Unveiling the Anti-Cholera and Active Diabetic Renoprotective Compounds of Maqian Essential Oil: A Computational and Molecular Dynamics Study
Abstract
:1. Introduction
2. Results
2.1. Molecular Docking Analysis of Ligands with Target Proteins
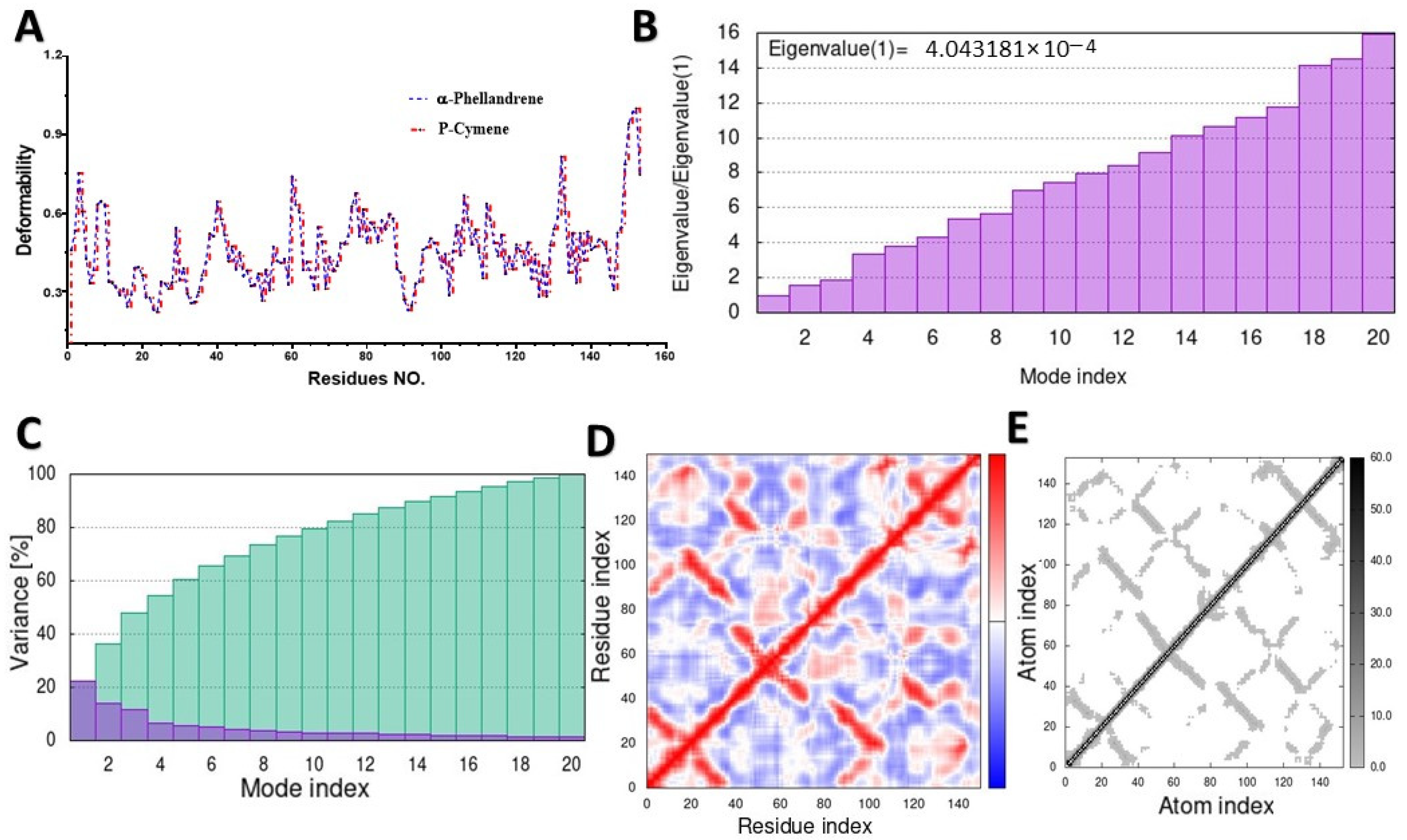
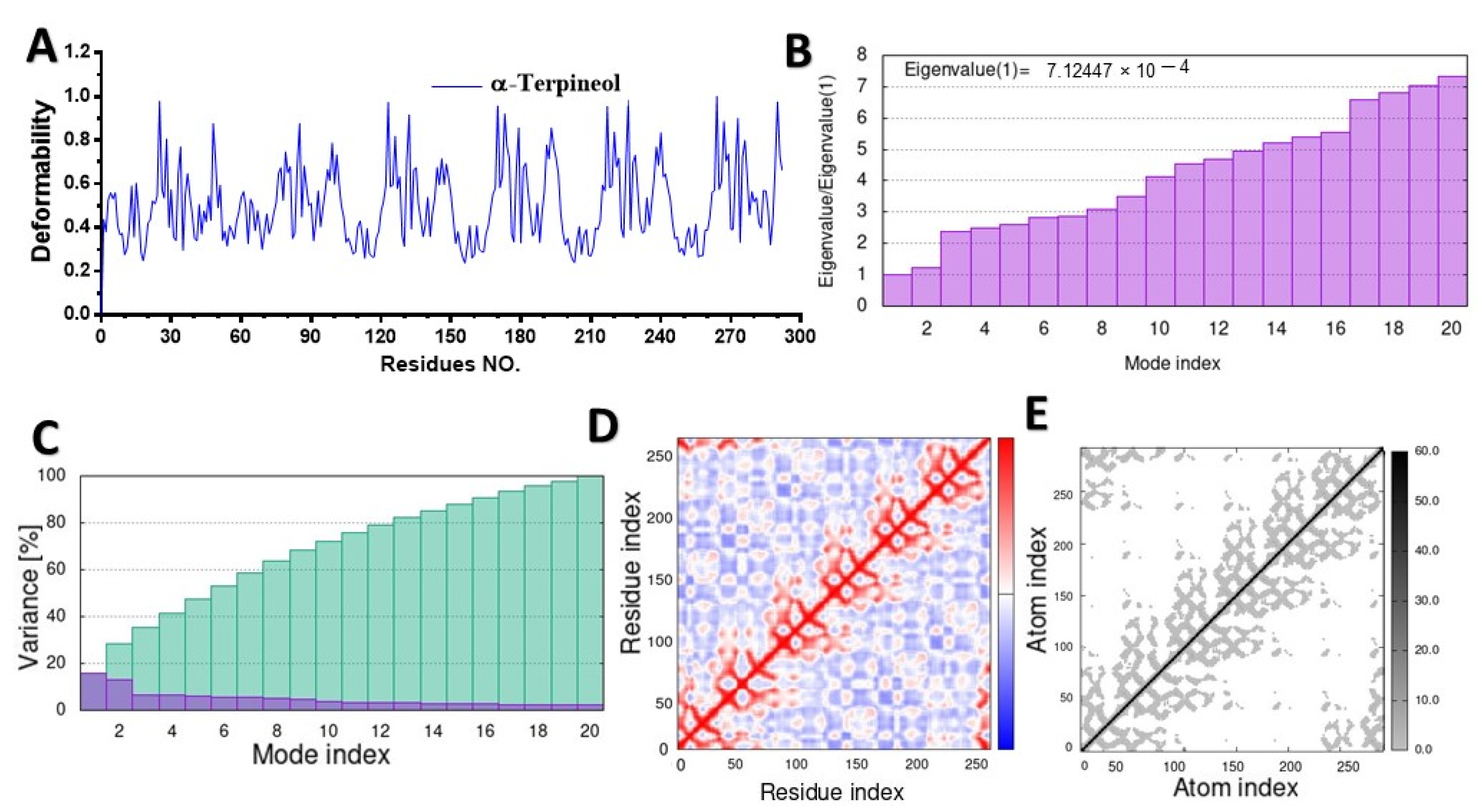
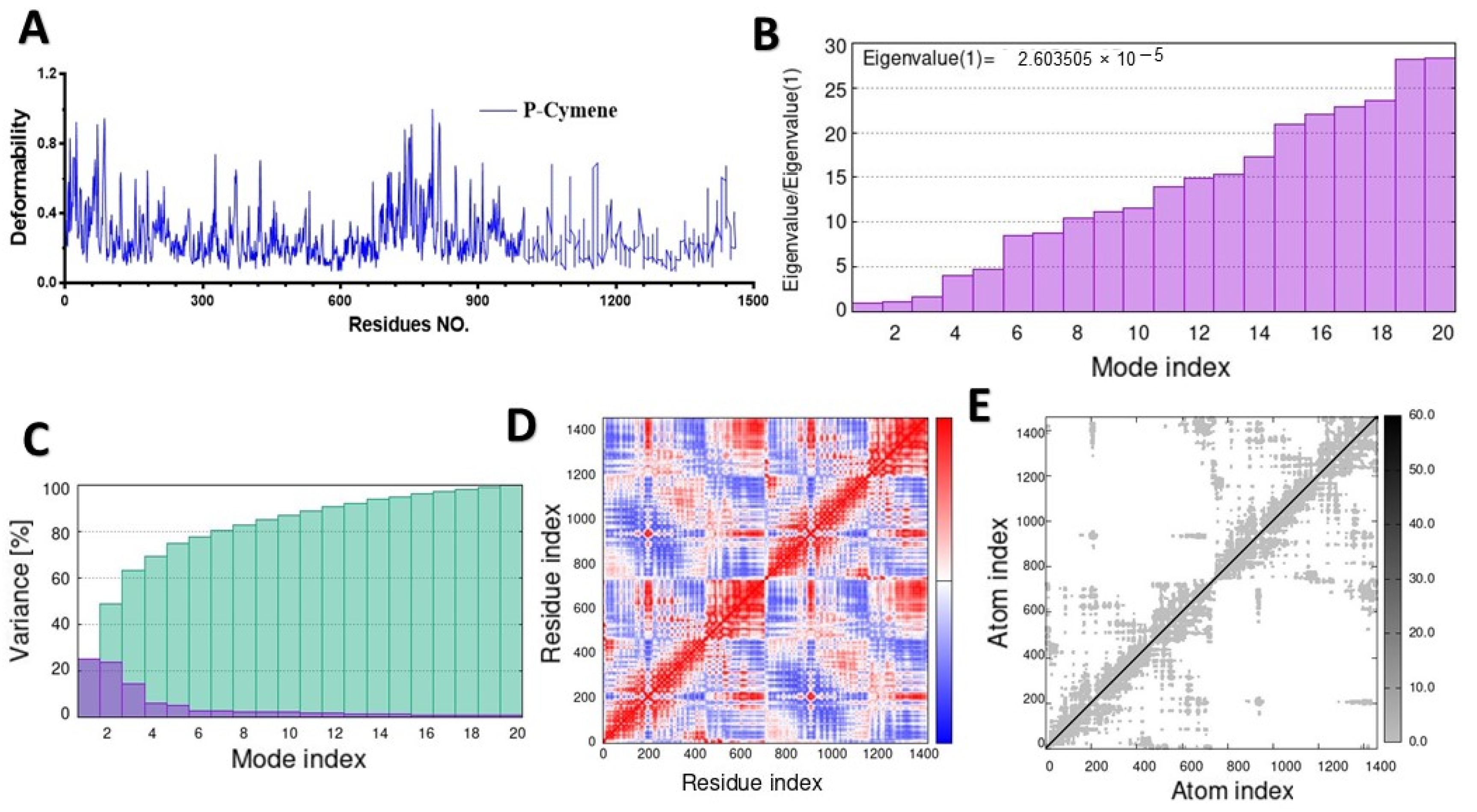
2.2. Molecular Dynamic Simulation (MD)
2.3. In Silico Prediction of ADME Properties and Toxicity Parameters of Selected Ligands
3. Discussion
4. Materials and Methods
4.1. Retrieval and Preparation of Target Receptor Proteins
4.2. Ligand Preparation
4.3. Molecular Docking
4.4. Molecular Dynamic Simulation
4.5. Prediction of ADME Property and Toxicity Parameters of Selected Ligands
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdallah, E.M. Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011, 1, 16–20. [Google Scholar]
- Boy, H.I.A.; Rutilla, A.J.H.; Santos, K.A.; Ty, A.M.T.; Alicia, I.Y.; Mahboob, T.; Tangpoong, J.; Nissapatorn, V. Recommended medicinal plants as source of natural products: A review. Digit. Chin. Med. 2018, 1, 131–142. [Google Scholar] [CrossRef]
- Medina, E.; Pieper, D.H. Tackling threats and future problems of multidrug-resistant bacteria. In How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives; Springer Cham: Berlin/Heidelberg, Germany, 2016; pp. 3–33. [Google Scholar]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Nateghizad, H.; Sajadi, R.; Shivaee, A.; Shirazi, O.; Sharifian, M.; Tadi, D.A.; Amini, K. Resistance of Vibrio cholera to antibiotics that inhibit cell wall synthesis: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1027277. [Google Scholar] [CrossRef] [PubMed]
- Basu, I.; Mukhopadhyay, C. Insights into binding of cholera toxin to GM1 containing membrane. Langmuir 2014, 30, 15244–15252. [Google Scholar] [CrossRef] [PubMed]
- Ndege, B.W. Acute Kidney Injury and Electrolyte Abnormalities Among Patients Admitted with Cholera in Kenyatta National Hospital in Nairobi, Kenya, In The Year 2017. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2018. [Google Scholar]
