Constructing In2S3/CdS/N-rGO Hybrid Nanosheets via One-Pot Pyrolysis for Boosting and Stabilizing Visible Light-Driven Hydrogen Evolution
Abstract
:1. Introduction
2. Results and Discussion
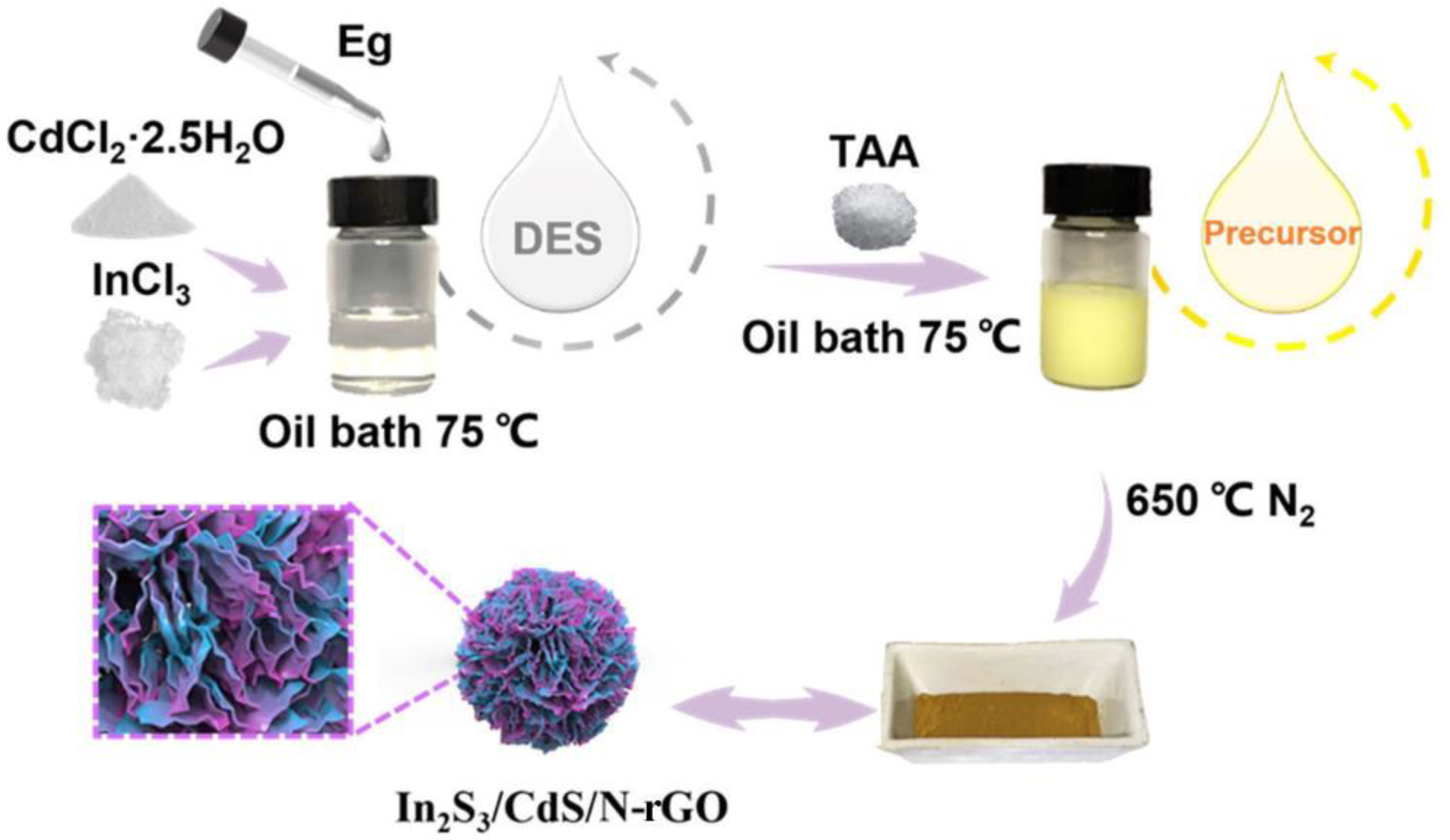
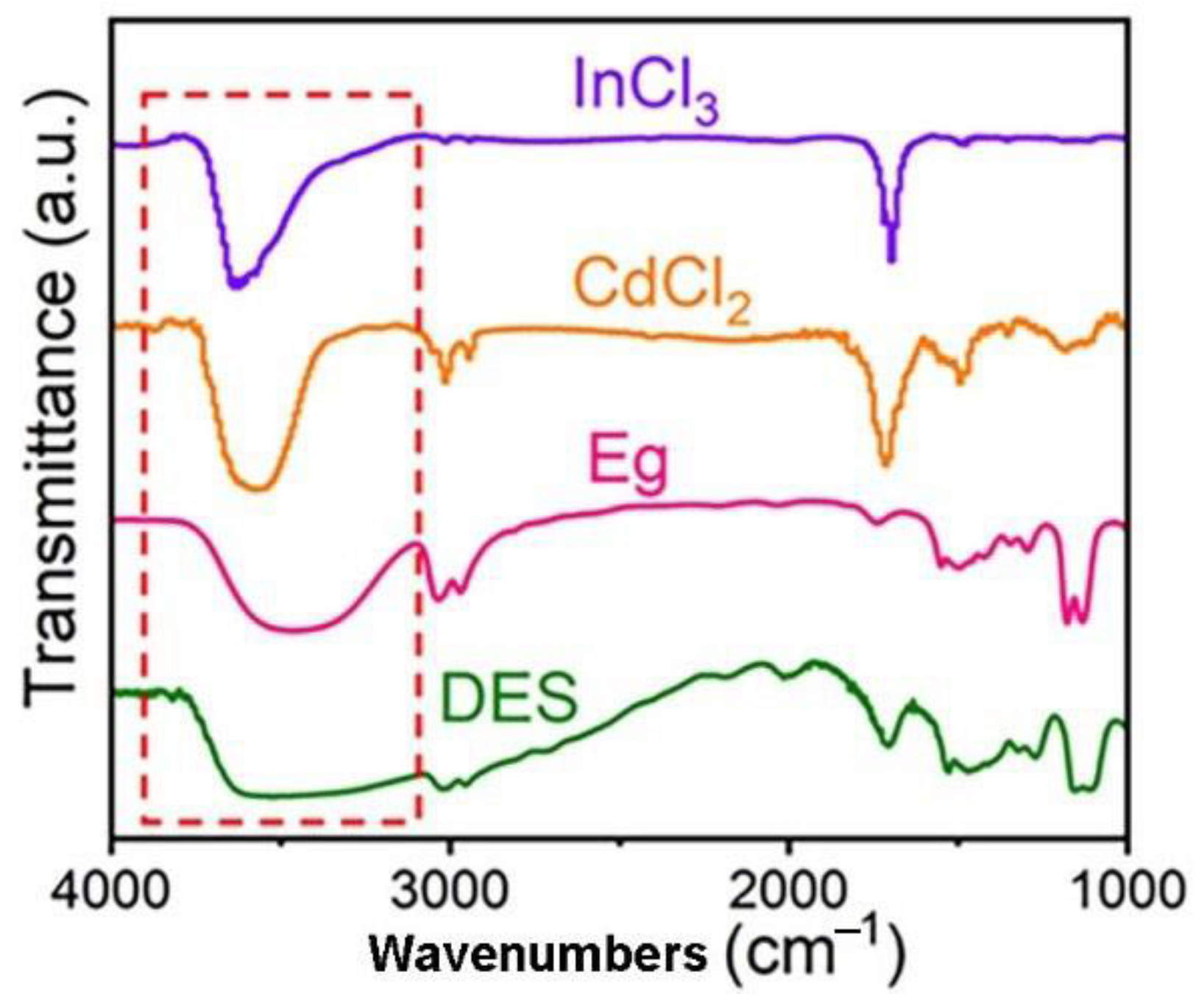
2.1. Formation of DES
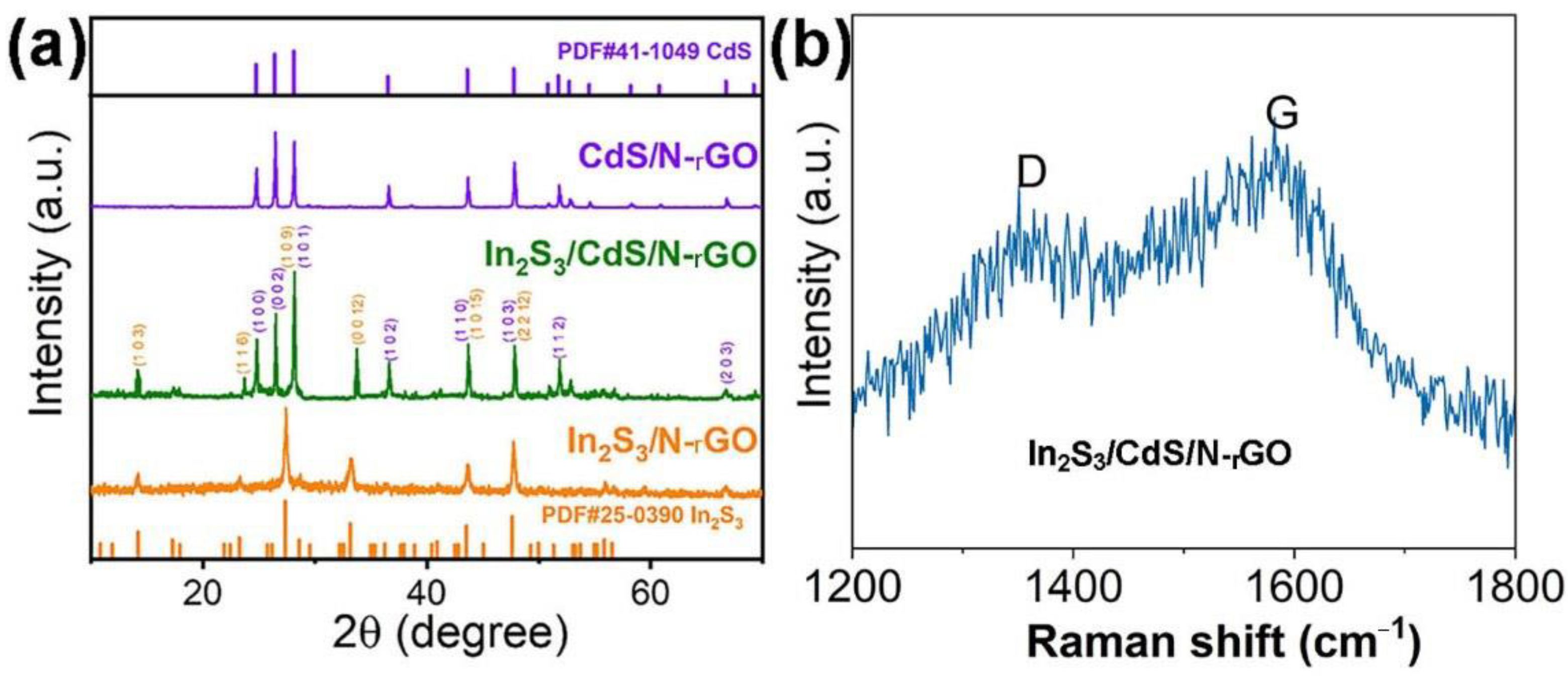
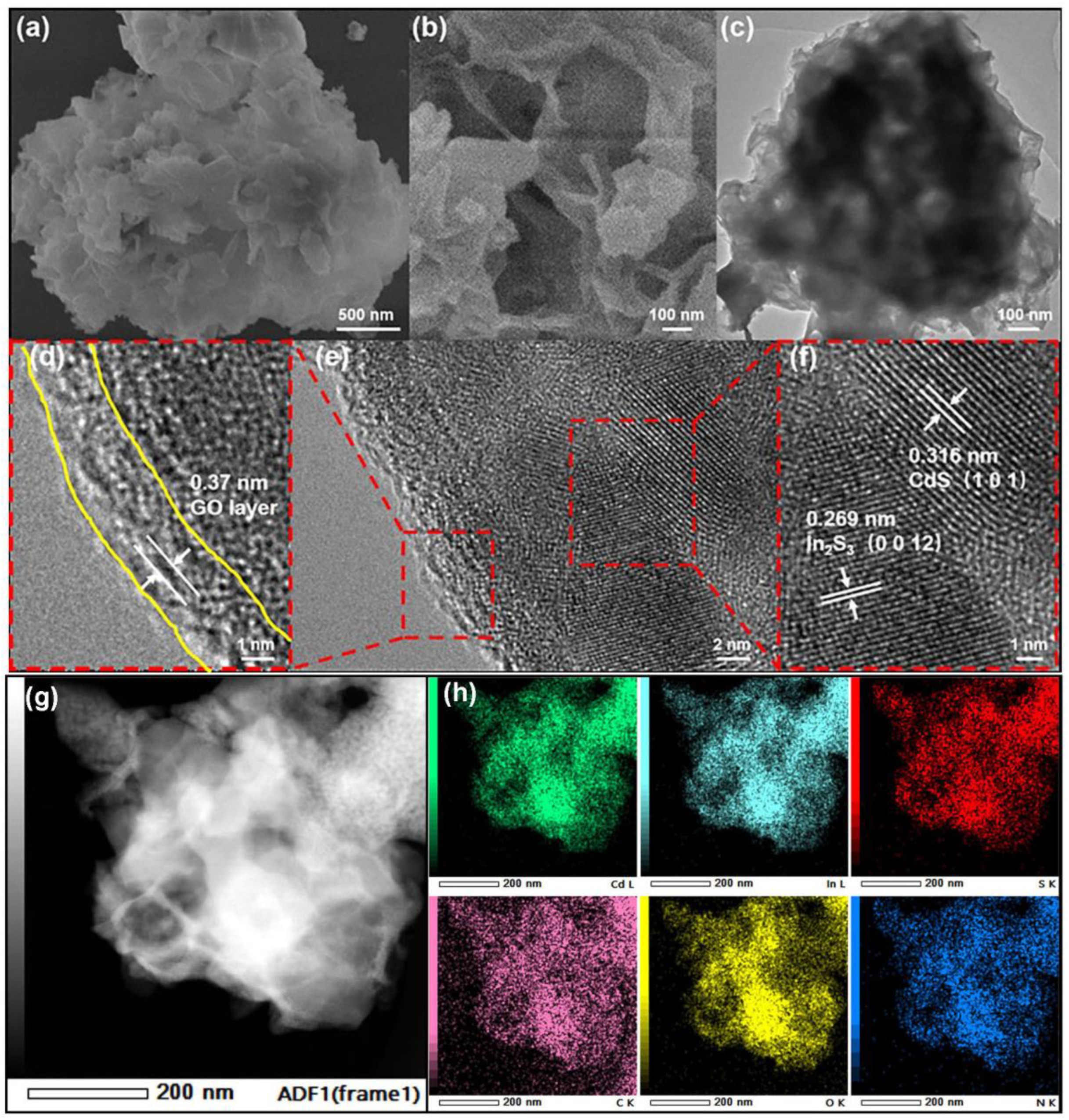
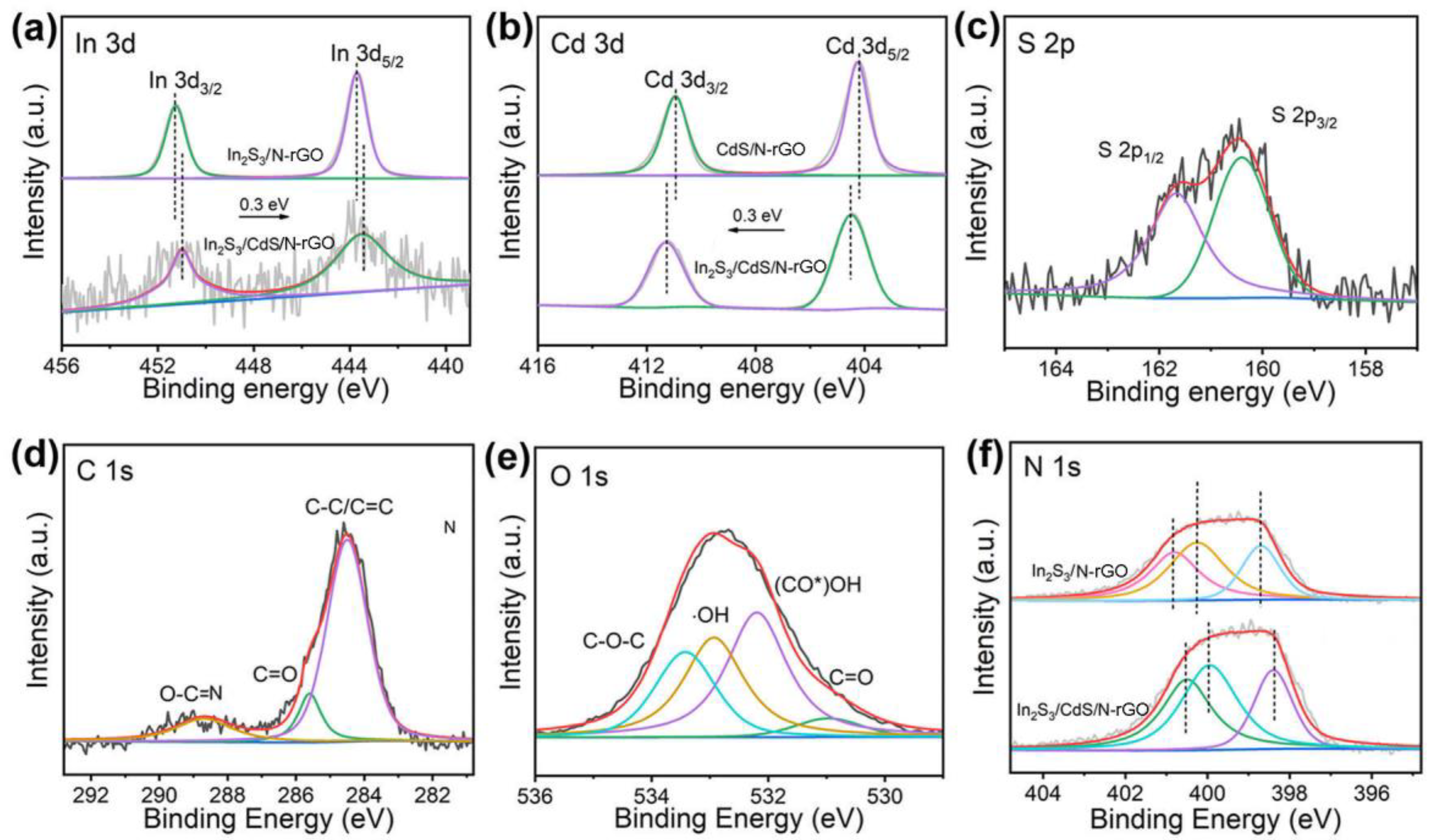
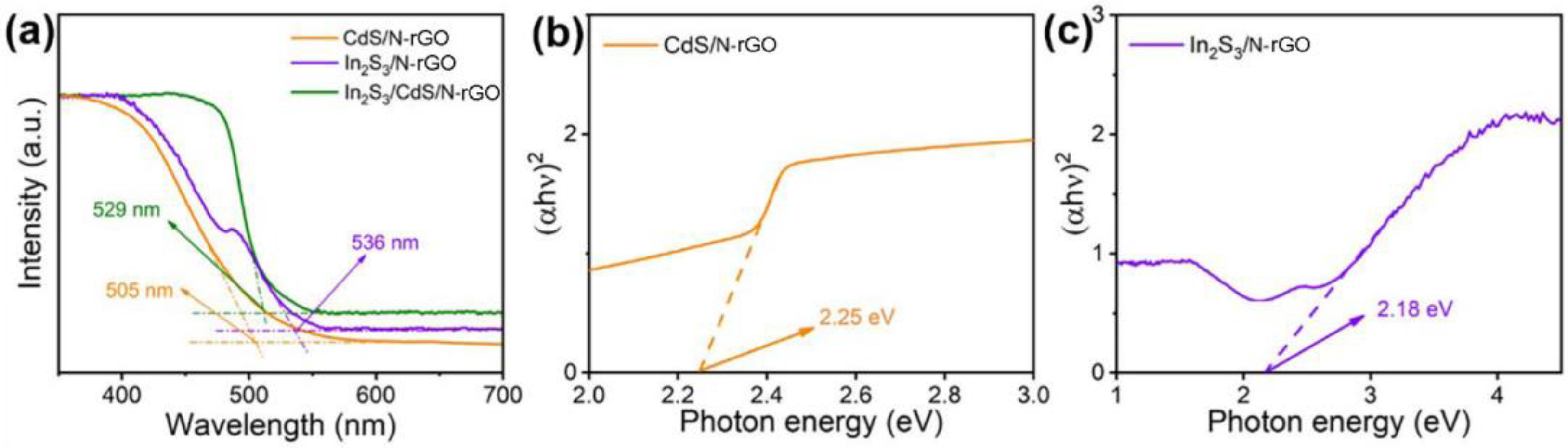
2.2. Material Characterization
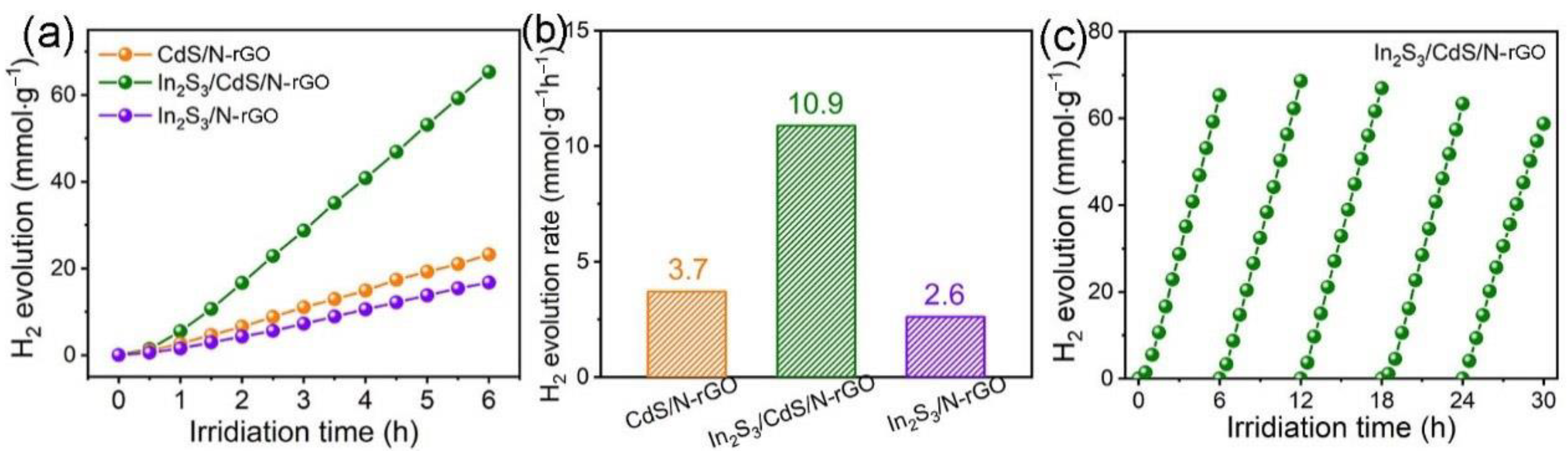
2.3. Photocatalytic Hydrogen Evolution
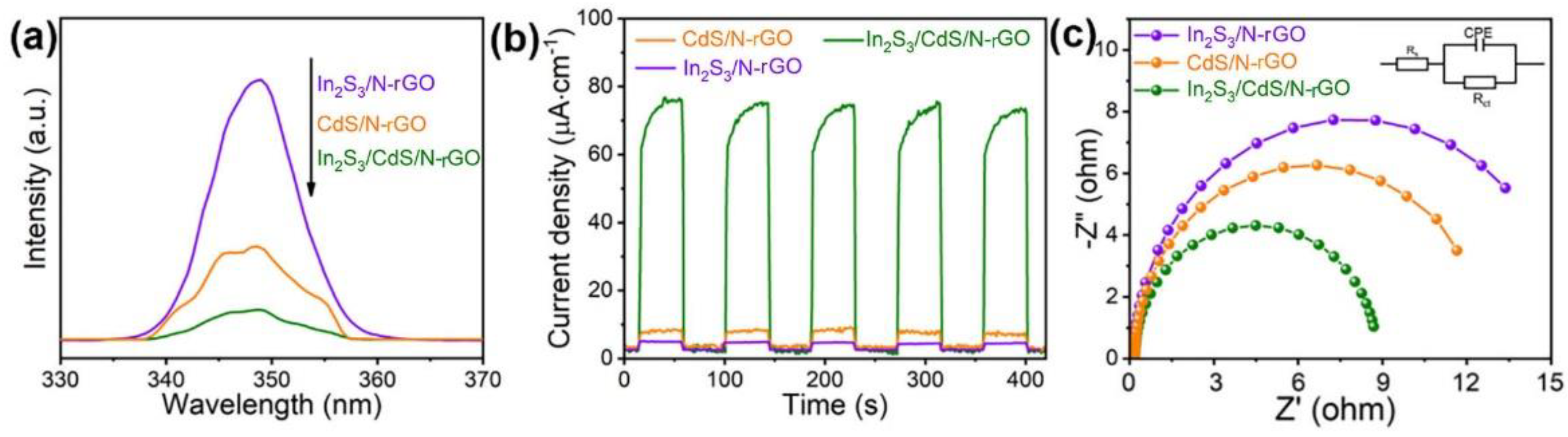
2.4. Photoelectrochemical Properties
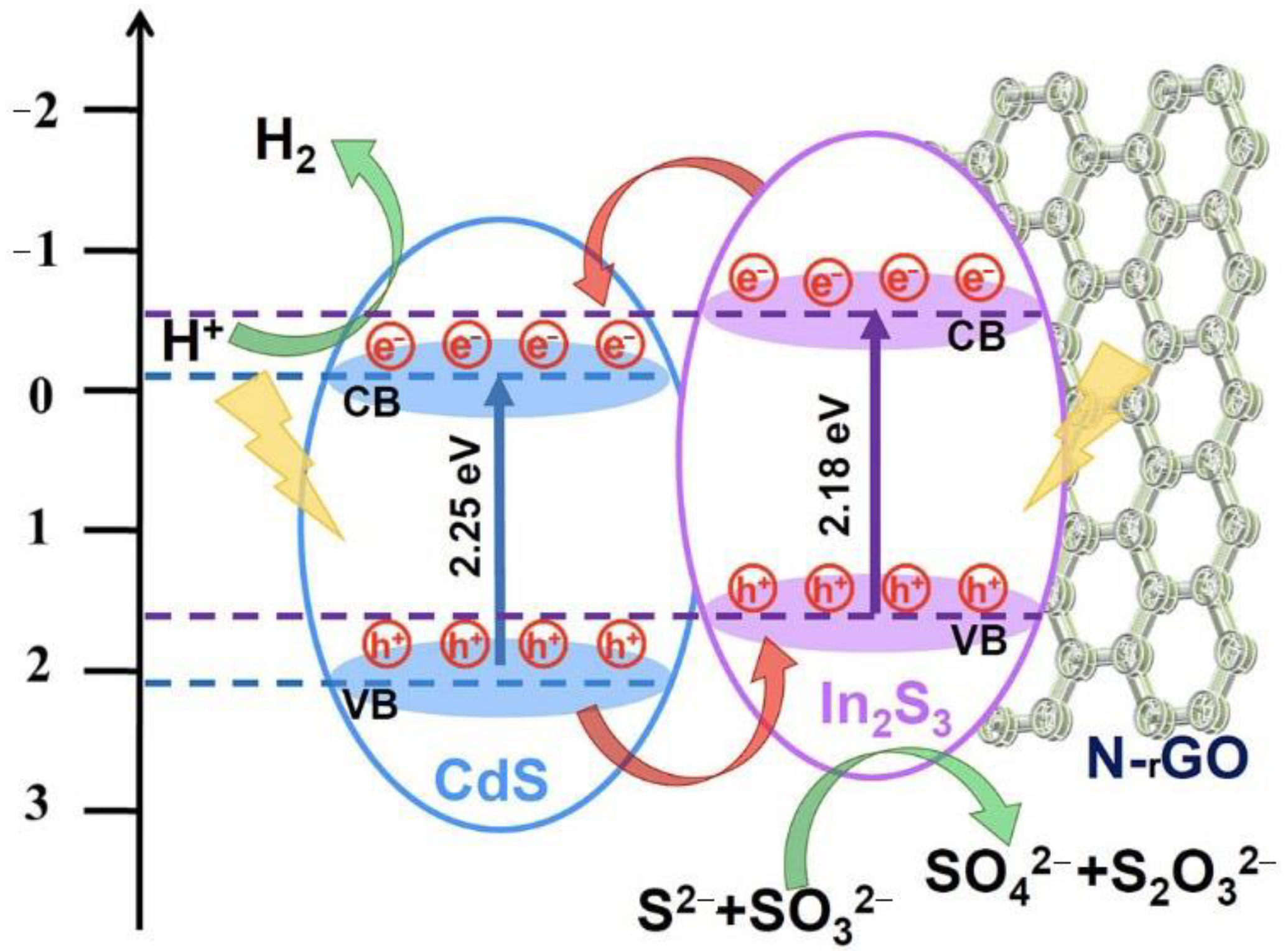
2.5. Photocatalytic Hydrogen Evolution Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of the Photocatalysts
3.3. Materials Characterization
3.4. Photocatalytic Reaction
3.5. Photoelectrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravi, P.; Noh, J. Photocatalytic water splitting: How far away are we from being able to industrially produce solar hydrogen? Molecules 2022, 27, 7176. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Ren, K.; Zheng, R.X.; Wang, L.M.; Wang, L. Ultrahigh carrier mobility in two-dimensional IV–VI semiconductors for photocatalytic water splitting. Molecules 2023, 28, 4126. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Rahman, A. Chalcogenides and chalcogenide-based heterostructures as photocatalysts for water splitting. Catalysts 2022, 12, 1338. [Google Scholar] [CrossRef]
- Poudel, M.B.; Logeshwaran, N.; Kim, A.R.; Karthikeyan, S.C.; Vijayapradeep, S.; Yoo, D.J. Integrated core-shell assembly of Ni3S2 nanowires and CoMoP nanosheets as highly efficient bifunctional electrocatalysts for overall water splitting. J. Alloys Compd. 2023, 960, 170678. [Google Scholar] [CrossRef]
- Poudel, M.B.; Logeshwaran, N.; Prabhakaran, S.; Kim, A.R.; Kim, D.H.; Yoo, D.J. Low-cost hydrogen production from alkaline/seawater over a single step synthesis of Mo3Se4-NiSe core-shell nanowire arrays. Adv. Mater. 2023, e2305813. [Google Scholar] [CrossRef]
