Application of Sorbent-Based Extraction Techniques in Food Analysis
Abstract
:1. Introduction
2. The Introduction to SBE Methods
3. SPE in Food Analysis—Main Fields of Applications
3.1. Lipid Analysis
3.2. Mycotoxins
3.3. Pesticide Residues
3.4. Environmental Contaminants in Food
3.5. Processing Contaminants
3.6. Flavor Analysis
3.7. Limitations of SPE for Food Analysis
4. SPME in Food Analysis—Main Fields of Applications
4.1. Volatile Compounds
4.2. Flavor Compounds
4.3. Processing Contaminants
4.4. Limitations of SPME in Food Analysis
4.5. Advances in Geometries of SPME
5. Other SBE Approaches in Food Analysis
5.1. Stir Bar Sorptive Extraction
5.2. Molecularly Imprinted Polymers (MIPs)
5.3. Needle Traps
5.4. Other SBE Solutions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Capuano, E.; Oliviero, T.; van Boekel, M.A.J.S. Modeling Food Matrix Effects on Chemical Reactivity: Challenges and Perspectives. Crit. Rev. Food Sci. Nutr. 2018, 58, 2814–2828. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.N.; Zhou, W.; Pawliszyn, J. Sequential Thin Film-Solid Phase Microextraction as a New Strategy for Addressing Displacement and Saturation Effects in Food Analysis. Food Chem. 2022, 389, 133038. [Google Scholar] [CrossRef] [PubMed]
- Rejczak, T.; Tuzimski, T. Recent Trends in Sample Preparation and Liquid Chromatography/Mass Spectrometry for Pesticide Residue Analysis in Food and Related Matrixes. J. AOAC Int. 2015, 98, 1143–1162. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Muzammil, K.; Nasir, N.; Khan, M.S.; Ahmad, M.F.; Khalid, M.; Ahmad, W.; Dawria, A.; Reddy, L.K.V.; Busayli, A.M. Review Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants 2022, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Chatterjee, S.; Kumar, V.; Variyar, P.S.; Sharma, A. Analysis of Free and Glycosidically Bound Compounds of Ash Gourd (Benincasa Hispida): Identification of Key Odorants. Food Chem. 2010, 122, 1327–1332. [Google Scholar] [CrossRef]
- Boland, A.B.; Buhr, K.; Giannouli, P.; van Ruth, S.M. Influence of Gelatin, Starch, Pectin and Artificial Saliva on the Release of 11 Flavour Compounds from Model Gel Systems. Food Chem. 2004, 86, 401–411. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, S.; Tian, H.; Sun, B. Research Progress in the Use of Liquid-Liquid Extraction for Food Flavour Analysis. Trends Food Sci. Technol. 2023, 132, 138–149. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep Eutectic Solvents as New Media for Green Extraction of Food Proteins: Opportunity and Challenges. Food Res. Int. 2022, 161, 111842. [Google Scholar] [CrossRef]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. New Advanced Materials and Sorbent-Based Microextraction Techniques as Strategies in Sample Preparation to Improve the Determination of Natural Toxins in Food Samples. Molecules 2020, 25, 702. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Gholami, M. Recent Advances and Trends in Applications of Solid-Phase Extraction Techniques in Food and Environmental Analysis. Chromatographia 2019, 82, 1207–1249. [Google Scholar] [CrossRef]
- Souza-Silva, É.A.; Gionfriddo, E.; Pawliszyn, J. A Critical Review of the State of the Art of Solid-Phase Microextraction of Complex Matrices II. Food Analysis. TrAC Trends Anal. Chem. 2015, 71, 236–248. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-Phase Extraction of Organic Compounds: A Critical Review. Part II. TrAC Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-Phase Extraction of Organic Compounds: A Critical Review (Part I). TrAC Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Rejczak, T.; Tuzimski, T. A Review of Recent Developments and trends in the QuEChERS Sample Preparation Approach. Open Chem. 2015, 13, 000010151520150109. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Su, Q.-Z.; Vera, P.; Nerín, C. Direct Immersion–Solid-Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry and Response Surface Methodology for Nontarget Screening of (Semi-) Volatile Migrants from Food Contact Materials. Anal. Chem. 2020, 92, 5577–5584. [Google Scholar] [CrossRef]
- Goryński, K.; Kiedrowicz, A.; Bojko, B. Development of SPME-LC–MS Method for Screening of Eight Beta-Blockers and Bronchodilators in Plasma and Urine Samples. J. Pharm. Biomed. Anal. 2016, 127, 147–155. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Pawliszyn, J. Development of Coated Blade Spray Ionization Mass Spectrometry for the Quantitation of Target Analytes Present in Complex Matrices. Angew. Chem. Int. Ed. 2014, 53, 14503–14507. [Google Scholar] [CrossRef]
- Kędziora-Koch, K.; Wasiak, W. Needle-Based Extraction Techniques with Protected Sorbent as Powerful Sample Preparation Tools to Gas Chromatographic Analysis: Trends in Application. J. Chromatogr. A 2018, 1565, 1–18. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest Applications of Molecularly Imprinted Polymers for Extraction of Contaminants from Environmental and Food Matrices: A Review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, A.A.; Álvarez-Romero, G.A.; Contreras-López, E.; Aguilar-Arteaga, K.; Castañeda-Ovando, A. Food Analysis by Microextraction Methods Based on the Use of Magnetic Nanoparticles as Supports: Recent Advances. Food Anal. Methods 2017, 10, 2974–2993. [Google Scholar] [CrossRef]