- Aminzare, M.; Hashemi, M.; Abbasi, Z.; Mohseni, M.; Amiri, E. Vibriosis phytotherapy: A review on the most important world medicinal plants effective on Vibrio spp. J. Appl. Pharm. Sci. 2018, 8, 170–177. [Google Scholar] [CrossRef]
- Al-Sharafi, B.; Alemad, S.; Algoby, M.; Al-Yousfi, N. Managing patients with Diabetes Mellitus after a cholera at-tack: A retrospective analysis in a tertiary center and specialty clinic in Sana’a Yemen. J. Diabetes Treat. 2020, 5, 1083. [Google Scholar]
- Park, S.-J.; Kim, Y.; Li, C.; Suh, J.; Sivapackiam, J.; Goncalves, T.M.; Jarad, G.; Zhao, G.; Urano, F.; Sharma, V. Blocking CHOP-dependent TXNIP shuttling to mitochondria attenuates albuminuria and mitigates kidney injury in nephrotic syndrome. Proc. Natl. Acad. Sci. USA 2022, 119, e2116505119. [Google Scholar] [CrossRef]
- Duran-Salgado, M.; Rubio-Guerra, A. Diabetic nephropathy and inflammation. World J. Diabetes 2014, 5, 393–398. [Google Scholar] [CrossRef]
- Sun, H.; Sun, R.; Hua, Y.; Lu, Q.; Shao, X. An update on the role of thioredoxin-interacting protein in diabetic kidney disease: A mini review. Front. Med. 2023, 10, 1153805. [Google Scholar] [CrossRef]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Shahin, D.H.H.; Sultana, R.; Farooq, J.; Taj, T.; Khaiser, U.F.; Alanazi, N.S.A.; Alshammari, M.K.; Alshammari, M.N.; Alsubaie, F.H.; Asdaq, S.M.B. Insights into the uses of traditional plants for diabetes nephropathy: A review. Curr. Issues Mol. Biol. 2022, 44, 2887–2902. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, A.M.E.; Abdallah, E.M.; Alanazi, N.A.; Ed-Dra, A.; Jamal, A.; Idriss, H.; Alshammari, A.S.; Shommo, S.A. Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential. Pharmaceuticals 2023, 16, 1451. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin ameliorates diabetic nephropathy by suppressing NLRP3 inflammasome signaling. BioMed Res. Int. 2017, 2017, 1516985. [Google Scholar] [CrossRef] [PubMed]
