Acidity Quantification and Structure Analysis of Amide-AlCl3 Liquid Coordination Complexes for C4 Alkylation Catalysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. 27Al NMR and Raman Spectroscopic Analysis
2.2. Experimental Measurement of LCC Acidity
2.3. DFT Calculations of FIA Values
2.4. Computed FIA, Δδ31Pexp, and Structure
3. Materials and Methods
3.1. Materials
3.2. Preparation of LCCs
3.3. Characterization
3.4. Computed FIA
3.5. The C4 Alkylation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, F.; Srinivasan, G.; Swadźba-Kwaśny, M. Liquid Coordination Complexes Formed by the Heterolytic Cleavage of Metal Halides. Angew. Chem. Int. Ed. 2013, 52, 12582–12586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108. [Google Scholar] [CrossRef] [PubMed]
- Kore, R.; Kelley, S.P.; Sawant, A.D.; Mishra, M.K.; Rogers, R.D. Are Ionic Liquids and Liquid Coordination Complexes Really Different?-Synthesis, Characterization, and Catalytic Activity of AlCl3base Catalysts. Chem. Commun. 2020, 56, 5362–5365. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.M.; Brown, L.C.; Matuszek, K.; Latos, P.; Chrobok, A.; Swadźba-Kwaśny, M. Liquid Coordination Complexes of Lewis Acidic Metal Chlorides: Lewis Acidity and Insights into Speciation. Dalt. Trans. 2017, 46, 11561–11574. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.; Hogg, J.M.; Swadźba-Kwaśny, M. Lewis Acidic Ionic Liquids. Top. Curr. Chem. 2017, 375, 78. [Google Scholar] [CrossRef]
- Kore, R.; Uppara, P.V.; Rogers, R.D. Replacing HF or AlCl3 in the Acylation of Isobutylbenzene with Chloroaluminate Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 10330–10334. [Google Scholar] [CrossRef]