- Montesano, D.; Albrizio, S.; Lucini, L.; Barba, F.J.; Gallo, M. Lipids and Food Quality. J. Food Qual. 2018, 2018, 4046381. [Google Scholar] [CrossRef]
- Kaluzny, M.A.; Duncan, L.A.; Merritt, M.V.; Epps, D.E. Rapid Separation of Lipid Classes in High Yield and Purity Using Bonded Phase Columns. J. Lipid Res. 1985, 26, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Bravi, E.; Marconi, O.; Sileoni, V.; Perretti, G. Determination of Free Fatty Acids in Beer. Food Chem. 2017, 215, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Budge, S.M. GC-MS Characterization of Hydroxy Fatty Acids Generated From Lipid Oxidation in Vegetable Oils. Eur. J. Lipid Sci. Technol. 2018, 120, 1700313. [Google Scholar] [CrossRef]
- Custodio-Mendoza, J.A.; Lorenzo, R.A.; Valente, I.M.; Almeida, P.J.; Lage, M.A.; Rodrigues, J.A.; Carro, A.M. Development of a Partitioned Liquid-Liquid Extraction- Dispersive Solid Phase Extraction Procedure Followed by Liquid Chromatography-Tandem Mass Spectrometry for Analysis of 3-Monochloropropane-1,2-Diol Diesters in Edible Oils. J. Chromatogr. A 2018, 1548, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.L.; Burrin, D.G.; Guthrie, G.; Thijs, C.; Te Velde, A.A.; Vreugdenhil, A.C.E.; et al. Plant-Based Sterols and Stanols in Health & Disease: “Consequences of Human Development in a Plant-Based Environment? ” Prog. Lipid Res. 2019, 74, 87–102. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and Their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Masri, O.A.; Chalhoub, J.M.; Sharara, A.I. Role of Vitamins in Gastrointestinal Diseases. World J. Gastroenterol. 2015, 21, 5191–5209. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Krupa-Kozak, U.; Abramowicz, P.; Jarocka-Cyrta, E. Beneficial Effect of Oligofructose-Enriched Inulin on Vitamin D and E Status in Children with Celiac Disease on a Long-Term Gluten-Free Diet: A Preliminary Randomized, Placebo-Controlled Nutritional Intervention Study. Nutrients 2018, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; D’Orazio, G.; Fanali, S.; Gentili, A. Advanced Analytical Techniques for Fat-Soluble Vitamin Analysis. TrAC Trends Anal. Chem. 2017, 87, 82–97. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Morales, H.; Soares, C.; Calado, T.; Vila-Chã, A.S.; Pereira, M.; Venâncio, A. A Review of Mycotoxins in Food and Feed Products in Portugal and Estimation of Probable Daily Intakes. Crit. Rev. Food Sci. Nutr. 2016, 56, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Solid Phase Extraction as Sample Treatment for the Determination of Ochratoxin A in Foods: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3405–3420. [Google Scholar] [CrossRef]
- Du, L.-J.; Chu, C.; Warner, E.; Wang, Q.-Y.; Hu, Y.-H.; Chai, K.-J.; Cao, J.; Peng, L.-Q.; Chen, Y.-B.; Yang, J.; et al. Rapid Microwave-Assisted Dispersive Micro-Solid Phase Extraction of Mycotoxins in Food Using Zirconia Nanoparticles. J. Chromatogr. A 2018, 1561, 1–12. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Sharma, B.; Borah, R.; Haque, S.; Mahmud, M.M.C.; Shah, A.K.; Rawal, D.; Bora, H.; Bui, S. Ochratoxins in Food and Feed: Occurrence and Its Impact on Human Health and Management Strategies. Toxicon 2020, 187, 151–162. [Google Scholar] [CrossRef]
- Paoloni, A.; Solfrizzo, M.; Bibi, R.; Pecorelli, I. Development and Validation of LC-MS/MS Method for the Determination of Ochratoxin A and Its Metabolite Ochratoxin α in Poultry Tissues and Eggs. J. Environ. Sci. Health Part B 2018, 53, 327–333. [Google Scholar] [CrossRef]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Russo, M.; Rastrelli, L. Rapid and Automated Analysis of Aflatoxin M1 in Milk and Dairy Products by Online Solid Phase Extraction Coupled to Ultra-High-Pressure-Liquid-Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1428, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Russo, M.; Valdés, A.; Ibáñez, C.; Rastrelli, L. A Fully Automated Method for Simultaneous Determination of Aflatoxins and Ochratoxin A in Dried Fruits by Pressurized Liquid Extraction and Online Solid-Phase Extraction Cleanup Coupled to Ultra-High-Pressure Liquid Chromatography–Tandem Mass Spectrometry. Anal. BioAnal. Chem. 2015, 407, 2899–2911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wei, D.; Wang, L.; Ma, S.; Du, Y.; Wang, M. Multiwalled Carbon Nanotube for One-Step Cleanup of 21 Mycotoxins in Corn and Wheat Prior to Ultraperformance Liquid Chromatography–Tandem Mass Spectrometry Analysis. Toxins 2018, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Wang, T.; Li, C.; Wu, Z. Effects of Ozone Treatment on Pesticide Residues in Food: A Review. Int. J. Food Sci. Technol. 2019, 54, 301–312. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lee, H.S.; Abd El-Aty, A.M.; Kabir, M.H.; Chung, H.S.; Park, J.-H.; Kim, M.-R.; Kim, J.; Shin, H.-C.; Shin, S.S.; et al. Determination of Endrin and δ-Keto Endrin in Five Food Products of Animal Origin Using GC-ΜECD: A Modified QuEChERS Approach to Traditional Detection. Food Chem. 2018, 263, 59–66. [Google Scholar] [CrossRef]