- Al Hroob, A.M.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Jing, W.; Qing, W.; Anxiang, S.; Mei, X.; Qin, L.; Qiuhui, H. Inhibitory effect of Zanthoxylum bungeanum essential oil (ZBEO) on Escherichia coli and intestinal dysfunction. Food Funct. 2017, 8, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-C.; Li, R.; Tan, J.; Jiang, Z.-T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef]
- Andima, M.; Coghi, P.; Yang, L.J.; Wong, V.K.W.; Ngule, C.M.; Heydenreich, M.; Ndakala, A.J.; Yenesew, A.; Derese, S. Antiproliferative activity of secondary metabolites from Zanthoxylum zanthoxyloides Lam: In vitro and in silico studies. Pharmacogn. Commun. 2020, 10, 44–51. [Google Scholar] [CrossRef]
- Bitchagno, G.T.M.; Tankeo, S.B.; Tsopmo, A.; Mpetga, J.D.S.; Tchinda, A.T.; Fobofou, S.A.T.; Wessjohann, L.A.; Kuete, V.; Tane, P. Lemairones A and B: Two new antibacterial tetraflavonoids from the leaves of Zanthoxylum lemairei (Rutaceae). Phytochem. Lett. 2015, 14, 1–7. [Google Scholar] [CrossRef]
- Goodman, C.D.; Hoang, A.T.; Diallo, D.; Malterud, K.E.; McFadden, G.I.; Wangensteen, H. Anti-plasmodial effects of Zanthoxylum zanthoxyloides. Planta Medica 2019, 85, 1073–1079. [Google Scholar] [CrossRef]
- Nurain, I.O.; Bewaji, C.O.; Johnson, J.S.; Davenport, R.D.; Zhang, Y. Potential of three ethnomedicinal plants as antisickling agents. Mol. Pharm. 2017, 14, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, J.-J.; Shi, Y.-X.; Zhao, M.; Ji, K.-L.; Zhang, P.; Xu, Y.-K.; Hu, H.-B. Chemical composition, antimicrobial and anti-inflammatory activities of the essential oil from Maqian (Zanthoxylum myriacanthum var. pubescens) in Xishuangbanna, SW China. J. Ethnopharmacol. 2014, 158, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Gan, X.-Q.; Fan, Q.-F.; Yang, J.-J.; Zhang, P.; Hu, H.-B.; Song, Q.-S. Chemical constituents and anti-inflammatory activities of Maqian (Zanthoxylum myriacanthum var. pubescens) bark extracts. Sci. Rep. 2017, 7, 45805. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.R.; Chandrashekar, B.; Lukose, O.; Ravindra, M.; Ravikumar, K. Evaluation of in-vitro antioxidant property and total phenolic content of Zanthoxylum rhetsa fruit extracts. J. Pharmacogn. Phytochem. 2019, 8, 1139–1144. [Google Scholar]