- Lv, S.; Sun, Y.; Liu, D.; Song, C.; Wang, D. Construction of S-Scheme heterojunction Ni11(HPO3)8(OH)6/CdS photocatalysts with open framework surface for enhanced H2 evolution activity. J. Colloid Inter. Sci. 2023, 634, 148–158. [Google Scholar] [CrossRef]
- Ahmad, I.; Ben Ahmed, S.; Shabir, M.; Imran, M.; Hassan, A.M.; Alatawi, N.S. Review on CdS-derived photocatalysts for solar photocatalytic applications–advances and challenges. J. Ind. Eng. Chem. 2023, in press. [Google Scholar] [CrossRef]
- Lv, S.H.; Liu, D.Z.; Sun, Y.Y.; Li, M.X.; Zhou, Y.H.; Song, C.X.; Wang, D.B. Graphene oxide coupled high-index facets CdZnS with rich sulfur vacancies for synergistic boosting visible-light-catalytic hydrogen evolution in natural seawater: Experimental and DFT study. J. Colloid Inter. Sci. 2022, 623, 34–43. [Google Scholar] [CrossRef]
- Tian, J.; Wen, X.; Hu, W.; Luo, L.; Wang, W.; Lin, K.; Zhan, H.; Ma, B. A highly efficient composite catalyst (Au/Ta3N5)/CdS for photocatalytic hydrogen production. Catalysts 2023, 13, 1103. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Mou, X.; Song, C.; Wang, D. Construction of S-scheme Mn0.1Cd0.9S/WO3 1D/0D heterojunction assemblies for visible-light driven high-efficient H2 evolution. J. Alloys Compd. 2022, 927, 167114. [Google Scholar] [CrossRef]
- Ning, X.; Lu, G. Photocorrosion Inhibition of CdS-Based Catalysts for Photocatalytic Overall Water Splitting. Nanoscale 2020, 12, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, T.; Li, Y.; Chen, Y.; Liu, M. Photocatalytic Activities of Copper Doped Cadmium Sulfide Microspheres Prepared by a Facile Ultrasonic Spray-Pyrolysis Method. Molecules 2016, 21, 735. [Google Scholar] [CrossRef] [PubMed]
- Nadikatla, S.K.; Chintada, V.B.; Gurugubelli, T.R.; Koutavarapu, R. Review of Recent Developments in the Fabrication of ZnO/CdS Heterostructure Photocatalysts for Degradation of Organic Pollutants and Hydrogen Production. Molecules 2023, 28, 4277. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Shen, J.; Guo, W.; Zhu, X.; Guo, L.; Wang, Y.; Li, F. The Synergistic Effect in CdS/g-C3N4 Nanoheterojunctions Improves Visible Light Photocatalytic Performance for Hydrogen Evolution Reactions. Molecules 2023, 28, 6412. [Google Scholar] [CrossRef]
