Iridium-Catalysed Transfer Hydrogenation of 1,8-Naphthyridine with Indoline: Access to Functionalized N-Heteroarenes
Abstract
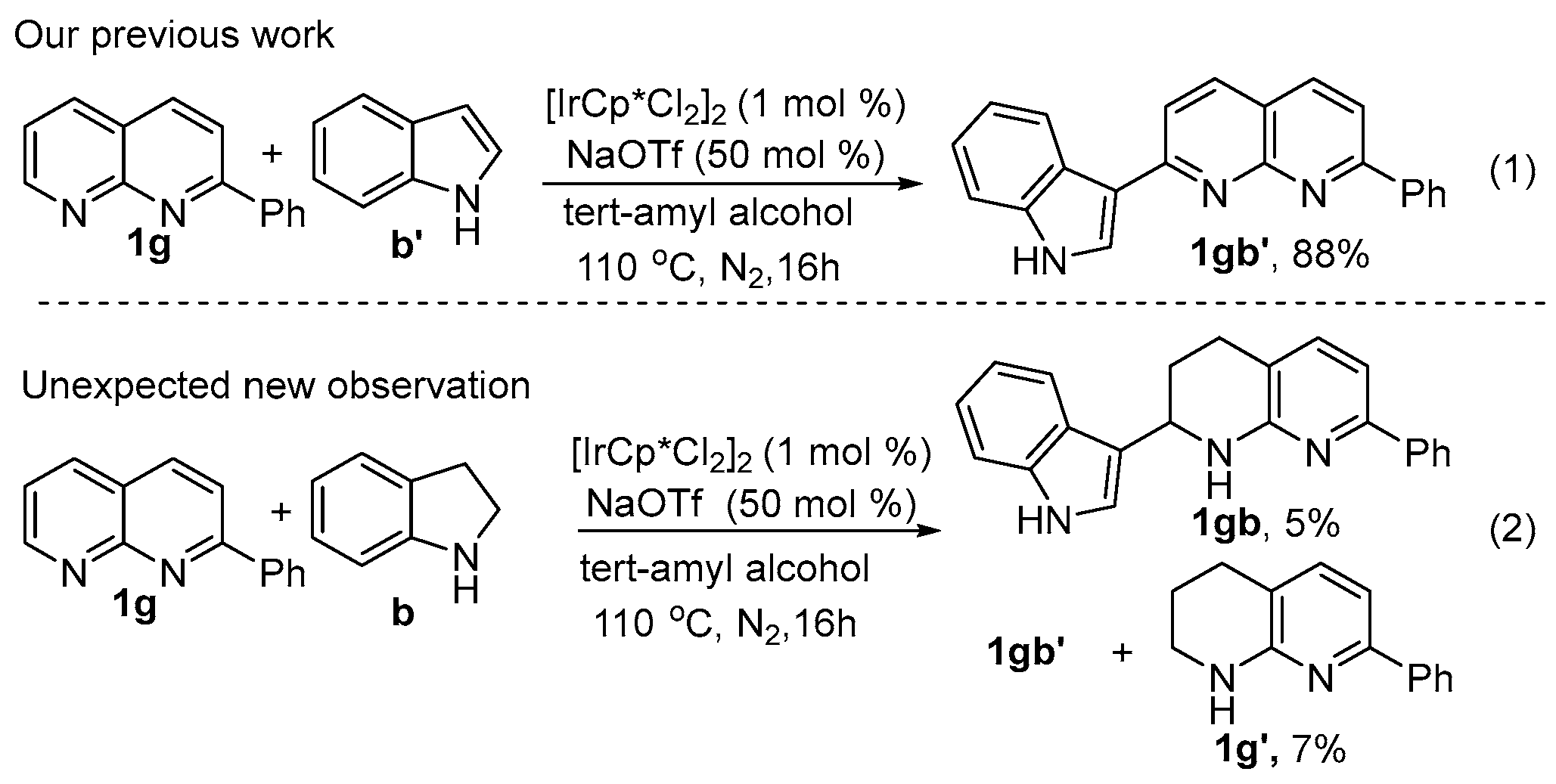
:1. Introduction
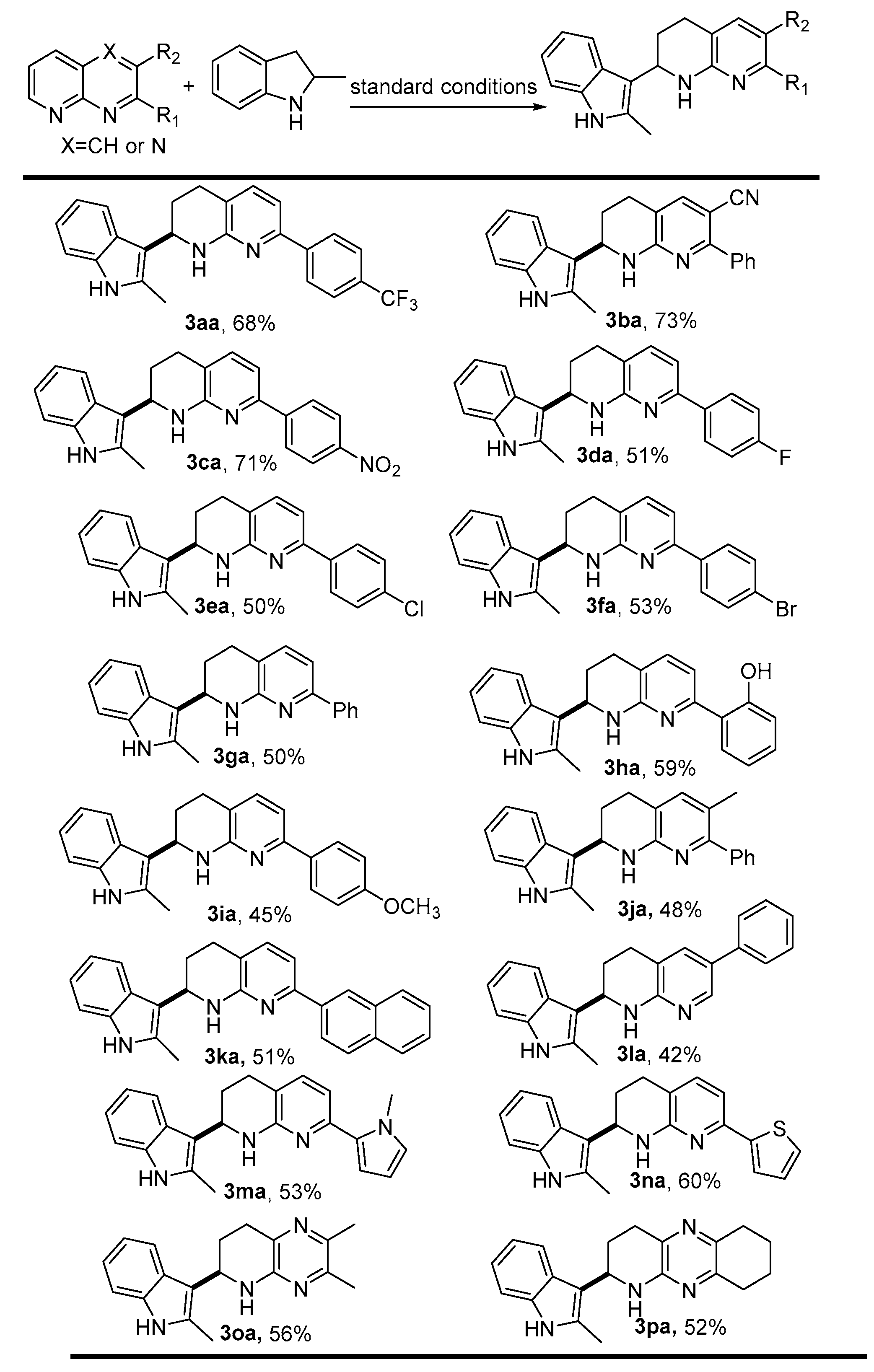
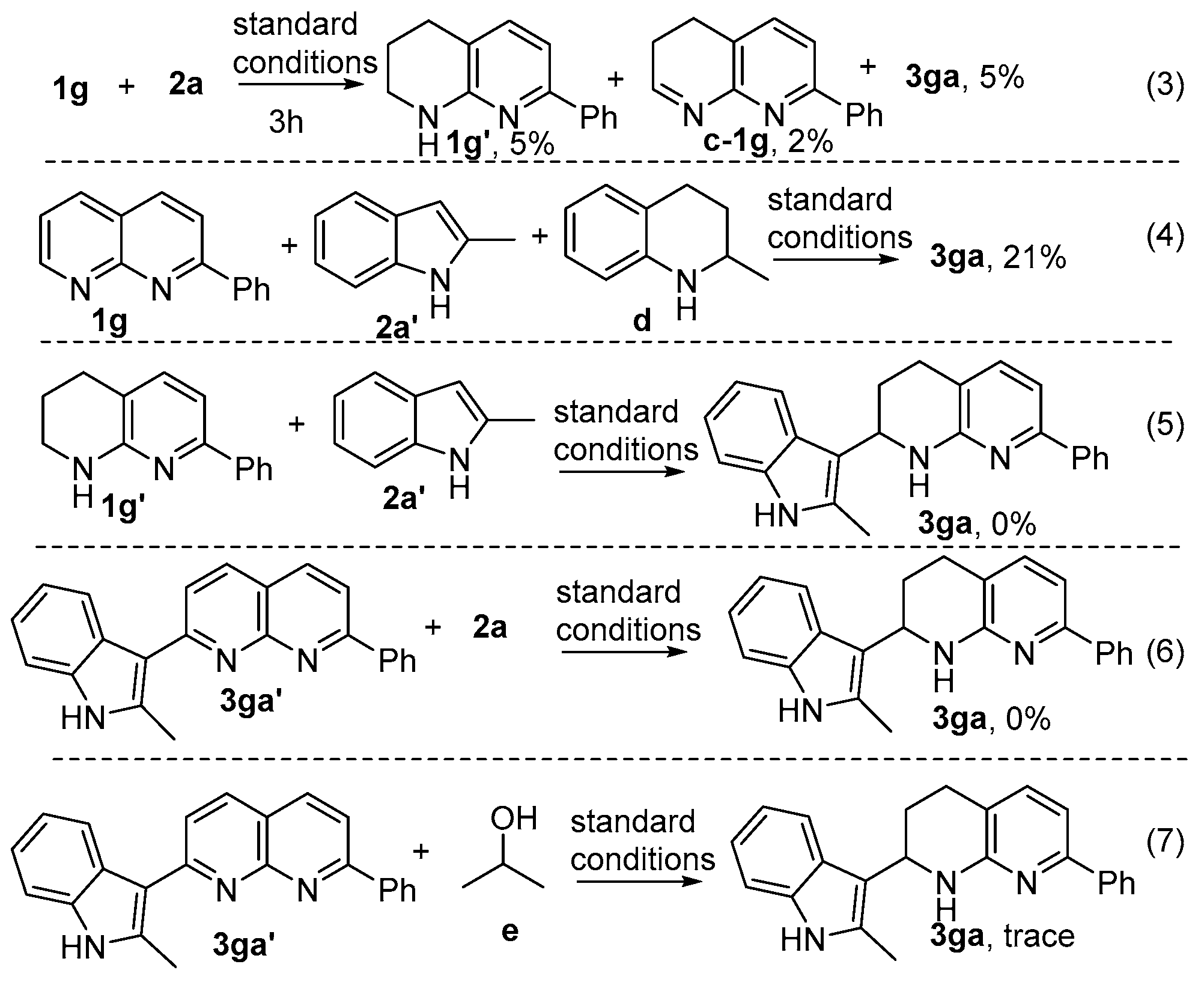
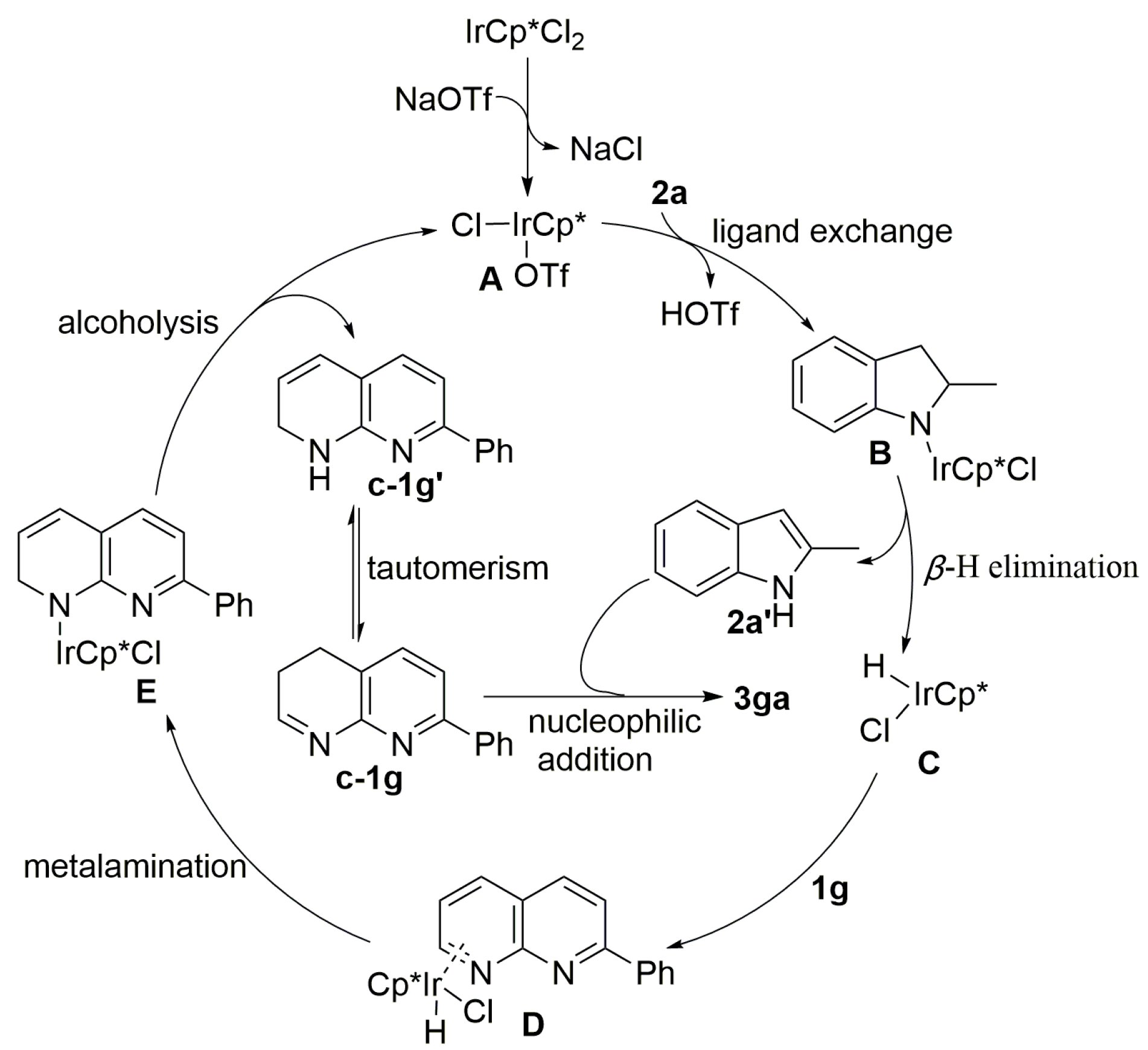
2. Results and Discussion
3. Experimental
3.1. General Information
3.2. Substrates Preparation
3.3. Typical Procedure for the Synthesis of Ester 3aa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malancona, S.; Donghi, M.; Ferrara, M.; Hernando, M.J.I.; Pompei, M.; Pesci, S.; Ontoria, J.M.; Koch, U.; Rowley, M.; Summa, V. Allosteric inhibitors of hepatitis C virus NS5B polymerase thumb domain site II: Structure-based design and synthesis of new templates. Bioorg. Med. Chem. 2010, 18, 2836. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Ikeda, C.; Takahashi, Y.; Fujita, K.I. Homogeneous Catalytic System for Reversible Dehydrogenation-Hydrogenation Reactions of Nitrogen Heterocycles with Reversible Interconversion of Catalytic Species. J. Am. Chem. Soc. 2009, 131, 8410. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Kim, M.H.; Kim, J. Cu-Catalyzed Aerobic Oxidation of Di-tert-butyl Hydrazodicarboxylate to Di-tert-butyl Azodicarboxylate and Its Application on Dehydrogenation of 1,2,3,4-Tetrahydroquinolines under Mild Conditions. Org. Lett. 2016, 18, 6300. [Google Scholar] [CrossRef] [PubMed]
- Iosub, A.V.; Stahl, V. Catalytic Aerobic Dehydrogenation of Nitrogen Heterocycles Using Heterogeneous Cobalt Oxide Supported on Nitrogen-Doped Carbon. Org. Lett. 2015, 17, 4404. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Hu, L.; Wang, J.; Li, X.; Qi, F.; Lu, J.; Cao, X.; Gu, H. Reversible Hydrogenation-Oxidative Dehydrogenation of Quinolines over a Highly Active Pt Nanowire Catalyst under Mild Conditions. ChemCatChem 2013, 5, 2183. [Google Scholar] [CrossRef]