- Sriwichai, T.; Sookwong, P.; Siddiqui, M.W.; Sommano, S.R. Aromatic profiling of Zanthoxylum myriacanthum (makwhaen) essential oils from dried fruits using different initial drying techniques. Ind. Crops Prod. 2019, 133, 284–291. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Li, S.; Peng, Z. Effects of pepper (Zanthoxylum bungeanum Maxim.) leaf extract on the antioxidant enzyme activities of salted silver carp (Hypophthalmichthys molitrix) during processing. J. Funct. Foods 2015, 18, 1179–1190. [Google Scholar] [CrossRef]
- Monteiro, M.B.; Santos-Bezerra, D.P.; Thieme, K.; Admoni, S.N.; Perez, R.V.; Machado, C.G.; Queiroz, M.S.; Nery, M.; Oliveira-Souza, M.; Woronik, V. Thioredoxin interacting protein expression in the urinary sediment associates with renal function decline in type 1 diabetes. Free. Radic. Res. 2016, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Y.; Kelly, D.J.; Tan, C.Y.; Gill, A.; Cheng, D.; Braet, F.; Park, J.-S.; Sue, C.M.; Pollock, C.A. Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci. Rep. 2016, 6, 29196. [Google Scholar] [CrossRef]
- Thielen, L.; Shalev, A. Diabetes pathogenic mechanisms and potential new therapies based upon a novel target called TXNIP. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 75. [Google Scholar] [CrossRef]
- Shah, A.; Xia, L.; Masson, E.A.; Gui, C.; Momen, A.; Shikatani, E.A.; Husain, M.; Quaggin, S.; John, R.; Fantus, I. Thioredoxin-interacting protein deficiency protects against diabetic nephropathy. J. Am. Soc. Nephrol. 2015, 26, 2963. [Google Scholar] [CrossRef]
- Dahab, M.; Fu, B.; Osman, E.; Xu, Y.-K.; Zhang, P. Zanthoxylum myriacanthum var. pubescens essential oil protective potential against diabetic mice nephropathy and its relevant oxidative stress. J. Essent. Oil Bear. Plants 2019, 22, 581–591. [Google Scholar] [CrossRef]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, M.A.; Nicholas, S.B.; Norris, K.C.; Vaziri, N.D. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol. Dial. Transplant. 2013, 28, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Min, X.; Xu, X.; Du, B.; Luo, P. Role of nuclear factor erythroid 2-related factor 2 in diabetic nephropathy. J. Diabetes Res. 2017, 2017, 2608. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Arulmozhiraja, S.; Matsuo, N.; Ishitsubo, E.; Okazaki, S.; Shimano, H.; Tokiwa, H. Comparative binding analysis of dipeptidyl peptidase IV (DPP-4) with antidiabetic drugs–an ab initio fragment molecular orbital study. PLoS ONE 2016, 11, e0166275. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, G.; Zhong, J.; Peters, M.; Rajagopalan, S. Incretin-based therapy for diabetes: What a cardiologist needs to know. J. Am. Coll. Cardiol. 2016, 67, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Kodera, R.; Shikata, K.; Takatsuka, T.; Oda, K.; Miyamoto, S.; Kajitani, N.; Hirota, D.; Ono, T.; Usui, H.K.; Makino, H. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem. Biophys. Res. Commun. 2014, 443, 828–833. [Google Scholar] [CrossRef]
- Panchapakesan, U.; Pollock, C. The role of dipeptidyl peptidase–4 inhibitors in diabetic kidney disease. Front. Immunol. 2015, 6, 443. [Google Scholar] [CrossRef]
- Swargiary, A.; Verma, A.K.; Singh, S.; Roy, M.K.; Daimari, M. Antioxidant and antiproliferative activity of selected medicinal plants of lower Assam, India: An in vitro and in silico study. Anti-Cancer Agents Med. Chem. 2021, 21, 267–277. [Google Scholar] [CrossRef]
- Perveen, S.; Chaudhary, H.S. In silico screening of antibacterial compounds from herbal sources against Vibrio cholerae. Pharmacogn. Mag. 2015, 11, S550. [Google Scholar] [PubMed]
- Al Sheebani, S.; Al-Kamarany, M.A.; Ghouth, A.B.; Kamal, A.; Alaq, M. Acute renal failure induced by cholera: Outbreak of Hodeidah, Yemen, 2017. Eur. J. Pharm. Med. Res. 2018, 5, 188–192. [Google Scholar]
- Trigiani-Fernandez, E.R. The Capsular Polysaccharide of Vibrio Cholerae O139 Bengal: Induction and Modulation of Host-Cell Mediated Immune Responses. Ph.D. Thesis, University of Maryland, Baltimore, MD, USA, 2001. [Google Scholar]