- Huang, M.; Yu, M.; Si, R.; Zhao, X.; Chen, S.; Liu, K.; Pan, X. Tailoring Morphology in Hydrothermally Synthesized CdS/ZnS Nanocomposites for Extraordinary Photocatalytic H2 Generation via Type-II Heterojunction. Catalysts 2023, 13, 1123. [Google Scholar] [CrossRef]
- Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M.D. Recent advances in In2S3-based photocatalysts for catalytic reduction of CO2. Chem. Phys. Impact 2023, 7, 100324. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Ruan, M. Combined CdS/In2S3 Heterostructures with Cocatalyst for Boosting Carriers Separation and Photoelectrochemical Water Splitting. Appl. Sur. Sci. 2021, 541, 148431. [Google Scholar] [CrossRef]
- Jiang, F.; Gunawan; Harada, T.; Kuang, Y.; Minegishi, T.; Domen, K.; Ikeda, S. Pt/In2S3/CdS/Cu2ZnSnS4 thin film as an efficient and stable photocathode for water reduction under sunlight radiation. J. Am. Chem. Soc. 2015, 137, 13691–13697. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, Y.; Yan, K.; Zhang, J. Dual-mode visible light-induced aptasensing platforms for bleomycin detection based on CdS-In2S3 heterojunction. Biosens. Bioelectron. 2019, 145, 111712. [Google Scholar] [CrossRef]
- Hu, J.; Wu, J.; Zhang, S.; Chen, W.; Xiao, W.; Hou, H.; Lu, X.; Liu, C.; Zhang, Q. One-Pot Fabrication of 2D/2D CdIn2S4/In2S3 Heterojunction for Boosting Photocatalytic Cr(VI) Reduction. Catalysts 2023, 13, 826. [Google Scholar] [CrossRef]
- Nawaz, M.; Akhtar, S.; Qureshi, F.; Almofty, S.A.; Nissapatron, V. Preparation of indium-cadmium sulfide nanoparticles with diverse morphologies: Photocatalytic and cytotoxicity study. J. Mol. Struct. 2022, 1253, 132288. [Google Scholar] [CrossRef]
- Wu, J.; Wei, J.; Lv, B.; Wang, M.J.; Wang, X.L.; Wang, W.Z. Enhanced solar-light-driven photoelectrochemical water splitting performance of type II 1D/0D CdS/In2S3 nanorod arrays. Chem. Phys. Lett. 2023, 830, 140776. [Google Scholar] [CrossRef]
