A tert-Butyldiphenylsilyl-Containing Polyimide-Based Chemosensor for Sequential Detection of Fluoride Ions and Trace Water in Organic Solvents
Abstract
:1. Introduction
2. Results and Discussion
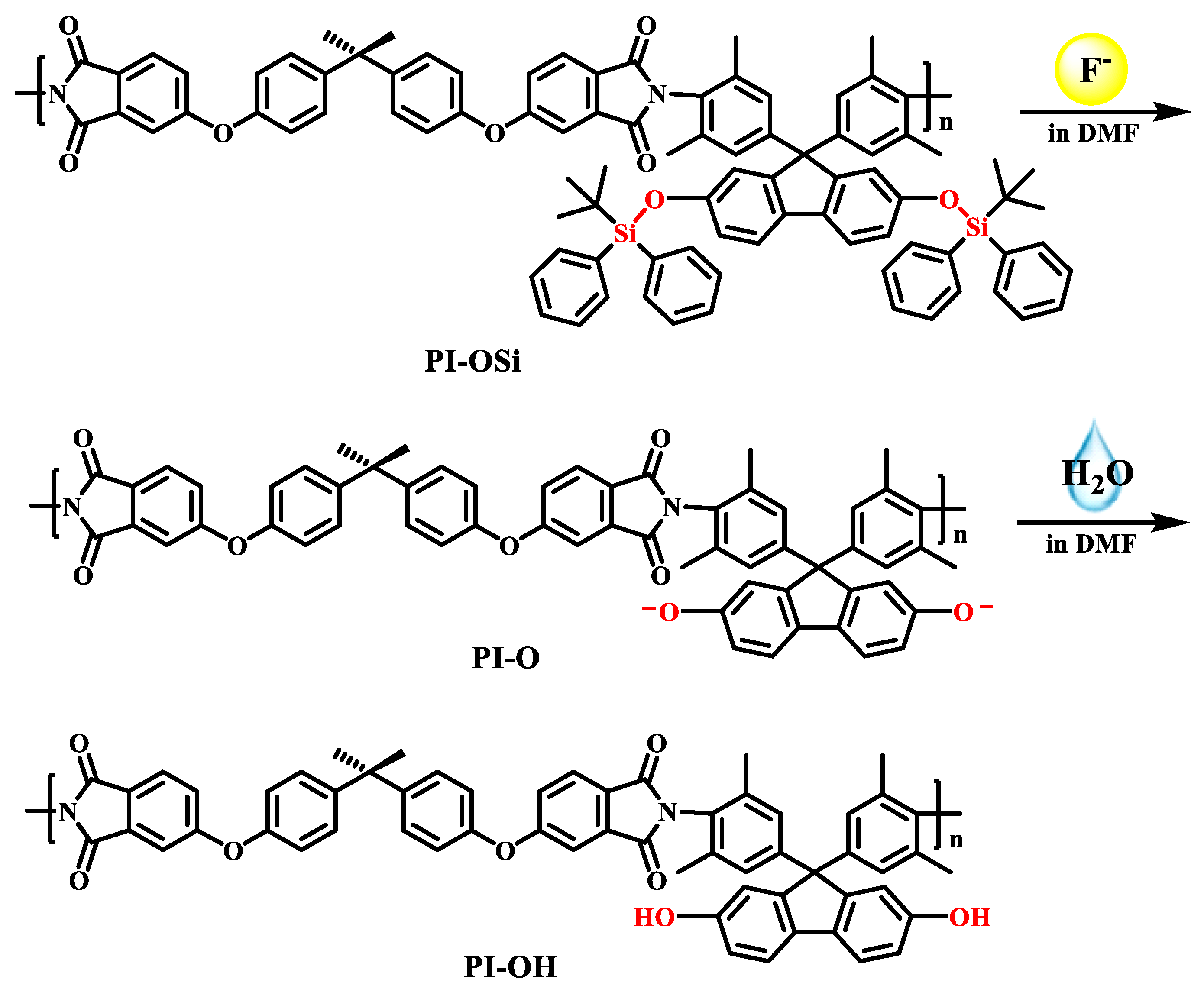
2.1. Synthesis and Characterization of PI-OH and PI-OSi
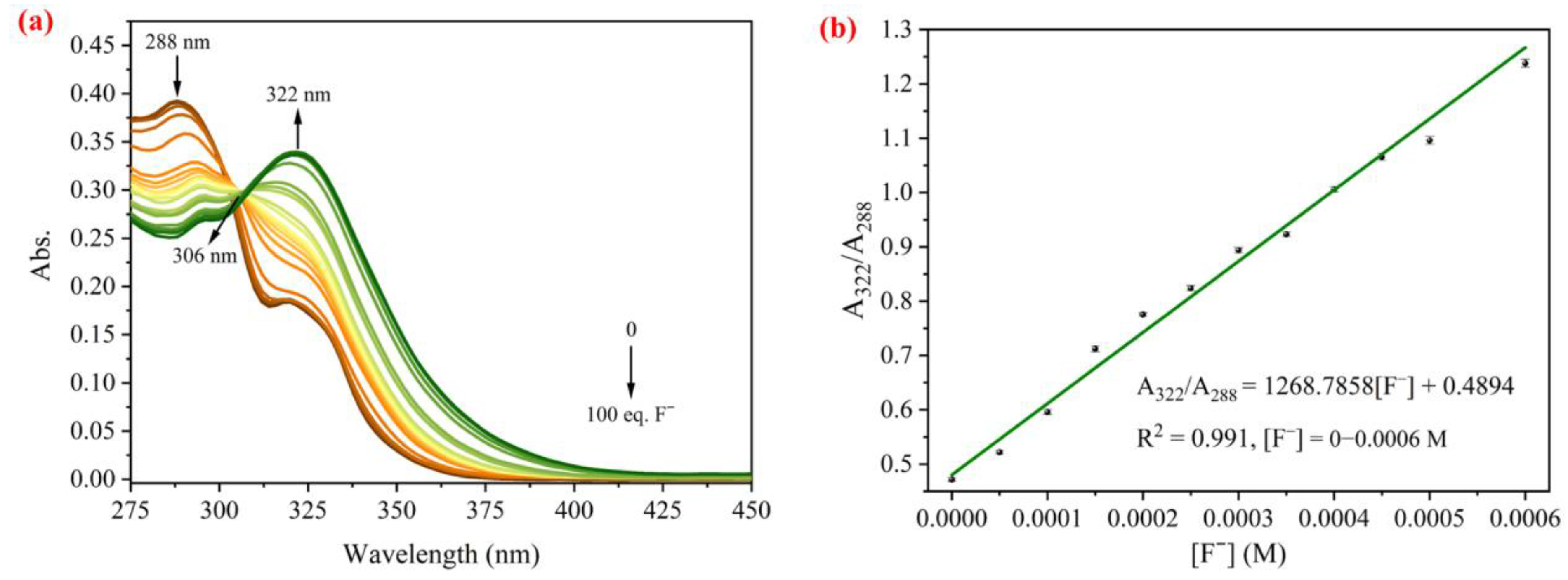
2.2. Spectral Sensing of F− by PI-OSi
2.3. Visual Sensing of F− by PI-OSi
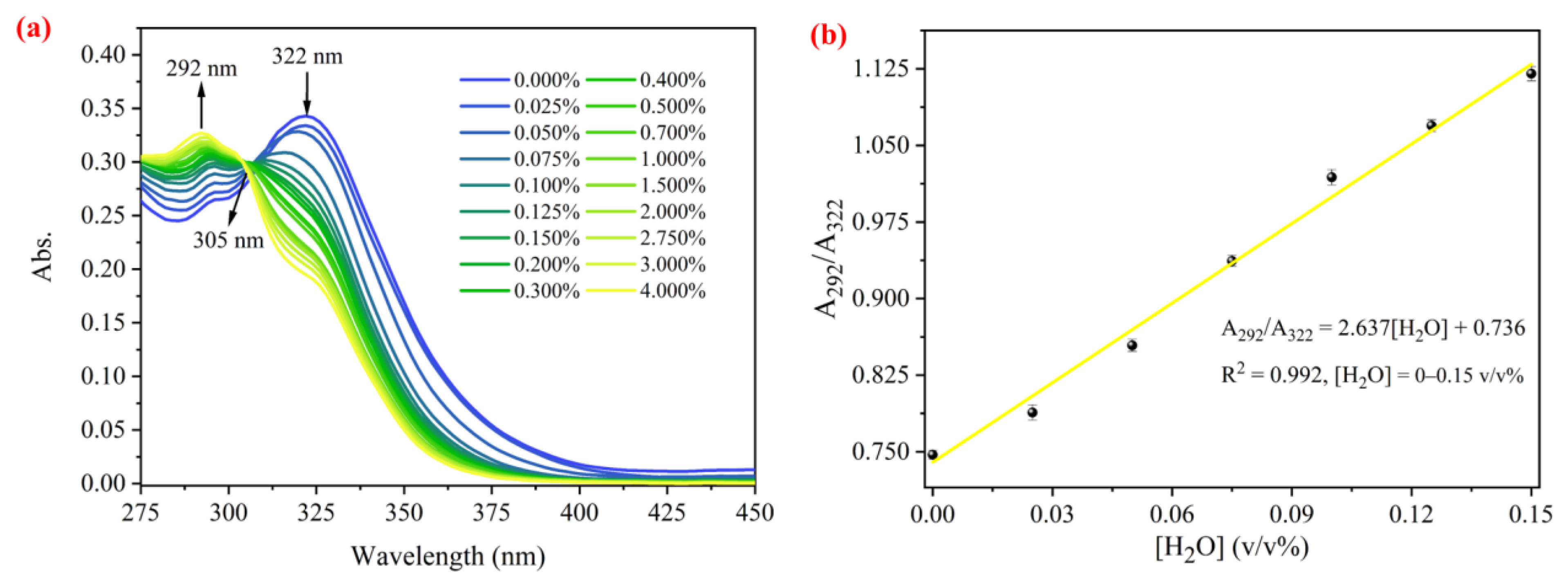
2.4. Spectral Sensing of Water by PI-OSi·F
2.5. Visual Sensing of Water by PI-OSi·F
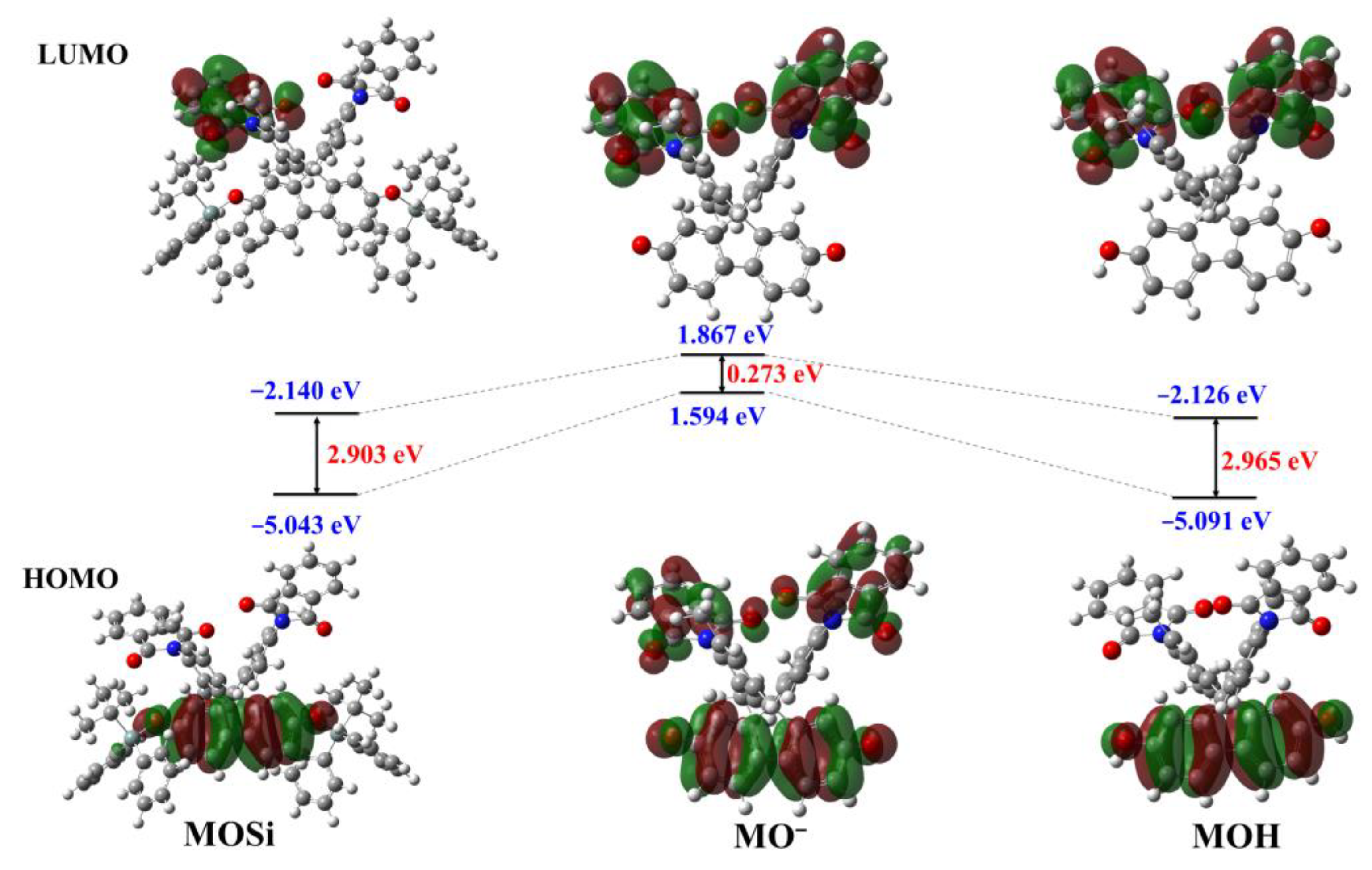
2.6. Reaction Mechanism
2.7. Portable Test Polyimide Film-Based Chemosensor Device
3. Experimental Setup
3.1. Reagents and Apparatus
3.2. General Procedure for UV-vis Absorption Spectra Measurements
3.3. Calculation Method for Detection Limit (DL)
3.4. Synthesis
3.4.1. Synthesis of PI-OH
3.4.2. Synthesis of PI-OSi
3.5. Preparation of PI-OSi Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental Fluoride Neurotoxicity: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Lacson, C.F.Z.; Lu, M.-C.; Huang, Y.-H. Fluoride network and circular economy as potential model for sustainable development—A review. Chemosphere 2020, 239, 124662. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kiss, L.; Mei, H.; Remete, A.M.; Ponikvar-Svet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical Aspects of Human and Environmental Overload with Fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef] [PubMed]
- Hana, J.; Zhang, J.; Gao, M.; Hao, H.; Xu, X. Recent advances in chromo-fluorogenic probes for fluoride detection. Dyes Pigments 2019, 162, 412–439. [Google Scholar] [CrossRef]
