Photoinduced Synthesis of Sulfonyl-Containing Phosphorothioates via a Three-Component Reaction
Abstract
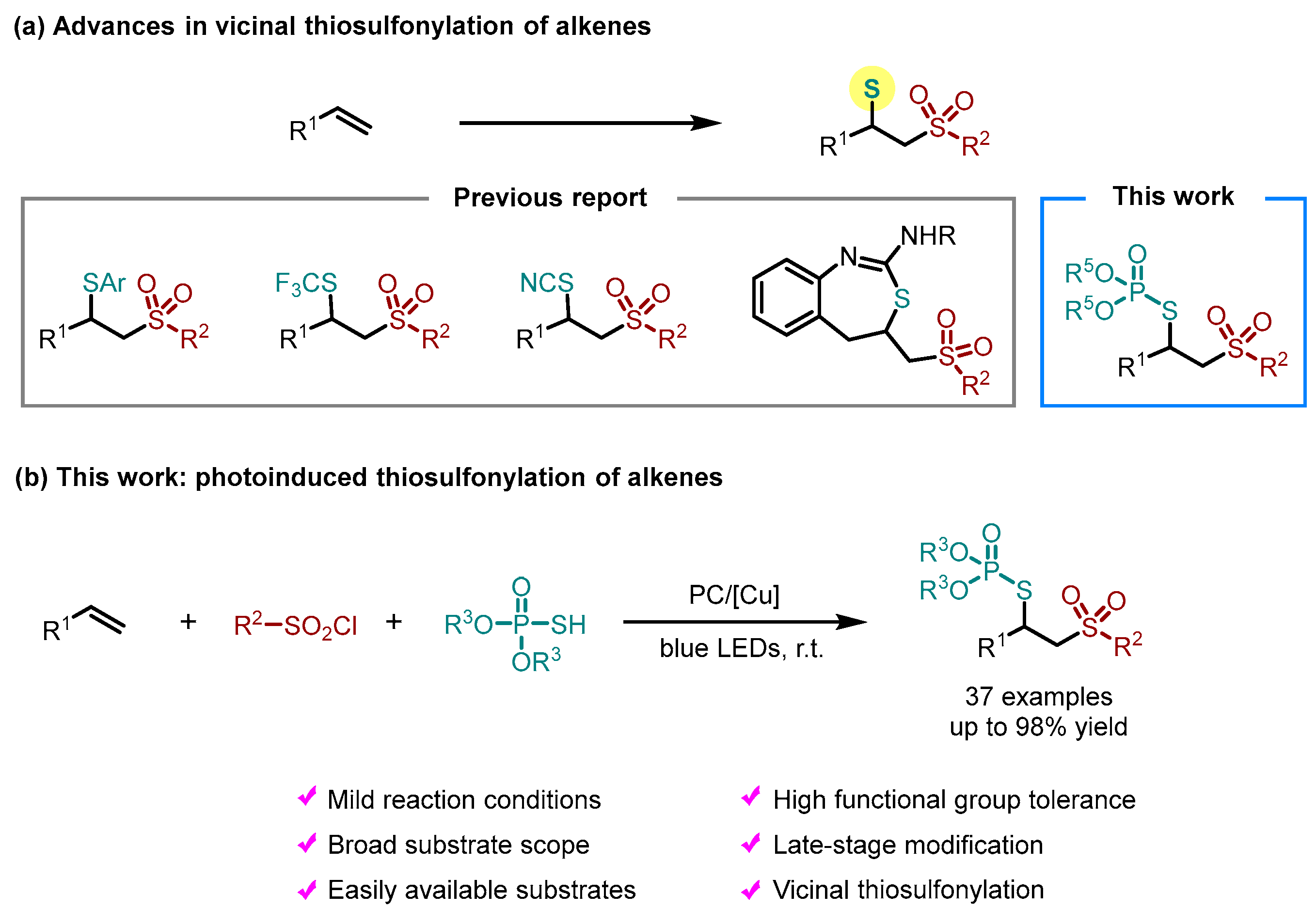
:1. Introduction
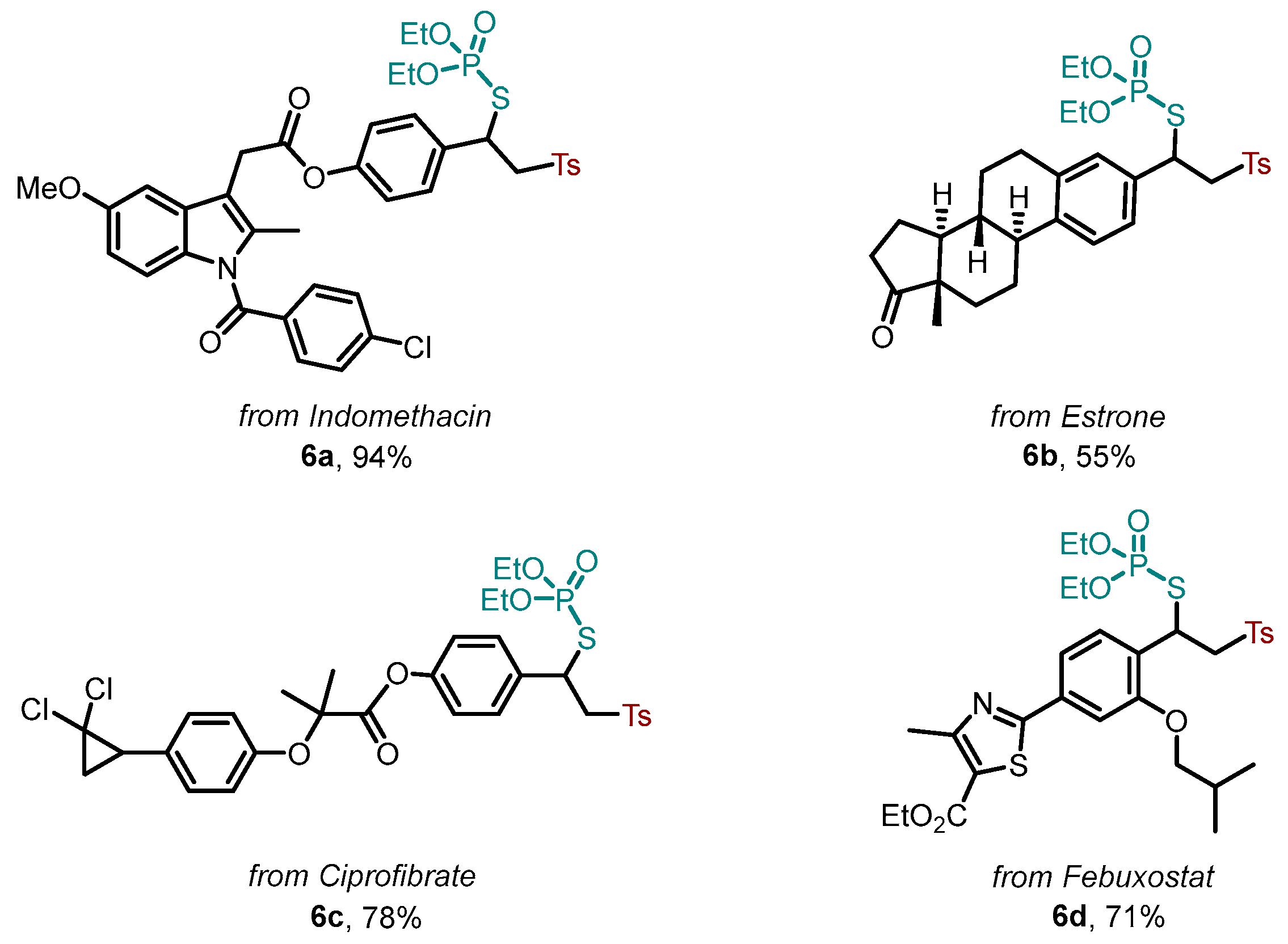
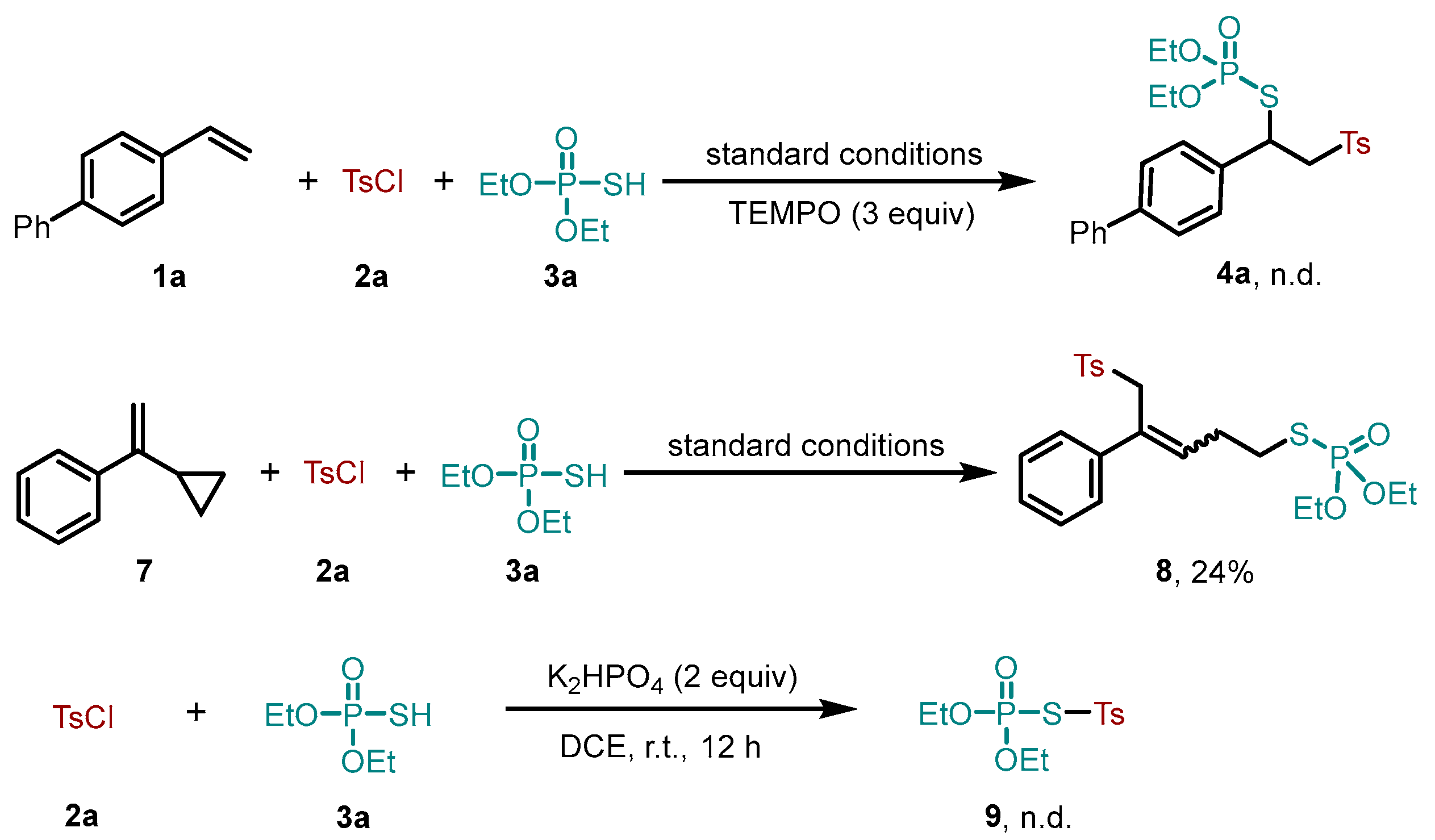
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Sulfonyl-Containing Phosphorothioates
3.3. General Procedure for the Scale-Up Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.S.; Frederiksen, J.K.; Piccirilli, J.A. Synthesis, Properties, and Applications of Oligonucleotides Containing an RNA Dinucleotide Phosphorothiolate Linkage. Acc. Chem. Res. 2011, 44, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.; Yang, T.; Mishra, S.; Cronin, C.; Chakraborty, S.; Shen, J.-B.; Liang, B.T.; Jacobson, K.A. 5′-Phosphate and 5′-Phosphonate Ester Derivatives of (N)-Methanocarba Adenosine with in Vivo Cardioprotective Activity. J. Med. Chem. 2013, 56, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, X.; KuoLee, R.; Chen, W. Synthesis and immunostimulatory properties of the phosphorothioate analogues of cdiGMP. Bioorg. Med. Chem. Lett. 2008, 18, 5631–5634. [Google Scholar] [CrossRef] [PubMed]
- Cogoi, S.; Rapozzi, V.; Quadrifoglio, F.; Xodo, L. Anti-gene Effect in Live Cells of AG Motif Triplex-Forming Oligonucleotides Containing an Increasing Number of Phosphorothioate Linkages. Biochemistry 2001, 40, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Milligan, J.F.; Uhlenbeck, O.C. Determination of RNA-protein contacts using thiophosphate substitutions. Biochemistry 1989, 28, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Murdock, L.L.; Hopkins, T.L. Insecticidal, anticholinesterase, and hydrolytic properties of O,O-dialkyl S-aryl phosphorothiolates in relation to structure. J. Agric. Food Chem. 1968, 16, 954–958. [Google Scholar] [CrossRef]