- Yang, L.; Gao, T.; Yuan, S.; Dong, Y.; Chen, Y.; Wang, X.; Chen, C.; Tang, L.; Ohno, T. Spatial charge separated two-dimensional/two-dimensional Cu-In2S3/CdS heterojunction for boosting photocatalytic hydrogen production. J. Colloid Inter. Sci. 2023, 652, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Jang, H.W. beta-In2S3 as water splitting photoanodes: Promise and challenges, electron. Mater. Lett. 2021, 17, 119–135. [Google Scholar]
- Chang, D.W.; Baek, J.-B. Nitrogen-doped graphene for photocatalytic hydrogen generation. Chem. Asian J. 2016, 11, 1125–1137. [Google Scholar] [CrossRef]
- Jia, L.; Wang, D.H.; Huang, Y.X.; Xu, A.W.; Yu, H.Q. Highly durable N-doped graphene/CdS nanocomposites with enhanced photocatalytic hydrogen evolution from water under visible light irradiation. J. Phys. Chem. C 2011, 115, 11466–11473. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Ren, Q.; Li, T.; Tu, G.; Zhong, S.; Zhao, Y.; Bai, S. Stacking design in photocatalysis: Synergizing cocatalyst roles and anti-corrosion functions of metallic MoS2 and graphene for remarkable hydrogen evolution over CdS. J. Mater. Chem. A 2021, 9, 1552–1562. [Google Scholar] [CrossRef]
- Nam, N.N.; Do, H.D.K.; Trinh, K.T.L.; Lee, N.Y. Design Strategy and Application of Deep Eutectic Solvents for Green Synthesis of Nanomaterials. Nanomaterials 2023, 13, 1164. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Y.; Gao, W.; Bai, T.; Cao, T.; Jin, J.; Xin, B. Deep Eutectic Solvent-Mediated Electrocatalysts for Water Splitting. Molecules 2022, 27, 8098. [Google Scholar] [CrossRef]
- Zhang, D.; Mou, H.; Lu, F.; Song, C.; Wang, D. A Novel Strategy for 2D/2D NiS/Graphene Heterostructures as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Appl. Catal. B Environ. 2019, 254, 471–478. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Amhamed, A. Review on carbon dioxide absorption by choline chloride/urea deep eutectic solvents. Adv. Chem. 2018, 2018, 2675659a. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Z.; Shi, J.-W.; Zhu, M.; Yu, H.; Zou, Y.; Lv, Y.; Sun, G.; Mao, S.; Cheng, Y. Cu-In2S3 Nanorod Induced the Growth of Cu&In Co-Doped Multi-Arm CdS Hetero-Phase Junction to Promote Photocatalytic H2 Evolution. Chem. Eng. J. 2020, 399, 125785. [Google Scholar] [CrossRef]
- Shen, R.; Ren, D.; Ding, Y.; Guan, Y.; Ng, Y.H.; Zhang, P.; Li, X. Nanostructured CdS for Efficient Photocatalytic H2 Evolution: A Review. Sci. China Mater. 2020, 63, 2153–2188. [Google Scholar] [CrossRef]
- Park, J.; Lee, T.H.; Kim, C.; Lee, S.A.; Choi, M.J.; Kim, H.; Yang, J.W.; Lim, J.; Jang, W.H. Hydrothermally Obtained Type-Ⅱ Heterojunction Nanostructures of In2S3/TiO2 for Remarkably Enhanced Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2021, 295, 120276. [Google Scholar] [CrossRef]
- He, B.; Bie, C.; Fei, X.; Cheng, B.; Yu, J.; Ho, W.; Al-Ghamdi, A.A.; Wangeh, S. Enhancement in the Photocatalytic H2 Production Activity of CdS NrS by Ag2S and NiS Dual Cocatalysts. Appl. Catal. B Environ. 2021, 288, 119994. [Google Scholar] [CrossRef]
- She, H.; Sun, Y.; Li, S.; Huang, J.; Wang, L.; Zhu, G.; Wang, Q. Synthesis of Non-Noble Metal Nickel Doped Sulfide Solid Solution for Improved Photocatalytic Performance. Appl. Catal. B Environ. 2019, 245, 439–447. [Google Scholar] [CrossRef]
- Marinoiu, A.; Raceanu, M.; Carcadea, E.; Varlam, M. Nitrogen-Doped Graphene Oxide as Efficient Metal-Free Electrocatalyst in PEM Fuel Cells. Nanomaterials 2023, 13, 1233. [Google Scholar] [CrossRef]
- Wang, H.J.; Wan, Y.; Li, B.R.; Ye, J.; Gan, J.; Liu, J.; Liu, X.; Song, X.; Zhou, W.; Li, X.; et al. Rational design of Ce-doped CdS/N-rGO photocatalyst enhanced interfacial charges transfer for high effective degradation of tetracycline. J. Mater. Sci. Technol. 2024, 173, 137–148. [Google Scholar] [CrossRef]
- Du, M.; Wang, Z.; Yang, C.; Deng, Y.; Wang, T.; Zhong, W.; Yin, W.; Jiao, Z.; Xia, W.; Jiang, B.; et al. N-doped reduced graphene oxide/Co0.85Se microflowers with high mass loading as battery-type materials for quasi-solid-state hybrid supercapacitors. J. Alloys Compd. 2021, 890, 161801. [Google Scholar] [CrossRef]