- Hu, R.; Li, R.; Wang, Y.; Wang, M.; Huang, H.; Hu, D.; Tian, M.; Shuai, Z.; Wei, Y. Ratiometric fluorescence probe for fluoride ion detection based on di-catechol substituted naphthalene scaffold. Dyes Pigments 2023, 213, 111156. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Q.; Xiang, W.; Li, Z.; He, Y.; Zhao, D. Amino-Functionalized Single-Lanthanide Metal-Organic Framework as a Ratiometric Fluorescent Sensor for Quantitative Visual Detection of Fluoride Ions. Inorg. Chem. 2022, 61, 13627–13636. [Google Scholar] [CrossRef] [PubMed]
- Drisya, V.; Shurooque, K.S.; Das, S.; Chakkumkumarath, L. Fmoc-phenylalanine-based fluorimetric and colorimetric dual-channel probes for the detection of Fe3+, Cu2+ and F−. J. Photochem. Photobiol. A 2023, 442, 114796. [Google Scholar] [CrossRef]
- Mathew, D.; Arunkumar, C.; Sujatha, S.; Parameswaran, P. Hydrogen-bonding receptor substituted BODIPYs as selective ON-OFF fluorimetric sensors for fluoride ions in polar aprotic organic solvents—A molecular-level understanding based on experimental and theoretical studies. J. Photochem. Photobiol. A 2023, 442, 114780. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.; You, S.; Lin, Z.; Chen, Z.; Huang, F.; Qiu, B. Colorimetric and fluorescent Dual-Modality sensing platform based on UiO-66 for fluorion detection. Microchem. J. 2023, 186, 108318. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Gurusamy, S.; Rajakumar, K.; Sathish, V.; Thanasekaran, P.; Mathavan, A. Aggregation induced emission (AIE), selective fluoride ion sensing and lysozyme interaction properties of Julolidinesulphonyl derived Schiff base. J. Photochem. Photobiol. A 2022, 427, 113822. [Google Scholar] [CrossRef]