- Kore, R.; Berton, P.; Kelley, S.P.; Aduri, P.; Katti, S.S.; Rogers, R.D. Group IIIA Halometallate Ionic Liquids: Speciation and Applications in Catalysis. ACS Catal. 2017, 7, 7014–7028. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Hogg, J.M.; Coleman, F.; Ferrer-Ugalde, A.; Atkins, M.P.; Swadźba-Kwaśny, M. Liquid Coordination Complexes: A New Class of Lewis Acids as Safer Alternatives to BF3 in Synthesis of Polyalphaolefins. Green Chem. 2015, 17, 1831–1841. [Google Scholar] [CrossRef]
- Atwood, D.A. Cationic Group 13 Complexes. Coord. Chem. Rev. 1998, 176, 407–430. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, R.; Meng, X.; Liu, H.; Xu, C.; Liu, Z. Structural and Spectroscopic Characterizations of Amide–AlCl 3 -Based Ionic Liquid Analogues. Inorg. Chem. 2016, 55, 2374–2380. [Google Scholar] [CrossRef]
- Hu, P.; Jiang, W.; Zhong, L.; Zhou, S.-F. Determination of the Lewis Acidity of Amide–AlCl3 Based Ionic Liquid Analogues by Combined in Situ IR Titration and NMR Methods. RSC Adv. 2018, 8, 13248–13252. [Google Scholar] [CrossRef] [PubMed]
- Abood, H.M.A.; Abbott, A.P.; Ballantyne, A.D.; Ryder, K.S. Do All Ionic Liquids Need Organic Cations? Characterisation of [AlCl2·nAmide]+ AlCl4− and Comparison with Imidazolium Based Systems. Chem. Commun. 2011, 47, 3523. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-S.; Kim, K.-W.; Cho, H.-G. Solvation of AlCl3 in Deuterated Acetonitrile Studied by Means of Vibrational Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 477–486. [Google Scholar] [CrossRef]
- Dalibart, M.; Derouault, J.; Granger, P.; Chapelle, S. Spectroscopic Investigations of Complexes between Acetonitrile and Aluminum Trichloride. 1. Aluminum Chloride-Acetonitrile Solutions. Inorg. Chem. 1982, 21, 1040–1046. [Google Scholar] [CrossRef]