- Chakraborty, S.; Brennessel, W.W.; Jones, W.D. A Molecular Iron Catalyst for the Acceptorless Dehydrogenation and Hydrogenation of N-Heterocycles. J. Am. Chem. Soc. 2014, 136, 8564. [Google Scholar] [CrossRef] [PubMed]
- Benkeser, R.A.; Robinson, R.E.; Landesman, H. The True Identity of the Solvated Free Radical “Triphenylsilicyl Ethylammine.” The Multiple Addition of Lithium to an Aromatic System in Ethylamine. J. Am. Chem. Soc. 1952, 74, 5699. [Google Scholar] [CrossRef]
- Benkeser, R.A.; Robinson, R.E.; Sauve, D.M.; Thomas, O.H. Reduction of Organic Compounds by Lithium in Low Molecular Weight Amines. I. Selective Reduction of Aromatic Hydrocarbons to Monoölefins. J. Am. Chem. Soc. 1955, 77, 3230. [Google Scholar] [CrossRef]
- Birch, A.J. Reduction by dissolving metals. Nature 1946, 158, 585. [Google Scholar] [CrossRef]
- Zimmerman, H.E. A Mechanistic Analysis of the Birch Reduction. Acc. Chem. Res. 2012, 45, 164. [Google Scholar] [CrossRef]
- Li, H.X.; Yin, H.; Zhang, F.; Li, H.; Huo, Y.N.; Lu, Y.F. Water-Medium Clean Organic Reactions over an Active Mesoporous Ru(II) Organometallic Catalyst. Environ. Sci. Technol. 2009, 43, 188. [Google Scholar] [CrossRef]
- He, W.; Ge, Y.C.; Tan, C.H. Halogen-Bonding-Induced Hydrogen Transfer to C=N Bond with Hantzsch Ester. Org. Lett. 2014, 16, 3244. [Google Scholar] [CrossRef] [PubMed]
- Grobas, J.; Bolivar, C.; Scott, C.E. Hydrodesulfurization of Benzothiophene and Hydrogenation of Cyclohexene, Biphenyl, and Quinoline, Assisted by Ultrasound, Using Formic Acid as Hydrogen Precursor. Energy Fuels 2007, 21, 19. [Google Scholar] [CrossRef]