- Mahishi, A.A.; Shet, S.M.; Mane, P.V.; Yu, J.; Sowriraajan, A.V.; Kigga, M.; Bhat, M.P.; Lee, K.-H.; Kurkur, M.D. Ratiometric colorimetric detection of fluoride ions using a Schiff base sensor: Enhancing selectivity and sensitivity for naked-eye analysis. Anal. Methods 2023, 15, 3259. [Google Scholar] [CrossRef] [PubMed]
- Shabashini, A.; Richard, S.; Panja, S.K.; Nandi, G.C. Tricyanopyrroline-based colorimetric NIR probe for rapid “naked-eye” fluoride ion detection. Dyes Pigments 2023, 216, 111376. [Google Scholar] [CrossRef]
- Hussein, A.S.; Lafzi, F.; Bayindir, S.; Toprak, M. The selective turn-on recognition of fluoride ions using 5-aryl-rhodanines: Colorimetric & fluorescent detection. J. Photochem. Photobiol. A 2023, 438, 114574. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Feng, Q.; Zhao, J. Improving anion sensing ability of the indolocarbazole-based fluorescence turn-on sensor by increasing salicylaldehyde response unit. Talanta 2023, 265, 124887. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, M.; Ruan, S.; Xu, X.; Wang, Z.; Wang, S. Highly efficient coumarin-derived colorimetric chemosensors for sensitive sensing of fluoride ions and their applications in logic circuits. Spectrochim. Acta A 2021, 255, 119718. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, P.; Weißhoff, H.; Kraus, W.; Rurack, K. Test-Strip-Based Fluorometric Detection of Fluoride in Aqueous Media with a BODIPY-Linked Hydrogen-Bonding Receptor. Angew. Chem. Int. Ed. 2014, 53, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, L.; Li, J.; Chen, X.; Chi, Z.; Xu, B.; Zhao, J. Light-up, colorimetric