- Kara, R.; Ince, S. Evaluation of Malathion and Malaoxon Contamination in Buffalo and Cow Milk from Afyonkarahisar Region, Turkey, Using Liquid Chromatography/Tandem Mass Spectrometry—A Short Report. Pol. J. Food Nutr. Sci. 2016, 66, 57–60. [Google Scholar] [CrossRef]
- Otles, S.; Kartal, C. Solid-Phase Extraction (SPE): Principles and Applications in Food Samples. Acta Sci. Pol. Technol. Aliment. 2016, 15, 5–15. [Google Scholar] [CrossRef]
- Lawal, A.; Wong, R.C.S.; Tan, G.H.; Abdulra’uf, L.B.; Alsharif, A.M.A. Recent Modifications and Validation of QuEChERS-DSPE Coupled to LC–MS and GC–MS Instruments for Determination of Pesticide/Agrochemical Residues in Fruits and Vegetables: Review. J. Chromatogr. Sci. 2018, 56, 656–669. [Google Scholar] [CrossRef]
- Bucur, B.; Munteanu, F.D.; Marty, J.L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, M.; Hao, X.; Han, L.; Song, S.; Yao, W. Evaluation of Cleanup Procedures in Pesticide Multi-Residue Analysis with QuEChERS in Cinnamon Bark. Food Chem. 2019, 276, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tong, M.; Tang, J.; Bian, H.; Wan, X.; He, L.; Hou, R. Analysis of Multiple Pesticide Residues in Polyphenol-Rich Agricultural Products by UPLC-MS/MS Using a Modified QuEChERS Extraction and Dilution Method. Food Chem. 2019, 274, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Hakme, E.; Lozano, A.; Ferrer, C.; Díaz-Galiano, F.J.; Fernández-Alba, A.R. Analysis of Pesticide Residues in Olive Oil and Other Vegetable Oils. TrAC Trends Anal. Chem. 2018, 100, 167–179. [Google Scholar] [CrossRef]
- Castro, G.; Pérez-Mayán, L.; Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Cela, R. Multianalyte, High-Throughput Liquid Chromatography Tandem Mass Spectrometry Method for the Sensitive Determination of Fungicides and Insecticides in Wine. Anal BioAnal. Chem. 2018, 410, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, B.; Abu Alnaser, A.; Ghanem, I.; Islam, M.; Cesio, V.; Heinzen, H.; Kelly, S.; Cannavan, A. Validation of an Analytical Method for the Determination of Pesticide Residues in Vine Leaves by GC-MS/MS. J. Agric. Food Chem. 2018, 66, 6421–6430. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhu, K.; Han, L.; Sapozhnikova, Y.; Zhang, Z.; Yao, W. Residue Analysis of 60 Pesticides in Red Swamp Crayfish Using QuEChERS with High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 5031–5038. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.M.; Muzolf-Panek, M.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the Botanical Origin of Honeys with Chemometric Analysis According to Their Antioxidant and Physicochemical Properties. Pol. J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Gaweł, M.; Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Goliszek, M.; Burek, O.; Posyniak, A. Determination of Neonicotinoids and 199 Other Pesticide Residues in Honey by Liquid and Gas Chromatography Coupled with Tandem Mass Spectrometry. Food Chem. 2019, 282, 36–47. [Google Scholar] [CrossRef]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The Sources of Chemical Contaminants in Food and Their Health Implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef]
- Guerrero Esperanza, M.; Yanez Barrientos, E.; Wrobel, K.; Acevedo Aguilar, F.J.; Corrales Escobosa, A.R.; Wrobel, K. Determination of Total Arsenic and Speciation Analysis in Mexican Maize Tortillas by Hydride Generation—Microwave Plasma Atomic Emission Spectrometry and High Performance Liquid Chromatography—Inductively Coupled Plasma—Mass Spectrometry. Anal. Methods 2017, 9, 2059–2068. [Google Scholar] [CrossRef]
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic Nanoparticle Based Solid-Phase Extraction of Heavy Metal Ions: A Review on Recent Advances. Microchim. Acta 2018, 185, 160. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Lin, Y.; Feng, C.; Wang, D.; Qiu, X.; Jin, Y.; Xiong, L.; Jin, Y.; Wang, G. Determination of Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls in Fishery and Aquaculture Products Using Sequential Solid Phase Extraction and Large Volume Injection Gas Chromatography/Tandem Mass Spectrometry. J. Chromatogr. B 2014, 945–946, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; García-Bermejo, Á.; Malarvannan, G.; Gómara, B.; Neels, H.; Covaci, A. Multi-Contaminant Analysis of Organophosphate and Halogenated Flame Retardants in Food Matrices Using Ultrasonication and Vacuum Assisted Extraction, Multi-Stage Cleanup and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2015, 1401, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, I.; Martín, J.; Abril, C.; Santos, J.L.; Alonso, E. Determination of Household and Industrial Chemicals, Personal Care Products and Hormones in Leafy and Root Vegetables