- Kasagami, T.; Miyamoto, T.; Yamamoto, I. Activated transformations of organophosphorus insecticides in the case of non-AChE inhibitory oxons. Pest Manag. Sci. 2002, 58, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Duschmale, J.; Hansen, H.F.; Duschmale, M.; Koller, E.; Albaek, N.; Moller, M.R.; Jensen, K.; Koch, T.; Wengel, J.; Bleicher, K. In Vitro and in Vivo Properties of Therapeutic Oligonucleotides Containing Non-Chiral 3′ and 5′ Thiophosphate Linkages. Nucleic Acids Res. 2020, 48, 63–74. [Google Scholar] [CrossRef]
- Jones, D.J.; O’Leary, E.M.; O’Sullivan, T.P. Synthesis and application of phosphonothioates, phosphonodithioates, phosphorothioates, phosphinothioates and related compounds. Tetrahedron Lett. 2018, 59, 4279–4292. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Yang, R.; Song, X.; Xiao, Q. Recent Advances in the Direct Synthesis of Sulfur-Containing Organophosphorus Compounds via Radical Processes. Adv. Syn. Catal. 2023, 365, 2280–2298. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5–39. [Google Scholar] [CrossRef] [PubMed]
- Man, H.-W.; Schafer, P.; Wong, L.M.; Patterson, R.T.; Corral, L.G.; Raymon, H.; Blease, K.; Leisten, J.; Shirley, M.A.; Tang, Y.; et al. Discovery of (S)-N-[2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl] acetamide (apremilast), A Potent and Orally Active Phosphodiesterase 4 and Tumor Necrosis Factor-alpha Inhibitor. J. Med. Chem. 2009, 52, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Wu, H.; Kobayashi, J.; Ohizumi, Y.; Hirata, Y.; Higashijima, T.; Miyazawa, T. Agelasidine-A, A Novel Sesquiterpene Possessing Antispasmodic Activity from the Okinawa Sea Sponge Agelas sp. Tetrahedron Lett. 1983, 24, 4105–4108. [Google Scholar] [CrossRef]