- Campos, T.B.C.; da Silva, E.F.; Alves, W.A. A Raman Study on the Coordination Sites and Stability of the [Al(Formamide)5]Cl3 Complex. Vib. Spectrosc. 2013, 65, 24–27. [Google Scholar] [CrossRef]
- Alves, C.C.; Campos, T.B.C.; Alves, W.A. FT-Raman Spectroscopic Analysis of the Most Probable Structures in Aluminum Chloride and Tetrahydrofuran Solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Kore, R.; Scurto, A.M.; Shiflett, M.B. Review of Isobutane Alkylation Technology Using Ionic Liquid-Based Catalysts–Where Do We Stand? Ind. Eng. Chem. Res. 2020, 59, 15811–15838. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, G.; Hu, R.; Gao, G. Effects of Aromatics on Ionic Liquids for C4 Alkylation Reaction: Insights from Scale-up Experiment and Molecular Dynamics Simulation. Chem. Eng. J. 2020, 402, 126252. [Google Scholar] [CrossRef]
- Liu, G.; Wu, G.; Liu, Y.; Hu, R.; Gao, G. Theoretical Study on the C4 Alkylation Mechanism Catalyzed by Cu-Containing Chloroaluminate Ionic Liquids. Fuel 2022, 310, 122379. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, R.; Xu, C.; Su, H. Alkylation of Isobutene with 2-Butene Using Composite Ionic Liquid Catalysts. Appl. Catal. A Gen. 2008, 346, 189–193. [Google Scholar] [CrossRef]
- Sanchez, B.; Campodónico, P.R.; Contreras, R. Gutmann’s Donor and Acceptor Numbers for Ionic Liquids and Deep Eutectic Solvents. Front. Chem. 2022, 10, 861379. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, P.; Greb, L. Multidimensional Lewis Acidity: A Consistent Data Set of Chloride, Hydride, Methide, Water and Ammonia Affinities for 183 p-Block Element Lewis Acids. ChemPhysChem 2021, 22, 935–943. [Google Scholar] [CrossRef]