- Hussein, B.A.; Karimi, I.; Yousofvand, N. Computational insight to putative anti-acetylcholinesterase activity of Commiphora myrrha (Nees), Engler, Burseraceae: A lessen of archaeopharmacology from Mesopotamian Medicine I. Silico Pharmacol. 2019, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Rabbani, S.A. Acute Kidney Injury Associated with Cholera. Cureus 2023, 15, e34101. [Google Scholar] [CrossRef] [PubMed]
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: Role of NF-κB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199. [Google Scholar] [CrossRef] [PubMed]
- De Marinis, Y.; Cai, M.; Bompada, P.; Atac, D.; Kotova, O.; Johansson, M.E.; Garcia-Vaz, E.; Gomez, M.F.; Laakso, M.; Groop, L. Epigenetic regulation of the thioredoxin-interacting protein (TXNIP) gene by hyperglycemia in kidney. Kidney Int. 2016, 89, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Widjaja, J.; Hong, J.; Dolo, P.R.; Zhu, X.; Yao, L. Effect of sleeve gastrectomy on plasma thioredoxin-interacting protein (TXNIP). Obes. Surg. 2021, 31, 4829–4835. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.; Liu, N.; Liu, Z.; Shi, B.; Sun, H. Association of Circulating TXNIP levels with fatty liver in newly diagnosed type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 225–233. [Google Scholar] [CrossRef]
- Dahab, M.; Xu, Y.-K.; Zhang, P. Hepatoprotective and Antioxidant Activities of the Essential Oil from Maqian (Zanthoxylum myriacanthum var. pubescens) in Streptozotocin-Induced Diabetic Mice. In Proceedings of the 17th International Conference on Medicine Sciences and Bioengineering, Xiamen, China, 20–22 October 2017. [Google Scholar]
- Daza-Arnedo, R.; Rico-Fontalvo, J.-E.; Pájaro-Galvis, N.; Leal-Martínez, V.; Abuabara-Franco, E.; Raad-Sarabia, M.; Montejo-Hernández, J.; Cardona-Blanco, M.; Cabrales-Juan, J.; Uparella-Gulfo, I. Dipeptidyl peptidase-4 inhibitors and diabetic kidney disease: A narrative review. Kidney Med. 2021, 3, 1065–1073. [Google Scholar] [CrossRef]