- Woo, S.Y.; Kim, J.H.; Moon, M.K.; Han, S.H.; Yeon, S.K.; Choi, J.W.; Jang, B.K.; Song, H.J.; Kang, Y.G.; Kim, J.W.; et al. Discovery of vinyl sulfones as a novel class of neuroprotective agents toward Parkinson’s disease therapy. J. Med. Chem. 2014, 57, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, J.; Li, X.; Zhu, J.; Volodine, A.; Wang, X.; Yang, J.; Van Puyvelde, P.; Van der Bruggen, B. New Promising Polymer for Organic Solvent Nanofiltration: Oxidized Poly (Arylene Sulfide Sulfone). J. Membr. Sci. 2018, 549, 438–445. [Google Scholar] [CrossRef]
- Kausar, A.; Zulfiqar, S.; Sarwar, M.I. Recent Developments in Sulfur-Containing Polymers. Polym. Rev. 2014, 54, 185–267. [Google Scholar] [CrossRef]
- Julia, M.; Paris, J.-M. Syntheses a l’aide de Sulfones v(+)-Methode de Synthese Generale de Doubles Liaisons. Tetrahedron Lett. 1973, 14, 4833–4836. [Google Scholar] [CrossRef]
- Joseph, D.; Idris, M.A.; Chen, J.; Lee, S. Recent Advances in the Catalytic Synthesis of Arylsulfonyl Compounds. ACS Catal. 2021, 11, 4169–4204. [Google Scholar] [CrossRef]
- Qiu, G.; Zhou, K.; Gao, L.; Wu, J. Insertion of sulfur dioxide via a radical process: An efficient route to sulfonyl compounds. Org. Chem. Front. 2018, 5, 691–705. [Google Scholar] [CrossRef]
- Ge, D.; Chen, J.-W.; Xu, P.; Pan, J.; Chu, X.-Q. 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents. Chin. Chem. Lett. 2022, 33, 4732–4739. [Google Scholar] [CrossRef]
- Zhen, J.; Du, X.; Xu, X.; Li, Y.; Yuan, H.; Xu, D.; Xue, C.; Luo, Y. Visible-Light-Mediated Late-Stage Sulfonylation of Boronic Acids via N–S Bond Activation of Sulfonamides. ACS Catal. 2022, 12, 1986–1991. [Google Scholar] [CrossRef]