- Erdmann, P.; Greb, L. What Distinguishes the Strength and the Effect of a Lewis Acid: Analysis of the Gutmann–Beckett Method. Angew. Chemie 2022, 134, e202114550. [Google Scholar] [CrossRef]
- Mayer, U.; Gutmann, V.; Gerger, W. The Acceptor Number—A Quantitative Empirical Parameter for the Electrophilic Properties of Solvents. Monatshefte Für Chem. 1975, 106, 1235–1257. [Google Scholar] [CrossRef]
- Gutmann, V. The Donor-Acceptor Approach to Molecular Interactions; Springer: Boston, MA, USA, 1978; pp. 12–29. ISBN 978-1-4615-8827-6. [Google Scholar]
- Beckett, M.A.; Strickland, G.C.; Holland, J.R.; Sukumar Varma, K. A Convenient n.m.r. Method for the Measurement of Lewis Acidity at Boron Centres: Correlation of Reaction Rates of Lewis Acid Initiated Epoxide Polymerizations with Lewis Acidity. Polymer 1996, 37, 4629–4631. [Google Scholar] [CrossRef]
- Kerridge, D.H. The Chemistry of Molten Acetamide and Acetamide Complexes. Chem. Soc. Rev. 1988, 17, 181–227. [Google Scholar] [CrossRef]
- Jupp, A.R.; Johnstone, T.C.; Stephan, D.W. The Global Electrophilicity Index as a Metric for Lewis Acidity. Dalt. Trans. 2018, 47, 7029–7035. [Google Scholar] [CrossRef]
- Böhrer, H.; Trapp, N.; Himmel, D.; Schleep, M.; Krossing, I. From Unsuccessful H2-Activation with FLPs Containing B(Ohfip)3 to a Systematic Evaluation of the Lewis Acidity of 33 Lewis Acids Based on Fluoride, Chloride, Hydride and Methyl Ion Affinities. Dalt. Trans. 2015, 44, 7489–7499. [Google Scholar] [CrossRef]
