%) | 1.83 | 3.22 | 1.44 | 2.51 |
| Atomic (%) | 1.32 | 2.25 | 1.05 | 1.82 | |
| Chlorine (Cl) | Weight (%) | 25.95 | 35.86 | 43.85 | 42.35 |
| Atomic (%) | 16.96 | 22.68 | 28.9 | 27.8 | |
| Potassium (K) | Weight (%) | 0.57 | 0.99 | 0.58 | 0.45 |
| Atomic (%) | 0.34 | 0.57 | 0.35 | 0.27 | |
| Calcium (Ca) | Weight (%) | 0.32 | 0.61 | 0 | 0 |
| Atomic (%) | 0.19 | 0.34 | 0 | 0 | |
| Platinum (Pt) | Weight (%) | 11.5 | 0 | 0 | 0 |
| Atomic (%) | 1.37 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Park, Y.; Jung, Y.M. Investigation of Minerals Extracted during Seawater Desalination Using Two-Dimensional Correlation Spectroscopy. Molecules 2023, 28, 7852. https://doi.org/10.3390/molecules28237852
Ji H, Park Y, Jung YM. Investigation of Minerals Extracted during Seawater Desalination Using Two-Dimensional Correlation Spectroscopy. Molecules. 2023; 28(23):7852. https://doi.org/10.3390/molecules28237852
Chicago/Turabian StyleJi, Ho, Yeonju Park, and Young Mee Jung. 2023. "Investigation of Minerals Extracted during Seawater Desalination Using Two-Dimensional Correlation Spectroscopy" Molecules 28, no. 23: 7852. https://doi.org/10.3390/molecules28237852
APA StyleJi, H., Park, Y., & Jung, Y. M. (2023). Investigation of Minerals Extracted during Seawater Desalination Using Two-Dimensional Correlation Spectroscopy. Molecules, 28(23), 7852. https://doi.org/10.3390/molecules28237852