- He, F.-S.; Bao, P.; Yu, F.; Zeng, L.-H.; Deng, W.-P.; Wu, J. Copper-Catalyzed Regioselective 1,4-Selenosulfonylation of 1,3-Enynes to Access Cyanoalkylsulfonylated Allenes. Org. Lett. 2021, 23, 7472–7476. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, W.; Xie, W.; Chen, Q.; Wu, J. Generation of (E)-β-trifluoromethyl vinylsulfonohydrazides under photocatalysis and their anti-bacteria activity. Chin. Chem. Lett. 2023, 34, 107984. [Google Scholar] [CrossRef]
- He, F.-S.; Bao, P.; Tang, Z.; Yu, F.; Deng, W.-P.; Wu, J. Photoredox-catalyzed α-sulfonylation of ketones from sulfur dioxide and thianthrenium salts. Org. Lett. 2022, 24, 2955–2960. [Google Scholar] [CrossRef] [PubMed]
- Beller, M.; Seayad, J.; Tillack, A.; Jiao, H. Catalytic Markovnikov and anti-Markovnikov Functionalization of Alkenes and Alkynes: Recent Developments and Trends. Angew. Chem. Int. Ed. 2004, 43, 3368–3398. [Google Scholar] [CrossRef] [PubMed]
- He, F.-S.; Ye, S.; Wu, J. Recent Advances in Pyridinium Salts as Radical Reservoirs in Organic Synthesis. ACS Catal. 2019, 9, 8943–8960. [Google Scholar] [CrossRef]
- Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 2020, 49, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Studer, A. Intermolecular Radical Carboamination of Alkenes. Chem. Soc. Rev. 2020, 49, 1790–1811. [Google Scholar] [CrossRef]
- Derosa, J.; Apolinar, O.; Kang, T.; Tran, V.T.; Engle, K.M. Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci. 2020, 11, 4287–4296. [Google Scholar] [CrossRef]
- Zhang, P.; Li, W.; Zhu, X.; Li, Y.; Zhao, X.; Shi, S.; Zhu, F.; Lin, J.; Gao, X. Photoredox and Copper-Catalyzed Sulfonylphosphorothiolation of Alkenes toward β-Sulfonyl Phosphorothioates. Adv. Synth. Catal. 2022, 364, 3316–3320. [Google Scholar] [CrossRef]
