Nucleotides as Bacterial Second Messengers
Abstract
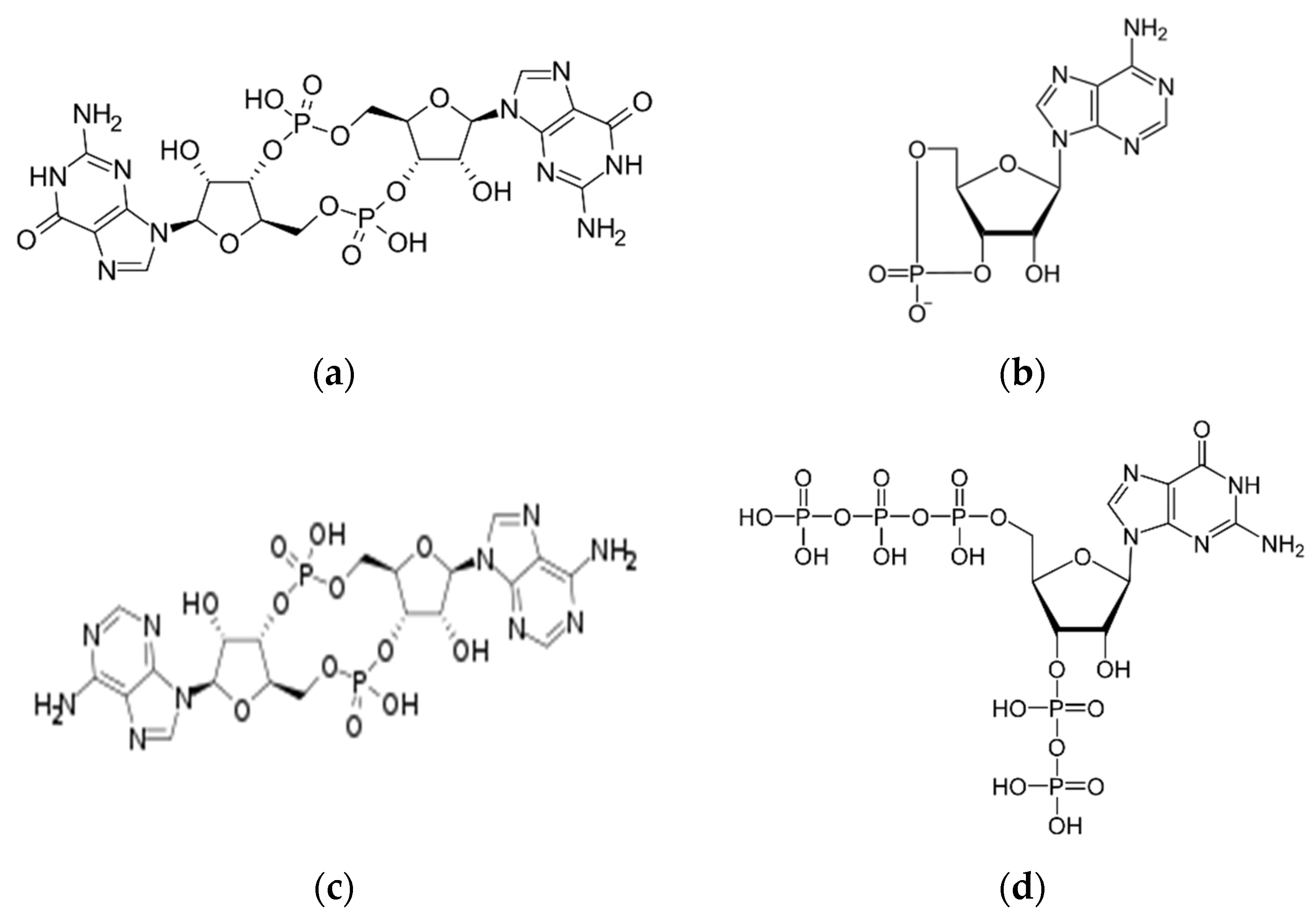
:1. Introduction
Similarities and Differences between c-di-Nucleotides
2. The c-di-GMP Nucleotide
2.1. Types of c-di-GMP Receptors
2.2. Processes of c-di-GMP Signaling
2.3. Cell Motility
2.4. Regulation of Biofilms
2.5. Pili as c-di-GMP Targets
2.6. Adhesins as a Target of c-di-GMP
2.7. Exopolysaccharide as a Target of c-di-GMP
2.8. Alginate Polysaccharides, Pel, and Psl as Targets of c-di-GMP
2.9. Cyclic di-GMP and Quorum Sensing
2.10. Cellulose Biosynthesis
2.11. Cell Cycle Regulation and Differentiation
2.12. Cell Differentiation in Multicellular Bacteria
2.13. Virulence
2.14. Cyclic di-GMP as an Immunomodulator
3. The Novel Cyclic Dinucleotide Second Messengers
4. The cAMP Nucleotide
4.1. cAMP Binds to cAMP Receptor Protein (CRP)
4.2. cAMP Signaling in Pathogenic Bacteria
4.3. Pseudomonas aeruginosa
4.4. Vibrio cholerae
4.5. Mycobacterium tuberculosis
5. The c-di-AMP Nucleotide
5.1. c-di-AMP Receptors
5.2. Cellular Processes Regulated by c-di-AMP
5.2.1. Cell Wall Homeostasis
5.2.2. Sensing of DNA Damage
5.3. c-di-AMP in Eukaryotic Host Cell
6. The pppGpp or ppGpp Nucleotide
Interactions between ppGpp, c-di-GMP, and c-di-AMP
7. Clinical Application of di-Nucleotides
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonough, K.A.; Rodriguez, A. The myriad roles of cyclic AMP in microbial pathogens: From signal to sword. Nat. Rev. Microbiol. 2012, 10, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Swanson, M.S. ppGpp: Magic beyond RNA polymerase. Nat. Rev. Microbiol. 2012, 10, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Makman, R.S.; Sutherland, E.W. Adenosine 3′,5′-phosphate in Escherichia coli. J. Biol. Chem. 1965, 240, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.C.; Yildiz, F.H. Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J. Bacteriol. 2008, 190, 6646–6659. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.J.; Ryjenkov, D.A.; Gomelsky, M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: Enzymatically active and inactive EAL domains. J. Bacteriol. 2005, 187, 4774–4781. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Christen, B.; Folcher, M.; Schauerte, A.; Jenal, U. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 2005, 280, 30829–30837. [Google Scholar] [CrossRef] [PubMed]
- Simm, R.; Morr, M.; Kader, A.; Nimtz, M.; Romling, U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004, 53, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Stulke, J.; Kruger, L. Cyclic di-AMP signaling in bacteria. Annu. Rev. Microbiol. 2020, 74, 159–179. [Google Scholar] [CrossRef]
- He, J.; Yin, W.; Galperin, M.Y.; Chou, S.H. Cyclic di-AMP, a second messenger of primary importance: Tertiary structures and binding mechanisms. Nucleic Acids Res. 2020, 48, 2807–2829. [Google Scholar] [CrossRef]
- Pham, H.T.; Nhiep, N.T.H.; Vu, T.N.M.; Huynh, T.N.; Zhu, Y.; Huynh, A.L.D.; Chakrabortti, A.; Marcellin, E.; Lo, R.; Howard, C.B.; et al. Enhanced uptake of potassium or glycine betaine or export of cyclic-diAMP restores osmoresistance in a high cyclic-di-AMP Lactococcus lactis mutant. PLoS Genet. 2018, 14, e1007574. [Google Scholar] [CrossRef]
- Römling, U.; Gomelsky, M.; Galperin, M.Y. c-di-GMP: The dawning of a novel bacterial signalling system. Mol. Microbiol. 2005, 57, 629–639. [Google Scholar] [CrossRef]
- Rao, F.; See, R.Y.; Zhang, D.; Toh, D.C.; Ji, Q.; Liang, Z.X. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J. Biol. Chem. 2010, 285, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.; Tuckerman, J.R.; Gonzalez, G.; Mayer, R.; Weinhouse, H.; Volman, G.; Amikam, D.; Benziman, M.; Gilles-Gonzalez, M.A. Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry 2001, 40, 3420–3426. [Google Scholar] [CrossRef]