- Chatterjee, I.; Oestreich, M. B(C6F5)3-Catalyzed Transfer Hydrogenation of Imines and Related Heteroarenes Using Cyclohexa-1,4-dienes as a Dihydrogen Source. Angew. Chem. Int. Ed. 2015, 54, 1965. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Perez, F.; Krische, M.J. Ruthenium(0)-Catalyzed [4+2] Cycloaddition of Acetylenic Aldehydes with α-Ketols: Convergent Construction of Angucycline Ring Systems. Angew. Chem. Int. Ed. 2016, 55, 1493. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Kasun, Z.A.; Krische, M.J. Enantioselective Alcohol C-H Functionalization for Polyketide Construction: Unlocking Redox-Economy and Site-Selectivity for Ideal Chemical Synthesis. J. Am. Chem. Soc. 2016, 138, 5467. [Google Scholar] [CrossRef] [PubMed]
- Garza, V.J.; Krische, M.J. Hydroxymethylation beyond Carbonylation: Enantioselective Iridium-Catalyzed Reductive Coupling of Formaldehyde with Allylic Acetates via Enantiotopic π-Facial Discrimination. J. Am. Chem. Soc. 2016, 138, 3655. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Krische, M.J. Iridium-Catalyzed C-C Coupling of a Simple Propargyl Ether with Primary Alcohols: Enantioselective Homoaldol Addition via Redox-Triggered (Z)-Siloxyallylation. J. Am. Chem. Soc. 2015, 137, 16024. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Herkommerand, D.; Krische, M.J. Ruthenium-BINAP Catalyzed Alcohol C-H tert-Prenylation via 1,3-Enyne Transfer Hydrogenation: Beyond Stoichiometric Carbanions in Enantioselective Carbonyl Propargylation. J. Am. Chem. Soc. 2016, 138, 5238. [Google Scholar] [CrossRef]
- Perez, F.; Waldeck, A.R.; Krische, M.J. Total Synthesis of Cryptocaryol A by Enantioselective Iridium-Catalyzed Alcohol C-H Allylation. Angew. Chem. Int. Ed. 2016, 55, 5049. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, H.; Girard, S.A.; Wang, F.; Chen, N.; Li, C.J. Formal Direct Cross-Coupling of Phenols with Amines. Angew. Chem. Int. Ed. 2015, 54, 14487. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, H.; Gong, H.; Wang, H.; Li, C.J. Palladium-catalyzed reductive coupling of phenols with anilines and amines: Efficient conversion of phenolic lignin model monomers and analogues to cyclohexylamines. Chem. Sci. 2015, 6, 4174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Breslin, M.J.; Coleman, P.J.; Duggan, M.E.; Hunt, C.A.; Hutchinson, J.H.; Leu, C.T.; Rodan, S.B.; Rodan, G.A.; Duong, L.T.; et al. Non-peptide αvβ3 antagonists. Part 7: 3-Substituted tetrahydro-1, 8naphthyridine derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Seefeld, M.A.; Miller, W.H.; Newlander, K.A.; Burgess, W.J.; DeWolf, W.E.; Elkins, P.A.; Head, M.S.; Jakas, D.R.; Janson, C.A.; Keller, P.M.; et al. Indole Naphthyridinones as Inhibitors of Bacterial Enoyl-ACP Reductases FabI and FabK. J. Med. Chem. 2003, 46, 1627. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; Rector, C.L.; Kim, H.Y.; Sonnen, A.F.P.; Meyer, R.; Nau, W.M.; Atkinson, J.; Rintoul, J.; Pratt, D.A.; Porter, N.A. Tetrahydro-1,8-naphthyridinol Analogues of α-Tocopherol as Antioxidants in Lipid Membranes and Low-Density Lipoproteins. J. Am. Chem. Soc. 2007, 129, 10211. [Google Scholar] [CrossRef]
- Lv, W.; Xiong, B.; Jiang, H.F.; Zhang, M. Synthesis of 2-Alkylaminoquinolines and 1,8-Naphthyridines by Successive Ruthenium-Catalyzed Dehydrogenative Annulation and N-Alkylation Processes. Adv. Synth. Catal. 2017, 359, 1202. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, S.D.; Jiang, H.F.; Zhang, M. Hydrogen-Transfer-Mediated Direct β-Alkylation of Aryl-1,8-naphthyridines with Alcohols under Transition Metal Catalyst Free Conditions. Org. Lett. 2016, 18, 724. [Google Scholar] [CrossRef]
- Tan, Z.D.; Jiang, H.F.; Zhang, M. Ruthenium-Catalyzed Dehydrogenative β-Benzylation of 1,2,3,4-Tetrahydroquinolines with Aryl Aldehydes: Access to Functionalized Quinolines. Org. Lett. 2016, 18, 3174. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, S.D.; Chen, L.; Li, B.; Jiang, H.F.; Zhang, M. An annulative transfer hydrogenation strategy enables straightforward access to tetrahydro fused-pyrazine derivatives. Chem. Commun. 