anion sensing and fingerprint visualization using the salicylaldehyde-based aggregation-induced emission-active phosphorescent Pt(II) complexes formed by restricting the molecular configuration transformations. Dyes Pigments 2023, 209, 110912. [Google Scholar] [CrossRef]
- Mohammadi, A.; Dehghan, Z.; Rassa, M.; Chaibakhsh, N. Colorimetric probes based on bioactive organic dyes for selective sensing of cyanide and fluoride ions. Sens. Actuators B Chem. 2016, 230, 388–397. [Google Scholar] [CrossRef]
- Chen, P.; Bai, W.; Bao, Y. The Fluorescent Chemodosimeters for Fluoride Ion via Silicon-Fluorine Chemistry: 20 Years of Progress. J. Mater. Chem. C 2019, 7, 11731–11746. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, H.; Kong, L.; Xu, X.; Tian, Y.; Yang, J. Multifunctional behavior of a novel tetraphenylethylene derivative: Mechanochromic luminescence, detection of fluoride ions and trace water in aprotic solvents. Dyes Pigments 2020, 172, 107832. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Ma, C. A novel ICT-based chemosensor for F− and its application in real samples and bioimaging. J. Hazard. Mater. 2021, 413, 125384. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhou, W.-F.; Zhao, Y.; Shen, L.; Chang, W.-Y.; Musendo, R.-K.; Chen, E.-Q.; Song, Y.-L.; Ren, X.-K. Polyhedral oligosilsesquioxane tethered perylene diimide for application in optical limiting and rapid detection of fluoride ions. Chem. Commun. 2019, 55, 3012–3014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, Y.; Bian, Y.; He, S.; Wang, X.; Liu, X.; Li, C.; Wu, Q.; Zeng, X.; Wang, H.; et al. A 2-(benzo[d]thiazol-2-yl)phenol based on conjugated polymer: Highly selective colorimetric fluorescent chemosensor for F− depending on Si-O bond cleavage reaction. High Perform. Polym. 2020, 32, 344–356. [Google Scholar] [CrossRef]