by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2018, 1533, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lei, C.; Wang, N.; Jiang, X.; Zeng, Y.; Fu, Z.; Zou, L.; He, L.; Liu, S.; Ao, X.; et al. Preparation of Magnetic Molecularly Imprinted Polymers with Double Functional Monomers for the Extraction and Detection of Chloramphenicol in Food. J. Chromatogr. B 2018, 1100–1101, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Nerín, C.; Aznar, M.; Carrizo, D. Food Contamination during Food Process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- Yoshioka, T.; Nagatomi, Y.; Harayama, K.; Bamba, T. Development of an Analytical Method for Polycyclic Aromatic Hydrocarbons in Coffee Beverages and Dark Beer Using Novel High-Sensitivity Technique of Supercritical Fluid Chromatography/Mass Spectrometry. J. Biosci. Bioeng. 2018, 126, 126–130. [Google Scholar] [CrossRef]
- Zacs, D.; Rozentale, I.; Reinholds, I.; Bartkevics, V. Multi-Walled Carbon Nanotubes as Effective Sorbents for Rapid Analysis of Polycyclic Aromatic Hydrocarbons in Edible Oils Using Dispersive Solid-Phase Extraction (d-SPE) and Gas Chromatography—Tandem Mass Spectrometry (GC-MS/MS). Food Anal. Methods 2018, 11, 2508–2517. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, T.M.; García de Llasera, M.P. On-Line MSPD-SPE-HPLC/FLD Analysis of Polycyclic Aromatic Hydrocarbons in Bovine Tissues. Food Chem. 2017, 223, 82–88. [Google Scholar] [CrossRef]
- Hu, G.; Hernandez, M.; Zhu, H.; Shao, S. An Efficient Method for the Determination of Furan Derivatives in Apple Cider and Wine by Solid Phase Extraction and High Performance Liquid Chromatography—Diode Array Detector. J. Chromatogr. A 2013, 1284, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Constantin, O.E.; Kukurová, K.; Daško, Ľ.; Stănciuc, N.; Ciesarova, Z.; Croitoru, C.; Rapeanu, G. Effect of Thermal Processing on Simultaneous Formation of Acrylamide and Hydroxymethylfurfural in Plum Purée. Pol. J. Food Nutr. Sci. 2019, 69, 179–189. [Google Scholar] [CrossRef]
- Ferrer-Aguirre, A.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Simple and Fast Determination of Acrylamide and Metabolites in Potato Chips and Grilled Asparagus by Liquid Chromatography Coupled to Mass Spectrometry. Food Anal. Methods 2016, 9, 1237–1245. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass Spectrometry-Based Metabolomics of Volatiles as a New Tool for Understanding Aroma and Flavour Chemistry in Processed Food Products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, K.; Jeon, I.J. An Overview of Solid-phase Extraction of Food Flavor Compounds and Chemical Residues. Food Rev. Int. 1996, 12, 131–151. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea Aroma Formation from Six Model Manufacturing Processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- van Boekel, M.A.J.S. Formation of Flavour Compounds in the Maillard Reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef]
- Ebeler, S.E. Analytical Chemistry: Unlocking the Secrets of Wine Flavor. Food Rev. Int. 2001, 17, 45–64. [Google Scholar] [CrossRef]
- Stój, A.; Czernecki, T.; Sosnowska, B.; Niemczynowicz, A.; Matwijczuk, A. Impact of Grape Variety, Yeast and Malolactic Fermentation on Volatile Compounds and Fourier Transform Infrared Spectra in Red Wines. Pol. J. Food Nutr. Sci. 2022, 72, 39–55. [Google Scholar] [CrossRef]
- Picard, M.; Franc, C.; de Revel, G.; Marchand, S. Dual Solid-Phase and Stir Bar Sorptive Extraction Combined with Gas Chromatography-Mass Spectrometry Analysis Provides a Suitable Tool for Assaying Limonene-Derived Mint Aroma Compounds in Red Wine. Anal. Chim. Acta 2018, 1001, 168–178. [Google Scholar] [CrossRef]
- Uckun, O.; Selli, S. Characterization of Key Aroma Compounds in a Representative Aromatic Extracts from Citrus and Astragalus Honeys Based on Aroma Extract Dilution Analyses. J. Food Meas. Charact. 2017, 11, 512–522. [Google Scholar] [CrossRef]
- Zheng, S.-J.; Wang, Y.-L.; Liu, P.; Zhang, Z.; Yu, L.; Yuan, B.-F.; Feng, Y.-Q. Stable Isotope Labeling-Solid Phase Extraction-Mass Spectrometry Analysis for Profiling of Thiols and Aldehydes in Beer. Food Chem. 2017, 237, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Mrduljaš, N.; Krešić, G.; Bilušić, T. Polyphenols: Food Sources and Health Benefits. In Functional Food; Hueda, M.C., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 2; pp. 23–41. ISBN 978-953-51-3440-4. [Google Scholar]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Malacarne, M.; Larcher, R. Identification and Quantification of 56 Targeted Phenols in Wines, Spirits, and Vinegars by Online Solid-Phase Extraction—Ultrahigh-Performance Liquid Chromatography—Quadrupole-Orbitrap Mass Spectrometry. J. Chromatogr. A 2015, 1423, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern Trends in Solid Phase Extraction: New Sorbent Media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Drabińska, N.; Jeleń, H.H. Optimisation of Headspace Solid-Phase Microextraction with Comprehensive Two-Dimensional Gas Chromatography–Time of Flight Mass Spectrometry (HS-SPME–GC × GC–ToFMS) for Quantitative Analysis of Volatile Compounds in Vegetable Oils Using Statistical Experimental Design. J. Food Compos. Anal. 2022, 110, 104595. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Průchová, K.; Grégrová, A.; Helísková, H.; Kružík, V.; Čížková, H. Enantioselective HS-SPME-GC-MS for Authentication of Natural and Synthetic Strawberry Flavour in Syrups. Pol. J. Food Nutr. Sci. 2022, 72, 305–317. [Google Scholar] [CrossRef]