2016, 52, 10636. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, M.; Jiang, H.F.; Chen, M.M.; Lv, W.; Zheng, A.B.; Jian, X.J. Efficient synthesis of quinoxalines from 2-nitroanilines and vicinal diols via a ruthenium-catalyzed hydrogen transfer strategy. Green Chem. 2015, 17, 279. [Google Scholar] [CrossRef]
- Xiong, B.; Li, Y.; Lv, W.; Tan, Z.D.; Jiang, H.F.; Zhang, M. Ruthenium-Catalyzed Straightforward Synthesis of 1,2,3,4-Tetrahydronaphthyridines via Selective Transfer Hydrogenation of Pyridyl Ring with Alcohols. Org. Lett. 2015, 17, 4054. [Google Scholar] [CrossRef]
- Tan, Z.D.; Jiang, H.F.; Zhang, M. A novel iridium/acid co-catalyzed transfer hydrogenative C(sp3)–H bond alkylation to access functionalized N-heteroaromatics. Chem. Commun. 2016, 52, 9359. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, M.; Chen, M.M.; Lv, W.; Jiang, H.F. Convenient Synthesis of Quinolines from α-2-Nitroaryl Alcohols and Alcohols via a Ruthenium-catalyzed Hydrogen Transfer Strategy. ChemCatChem 2015, 7, 349. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Zhao, H.; Jiang, H.; Zhang, M. Direct Access to Nitrogen Bi-heteroarenes via Iridium-Catalyzed Hydrogen-Evolution Cross-Coupling Reaction. Org. Lett. 2017, 19, 3390. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Xiong, B.; Yang, J.; Ci, C.; Jiang, H.; Zhang, M. Selective reductive cross-coupling of N-heteroarenes by an unsymmetrical PNP-ligated manganese catalyst. J. Catal. 2020, 392, 135. [Google Scholar] [CrossRef]
- Modha, S.G.; Greaney, M.F. Atom-Economical Transformation of Diaryliodonium Salts: Tandem C-H and N-H Arylation of Indoles. J. Am. Chem. Soc. 2015, 137, 1416. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.H.; Zhang, B.; Sun, Y.; Wang, L.; Chen, J.B.; Cheng, J. Copper-mediated C3-cyanation of indoles by the combination of amine and ammonium. Chem. Commun. 2014, 50, 2315. [Google Scholar] [CrossRef]