- Ryjenkov, D.A.; Simm, R.; Römling, U.; Gomelsky, M. The PilZ domain is a receptor for the second messenger c-di-GMP. The PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 2006, 281, 30310–30314. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.T.; Tamayo, R.; Tischler, A.D.; Camilli, A. PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae. J. Biol. Chem. 2007, 282, 12860–12870. [Google Scholar] [CrossRef] [PubMed]
- Duerig, A.; Abel, S.; Folcher, M.; Nicollier, M.; Schwede, T.; Amiot, N.; Giese, B.; Jenal, U. Second messenger-mediated spatiotemporal control of protein degradation regulates bacterial cell cycle progression. Genes Dev. 2009, 23, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Hobley, L.; Fung, R.K.; Lambert, C.; Harris, M.A.; Dabhi, J.M.; King, S.S.; Basford, S.M.; Uchida, K.; Till, R.; Ahmad, R.; et al. Discrete cyclic di-GMP-dependent control of bacterial predation versus axenic growth in Bdellovibrio bacteriovorus. PLoS Pathog. 2012, 8, e1002493. [Google Scholar] [CrossRef]
- Newell, P.D.; Monds, R.D.; O’Toole, G.A. LapD is a bis-(3′, 5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc. Natl. Acad. Sci. USA 2009, 106, 3461–3466. [Google Scholar] [CrossRef]
- Barrick, J.E.; Breaker, R.R. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol. 2007, 8, R239. [Google Scholar] [CrossRef]
- Lee, E.R.; Baker, J.L.; Weinberg, Z.; Sudarsan, N.; Breaker, R.R. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science 2010, 329, 845–848. [Google Scholar] [CrossRef]
- Bobrov, A.G.; Kirillina, O.; Ryjenkov, D.A.; Waters, C.M.; Price, P.A.; Fetherston, J.D.; Mack, D.; Goldman, W.E.; Gomelsky, M.; Perry, R.D. Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol. Microbiol. 2011, 79, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Bharati, B.K.; Sharma, I.M.; Kasetty, S.; Kumar, M.; Mukherjee, R.; Chatterji, D. A full length bifunctional protein involved in c-di-GMP turnover is required for long term survival under nutrient starvation in Mycobacterium smegmatis. Microbiology 2012, 158, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.Z.; Pitzer, J.E.; Boquoi, T.; Hobbs, G.; Miller, M.R.; Motaleb, M.A. Analysis of the HD-GYP domain cyclic dimeric GMP phosphodiesterase reveals a role in motility and the enzootic life cycle of Borrelia burgdorferi. Infect. Immun. 2011, 79, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Girgis, H.S.; Liu, Y.; Ryu, W.S.; Tavazoie, S. A comprehensive genetic characterization of bacterial motility. PLoS Genet. 2007, 3, 1644–1660. [Google Scholar] [CrossRef] [PubMed]
- Jyot, J.; Dasgupta, N.; Ramphal, R. FleQ, the major flagellar gene regulator in Pseudomonas aeruginosa, binds to enhancer sites located either upstream or atypically downstream of the RpoN binding site. J. Bacteriol. 2002, 184, 5251–5260. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Remminghorst, U.; Rehm, B.H. MucR, a novel membrane associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2009, 75, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Römling, U. Cyclic di-GMP, an established secondary messenger still speeding up. Environ. Microbiol. 2012, 14, 1817–1829. [Google Scholar] [CrossRef]
- Johnson, J.G.; Clegg, S. Role of MrkJ, a phosphodiesterase, in type 3 fimbrial expression and biofilm formation in Klebsiella pneumoniae. J. Bacteriol. 2010, 192, 3944–3950. [Google Scholar] [CrossRef]