- Kim, T.-H.; Swager, T.M. A Fluorescent Self-Amplifying Wavelength-Responsive Sensory Polymer for Fluoride Ions. Angew. Chem. 2003, 115, 4951–4954. [Google Scholar] [CrossRef]
- Kumar, P.; Ghosh, A.; Jose, D.A. Chemical Sensors for Water Detection in Organic Solvents and their Applications. ChemistrySelect 2021, 6, 820–842. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, A.K. Optical sensors for water and humidity and their further applications. Coord. Chem. Rev. 2021, 445, 214063. [Google Scholar] [CrossRef]
- Yang, D.; Wu, X.-T.; Cao, X.-J.; Zhao, B.-X. A reversible ratiometric fluorescence probe for fast detection of trace water in different organic solvents. Dyes Pigments 2019, 170, 107558. [Google Scholar] [CrossRef]
- Enoki, T.; Ooyama, Y. Colorimetric and ratiometric fluorescence sensing of water based on 9-methyl pyrido[3,4-b]indole-boron trifluoride complex. Dalton Trans. 2019, 48, 2086–2092. [Google Scholar] [CrossRef]
- Keiichi, I.; Enoki, T.; Ooyama, Y. Development of an intramolecular charge transfer-type colorimetric and fluorescence sensor for water by fusion with a juloidine structure and complexation with boron trifluoride. RSC Adv. 2019, 9, 31466. [Google Scholar] [CrossRef]
- Kumar, P.; Jose, D.A. Naked eye detection of moisture in organic solvents and development of alginate polymer beads and test cassettes as a portable kit. Anal. Chim. Acta 2020, 1136, 178–186. [Google Scholar] [CrossRef]