- Erdmann, P.; Leitner, J.; Schwarz, J.; Greb, L. An Extensive Set of Accurate Fluoride Ion Affinities for p -Block Element Lewis Acids and Basic Design Principles for Strong Fluoride Ion Acceptors. ChemPhysChem 2020, 21, 987–994. [Google Scholar] [CrossRef]
- Meek, D.W.; Drago, R.S. A Coördination Model as an Alternative to the Solvent System Concept in Some Oxychloride Solvents. I. Similarity in the Behavior of Phosphorus Oxychloride and Triethyl Phosphate as Non-Aqueous Solvents. J. Am. Chem. Soc. 1961, 83, 4322–4325. [Google Scholar] [CrossRef]
- Abbott, A.P.; Al-Barzinjy, A.A.; Abbott, P.D.; Frisch, G.; Harris, R.C.; Hartley, J.; Ryder, K.S. Speciation, Physical and Electrolytic Properties of Eutectic Mixtures Based on CrCl3·6H2O and Urea. Phys. Chem. Chem. Phys. 2014, 16, 9047–9055. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.; Fraile, J.M. Study of Interactions between Brønsted Acids and Triethylphosphine Oxide in Solution by 31P NMR: Evidence for 2:1 Species. Phys. Chem. Chem. Phys. 2020, 22, 24351–24358. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Frye, J.S.; Reynolds, G.F. 27A1 and 13C NMR Studies of Aluminum Chloride-Dialkylimidazolium Chloride Molten Salts. Inorg. Chem. 1983, 22, 3870–3872. [Google Scholar] [CrossRef]
- Cui, J.; De With, J.; Klusener, P.A.A.; Su, X.; Meng, X.; Zhang, R.; Liu, Z.; Xu, C.; Liu, H. Identification of Acidic Species in Chloroaluminate Ionic Liquid Catalysts. J. Catal. 2014, 320, 26–32. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Liu, H.; Meng, X.; Xu, C.; Liu, Z.; Klusener, P.A.A. Quantitative Characterization of Lewis Acidity and Activity of Chloroaluminate Ionic Liquids. Ind. Eng. Chem. Res. 2016, 55, 11878–11886. [Google Scholar] [CrossRef]