- Meissner, A.; Wild, V.; Simm, R.; Rohde, M.; Erck, C.; Bredenbruch, F.; Morr, M.; Römling, U.; Häussler, S. Pseudomonas aeruginosa cupA encoded fimbriae expression is regulated by a GGDEF and EAL domain dependent modulation of the intracellular level of cyclic diguanylate. Environ. Microbiol. 2007, 9, 2475–2485. [Google Scholar] [CrossRef]
- Huang, B.; Whitchurch, C.B.; Mattick, J.S. FimX, a multidomain protein connecting environmental signals to twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 2003, 185, 7068–7076. [Google Scholar] [CrossRef]
- Boehm, A.; Steiner, S.; Zaehringer, F.; Casanova, A.; Hamburger, F.; Ritz, D.; Keck, W.; Ackermann, M.; Schirmer, T.; Jenal, U. Second messenger signalling governs Escherichia coli biofilm induction upon ribosomal stress. Mol. Microbiol. 2009, 72, 1500–1516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Preston, J.F., 3rd; Romeo, T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 2004, 186, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.M.; O’Donnell, S.T.; Ryjenkov, D.A.; Gomelsky, L.; Slater, S.R.; Fey, P.D.; Gomelsky, M.; O’Gara, J.P. A staphylococcal GGDEF domain protein regulates biofilm formation independently of c-di-GMP. J. Bacteriol. 2008, 190, 5178–5189. [Google Scholar] [CrossRef] [PubMed]
- Güvener, Z.T.; Harwood, C.S. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Mol. Microbiol. 2007, 66, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Baraquet, C.; Murakami, K.; Parsek, M.R.; Harwood, C.S. The FleQ protein from Pseudomonas aeruginosa functions as both a repressor and an activator to control gene expression from the pel operon promoter in response to c-di-GMP. Nucleic Acids Res. 2012, 40, 7207–7218. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Borlee, B.R.; O’Connor, J.R.; Hill, P.J.; Harwood, C.S.; Wozniak, D.J.; Parsek, M.R. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2012, 109, 20632–20636. [Google Scholar] [CrossRef] [PubMed]
- Merighi, M.; Lee, V.T.; Hyodo, M.; Hayakawa, Y.; Lory, S. The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 876–895. [Google Scholar] [CrossRef] [PubMed]
- Beyhan, S.; Yildiz, F.H. Smooth to rugose phase variation in Vibrio cholerae can be mediated by a single nucleotide change that targets c-diGMP signalling pathway. Mol. Microbiol. 2007, 63, 995–1007. [Google Scholar] [CrossRef]
- Ueda, A.; Wood, T.K. Tyrosine phosphatase TpbA of Pseudomonas aeruginosa controls extracellular DNA via cyclic diguanylic acid concen- trations. Environ. Microbiol. Rep. 2010, 2, 449–455. [Google Scholar] [CrossRef]
- Hammer, B.K.; Bassler, B.L. Distinct sensory pathways in Vibrio cholerae El Tor and classical biotypes modulate c-di-GMP levels to control biofilm formation. J. Bacteriol. 2008, 191, 169–177. [Google Scholar] [CrossRef]
- Monteiro, C.; Saxena, I.; Wang, X.; Kade, A.; Bokranz, W.; Simm, R.; Nobles, D.; Chromek, M.; Brauner, A.; Brown, R.M., Jr.; et al. Characterization of cellulose production in Escherichia coli Nissle 1917 and its biological consequences. Environ. Microbiol. 2009, 11, 1105–1116. [Google Scholar] [CrossRef]