- Chen, S.P.; Liao, Y.F.; Zhao, F.; Qi, H.R.; Liu, S.W.; Deng, G.J. Palladium-Catalyzed Direct Arylation of Indoles with Cyclohexanones. Org. Lett. 2014, 16, 1618. [Google Scholar] [CrossRef]
- Li, Y.; Yan, T.; Junge, K.; Beller, M. Catalytic Methylation of C–H Bonds Using CO2 and H2. Angew. Chem. Int. Ed. 2014, 126, 10644. [Google Scholar] [CrossRef]
- Wu, J.C.; Song, R.J.; Wang, Z.Q.; Huang, X.C.; Xie, Y.X.; Li, J.H. Copper-Catalyzed C-H Oxidation/Cross-Coupling of α-Amino Carbonyl Compounds. Angew. Chem. Int. Ed. 2012, 51, 3453. [Google Scholar] [CrossRef]
- Leskinen, M.V.; Yip, K.T.; Valkonen, A.; Pihko, P.M. Palladium-Catalyzed Dehydrogenative β′-Functionalization of β-Keto Esters with Indoles at Room Temperature. J. Am. Chem Soc. 2012, 134, 5750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Rawal, V.H. Palladium-Catalyzed C3-Benzylation of Indoles. J. Am. Chem. Soc. 2012, 134, 111. [Google Scholar] [CrossRef]
- Wu, W.; Su, W. Mild and Selective Ru-Catalyzed Formylation and Fe-Catalyzed Acylation of Free (N-H) Indoles Using Anilines as the Carbonyl Source. J. Am. Chem. Soc. 2011, 133, 11924. [Google Scholar] [CrossRef]
- Zeng, Z.; Deng, Y.; Li, L.; Li, C.; Zhong, M. Hydrogen Transfer Coupling with 100% Atom Economy: Synthesis of 2-Indolyltetrahydronaphthyridine Derivatives. J. Org. Chem. 2022, 87, 12257. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wang, X.; Tong, Y.; Qiu, X.; Zeng, X.; Xiong, B. Ruthenium-catalyzed acceptorless dehydrogenative coupling of amino alcohols and ynones to access 3-acylpyrroles. Chem. Commun. 2022, 58, 2379. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Wang, Y.; Liu, Y.; Bao, Y.; Liu, Z.; Zhang, Y.; Ling, Y. Straightforward synthesis of quinolines from enones and 2-aminobenzyl alcohols using an iridium-catalyzed transfer hydrogenative strategy. Org. Biomol. Chem. 2018, 16, 5707. [Google Scholar] [CrossRef] [PubMed]