- Andersen, E.S.; Deacon, C.F.; Holst, J.J. Do we know the true mechanism of action of the DPP-4 inhibitors? Diabetes Obes. Metab. 2018, 20, 34–41. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.-L.; Gan, X.-Q.; Xu, Y.-K.; Li, X.-F.; Guo, J.; Dahab, M.M.; Zhang, P. Protective effect of the essential oil of Zanthoxylum myriacanthum var. pubescens against dextran sulfate sodium-induced intestinal inflammation in mice. Phytomedicine 2016, 23, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.; Ludowieg, H.; Autschbach, J.; Zurek, E. Extended Hückel Calculations on Solids Using the Avogadro Molecular Editor and Visualizer; ACS Publications: Washington, DC, USA, 2018. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Liu, Y.; Grimm, M.; Dai, W.-T.; Hou, M.-C.; Xiao, Z.-X.; Cao, Y. CB-Dock: A web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol. Sin. 2020, 41, 138–144. [Google Scholar] [CrossRef]
- López-Blanco, J.R.; Aliaga, J.I.; Quintana-Ortí, E.S.; Chacón, P. iMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
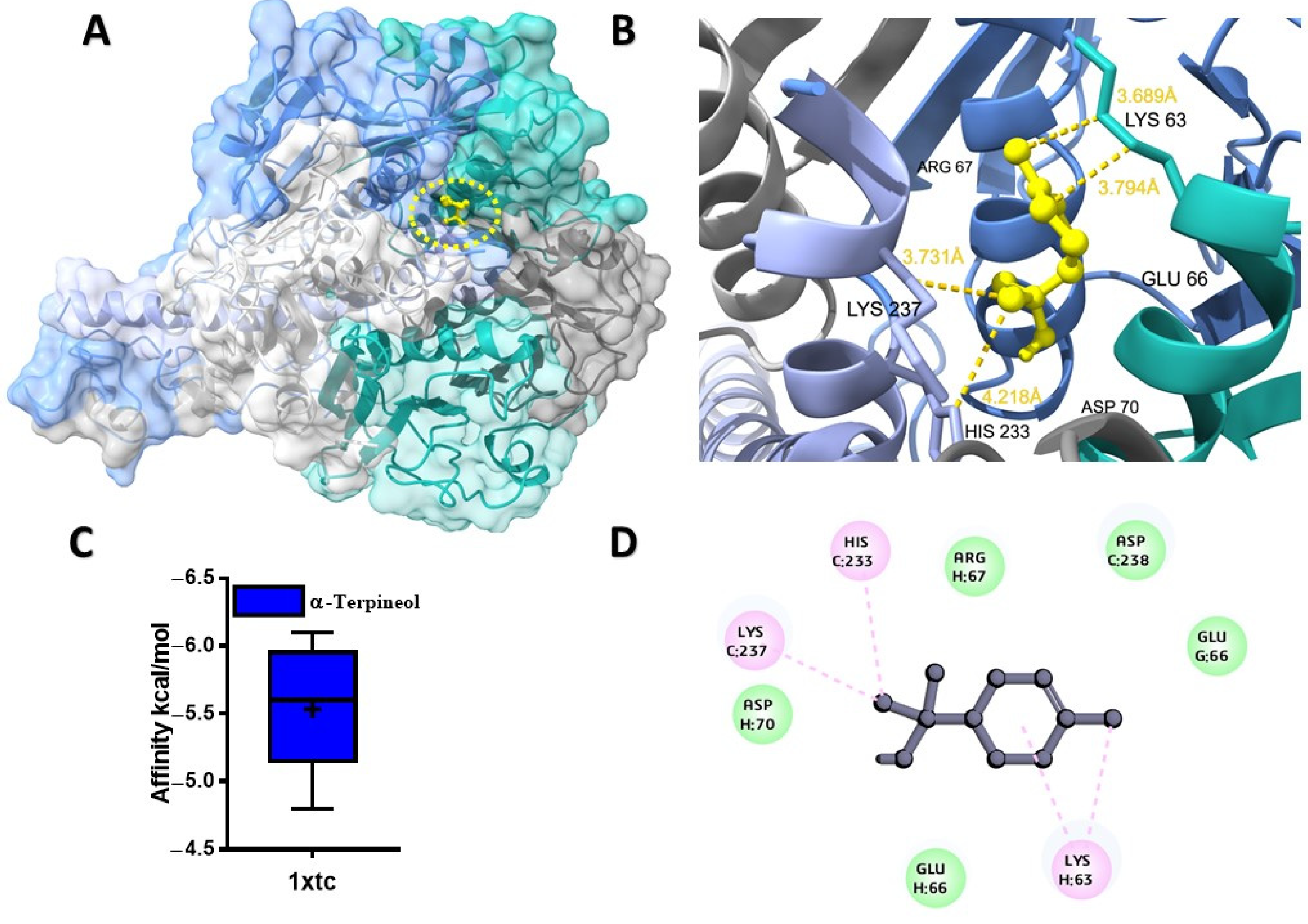


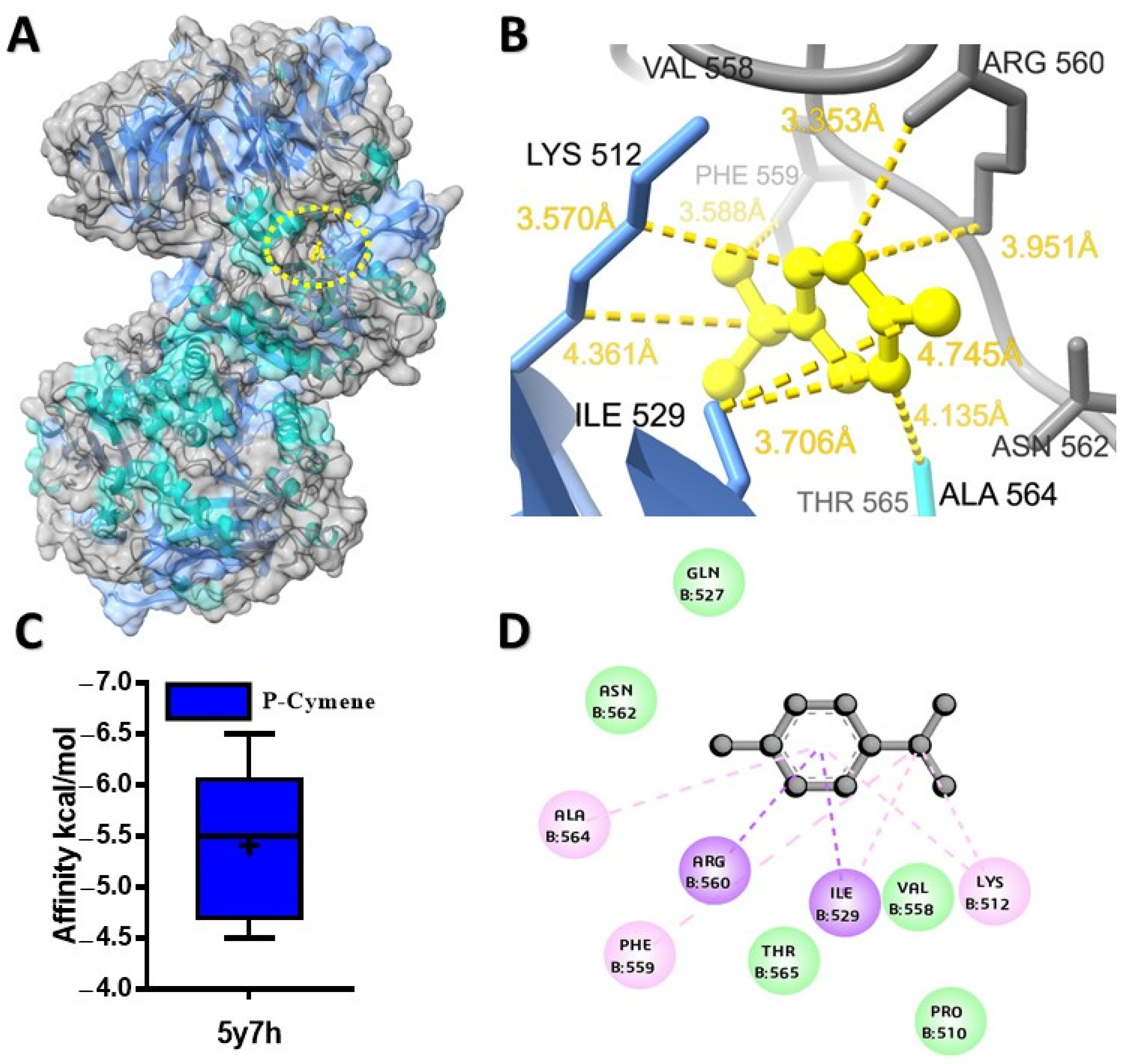
| Compound Name | 4gfx * | 5cgj ** | 5y7h *** | 6bie **** | 1xtc ***** |
|---|---|---|---|---|---|
| α-pinene | −4.3 | −5.2 | −4.8 | −4.9 | −5.7 |
| β-pinene | −4.2 | −5.1 | −5 | −5 | −5.6 |
| α-phellandrene | −5.6 | −4.9 | −5.3 | −5.5 | −5.8 |
| 3-carene | −4.4 | −5.1 | −5.1 | −4.2 | −5.9 |
| p-cymene | −5.6 | −5.1 | −6.5 | −5.4 | −5.8 |
| d-limonene | −5.2 | −5.1 | −6.2 | −5.5 | −5.6 |
| β-phellandrene | −5.5 | −4.9 | −6.2 | −5 | −5.7 |
| cis-β-ocimene | −4.6 | −4.7 | −5.6 | −4.6 | −5 |
| trans-β-ocimene | −4.7 | −4.8 | −5.4 | −4.9 | −5.1 |
| α-terpineol | −5 | −5.7 | −6.3 | −5.7 | −6.1 |
| n-decanal | −4.3 | −4.3 | −5.1 | −4.6 | −4.6 |
| acetic acid octyl ester | −4.5 | −4.5 | −5.2 | −4.2 | −5.1 |
| Compound Name | Molecular Weight | Hydrogen Bonds | Rotatable Bonds | LogP * (iLogPo/w) | Molar Refractivity | RO5 Violation ** | |
|---|---|---|---|---|---|---|---|
| Donor | Acceptor | ||||||
| α-pinene | 136 | 0 | 0 | 0 | 2.69 | 45.99 | 1 |