- Paul, R.; Jaeger, T.; Abel, S.; Wiederkehr, I.; Folcher, M.; Biondi, E.G.; Laub, M.T.; Jenal, U. Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell 2008, 133, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Chien, P.; Wassmann, P.; Schirmer, T.; Kaever, V.; Laub, M.T.; Baker, T.A.; Jenal, U. Regulatory cohesion of cell cycle and cell differentiation through interlinked phosphorylation and second messenger networks. Mol. Cell 2011, 43, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Neunuebel, M.R.; Golden, J.W. The Anabaena sp. strain PCC 7120 gene all2874 encodes a diguanylate cyclase and is required for normal heterocyst development under high-light growth conditions. J. Bacteriol. 2008, 190, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- den Hengst, C.D.; Tran, N.T.; Bibb, M.J.; Chandra, G.; Leskiw, B.K.; Buttner, M.J. Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol. Microbiol. 2010, 78, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Tischler, A.D.; Camilli, A. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 2005, 73, 5873–5882. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ouyang, Z.; Troxell, B.; Xu, H.; Moh, A.; Piesman, J.; Norgard, M.V.; Gomelsky, M.; Yang, X.F. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 2011, 7, e1002133. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Garcia, B.; Latasa, C.; Toledo-Arana, A.; Zorraquino, V.; Valle, J.; Casals, J.; Pedroso, E.; Lasa, I. Genetic reductionist approach for dissecting individual roles of GGDEF proteins within the c-di-GMP signaling network in Salmonella. Proc. Natl. Acad. Sci. USA 2009, 106, 7997–8002. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Chaudhuri, P.; Gourley, C.; Harms, J.; Splitter, G. Brucella melitensis cyclic di-GMP phosphodiesterase BpdA controls expression of flagellar genes. J. Bacteriol. 2011, 193, 5683–5691. [Google Scholar] [CrossRef]
- Zogaj, X.; Wyatt, G.C.; Klose, K.E. Cyclic di-GMP stimulates biofilm formation and inhibits virulence of Francisella novicida. Infect. Immun. 2012, 80, 4239–4247. [Google Scholar] [CrossRef]
- Amikam, D.; Steinberger, O.; Shkolnik, T.; Ben-Ishai, Z. The novel cyclic dinucleotide 3′-5′ cyclic diguanylic acid binds to p21ras and enhances DNA synthesis but not cell replication in the Molt 4 cell line. Biochem. J. 1995, 311, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Karaolis, D.K.; Cheng, K.; Lipsky, M.; Elnabawi, A.; Catalano, J.; Hyodo, M.; Hayakawa, Y.; Raufman, J.P. 3′, 5′-Cyclic diguanylic acid (c-diGMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem. Biophys. Res. Commun. 2005, 329, 40–45. [Google Scholar] [CrossRef]
- Hu, D.L.; Narita, K.; Hyodo, M.; Hayakawa, Y.; Nakane, A.; Karaolis, D.K. c-di-GMP as a vaccine adjuvant enhances protection against systemic methicillin-resistant Staphylococcus aureus (MRSA) infection. Vaccine 2009, 27, 4867–4873. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, A.T.; Eaglesham, J.B.; de Oliveira Mann, C.C.; Morehouse, B.R.; Lowey, B.; Nieminen, E.A.; Danilchanka, O.; King, D.S.; Lee, A.S.Y.; Mekalanos, J.J.; et al. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 2019, 567, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.W.; Bogard, R.W.; Young, T.S.; Mekalanos, J.J. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 2012, 149, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhu, B. The cyclic oligoadenylate signaling pathway of type III CRISPR-Cas systems. Front. Microbiol. 2021, 11, 602789. [Google Scholar] [CrossRef]
- Kamenetsky, M.; Middelhaufe, S.; Bank, E.M.; Levin, L.R.; Buck, J.; Steegborn, C. Molecular details of cAMP generation in mammalian cells: A tale of two systems. J. Mol. Biol. 2006, 362, 623–639. [Google Scholar] [CrossRef]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef]
- Kolb, A.; Busby, S.; Buc, H.; Garges, S.; Adhya, S. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 1993, 62, 749–795. [Google Scholar] [CrossRef]