- Cesoniene, L.; Daubaras, R.; Bogacioviene, S.; Maruska, A.S.; Stankevicius, M.; Valatavicius, A.; Zych, M.; Ercisli, S.; Ilhan, G. Investigations of Volatile Organic Compounds in Berries of Different Actinidia Kolomikta (Rupr. & Maxim.) Maxim. Accessions. Pol. J. Food Nutr. Sci. 2020, 70, 291–300. [Google Scholar] [CrossRef]
- Mehta, P.K.; de Sousa Galvão, M.; Soares, A.C.; Nogueira, J.P.; Narain, N. Volatile Constituents of Jambolan (Syzygium cumini L.) Fruits at Three Maturation Stages and Optimization of HS-SPME GC-MS Method Using a Central Composite Design. Food Anal. Methods 2018, 11, 733–749. [Google Scholar] [CrossRef]
- Karadag, A.; Bozkurt, F.; Bekiroglu, H.; Sagdic, O. Use of Principal Component Analysis and Cluster Analysis for Differentiation of Traditionally-Manufactured Vinegars Based on Phenolic and Volatile Profiles, and Antioxidant Activity. Pol. J. Food Nutr. Sci. 2020, 70, 347–360. [Google Scholar] [CrossRef]
- Drabińska, N.; Nogueira, M.; Szmatowicz, B. Valorisation of Broccoli By-Products: Technological, Sensory and Flavour Properties of Durum Pasta Fortified with Broccoli Leaf Powder. Molecules 2022, 27, 4672. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.N.; Jeleń, H.H. Volatile Compounds of Selected Raw and Cooked Brassica Vegetables. Molecules 2019, 24, 391. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Drabińska, N. Flavour Generation during Lactic Acid Fermentation of Brassica Vegetables—Literature Review. Appl. Sci. 2022, 12, 5598. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, J.; Ke, Y.; Huang, S.; Zeng, F.; Luan, T.; Ouyang, G. Applications of in Vivo and in Vitro Solid-Phase Microextraction Techniques in Plant Analysis: A Review. Anal. Chim. Acta 2013, 794, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Antignac, J.-P.; Courant, F.; Pinel, G.; Bichon, E.; Monteau, F.; Elliott, C.; Le Bizec, B. Mass Spectrometry-Based Metabolomics Applied to the Chemical Safety of Food. TrAC Trends Anal. Chem. 2011, 30, 292–301. [Google Scholar] [CrossRef]
- Esslinger, S.; Riedl, J.; Fauhl-Hassek, C. Potential and Limitations of Non-Targeted Fingerprinting for Authentication of Food in Official Control. Food Res. Int. 2014, 60, 189–204. [Google Scholar] [CrossRef]
- Gallo, M.; Ferranti, P. The Evolution of Analytical Chemistry Methods in Foodomics. J. Chromatogr. A 2016, 1428, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Pereira, J.A.; Silva, P.; Perestrelo, R.; Câmara, J.S. Food Fingerprints—A Valuable Tool to Monitor Food Authenticity and Safety. Food Chem. 2019, 278, 144–162. [Google Scholar] [CrossRef]
- Marsili, R.T. Shelf-Life Prediction of Processed Milk by Solid-Phase Microextraction, Mass Spectrometry, and Multivariate Analysis. J. Agric. Food Chem. 2000, 48, 3470–3475. [Google Scholar] [CrossRef]
- Pérès, C.; Viallon, C.; Berdagué, J.-L. Solid-Phase Microextraction-Mass Spectrometry: A New Approach to the Rapid Characterization of Cheeses. Anal. Chem. 2001, 73, 1030–1036. [Google Scholar] [CrossRef]
- Augusto, F.; Leite e Lopes, A.; Zini, C.A. Sampling and Sample Preparation for Analysis of Aromas and Fragrances. TrAC Trends Anal. Chem. 2003, 22, 160–169. [Google Scholar] [CrossRef]
- He, X.; Gbiorczyk, K.; Jeleń, H.H. Can Volatiles Fingerprints Be an Alternative to Isotope Ratio Mass Spectrometry in the Botanical Origin Determination of Spirits? J. Agric. Food Chem. 2023, 71, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Marsol-Vall, A.; Kortesniemi, M.; Karhu, S.T.; Kallio, H.; Yang, B. Profiles of Volatile Compounds in Blackcurrant (Ribes Nigrum) Cultivars with a Special Focus on the Influence of Growth Latitude and Weather Conditions. J. Agric. Food Chem. 2018, 66, 7485–7495. [Google Scholar] [CrossRef] [PubMed]
- Ruiz del Castillo, M.L.; Caja, M.M.; Herraiz, M. Use of the Enantiomeric Composition for the Assessment of the Authenticity of Fruit Beverages. J. Agric. Food Chem. 2003, 51, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Piasenzotto, L.; Gracco, L.; Conte, L. Solid Phase Microextraction (SPME) Applied to Honey Quality Control. J. Sci. Food Agric. 2003, 83, 1037–1044. [Google Scholar] [CrossRef]
- Cajka, T.; Hajslova, J.; Pudil, F.; Riddellova, K. Traceability of Honey Origin Based on Volatiles Pattern Processing by Artificial Neural Networks. J. Chromatogr. A 2009, 1216, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction Techniques in the Analysis of Food Flavor Compounds: A Review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef]