- Gale, R.J.; Gilbert, B.; Osteryoung, R.A. Raman Spectra of Molten Aluminum Chloride: 1-Butylpyridinium Chloride Systems at Ambient Temperatures. Inorg. Chem. 1978, 17, 2728–2729. [Google Scholar] [CrossRef]
- Wicelinski, S.P.; Gale, R.J.; Williams, S.D.; Mamantov, G. Raman Spectra of Molten Gallium Chloride: 1-Methyl-3-Ethylimidazolium Chloride at Ambient Temperatures. Spectrochim. Acta Part A Mol. Spectrosc. 1989, 45, 759–762. [Google Scholar] [CrossRef]
- Corma, A.; Martínez, A. Chemistry, Catalysts, and Processes for Isoparaffin–Olefin Alkylation: Actual Situation and Future Trends. Catal. Rev. Sci. Eng. 1993, 35, 483–570. [Google Scholar] [CrossRef]
- Estager, J.; Oliferenko, A.A.; Seddon, K.R.; Swadźba-Kwaśny, M. Chlorometallate(III) Ionic Liquids as Lewis Acidic Catalysts—A Quantitative Study of Acceptor Properties. Dalt. Trans. 2010, 39, 11375. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Purvis, G.D.; Bartlett, R.J. A Full Coupled-Cluster Singles and Doubles Model: The Inclusion of Disconnected Triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Grant, D.J.; Dixon, D.A.; Camaioni, D.; Potter, R.G.; Christe, K.O. Lewis Acidities and Hydride, Fluoride, and X- Affinities of the BH3-NXn Compounds for (X = F, Cl, Br, I, NH2, OH, and SH) from Coupled Cluster Theory. Inorg. Chem. 2009, 48, 8811–8821. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Liu, G.; Hu, R.; Gao, G. Role of Aromatics in Isobutane Alkylation of Chloroaluminate Ionic Liquids: Insights from Aromatic—Ion Interaction. J. Catal. 2021, 396, 54–64. [Google Scholar] [CrossRef]
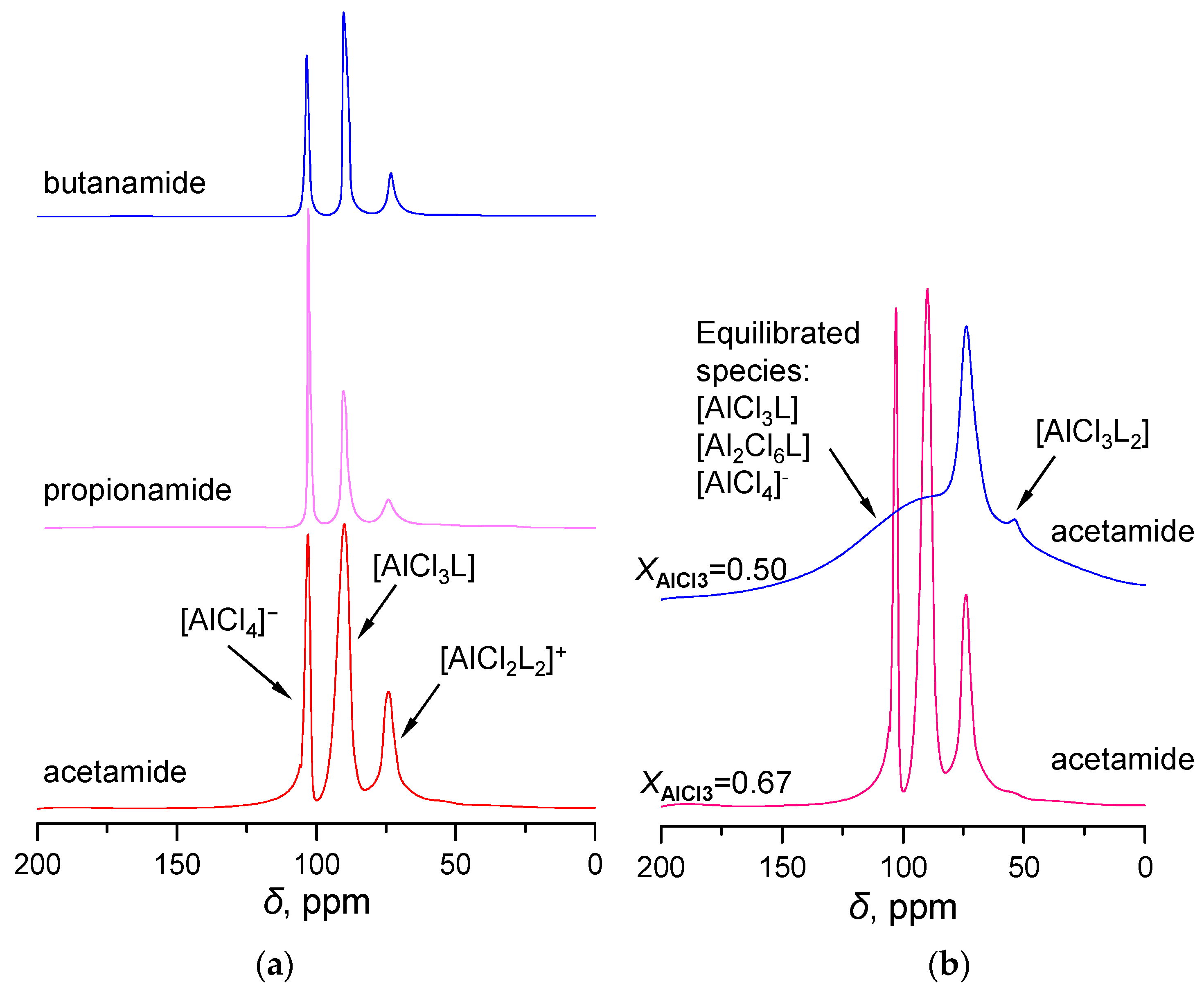
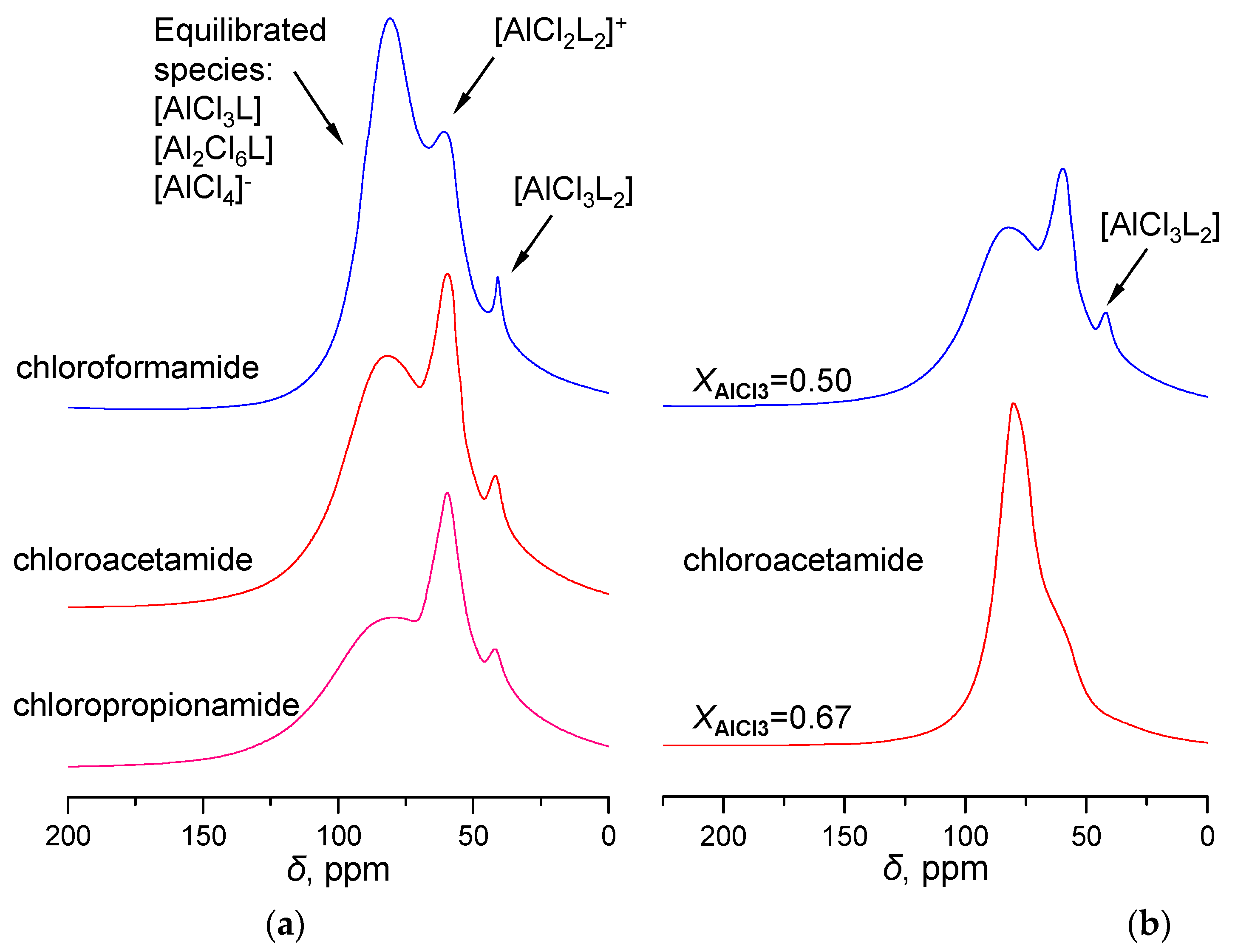
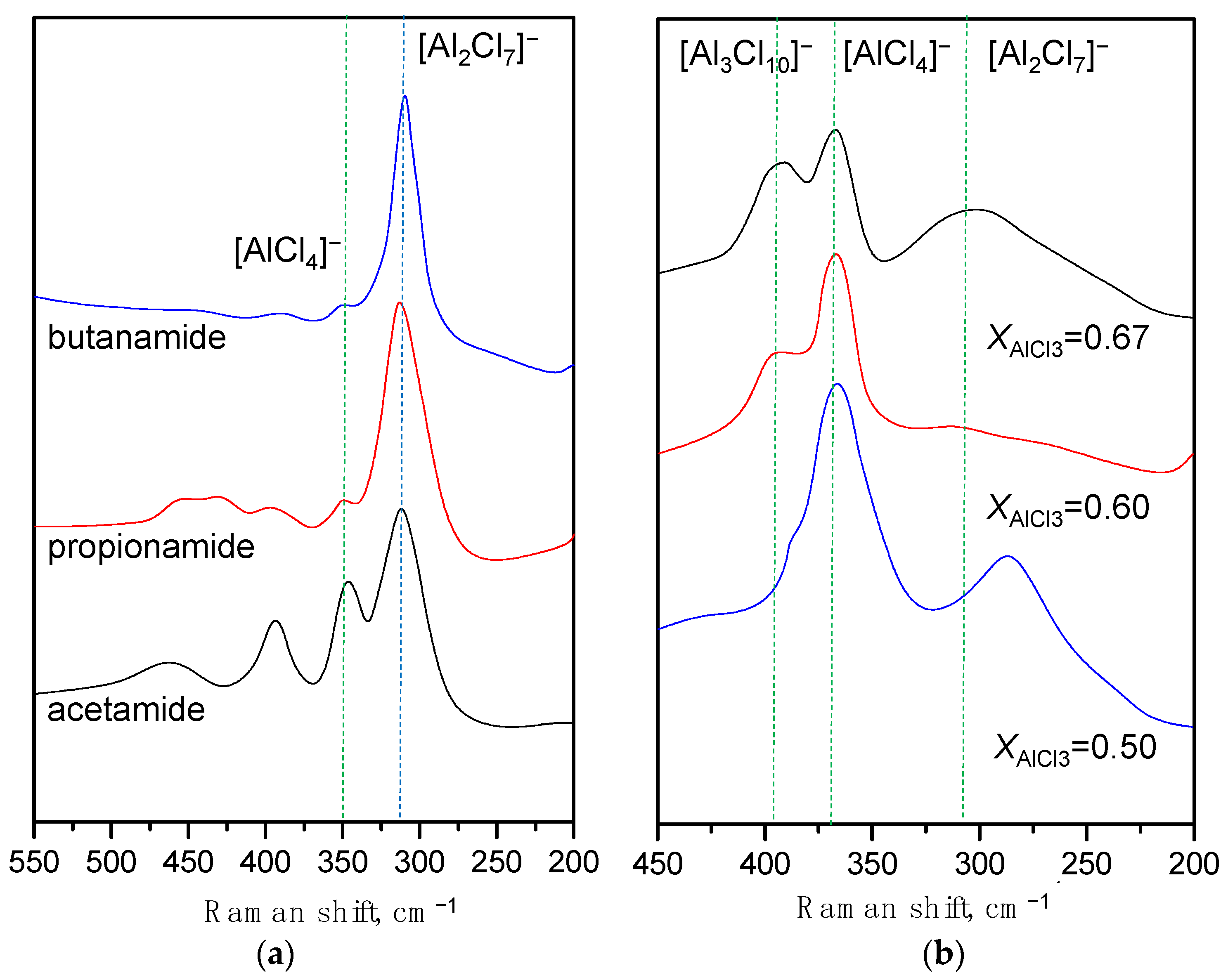
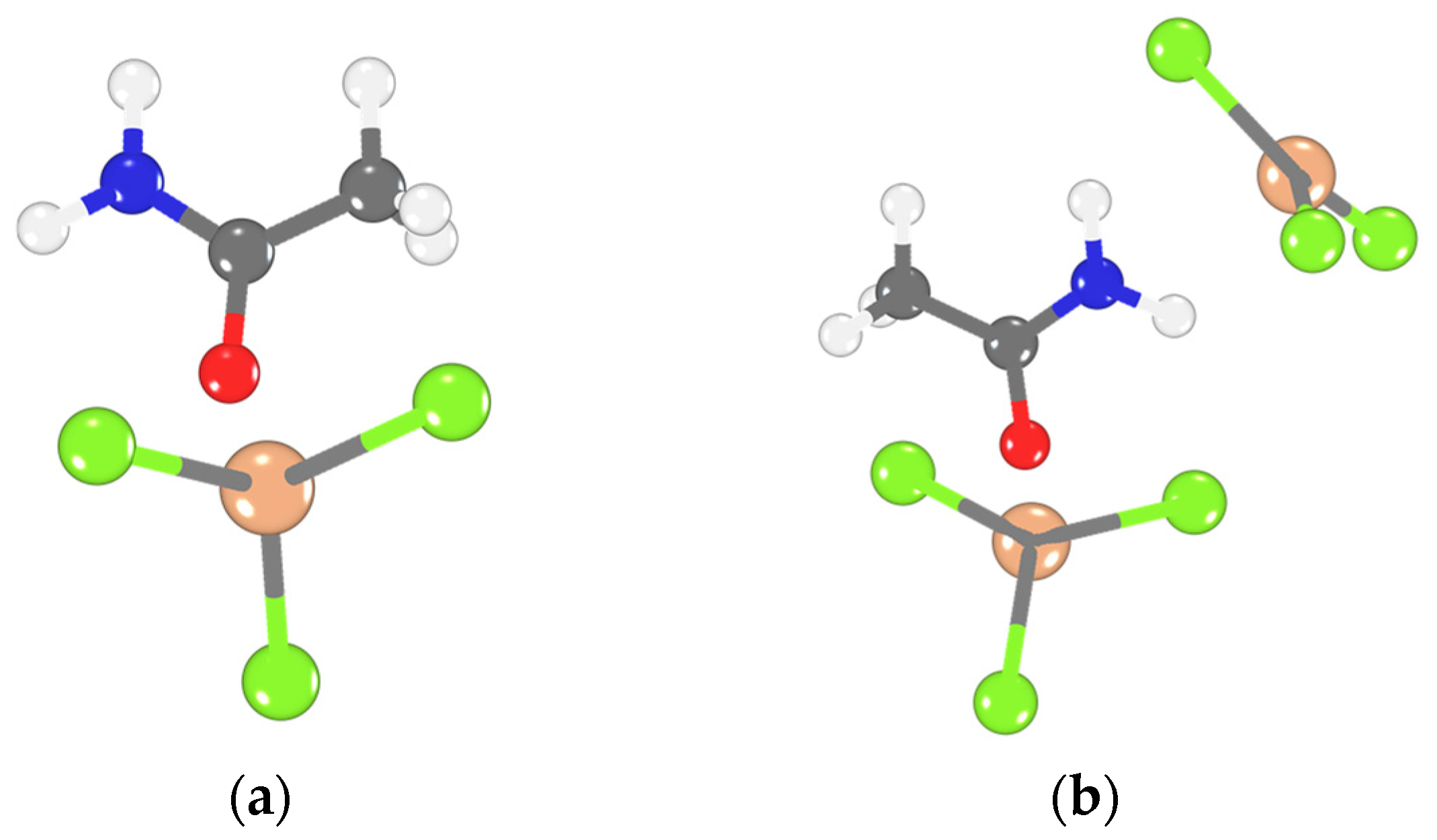