- Liang, R.-B.; Zhu, C.-M.; Song, P.-Q.; Zhao, L.-M.; Tong, Q.-X.; Zhong, J.-J. External oxidant-free and selective thiofunctionalization of alkenes enabled by photoredox-neutral catalysis. Org. Chem. Front. 2022, 9, 4536–4541. [Google Scholar] [CrossRef]
- Lu, M.; Liang, R.-B.; Zhu, C.-M.; Tong, Q.-X.; Zhong, J.-J. Photoredox Synthesis of Thio-Functionalized Cyclic Ethers Using N-Sulfenyl Phthalimides as a Thiyl-Radical Precursor. Chin. J. Chem. 2023, 41, 1823–1828. [Google Scholar] [CrossRef]
- Du, X.; Zhen, J.-S.; Xu, X.-H.; Yuan, H.; Li, Y.-H.; Zheng, Y.; Xue, C.; Luo, Y. Hydrosulfonylation of Alkenes with Sulfonyl Imines via Ir/Cu Dual Photoredox Catalysis. Org. Lett. 2022, 24, 3944–3949. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, J.; Wei, F.; Qi, Y.; Wang, H.; Liu, Z.; Lei, A. Aerobic Oxysulfonylation of Alkenes Leading to Secondary and Tertiary β-Hydroxysulfones. Angew. Chem. Int. Ed. 2013, 52, 7156–7159. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.-L.; Deng, Y.-X.; Li, Z.-J.; Zhao, S.-Y. Copper(I)-Catalyzed Three-Component Selenosulfonation of Maleimides with Sulfonyl Hydrazides and Diselenides via Radical Relay. J. Org. Chem. 2022, 87, 15661–15669. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shan, C.; Tung, C.-H.; Xu, Z. Dual gold and photoredox catalysis: Visible light-mediated intermolecular atom transfer thiosulfonylation of alkenes. Chem. Sci. 2017, 8, 2610–2615. [Google Scholar] [CrossRef]
- Gadde, K.; Mampuys, P.; Guidetti, A.; Ching, H.Y.V.; Herrebout, W.A.; Doorslaer, S.V.; Tehrani, K.A.; Maes, B.U.W. Thiosulfonylation of Unactivated Alkenes with Visible-Light Organic Photocatalysis. ACS Catal. 2020, 10, 8765–8779. [Google Scholar] [CrossRef]
- Jia, X.; Luo, L.; Huang, C.; Zhang, X.; Lian, Z. Iron-Catalyzed Sulfonylthiocyanation of α, β-Unsaturated Amides/Esters via the Insertion of Sulfur Dioxide. Org. Lett. 2022, 24, 7560–7565. [Google Scholar] [CrossRef]