- Bai, G.; Knapp, G.S.; McDonough, K.A. Cyclic AMP signalling in mycobacteria: Redirecting the conversation with a common currency. Cell Microbiol. 2011, 13, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, S.J.; Choi, S.H.; Lee, K.H. Vibrio vulnificus rpoS expression is repressed by direct binding of cAMP-cAMP receptor protein complex to its two promoter regions. J. Biol. Chem. 2008, 283, 30438–30450. [Google Scholar] [CrossRef] [PubMed]
- Yahr, T.L.; Vallis, A.J.; Hancock, M.K.; Barbieri, J.T.; Frank, D.W. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 1998, 95, 13899–13904. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, M.C.; Lee, V.T.; Gilmore, M.E.; Lory, S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell. 2003, 4, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Wolfgang, M.C.; Lory, S. An adenylate cyclase-controlled signaling network regulates Pseudomonas aeruginosa virulence in a mouse model of acute pneumonia. Infect. Immun. 2004, 72, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.L.; Brutinel, E.D.; Jones, A.K.; Fulcher, N.B.; Urbanowski, M.L.; Yahr, T.L.; Wolfgang, M.C. The Pseudomonas aeruginosa Vfr regulator controls global virulence factor expression through cyclic AMP-dependent and -independent mechanisms. J. Bacteriol. 2010, 192, 3553–3564. [Google Scholar] [CrossRef] [PubMed]
- Skorupski, K.; Taylor, R.K. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1997, 94, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Pascual-Montano, A.; Silva, A.J.; Benitez, J.A. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology 2007, 153, 2964–2975. [Google Scholar] [CrossRef]
- Agarwal, N.; Lamichhane, G.; Gupta, R.; Nolan, S.; Bishai, W.R. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature 2009, 460, 98–102. [Google Scholar] [CrossRef]
- Rickman, L.; Scott, C.; Hunt, D.M.; Hutchinson, T.; Menéndez, M.C.; Whalan, R.; Hinds, J.; Colston, M.J.; Green, J.; Buxton, R.S. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol. Microbiol. 2005, 56, 1274–1286. [Google Scholar] [CrossRef]
- Gazdik, M.A.; Bai, G.; Wu, Y.; McDonough, K.A. Rv1675c (cmr) regulates intramacrophage and cyclic AMP-induced gene expression in Mycobacterium tuberculosis-complex mycobacteria. Mol. Microbiol. 2009, 71, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Witte, G.; Hartung, S.; Buttner, K.; Hopfner, K.P. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell 2008, 30, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 2010, 328, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer-Shaanan, Y.; Wexselblatt, E.; Katzhendler, J.; Yavin, E.; Ben-Yehuda, S. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep. 2011, 12, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.R.; Koestler, B.J.; Carpenter, V.K.; Burdette, D.L.; Waters, C.M.; Vance, R.E.; Valdivia, R.H. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. mBio 2013, 4, e00018-13. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.M.; Abbott, J.C.; Burhenne, H.; Kaever, V.; Grundling, A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role incontrolling cell size and envelope stress. PLoS Pathog. 2011, 7, e1002217. [Google Scholar] [CrossRef] [PubMed]
- Kamegaya, T.; Kuroda, K.; Hayakawa, Y. Identification of a S treptococcus pyogenes SF370 gene involved in production of c-di-AMP. Nagoya J. Med. Sci. 2011, 73, 49–57. [Google Scholar] [PubMed]
- Corrigan, R.M.; Campeotto, I.; Jeganathan, T.; Lee, V.T.; Gründling, A. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 9084–9089. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; He, Z.G. DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in Mycobacterium smegmatis. J. Biol. Chem. 2013, 288, 3085–3096. [Google Scholar] [CrossRef]