- Moya, S.A.; Gajardo, J.; Araya, J.C.; Cornejo, J.J.; Guerchais, V.; Le Bozec, H.; Bayón, J.C.; Pardey, A.J.; Aguirre, P. Synthesis and characterization of new complexes of the type [Ru(CO)2Cl2(2-phenyl-1,8-naphthyridine-kN) (2-phenyl-1,8-naphthyridine-kN′)]. Preliminary applications in homogeneous catalysis. Appl. Organomet. Chem. 2008, 22, 471–478. [Google Scholar] [CrossRef]
- Hawes, E.M.; Gorecki, D.K.J.; Gedir, R.G. 2,3-Disubstituted 1,8-naphthyridines as potential diuretic agents. 2. 5,7-Dimethyl derivatives. J. Med. Chem. 1977, 20, 838–841. [Google Scholar] [CrossRef]
- Reddy, K.V.; Sreenivasulu, B. A Facile One-Step Synthesis of 2-Aryl and Heteryl-1,8-Naphthyridines. Curr. Sci. 1977, 46, 597–598. [Google Scholar]
- Mogilaiah, K.; Kumar, K.S.; Reddy, N.V. Potassium triiodide catalyzed Friedlander synthesis of 1,8-naphthyridines in aqueous media. Indian J. Chem. 2010, 49, 253–255. [Google Scholar]
- Galatsis, P.; Yamagata, K.; Wendt, J.A.; Connolly, C.J.; Mickelson, J.W.; Milbank, J.B.J.; Bove, S.E.; Knauer, C.S.; Brooker, R.M.; Augelli-Szafran, C.E.; et al. Synthesis and SAR comparison of regioisomeric aryl naphthyridines as potent mGlu5 receptor antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 6525–6528. [Google Scholar] [CrossRef]
- Chen, X.W.; Zhao, H.; Xiong, B.; Jiang, H.F.; Dixneuf, P.H.; Zhang, M. Selective synthesis of nitrogen bi-heteroarenes by a hydrogen transfer-mediated direct α,β-coupling reaction. Org. Biomol. Chem. 2017, 15, 6093–6097. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Zhao, H.; Chen, C.L.; Jiang, H.F.; Zhang, M. Hydrogen-Transfer-Mediated α-Functionalization of 1,8-Naphthyridines by a Strategy Overcoming the Over-Hydrogenation Barrier. Angew. Chem. Int. Ed. 2017, 56, 14232–14236. [Google Scholar] [CrossRef]