- He, Y.; Xu, G.; Wang, C.; Xu, L.; Zhang, K. Horsetail-Derived Si@N-Doped Carbon as Low-Cost and Long Cycle Life Anode for Li-Ion Half/Full Cells. Electrochim. Acta 2018, 264, 173–182. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Dong, X.; Zheng, N.; Ma, H.; Zhang, X. Fabrication of In2O3/In2S3 Microsphere Heterostructures for Efficient and Stable Photocatalytic Nitrogen Fixation. Appl. Catal. B Environ. 2019, 257, 117932. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X.; Wang, W.; Yang, Y.; Zhu, C.; Li, C.; Lin, W.; Tian, Q.; Liu, Y. CdS-Cu1.81S Heteronanorods with Continuous Sublattice for Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2022, 303, 120909. [Google Scholar] [CrossRef]
- Liu, S.; Ma, y.; Chi, D.; Sun, Y.; Chen, Q.; Zhang, J.; He, Z.; He, L.; Zhang, K.; Liu, B. Hollow Heterostructure CoS/CdS Photocatalysts with Enhanced Charge Transfer for Photocatalytic Hydrogen Production from Seawater. Int. J. Hydrog. Energ. 2022, 47, 9220–9229. [Google Scholar] [CrossRef]
- Shi, Y.; Lei, X.; Xia, L.; Wu, Q.; Yao, W. Enhanced Photocatalytic Hydrogen Production Activity of CdS Coated with Zn-Anchored Carbon Layer. Chem. Eng. J. 2020, 393, 124751. [Google Scholar] [CrossRef]
- Huang, X.; Lei, R.; Yuan, J.; Gao, F.; Jiang, C.; Feng, W.; Zhuang, J.; Liu, P. Insight into the Piezo-Photo Coupling Effect of PbTiO3/CdS Composites for Piezo-Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2021, 282, 119586. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Bai, Y.; Lian, J.; Huang, H.; Li, Z.; Lei, Z.; Shangguan, W. Photochemical Preparation of Cd/CdS Photocatalysts and Their Efficient Photocatalytic Hydrogen Production under Visible Light Irradiation. Green. Chem. 2014, 16, 2728–2735. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Ji, Y.; Bian, R.; Li, J.; Zhang, X.; Tian, J.; Yang, Q.; Shi, F. Ti3C2 Mxene Coupled with CdS Nanoflowers as 2D/3D Heterostructures for Enhanced Photocatalytic Hydrogen Production Activity. Int. J. Hydrog. Energ. 2022, 47, 22045–22053. [Google Scholar] [CrossRef]