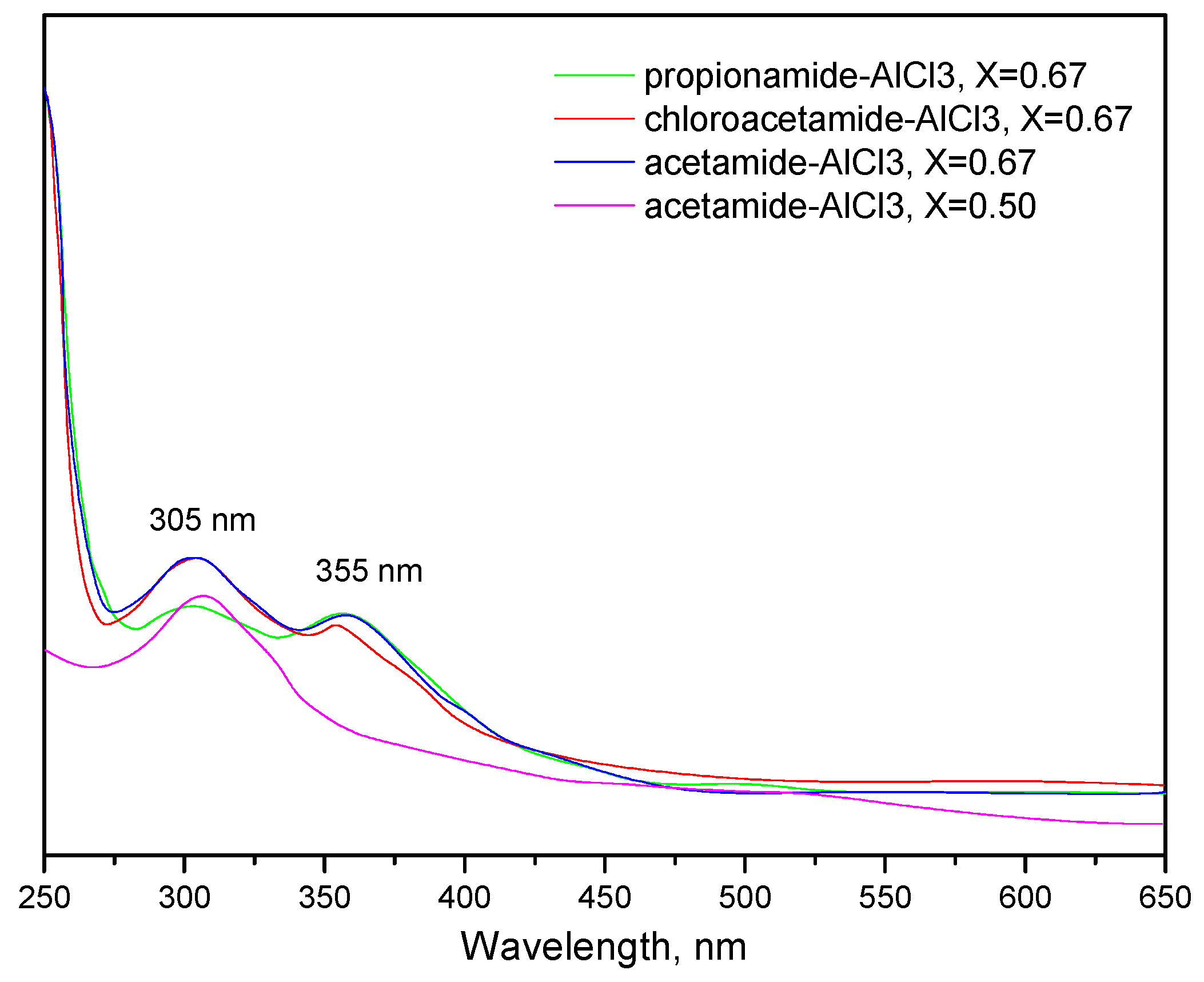
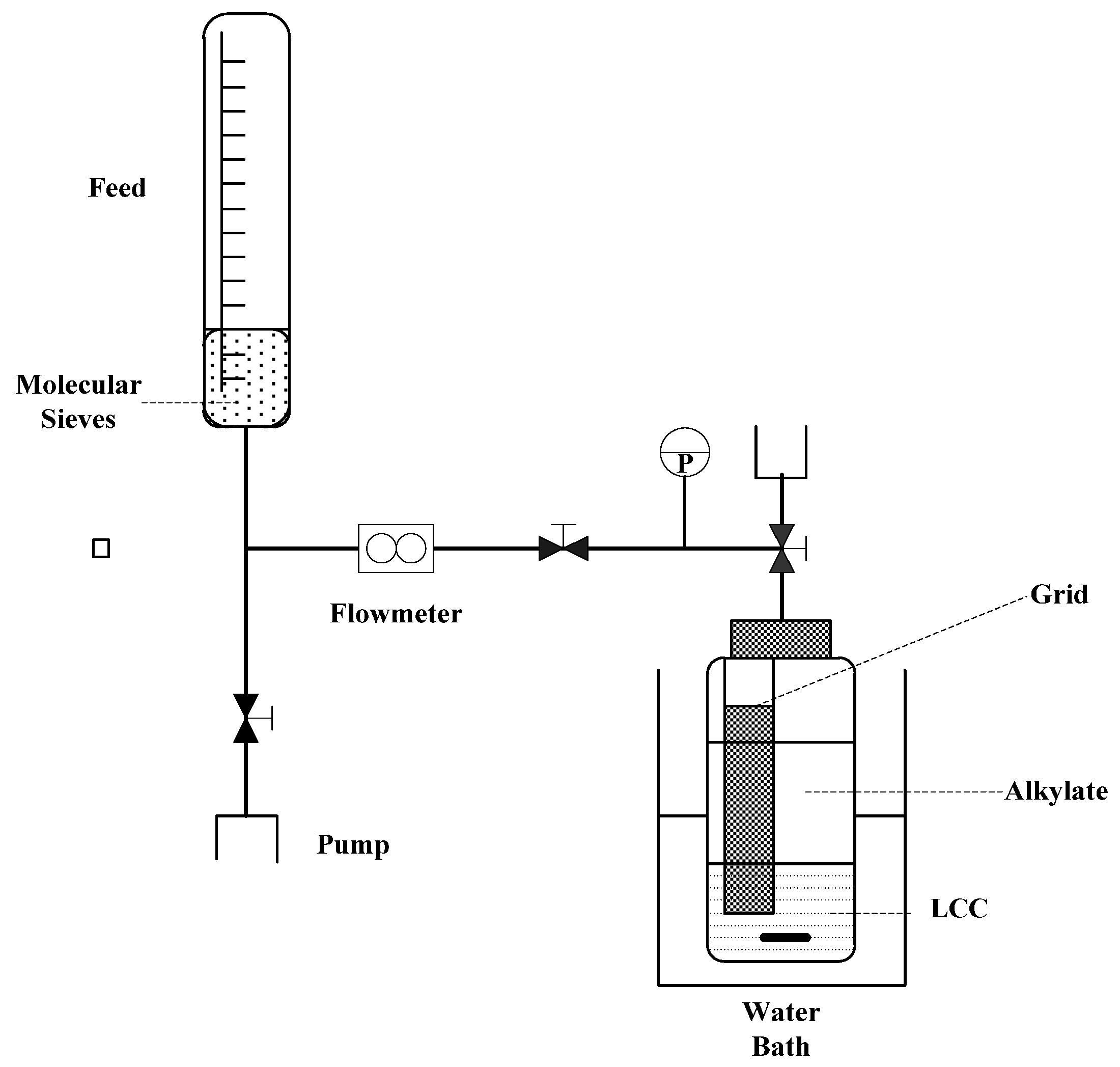
| No. | Catalysts | AN | XAlCl3 | Composition of Alkylate, wt% | ||
|---|---|---|---|---|---|---|
| C5–7 | C8 1 | C9+ | ||||
| 1 | [(C2H5)3NH]Cl−AlCl3 | 70.7 | 0.50 | - | - | - |
| 2 | [(C2H5)3NH]Cl−AlCl3 | 92.2 | 0.67 | 30.2 | 34.2 | 35.6 |
| 3 | [BMIm]Cl−AlCl3 | 91.7 | 0.67 | 23.5 | 42.0 | 34.5 |
| 4 | acetamide−AlCl3 | 82.2 | 0.50 | 2.2 | 84.1 2 | 13.7 |
| 5 | butanamide−AlCl3 | 96.4 | 0.67 | 20.4 | 45.6 | 34.0 |
| 6 | propionamide−AlCl3 | 97.0 | 0.67 | 20.2 | 48.3 | 31.5 |
| 7 | acetamide−AlCl3 | 97.6 | 0.67 | 20.1 | 43.4 | 36.5 |
| 8 | chloropropionamide−AlCl3 | 98.2 | 0.67 | 25.4 | 48.2 | 26.4 |
| 9 | chloroacetamide−AlCl3 | 99.1 | 0.67 | 24.2 | 49.6 | 26.2 |
| 10 | chloroformamide−AlCl3 | 102.0 | 0.67 | 37.5 | 44.7 | 17.8 |
| Lewis Acids | GB | Constructed Species Model, FIA | ||
|---|---|---|---|---|
| I | II | III | ||
| [(C2H5)3NH]Cl−AlCl3 1 | 22.9 | (C2H5)3NH+/AlCl4−, 345 | ||
| [(C2H5)3NH]Cl−AlCl3 | 32.6 | (C2H5)3NH+/Al2Cl7−, 500 | ||
| [BMIm]Cl−AlCl3 | 32.4 | BMIm+/Al2Cl7−, 490 | ||
| acetamide−AlCl3 1 | 23.1 | AlCl2L2+/AlCl4−, 426 | AlCl3L2/AlCl3L/Al2Cl6L, 517 | |
| butanamide−AlCl3 | 34.5 | AlCl2L2+/Al2Cl7−, 505 | AlCl3L/Al2Cl6L, 531 | AlCl3L2/AlCl3L/Al2Cl6L, 589 |
| propionamide−AlCl3 | 34.8 | AlCl2L2+/Al2Cl7−, 515 | AlCl3L/Al2Cl6L, 539 | AlCl3L2/AlCl3L/Al2Cl6L, 594 |
| acetamide−AlCl3 | 35.0 | AlCl2L2+/Al2Cl7−, 520 | AlCl3L/Al2Cl6L, 550 | AlCl3L2/AlCl3L/Al2Cl6L, 603 |
| chloropropionamide−AlCl3 | 35.3 | AlCl2L2+/Al2Cl7−, 530 | AlCl3L/Al2Cl6L, 538 | AlCl3L2/AlCl3L/Al2Cl6L, 600 |
| chloroacetamide−AlCl3 | 35.7 | AlCl2L2+/Al2Cl7−, 547 | AlCl3L/Al2Cl6L, 580 | AlCl3L2/AlCl3L/Al2Cl6L, 602 |