- Luo, Y.; Helmann, J.D. Analysis of the role of Bacillus subtilis σM in β-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol. Microbiol. 2012, 83, 623–639. [Google Scholar] [CrossRef]
- Mehne, F.M.; Gunka, K.; Eilers, H.; Herzberg, C.; Kaever, V.; Stülke, J. Cyclic di-AMP homeostasis in Bacillus subtilis: Both lack and high-level accumulation of the nucleotide are detrimental for cell growth. J. Biol. Chem. 2013, 288, 2004–2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Youn, S.J.; Kim, S.O.; Ko, J.; Lee, J.O.; Choi, B.S. Structural studies of potassium transport protein KtrA regulator of conductance of K (RCK) C domain in complex with cyclic diadenosine monophosphate (c-di-AMP). J. Biol. Chem. 2015, 290, 16393–16402. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.H.; Liang, J.M.; Yang, J.G.; Shih, M.S.; Tu, Z.L.; Wang, Y.C.; Sun, X.H.; Hu, N.J.; Liang, Z.X.; Dow, J.M.; et al. Structural insights into the distinct binding mode of cyclic di-AMP with SaCpaA_RCK. Biochemistry 2015, 54, 4936–4951. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, I.; Tholema, N.; Kröning, N.; Vor der Brüggen, M.; Wunnicke, D.; Bakker, E.P. KtrB, a member of the superfamily of K+ transporters. Eur. J. Cell Biol. 2011, 90, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Gries, C.M.; Bose, J.L.; Nuxoll, A.S.; Fey, P.D.; Bayles, K.W. The Ktr potassium transport system in Staphylococcus aureus and its role in cell physiology, antimicrobial resistance and pathogenesis. Mol. Microbiol. 2013, 89, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Freeman, Z.N.; Dorus, S.; Waterfield, N.R. The KdpD/KdpE two-component system: Integrating K+ homeostasis and virulence. PLoS Pathog. 2013, 9, e1003201. [Google Scholar] [CrossRef] [PubMed]
- Epstein, W. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 2003, 75, 293–320. [Google Scholar]
- Müller, M.; Hopfner, K.P.; Witte, G. c-di-AMP recognition by Staphylococcus aureus PstA. FEBS Lett. 2015, 589, 45–51. [Google Scholar] [CrossRef]
- Bowman, L.; Zeden, M.S.; Schuster, C.F.; Kaever, V.; Gründling, A. New insights into the cyclic di-adenosine monophosphate (c-diAMP) degradation pathway and the requirement of the cyclic dinucleotide for acid stress resistance in Staphylococcus aureus. J. Biol. Chem. 2016, 291, 26970–26986. [Google Scholar] [CrossRef]
- Smith, W.M.; Pham, T.H.; Lei, L.; Dou, J.; Soomro, A.H.; Beatson, S.A.; Dykes, G.A.; Turner, M.S. Heat resistance and salt hypersensitivity in Lactococcus lactis due to spontaneous mutation of llmg_1816 (gdpP) induced by high-temperature growth. Appl. Environ. Microbiol. 2012, 78, 7753–7759. [Google Scholar] [CrossRef]
- Witte, C.E.; Whiteley, A.T.; Burke, T.P.; Sauer, J.D.; Portnoy, D.A.; Woodward, J.J. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. mBio 2013, 4, e00282-13. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.D.; Sotelo-Troha, K.; von Moltke, J.; Monroe, K.M.; Rae, C.S.; Brubaker, W.; Hyodo, M.; Hayakawa, Y.; Woodward, J.J.; Portnoy, D.A.; et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 2010, 79, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Hayashi, T.; Takahara, K.; Satoh, T.; Lee, H.; Matsunaga, K.; Kageyama, S.; Omori, H.; Noda, T.; et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl. Acad. Sci. USA 2009, 106, 20842–20846. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, K.; Cashel, M. (p)ppGpp: Still magical? Annu. Rev. Microbiol. 2008, 62, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Haugen, S.P.; Ross, W.; Gourse, R.L. Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat. Rev. Microbiol. 2008, 6, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, S.; Del Peso-Santos, T.; Shingler, V. Regulation of alternative sigma factor use. Annu. Rev. Microbiol. 2011, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Hengge-Aronis, R. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 2002, 66, 373–395. [Google Scholar] [CrossRef]