- Deng, C.; Ye, F.; Wang, T.; Ling, X.; Peng, L.; Yu, H.; Ding, K.; Hu, H.; Dong, Q.; Le, H.; et al. Developing Hierarchical CdS/NiO Hollow Heterogeneous Architectures for Boosting Photocatalytic Hydrogen Generation. Nano Res. 2022, 15, 2003–2012. [Google Scholar] [CrossRef]
- Yuan, C.; Lv, H.; Zhang, Y.; Fei, Q.; Xiao, D.; Yin, H.; Lu, Z.; Zhang, Y. Three-Dimensional Nanoporous Heterojunction of CdS/np-rGO for Highly Efficient Photocatalytic Hydrogen Evolution under Visible Light. Carbon 2023, 206, 237–245. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Li, N.; Ge, L. In-Situ Constructing Cobalt Incorporated Nitrogen-Doped Carbon/CdS Heterojunction with Efficient Interfacial Charge Transfer for Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2022, 47, 27961–27972. [Google Scholar] [CrossRef]
- Xu, M.; Kang, Y.; Jiang, L.; Jiang, L.; Tremblay, P.-L.; Zhang, T. The One-Step Hydrothermal Synthesis of CdS Nanorods Modified with Carbonized Leaves from Japanese Raisin Trees for Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2022, 47, 15516–15527. [Google Scholar] [CrossRef]
- Yu, G.; Gong, K.; Xing, C.; Hu, L.; Huang, H.; Gao, L.; Wang, D.; Li, X. Dual P-doped-site modified porous g-C3N4 achieves high dissociation and mobility efficiency for photocatalytic H2O2 production. Chem. Eng. J. 2023, 461, 142140. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, T.; Wen, X.; Chu, D.; Jiang, Y. Tunable Type I and II heterojunction of CoOx nanoparticles confined in g-C3N4 nanotubes for photocatalytic hydrogen production. Appl. Catal. B Environ. 2019, 244, 814–822. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.J. Nanostructured 3D zinc cobaltite/nitrogen-doped reduced graphene oxide composite electrode for supercapacitor applications. J. Ind. Eng. Chem. 2017, 54, 205–217. [Google Scholar] [CrossRef]
- Yang, H.; Tang, J.; Luo, Y.; Zhan, X.; Liang, Z.; Jiang, L.; Hou, H.; Yang, W. MOFs-derived fusiform In2O3 mesoporous nanorods anchored with ultrafine CdZnS nanoparticles for boosting visible-light photocatalytic hydrogen evolution. Small 2021, 17, 2102307. [Google Scholar] [CrossRef]