- Resta, I.M.; Galindo, F. Phenol-based styrylpyrylium dyes for trace water detection via chromogenic and fluorogenic responses. Dyes Pigments 2022, 197, 109908. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, Q.; Liu, W.; Xiong, X.; Wang, Z.; Wang, H. A smartphone-available colorimetric and near-infrared fluorescence sensor for trace amounts of water detection in highly polar organic solvents. Dyes Pigments 2023, 213, 111186. [Google Scholar] [CrossRef]
- Kumar, P.; Ghosh, A.; Jose, D.A. A simple colorimetric sensor for the detection of moisture in organic solvents and building materials: Applications in rewritable paper and fingerprint imaging. Analyst 2019, 144, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Pati, C.; Raza, R.; Ghosh, K. Adenine-linked naphthalimide: A case of selective colorimetric as well as fluorometric sensing of F− and anion-activated moisture detection in organic solvents and CO2-sensing. Spectrochim. Acta A 2020, 229, 117910. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, F.; Li, Y.; Yan, M.; Cui, Y.; Sun, G. Three indole derived azo-azomethine dyes as effective chemosensors for F− ion and trace water detection. Bull. Chem. Soc. Jpn. 2020, 93, 870–879. [Google Scholar] [CrossRef]
- Yoon, Y.; Jo, S.; Lee, D.H.; Lee, T.S. Synthesis of fluorescent, ortho-azonaphthol-containing conjugated polymer for ratiometric fluoride ion sensing. Polymer 2022, 261, 125421. [Google Scholar] [CrossRef]