- Bougdour, A.; Wickner, S.; Gottesman, S. Modulating RssB activity: IraP, a novel regulator of σS stability in Escherichia coli. Genes Dev. 2006, 20, 884–897. [Google Scholar] [CrossRef]
- Siculella, L.; Damiano, F.; di Summa, R.; Tredici, S.M.; Alduina, R.; Gnoni, G.V.; Alifano, P. Guanosine 5′-diphosphate 3′-diphosphate (ppGpp) as a negative modulator of polynucleotide phosphorylase activity in a ‘rare’ actinomycete. Mol. Microbiol. 2010, 77, 716–729. [Google Scholar] [CrossRef]
- Huynh, T.N.; Luo, S.; Pensinger, D.; Sauer, J.D.; Tong, L.; Woodward, J.J. An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence. Proc. Natl. Acad. Sci. USA. 2015, 112, E747–E756. [Google Scholar] [CrossRef]
- Sahr, T.; Brüggemann, H.; Jules, M.; Lomma, M.; Albert-Weissenberger, C.; Cazalet, C.; Buchrieser, C. Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 2009, 72, 741–762. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.W.; Miller, V.L. Regulation of virulence by members of the MarR/SlyA family. Curr. Opin. Microbiol. 2006, 9, 153–159. [Google Scholar] [CrossRef]
- Zhao, G.; Weatherspoon, N.; Kong, W.; Curtiss, R.; Shi, Y. A dual-signal regulatory circuit activates transcription of a set of divergent operons in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 2008, 105, 20924–20929. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Sambanthamoorthy, K.; Sloup, R.E.; Parashar, V.; Smith, J.M.; Kim, E.E.; Semmeelhack, M.F.; Neiditch, M.B.; Waters, C.M. Identification of small molecules that antagonize diguanylate cyclase enzymes to inhibit biofilm formation. Antimicrob. Agents Chemother. 2012, 56, 5202–5211. [Google Scholar] [CrossRef]
- Antoniani, D.; Rossi, E.; Rinaldo, S.; Bocci, P.; Lolicato, M.; Paiardini, A.; Raffaelli, N.; Cutruzzola, F.; Landini, P. The immunosuppressive drug azathioprine inhibits biosynthesis of the bacterial signal molecule cyclic-di-GMP by interfering with intracellular nucleotide pool availability. Appl. Microbiol. Biotechnol. 2013, 97, 7325–7336. [Google Scholar] [CrossRef] [PubMed]
- Migliore, F.; Macchi, R.; Landini, P.; Paroni, M. Phagocytosis and epithelial cell invasion by Crohn’s disease-associated adherent-invasive Escherichia coli are inhibited by the anti-inflammatory drug 6-mercaptopurine. Front. Microbiol. 2018, 9, 964. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.S.; Choi, H.Y.; Yoon, S.S.; Kim, W.G. Terrein is an inhibitor of quorum sensing and c-di-GMP in Pseudomonas aeruginosa: A connection between quorum sensing and c-di-GMP. Sci. Rep. 2018, 8, 8617. [Google Scholar] [CrossRef]
- Zhou, J.; Watt, S.; Wang, J.; Nakayama, S.; Sayre, D.A.; Lam, Y.-F.; Lee, V.Y.; Sintim, H.O. Potent suppression of c-di-GMP synthesis via I-site allosteric inhibition of diguanylate cyclases with 2′-F-c-di-GMP. Bioorg. Med. Chem. 2013, 21, 4396–4404. [Google Scholar] [CrossRef]
- Lieberman, O.J.; Orr, M.W.; Wang, Y.; Lee, V.T. High-throughput screening using the differential radial capillary action of ligand assay identifies ebselen as an inhibitor of diguanylate cyclases. ACS Chem. Biol. 2014, 9, 183–192. [Google Scholar] [CrossRef]