- Xu, M.; Jin, Z.; Lan, Y.; Rao, J.; Chen, B. HS-SPME-GC-MS/Olfactometry Combined with Chemometrics to Assess the Impact of Germination on Flavor Attributes of Chickpea, Lentil, and Yellow Pea Flours. Food Chem. 2019, 280, 83–95. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. Quantitation of Selected Odor-Active Constituents in Dry Fermented Sausages Prepared with Different Curing Salts. J. Agric. Food Chem. 2007, 55, 3058–3065. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Wu, S.; Jin, R.; Ren, W.; Sun, T. Profiles of Volatile Flavor Compounds in Milk Fermented with Different Proportional Combinations of Lactobacillus Delbrueckii Subsp. Bulgaricus and Streptococcus Thermophilus. Molecules 2017, 22, 1633. [Google Scholar] [CrossRef]
- Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J.; Puertas, B.; Cantos-Villar, E.; Moreno-Rojas, J.M. Multivariate Optimization of Headspace Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry for the Analysis of Terpenoids in Sparkling Wines. Talanta 2020, 208, 120483. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Peppard, T. Solid-Phase Microextraction for Flavor Analysis. J. Agric. Food Chem. 1994, 42, 1925–1930. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Wieczorek, M.N. Commentary: “Quantitative” vs Quantitative Headspace Solid-Phase Microextraction (HS-SPME) in Food Volatile and Flavor Compounds Analysis. J. Food Compos. Anal. 2023, 115, 104955. [Google Scholar] [CrossRef]
- Roberts, D.D.; Pollien, P.; Milo, C. Solid-Phase Microextraction Method Development for Headspace Analysis of Volatile Flavor Compounds. J. Agric. Food Chem. 2000, 48, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, K.; Lalljie, S.P.D.; Smith, R.M. Analysis of Food Taints and Off-Flavours: A Review. Food Addit. Contam. Part A 2010, 27, 146–168. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.A.; Jeleń, H.H. Role of Sulfur Compounds in Vegetable and Mushroom Aroma. Molecules 2022, 27, 6116. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.H. Solid-Phase Microextraction in the Analysis of Food Taints and Off-Flavors. J. Chromatogr. Sci. 2006, 44, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Majcher, M.A. Analysis of the Ability to Produce Pleasant Aromas on Sour Whey and Buttermilk By-Products by Mold Galactomyces Geotrichum: Identification of Key Odorants. Molecules 2021, 26, 6239. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, W.; Qian, M.C. Characterization of Aroma Compounds in Apple Cider Using Solvent-Assisted Flavor Evaporation and Headspace Solid-Phase Microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar] [CrossRef]
- Majcher, M.; Jeleń, H.H. Comparison of Suitability of SPME, SAFE and SDE Methods for Isolation of Flavor Compounds from Extruded Potato Snacks. J. Food Compos. Anal. 2009, 22, 606–612. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Song, H. Comparison of SPME versus SAFE Processes for the Analysis of Flavor Compounds in Watermelon Juice. Food Anal. Methods 2018, 11, 1677–1689. [Google Scholar] [CrossRef]
- Linforth, R.S.T.; Taylor, A.J. Measurement of Volatile Release in the Mouth. Food Chem. 1993, 48, 115–120. [Google Scholar] [CrossRef]
- Cárdenes, L.; Ayala, J.H.; Afonso, A.M.; González, V. Solid-Phase Microextraction Coupled with High-Performance Liquid Chromatography for the Analysis of Heterocyclic Aromatic Amines. J. Chromatogr. A 2004, 1030, 87–93. [Google Scholar] [CrossRef]
- Sanz Alaejos, M.; Ayala, J.H.; González, V.; Afonso, A.M. Analytical Methods Applied to the Determination of Heterocyclic Aromatic Amines in Foods. J. Chromatogr. B 2008, 862, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Chang, L.-Y.; Dou, J. Determination of Acrylamide in Food by Solid-Phase Microextraction Coupled to Gas Chromatography–Positive Chemical Ionization Tandem Mass Spectrometry. Anal. Chim. Acta 2007, 582, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M.; Järvinen, T.; Järvenpää, E.; Tapanainen, H.; Virtanen, S.; Peltonen, K. Furan in the Baby-Food Samples Purchased from the Finnish Markets—Determination with SPME–GC–MS. Food Chem. 2009, 117, 522–528. [Google Scholar] [CrossRef]
- HUANG, M.; JIANG, G.; HE, B.; LIU, J.; ZHOU, Q.; FU, W.; WU, Y. Determination of 3-Chloropropane-1,2-Diol in Liquid Hydrolyzed Vegetable Proteins and Soy Sauce by Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry. Anal. Sci. 2005, 21, 1343–1347. [Google Scholar] [CrossRef]
- Lee, M.-R.; Chiu, T.-C.; Dou, J. Determination of 1,3-Dichloro-2-Propanol and 3-Chloro-1,2-Propandiol in Soy Sauce by Headspace Derivatization Solid-Phase Microextraction Combined with Gas Chromatography–Mass Spectrometry. Anal. Chim. Acta 2007, 591, 167–172. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Ferreira, V.; Escudero, A. Development of a Robust HS-SPME-GC-MS Method for the Analysis of Solid Food Samples. Analysis of Volatile Compounds in Fresh Raw Beef of Differing Lipid Oxidation Degrees. Food Chem. 2019, 281, 49–56. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Kaziur, W.; Salemi, A.; Jochmann, M.A.; Schmidt, T.C. Automated Determination of Picogram-per-Liter Level of Water Taste and Odor Compounds Using Solid-Phase Microextraction Arrow Coupled with Gas Chromatography-Mass Spectrometry. Anal BioAnal. Chem. 2019, 411, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting Molecules in Complex Matrices with Spme Arrows: A Review. Separations 2020, 7, 12. [Google Scholar] [CrossRef]