| Catalyst | Light Source | Scavengers | H2 Evolution Rate (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|
| In2S3/CdS/N-GO | λ ≥ 420 nm | Na2S and Na2SO3 | 10.9 | This work |
| CdS-Cu1.81S | λ ≥ 420 nm | Na2S and Na2SO3 | 2.714 | [42] |
| CoS/CdS | λ ≥ 420 nm | Na2S and Na2SO3 | 0.143 | [43] |
| CdS@Zn-C | λ ≥ 420 nm | Na2S and Na2SO3 | 6.6 | [44] |
| PbTiO3/CdS | λ ≥ 420 nm | Na2S and Na2SO3 | 0.849 | [45] |
| Cd/CdS | λ ≥ 420 nm | Na2S and Na2SO3 | 1.753 | [46] |
| Ti3C2@CdS | λ ≥ 420 nm | methanol | 0.088 | [47] |
| CdS/NiO | λ ≥ 420 nm | Na2S and Na2SO3 | 1.77 | [48] |
| CdS/np-rGO | λ ≥ 420 nm | Na2S and Na2SO3 | 2.171 | [49] |
| Co@NC/CdS | λ ≥ 420 nm | lactic acid | 8.2 | [50] |
| C/CdS | λ ≥ 420 nm | Na2S and triethanolamine | 5.71 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wu, X.; Liu, X.; Li, H.; Wang, Y.; Wang, D. Constructing In2S3/CdS/N-rGO Hybrid Nanosheets via One-Pot Pyrolysis for Boosting and Stabilizing Visible Light-Driven Hydrogen Evolution. Molecules 2023, 28, 7878. https://doi.org/10.3390/molecules28237878
Zhang M, Wu X, Liu X, Li H, Wang Y, Wang D. Constructing In2S3/CdS/N-rGO Hybrid Nanosheets via One-Pot Pyrolysis for Boosting and Stabilizing Visible Light-Driven Hydrogen Evolution. Molecules. 2023; 28(23):7878. https://doi.org/10.3390/molecules28237878
Chicago/Turabian StyleZhang, Minghao, Xiaoqun Wu, Xiaoyuan Liu, Huixin Li, Ying Wang, and Debao Wang. 2023. "Constructing In2S3/CdS/N-rGO Hybrid Nanosheets via One-Pot Pyrolysis for Boosting and Stabilizing Visible Light-Driven Hydrogen Evolution" Molecules 28, no. 23: 7878. https://doi.org/10.3390/molecules28237878
APA StyleZhang, M., Wu, X., Liu, X., Li, H., Wang, Y., & Wang, D. (2023). Constructing In2S3/CdS/N-rGO Hybrid Nanosheets via One-Pot Pyrolysis for Boosting and Stabilizing Visible Light-Driven Hydrogen Evolution. Molecules, 28(23), 7878. https://doi.org/10.3390/molecules28237878