- Deng, Q.; Li, Y.; Wu, J.; Liu, Y.; Fang, G.; Wang, S.; Zhang, Y. Highly sensitive fluorescent sensing for water based on poly(m-aminobenzoic acid). Chem. Commun. 2012, 48, 3009–3011. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Li, L.; Cao, D. The synthesis and highly sensitive detection of water content in THF using a novel solvatochromic AIE polymer containing diketopyrrolopyrrole and triphenylamine. New J. Chem. 2016, 40, 6706–6713. [Google Scholar] [CrossRef]
- Wu, Y.; Ji, J.; Zhou, Y.; Chen, Z.; Liu, S.; Zhao, J. Ratiometric and colorimetric sensors for highly sensitive detection of water in organic solvents based on hydroxyl-containing polyimide-fluoride complexes. Anal. Chim. Acta 2020, 1108, 37–45. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Huo, J.-P.; Cao, L.; Ding, S.; Wang, L.-Y.; Cao, D.; Wang, Z.-Y. Design and Application of Tri-benzimidazolyl Star-shape Molecules as Fluorescent Chemosensors for the Fast-response Detection of Fluoride Ion. Sens. Actuators B Chem. 2016, 237, 865–875. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, L.; Xie, L.; Liu, Z.; Li, Y.; Ning, R.; Zhang, G.; Gong, X.; Gao, B.; Liu, C.; et al. Colorimetric and On-Off fluorescent chemosensor for fluoride ion based on diketopyrrolopyrrole. Sens. Actuators B Chem. 2015, 207, 9–24. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, C.; Chen, Z.; Zhou, Y.; Liu, S.; Zhao, J. A novel hydroxyl-containing polyimide as a colorimetric and ratiometric chemosensor for the reversible detection of fluoride ions. Polym. Chem. 2019, 10, 1399–1406. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Yi, M.; Liu, C.; Wang, Y.; Li, J.; Li, Y.; Yang, R. Graphene oxide assisted fluorescent chemodosimeter for high-performance sensing and bioimaging of fluoride ions. ACS Appl. Mater. Interfaces 2014, 6, 9768–9775. [Google Scholar] [CrossRef] [PubMed]