- Song, N.-E.; Lee, J.-Y.; Lee, Y.-Y.; Park, J.-D.; Jang, H.W. Comparison of Headspace–SPME and SPME-Arrow–GC–MS Methods for the Determination of Volatile Compounds in Korean Salt–Fermented Fish Sauce. Appl. Biol. Chem. 2019, 62, 16. [Google Scholar] [CrossRef]
- Cha, J.; Chin, Y.W.; Lee, J.Y.; Kim, T.W.; Jang, H.W. Analysis of Volatile Compounds in Soju, a Korean Distilled Spirit, by SPME-Arrow-GC/MS. Foods 2020, 9, 1422. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.Y.; Choi, Y.S.; Sung, J.M.; Jang, H.W. Comparison of Different Types of SPME Arrow Sorbents to Analyze Volatile Compounds in Cirsium Setidens Nakai. Foods 2020, 9, 785. [Google Scholar] [CrossRef]
- Emmons, R.V.; Tajali, R.; Gionfriddo, E. Development, Optimization and Applications of Thin Film Solid Phase Microextraction (TF-SPME) Devices for Thermal Desorption: A Comprehensive Review. Separations 2019, 6, 39. [Google Scholar] [CrossRef]
- Khaled, A.; Gómez-Ríos, G.A.; Pawliszyn, J. Optimization of Coated Blade Spray for Rapid Screening and Quantitation of 105 Veterinary Drugs in Biological Tissue Samples. Anal. Chem. 2020, 92, 5937–5943. [Google Scholar] [CrossRef]
- Gruszecka, D.; Grandy, J.; Gionfriddo, E.; Singh, V.; Pawliszyn, J. Direct Immersion Thin Film Solid Phase Microextraction of Polychlorinated N-Alkanes in Cod Liver Oil. Food Chem. 2021, 353, 129244. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir Bar Sorptive Extraction (SBSE), a Novel Extraction Technique for Aqueous Samples: Theory and Principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Prieto, A.; Basauri, O.; Rodil, R.; Usobiaga, A.; Fernández, L.A.; Etxebarria, N.; Zuloaga, O. Stir-Bar Sorptive Extraction: A View on Method Optimisation, Novel Applications, Limitations and Potential Solutions. J. Chromatogr. A 2010, 1217, 2642–2666. [Google Scholar] [CrossRef] [PubMed]
- David, F.; Ochiai, N.; Sandra, P. Two Decades of Stir Bar Sorptive Extraction: A Retrospective and Future Outlook. TrAC Trends Anal. Chem. 2019, 112, 102–111. [Google Scholar] [CrossRef]
- Camino-Sánchez, F.J.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Santos-Fandila, A.; Vílchez, J.L. Stir Bar Sorptive Extraction: Recent Applications, Limitations and Future Trends. Talanta 2014, 130, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Takatsu, A.; Ito, R.; Nakazawa, H. Applications of Stir-Bar Sorptive Extraction to Food Analysis. TrAC Trends Anal. Chem. 2013, 45, 280–293. [Google Scholar] [CrossRef]
- He, M.; Chen, B.; Hu, B. Recent Developments in Stir Bar Sorptive Extraction. Anal BioAnal. Chem. 2014, 406, 2001–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Qian, Y.; Qian, M.C. Analysis of Volatile Phenols in Alcoholic Beverage by Ethylene Glycol-Polydimethylsiloxane Based Stir Bar Sorptive Extraction and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2015, 1390, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N.; Sasamoto, K.; Kishimoto, T. Development of a Method for the Quantitation of Three Thiols in Beer, Hop, and Wort Samples by Stir Bar Sorptive Extraction with in Situ Derivatization and Thermal Desorption–Gas Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 6698–6706. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N.; Sasamoto, K.; David, F.; Sandra, P. Recent Developments of Stir Bar Sorptive Extraction for Food Applications: Extension to Polar Solutes. J. Agric. Food Chem. 2018, 66, 7249–7255. [Google Scholar] [CrossRef]
- Nogueira, J.M.F. Novel Sorption-Based Methodologies for Static Microextraction Analysis: A Review on SBSE and Related Techniques. Anal. Chim. Acta 2012, 757, 1–10. [Google Scholar] [CrossRef]
- Bicchi, C.; Iori, C.; Rubiolo, P.; Sandra, P. Headspace Sorptive Extraction (HSSE), Stir Bar Sorptive Extraction (SBSE), and Solid Phase Microextraction (SPME) Applied to the Analysis of Roasted Arabica Coffee and Coffee Brew. J. Agric. Food Chem. 2002, 50, 449–459. [Google Scholar] [CrossRef]
- Tienpont, B.; David, F.; Bicchi, C.; Sandra, P. High Capacity Headspace Sorptive Extraction. J. Microcolumn Sep. 2000, 12, 577–584. [Google Scholar] [CrossRef]
- Kreck, M.; Scharrer, A.; Bilke, S.; Mosandl, A. Stir Bar Sorptive Extraction (SBSE)-Enantio-MDGC-MS—A Rapid Method for the Enantioselective Analysis of Chiral Flavour Compounds in Strawberries. Eur. Food Res. Technol. 2001, 213, 389–394. [Google Scholar] [CrossRef]
- Buettner, A.; Welle, F. Intra-Oral Detection of Potent Odorants Using a Modified Stir-Bar Sorptive Extraction System in Combination with HRGC–O, Known as the Buccal Odour Screening System (BOSS). Flavour Fragr. J. 2004, 19, 505–514. [Google Scholar] [CrossRef]