| chloroformamide−AlCl3 | 37.0 | AlCl2L2+/Al2Cl7−, 575 | AlCl3L/Al2Cl6L, 603 | AlCl3L2/AlCl3L/Al2Cl6L, 616 |
| Acids | FIA 1 | GB | LCC | GB | Predicted FIA 2 | Computed FIA 3 |
|---|---|---|---|---|---|---|
| AlMe3 | 368 | 16.7 | acetamide−AlCl3 4 | 23.1 | 427 | 426 |
| AlEt3 | 379 | 15.9 | butanamide−AlCl3 | 34.5 | 593 | 589 |
| [AlCalixMe]− | 402 | 18.5 | propionamide−AlCl3 | 34.8 | 595 | 594 |
| AlN(OC7H4)3 | 412 | 21.2 | acetamide−AlCl3 | 35.0 | 597 | 603 |
| AlCl3 | 565 | 32.2 | chloropropionamide−AlCl3 | 35.3 | 600 | 599 |
| AlBr3 | 577 | 33.1 | chloroacetamide−AlCl3 | 35.7 | 604 | 602 |
| AlI3 | 582 | 34.4 | chloroformamide−AlCl3 | 37.0 | 616 | 615 |
| Al(C6F5)3 | 596 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wu, Q.; Liu, Y.; Bao, J. Acidity Quantification and Structure Analysis of Amide-AlCl3 Liquid Coordination Complexes for C4 Alkylation Catalysis. Molecules 2023, 28, 7857. https://doi.org/10.3390/molecules28237857
Li H, Wu Q, Liu Y, Bao J. Acidity Quantification and Structure Analysis of Amide-AlCl3 Liquid Coordination Complexes for C4 Alkylation Catalysis. Molecules. 2023; 28(23):7857. https://doi.org/10.3390/molecules28237857
Chicago/Turabian StyleLi, Hao, Qiong Wu, Ying Liu, and Jinrong Bao. 2023. "Acidity Quantification and Structure Analysis of Amide-AlCl3 Liquid Coordination Complexes for C4 Alkylation Catalysis" Molecules 28, no. 23: 7857. https://doi.org/10.3390/molecules28237857
APA StyleLi, H., Wu, Q., Liu, Y., & Bao, J. (2023). Acidity Quantification and Structure Analysis of Amide-AlCl3 Liquid Coordination Complexes for C4 Alkylation Catalysis. Molecules, 28(23), 7857. https://doi.org/10.3390/molecules28237857