| β-pinene | 136 | 0 | 0 | 0 | 2.47 | 46.04 | 1 |
| α-phellandrene | 136 | 0 | 0 | 1 | 2.68 | 49.89 | 0 |
| 3-carene | 136 | 0 | 0 | 0 | 2.65 | 50.00 | 1 |
| p-cymene | 134 | 0 | 0 | 1 | 2.46 | 47.61 | 1 |
| d-limonene | 136 | 0 | 0 | 1 | 2.53 | 49.19 | 0 |
| β-phellandrene | 120 | 0 | 0 | 1 | 2.65 | 47.12 | 0 |
| cis-β-ocimene | 120 | 0 | 0 | 3 | 2.91 | 48.76 | 0 |
| trans-β-ocimene | 120 | 0 | 0 | 3 | 2.8 | 48.76 | 0 |
| α-terpineol | 154 | 1 | 1 | 1 | 2.59 | 53.05 | 0 |
| n-decanal | 152 | 0 | 1 | 8 | 3.05 | 54.98 | 0 |
| acetic acid octyl ester | 172 | 0 | 2 | 8 | 2.85 | 55.93 | 0 |
| Compound Name | LD50 (mg/kg) | Predicted Toxicity Class * | Average Similarity (%) | Prediction Accuracy (%) |
|---|---|---|---|---|
| α-pinene | 4700 | 5 | 100 | 100 |
| β-pinene | 4700 | 5 | 100 | 100 |
| α-phellandrene | 5700 | 6 | 100 | 100 |
| 3-carene | 4800 | 5 | 100 | 100 |
| p-cymene | 3 | 1 | 100 | 100 |
| d-limonene | 4400 | 5 | 100 | 100 |
| β-phellandrene | 5000 | 5 | 81.55 | 70.97 |
| cis-β-ocimene | 113 | 3 | 69.7 | 68.07 |
| trans-β-ocimene | 113 | 3 | 69.7 | 68.07 |
| α-terpineol | 2830 | 5 | 100 | 100 |
| n-decanal | 5000 | 5 | 100 | 100 |
| acetic acid octyl ester | 3000 | 5 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahab, M.; Zhang, P.; Al-Mijalli, S.H.; Abdallah, E.M. Unveiling the Anti-Cholera and Active Diabetic Renoprotective Compounds of Maqian Essential Oil: A Computational and Molecular Dynamics Study. Molecules 2023, 28, 7954. https://doi.org/10.3390/molecules28247954
Dahab M, Zhang P, Al-Mijalli SH, Abdallah EM. Unveiling the Anti-Cholera and Active Diabetic Renoprotective Compounds of Maqian Essential Oil: A Computational and Molecular Dynamics Study. Molecules. 2023; 28(24):7954. https://doi.org/10.3390/molecules28247954
Chicago/Turabian StyleDahab, Mahmoud, Ping Zhang, Samiah Hamad Al-Mijalli, and Emad M. Abdallah. 2023. "Unveiling the Anti-Cholera and Active Diabetic Renoprotective Compounds of Maqian Essential Oil: A Computational and Molecular Dynamics Study" Molecules 28, no. 24: 7954. https://doi.org/10.3390/molecules28247954