- Regal, P.; Díaz-Bao, M.; Barreiro, R.; Cepeda, A.; Fente, C. Application of Molecularly Imprinted Polymers in Food Analysis: Clean-up and Chromatographic Improvements. Open Chem. 2012, 10, 766–784. [Google Scholar] [CrossRef]
- He, Y.; Tan, S.; Abd EI-Aty, A.M.; Hacımüftüoğlu, A.; She, Y. Magnetic Molecularly Imprinted Polymers for the Detection of Aminopyralid in Milk Using Dispersive Solid-Phase Extraction. RSC Adv. 2019, 9, 29998–30006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; She, Y.; Zhang, C.; Wang, S.; Du, X.; Jin, F.; Jin, M.; Shao, H.; Zheng, L.; Wang, J. Selective Determination of Chloramphenicol in Milk Samples by the Solid-Phase Extraction Based on Dummy Molecularly Imprinted Polymer. Food Anal. Methods 2017, 10, 2566–2575. [Google Scholar] [CrossRef]
- Hu, X.; Pan, J.; Hu, Y.; Huo, Y.; Li, G. Preparation and Evaluation of Solid-Phase Microextraction Fiber Based on Molecularly Imprinted Polymers for Trace Analysis of Tetracyclines in Complicated Samples. J. Chromatogr. A 2008, 1188, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Gao, R.; Mu, H. A Novel Molecularly Imprinted Polymer Based on Carbon Nanotubes for Selective Determination of Dioctyl Phthalate from Beverage Samples Coupled with GC/MS. Food Anal. Methods 2016, 9, 2026–2035. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Li, Y.; Yang, J.; Jin, J.; Shah, S.M.; Chen, J. Novel Dummy Molecularly Imprinted Polymers for Matrix Solid-Phase Dispersion Extraction of Eight Fluoroquinolones from Fish Samples. J. Chromatogr. A 2014, 1359, 1–7. [Google Scholar] [CrossRef]
- Song, X.; Xu, S.; Chen, L.; Wei, Y.; Xiong, H. Recent Advances in Molecularly Imprinted Polymers in Food Analysis. J. Appl. Polym. Sci. 2014, 131, 109074. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Trefz, P.; Kischkel, S.; Hein, D.; James, E.S.; Schubert, J.K.; Miekisch, W. Needle Trap Micro-Extraction for VOC Analysis: Effects of Packing Materials and Desorption Parameters. J. Chromatogr. A 2012, 1219, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.; Gracka, A.; Myśków, B. Static Headspace Extraction with Compounds Trapping for the Analysis of Volatile Lipid Oxidation Products. Food Anal. Methods 2017, 10, 2729–2734. [Google Scholar] [CrossRef]
- Bicchi, C.; Cordero, C.; Liberto, E.; Rubiolo, P.; Sgorbini, B. Automated Headspace Solid-Phase Dynamic Extraction to Analyse the Volatile Fraction of Food Matrices. J. Chromatogr. A 2004, 1024, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Laaks, J.; Jochmann, M.A.; Schilling, B.; Schmidt, T.C. In-Tube Extraction of Volatile Organic Compounds from Aqueous Samples: An Economical Alternative to Purge and Trap Enrichment. Anal. Chem. 2010, 82, 7641–7648. [Google Scholar] [CrossRef] [PubMed]
- Laaks, J.; Jochmann, M.A.; Schilling, B.; Schmidt, T.C. Optimization Strategies of In-Tube Extraction (ITEX) Methods. Anal. BioAnal. Chem. 2015, 407, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J. The Effects of Temperature and Pressure on the Performance of Carboxen/PDMS Fibres during Solid Phase Microextraction (SPME) of Headspace Volatiles from Cooked and Raw Turkey Breast. Flavour Fragr. J. 2001, 16, 294–302. [Google Scholar] [CrossRef]
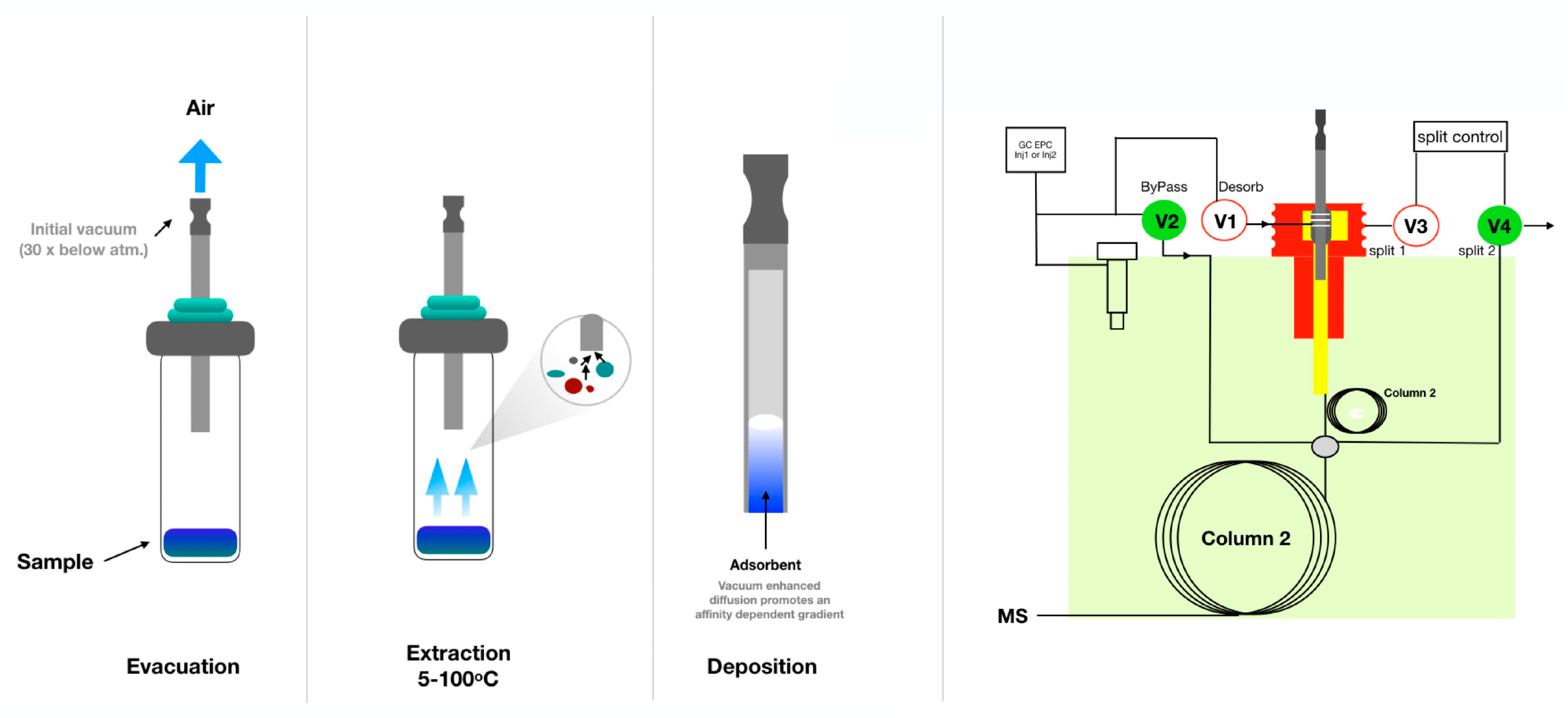
- Psillakis, E.; Yiantzi, E.; Sanchez-Prado, L.; Kalogerakis, N. Vacuum-Assisted Headspace Solid Phase Microextraction: Improved Extraction of Semivolatiles by Non-Equilibrium Headspace Sampling under Reduced Pressure Conditions. Anal. Chim. Acta 2012, 742, 30–36. [Google Scholar] [CrossRef]
- Psillakis, E. Vacuum-Assisted Headspace Solid-Phase Microextraction: A Tutorial Review. Anal. Chim. Acta 2017, 986, 12–24. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Marcinkowska, M.A.; Marek, M. Determination of Volatile Terpenes in Coriander Cold Pressed Oil by Vacuum Assisted Sorbent Extraction (Vase). Molecules 2021, 26, 884. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Gaca, A.; Marcinkowska, M. Use of Sorbent-Based Vacuum Extraction for Determination of Volatile Phenols in Beer. Food Anal. Methods 2018, 11, 3089–3094. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drabińska, N.; Marcinkowska, M.A.; Wieczorek, M.N.; Jeleń, H.H. Application of Sorbent-Based Extraction Techniques in Food Analysis. Molecules 2023, 28, 7985. https://doi.org/10.3390/molecules28247985
Drabińska N, Marcinkowska MA, Wieczorek MN, Jeleń HH. Application of Sorbent-Based Extraction Techniques in Food Analysis. Molecules. 2023; 28(24):7985. https://doi.org/10.3390/molecules28247985
Chicago/Turabian StyleDrabińska, Natalia, Monika A. Marcinkowska, Martyna N. Wieczorek, and Henryk H. Jeleń. 2023. "Application of Sorbent-Based Extraction Techniques in Food Analysis" Molecules 28, no. 24: 7985. https://doi.org/10.3390/molecules28247985
APA StyleDrabińska, N., Marcinkowska, M. A., Wieczorek, M. N., & Jeleń, H. H. (2023). Application of Sorbent-Based Extraction Techniques in Food Analysis. Molecules, 28(24), 7985. https://doi.org/10.3390/molecules28247985