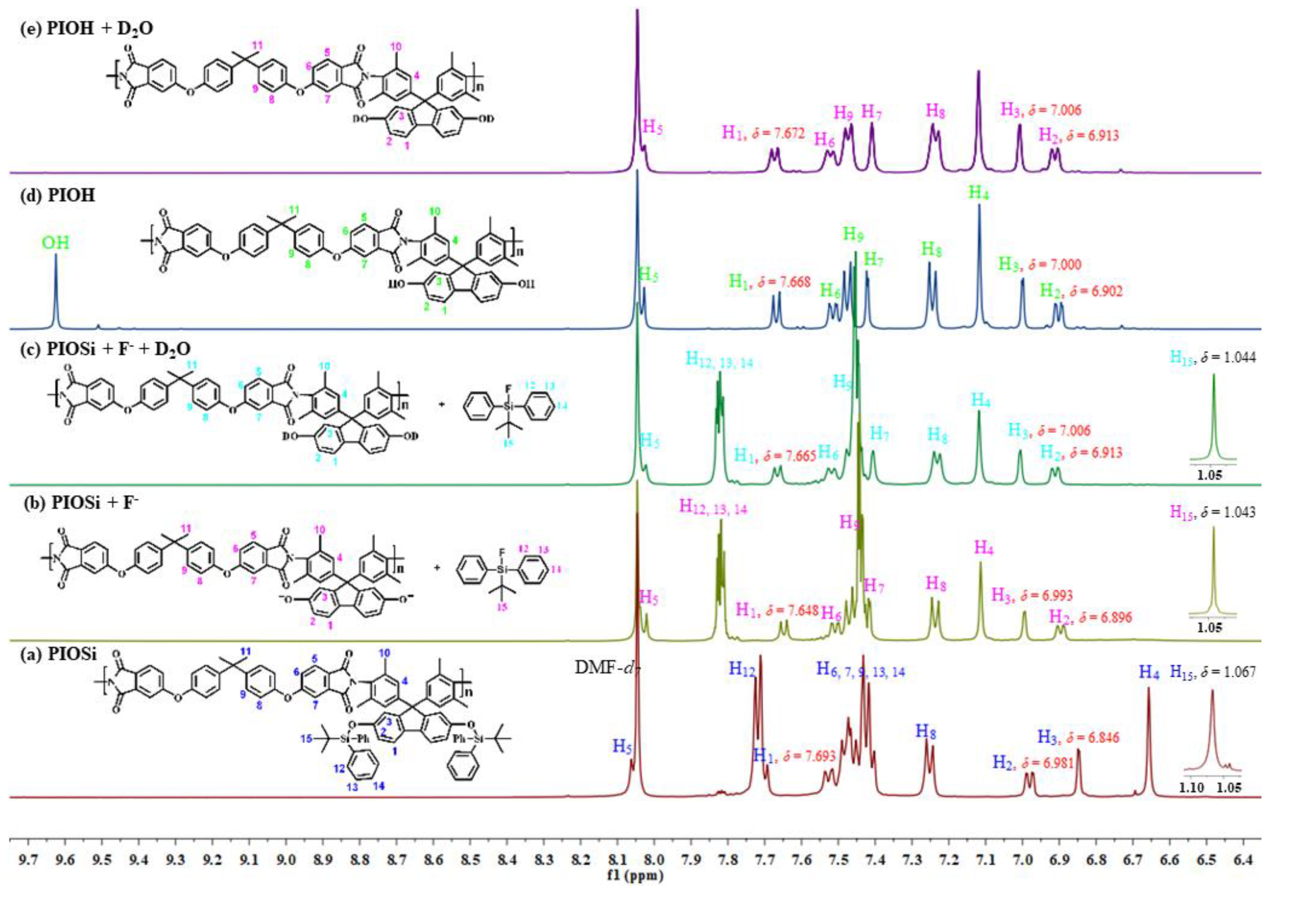

| Protons | PI-Osi (δ, ppm) | PI-OSi + F− (δ, ppm) | PI-OSi + F− + D2O (δ, ppm) | PI-OH (δ, ppm) | PI-OH + D2O (δ, ppm) |
|---|---|---|---|---|---|
| Ar-H1 | 7.693 | 7.648 | 7.665 | 7.668 | 7.672 |
| Ar-H2 | 6.981 | 6.896 | 6.913 | 6.902 | 6.913 |
| Ar-H3 | 6.846 | 6.993 | 7.006 | 7.000 | 7.006 |
| SiC-CH15 | 1.067 | 1.043 | 1.044 | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Lian, M.; Huang, G.; Zhang, Y.; Yi, N.; Tian, L.; Gan, F.; Ma, C. A tert-Butyldiphenylsilyl-Containing Polyimide-Based Chemosensor for Sequential Detection of Fluoride Ions and Trace Water in Organic Solvents. Molecules 2023, 28, 7987. https://doi.org/10.3390/molecules28247987
Wu Y, Lian M, Huang G, Zhang Y, Yi N, Tian L, Gan F, Ma C. A tert-Butyldiphenylsilyl-Containing Polyimide-Based Chemosensor for Sequential Detection of Fluoride Ions and Trace Water in Organic Solvents. Molecules. 2023; 28(24):7987. https://doi.org/10.3390/molecules28247987
Chicago/Turabian StyleWu, Yancheng, Manyu Lian, Guotao Huang, Yangfan Zhang, Ningbo Yi, Liyong Tian, Feng Gan, and Chunping Ma. 2023. "A tert-Butyldiphenylsilyl-Containing Polyimide-Based Chemosensor for Sequential Detection of Fluoride Ions and Trace Water in Organic Solvents" Molecules 28, no. 24: 7987. https://doi.org/10.3390/molecules28247987
APA StyleWu, Y., Lian, M., Huang, G., Zhang, Y., Yi, N., Tian, L., Gan, F., & Ma, C. (2023). A tert-Butyldiphenylsilyl-Containing Polyimide-Based Chemosensor for Sequential Detection of Fluoride Ions and Trace Water in Organic Solvents. Molecules, 28(24), 7987. https://doi.org/10.3390/molecules28247987