- Foletti, C.; Kramer, R.A.; Mauser, H.; Jenal, U.; Bleicher, K.H.; Wennemers, H. Functionalized proline-rich peptides bind the bacterial second messenger c-di-GMP. Angew. Chem. Int. Ed. Engl. 2018, 57, 7729–7733. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cha, E.; Kim, Y.; Jeon, Y.H.; Olson, B.H.; Byun, Y.; Park, H.D. Raffinose, a plant galactoside, inhibits Pseudomonas aeruginosa biofilm formation via binding to LecA and decreasing cellular cyclic diguanylate levels. Sci. Rep. 2016, 6, 25318. [Google Scholar] [CrossRef] [PubMed]
- De Smet, J.; Wagemans, J.; Hendrix, H.; Staes, I.; Visnapuu, A.; Horemans, B.; Aertsen, A.; Lavigne, R. Bacteriophage-mediated interference of the c-di-GMP signalling pathway in Pseudomonas aeruginosa. Microb. Biotechnol. 2021, 14, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Ebensen, T.; Debarry, J.; Pedersen, G.K.; Blazejewska, P.; Weissmann, S.; Schulze, K.; McCullough, K.C.; Cox, R.J.; Guzmán, C.A. Mucosal administration of cycle-di-nucleotide-adjuvanted virosomes efficiently induces protection against influenza H5N1 in mice. Front. Immunol. 2017, 8, 1223. [Google Scholar] [CrossRef] [PubMed]
- Landi, A.; Law, J.; Hockman, D.; Logan, M.; Crawford, K.; Chen, C.; Kundu, J.; Ebensen, T.; Guzman, C.A.; Deschatelets, L.; et al. Superior immunogenicity of HCV envelope glycoproteins when adjuvanted with cyclic- di-AMP, a STING activator or archaeosomes. Vaccine 2017, 35, 6949–6956. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kanuma, T.; Takahama, S.; Okamura, T.; Moriishi, E.; Ishii, K.J.; Terahara, K.; Yasutomi, Y. STING agonists activate latently infected cells and enhance SIV-specific responses ex vivo in naturally SIV controlled cynomolgus macaques. Sci. Rep. 2019, 9, 5917. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.J.; Dey, B.; Singh, A.K.; Praharaj, M.; Bishai, W. Bacillus Calmette-Guerin overexpressing an endogenous stimulator of interferon genes agonist provides enhanced protection against pulmonary tuberculosis. J. Infect. Dis. 2020, 221, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.N.; Cazorla, S.I.; Schulze, K.; Ebensen, T.; Guzmán, C.A.; Malchiodi, E.L. Immunization with Tc52 or its amino terminal domain adjuvanted with c-di-AMP induces Th17+Th1 specific immune responses and confers protection against Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2017, 11, e0005300. [Google Scholar] [CrossRef]
- Quintana, I.; Espariz, M.; Villar, S.R.; González, F.B.; Pacini, M.F.; Cabrera, G.; Bontempi, I.; Prochetto, E.; Stülke, J.; Perez, A.R.; et al. Genetic engineering of Lactococcus lactis co-producing antigen and the mucosal adjuvant 3′ 5′-cyclic di adenosine monophosphate (c-di-AMP) as a design strategy to develop a mucosal vaccine prototype. Front. Microbiol. 2018, 9, 2100. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancino-Diaz, M.E.; Guerrero-Barajas, C.; Betanzos-Cabrera, G.; Cancino-Diaz, J.C. Nucleotides as Bacterial Second Messengers. Molecules 2023, 28, 7996. https://doi.org/10.3390/molecules28247996
Cancino-Diaz ME, Guerrero-Barajas C, Betanzos-Cabrera G, Cancino-Diaz JC. Nucleotides as Bacterial Second Messengers. Molecules. 2023; 28(24):7996. https://doi.org/10.3390/molecules28247996
Chicago/Turabian StyleCancino-Diaz, Mario E., Claudia Guerrero-Barajas, Gabriel Betanzos-Cabrera, and Juan C. Cancino-Diaz. 2023. "Nucleotides as Bacterial Second Messengers" Molecules 28, no. 24: 7996. https://doi.org/10.3390/molecules28247996
APA StyleCancino-Diaz, M. E., Guerrero-Barajas, C., Betanzos-Cabrera, G., & Cancino-Diaz, J. C. (2023). Nucleotides as Bacterial Second Messengers. Molecules, 28(24), 7996. https://doi.org/10.3390/molecules28247996