- He, F.-S.; Wu, Y.; Zhang, J.; Xia, H.; Wu, J. Thiosulfonylation of alkenes with the insertion of sulfur dioxide under non-metallic conditions. Org. Chem. Front. 2018, 5, 2940–2944. [Google Scholar] [CrossRef]


 | ||
|---|---|---|
| Entry | Variation from Standard Conditions | Yield (%) b |
| 1 | None | 99 (93) c |
| 2 | Without Ir(ppy)3 | trace |
| 3 | In the dark | trace |
| 4 | Without Cu(OTf)2 | 59 |
| 5 | Ir(ppy)2(dtbbpy)PF6 instead of Ir(ppy)3 | 94 |
| 6 | CuCl2 instead of Cu(OTf)2 | 97 |
| 7 | Cu(CH3CN)4PF6 instead of Cu(OTf)2 | 93 |
| 8 | KHCO3 instead of K2HPO4 | 59 |
| 9 | K2CO3 instead of K2HPO4 | 33 |
| 10 | Na2CO3 instead of K2HPO4 | 66 |
| 11 | THF instead of DCE | 53 |
| 12 | CH3CN instead of DCE | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Chen, M.; Zheng, S.; Wu, J.; Liu, G.; He, F.-S. Photoinduced Synthesis of Sulfonyl-Containing Phosphorothioates via a Three-Component Reaction. Molecules 2023, 28, 7869. https://doi.org/10.3390/molecules28237869
Wu X, Chen M, Zheng S, Wu J, Liu G, He F-S. Photoinduced Synthesis of Sulfonyl-Containing Phosphorothioates via a Three-Component Reaction. Molecules. 2023; 28(23):7869. https://doi.org/10.3390/molecules28237869
Chicago/Turabian StyleWu, Xianda, Minghong Chen, Shuiyun Zheng, Jie Wu, Gang Liu, and Fu-Sheng He. 2023. "Photoinduced Synthesis of Sulfonyl-Containing Phosphorothioates via a Three-Component Reaction" Molecules 28, no. 23: 7869. https://doi.org/10.3390/molecules28237869
APA StyleWu, X., Chen, M., Zheng, S., Wu, J., Liu, G., & He, F.-S. (2023). Photoinduced Synthesis of Sulfonyl-Containing Phosphorothioates via a Three-Component Reaction. Molecules, 28(23), 7869. https://doi.org/10.3390/molecules28237869