| Entry | Catalyst (mol %) | Additive (mol %) | 3aa yield%b |
|---|---|---|---|
| 1 | [Cp*IrCl2]2 (1) | NaOTf (10) | 35 |
| 2 | [Cp*IrCl2]2 (1) | benzoic acid (50) | 27 |
| 3 | [Cp*IrCl2]2 (1) | CF3COOH (50) | 29 |
| 4 | [Cp*IrCl2]2 (1) | HOTf (50) | 25 |
| 5 | [Cp*IrCl2]2 (1) | Et3N (50) | 23 |
| 6 | [Cp*IrCl2]2 (1) | DBU (50) | - |
| 7 | [Cp*IrCl2]2 (1) | NaOH (50) | - |
| 8 | [Cp*IrCl2]2 (1) | - | 59 |
| 9 | [Cp*IrCl2]2 (1) | 68 c | |
| 10 | [Cp*IrCl2]2 (1) | - | (39, 47) d |
| 11 | - | - | - |
| 12 | [Cp*IrCl2]2 (1) | DPPB | - |
| 13 | [Cp*IrCl2]2 (1) | 1,10-Phenanthroline | - |
| 14 | [Cp*IrCl2]2 (1) | - | (51, 55) e |
| 15 | [Cp*IrCl2]2 (1) | - | (47, trace) f |
| 16 | [IrClCOD]2 (1) | - | trace |
| 17 | Pd(OAc)2 (5) | - | 17% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhang, J.; Fu, Y.; Chen, C.; Zhao, H. Iridium-Catalysed Transfer Hydrogenation of 1,8-Naphthyridine with Indoline: Access to Functionalized N-Heteroarenes. Molecules 2023, 28, 7886. https://doi.org/10.3390/molecules28237886
Zhou C, Zhang J, Fu Y, Chen C, Zhao H. Iridium-Catalysed Transfer Hydrogenation of 1,8-Naphthyridine with Indoline: Access to Functionalized N-Heteroarenes. Molecules. 2023; 28(23):7886. https://doi.org/10.3390/molecules28237886
Chicago/Turabian StyleZhou, Changjian, Jiahao Zhang, Yuqing Fu, Chunlian Chen, and He Zhao. 2023. "Iridium-Catalysed Transfer Hydrogenation of 1,8-Naphthyridine with Indoline: Access to Functionalized N-Heteroarenes" Molecules 28, no. 23: 7886. https://doi.org/10.3390/molecules28237886
APA StyleZhou, C., Zhang, J., Fu, Y., Chen, C., & Zhao, H. (2023). Iridium-Catalysed Transfer Hydrogenation of 1,8-Naphthyridine with Indoline: Access to Functionalized N-Heteroarenes. Molecules, 28(23), 7886. https://doi.org/10.3390/molecules28237886






