Antitumor and Phytochemical Properties of Ferula assa-foetida L. Oleo-Gum–Resin against HT-29 Colorectal Cancer Cells In Vitro and in a Xenograft Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis of Ferula assa-foetida L. OGR Extract
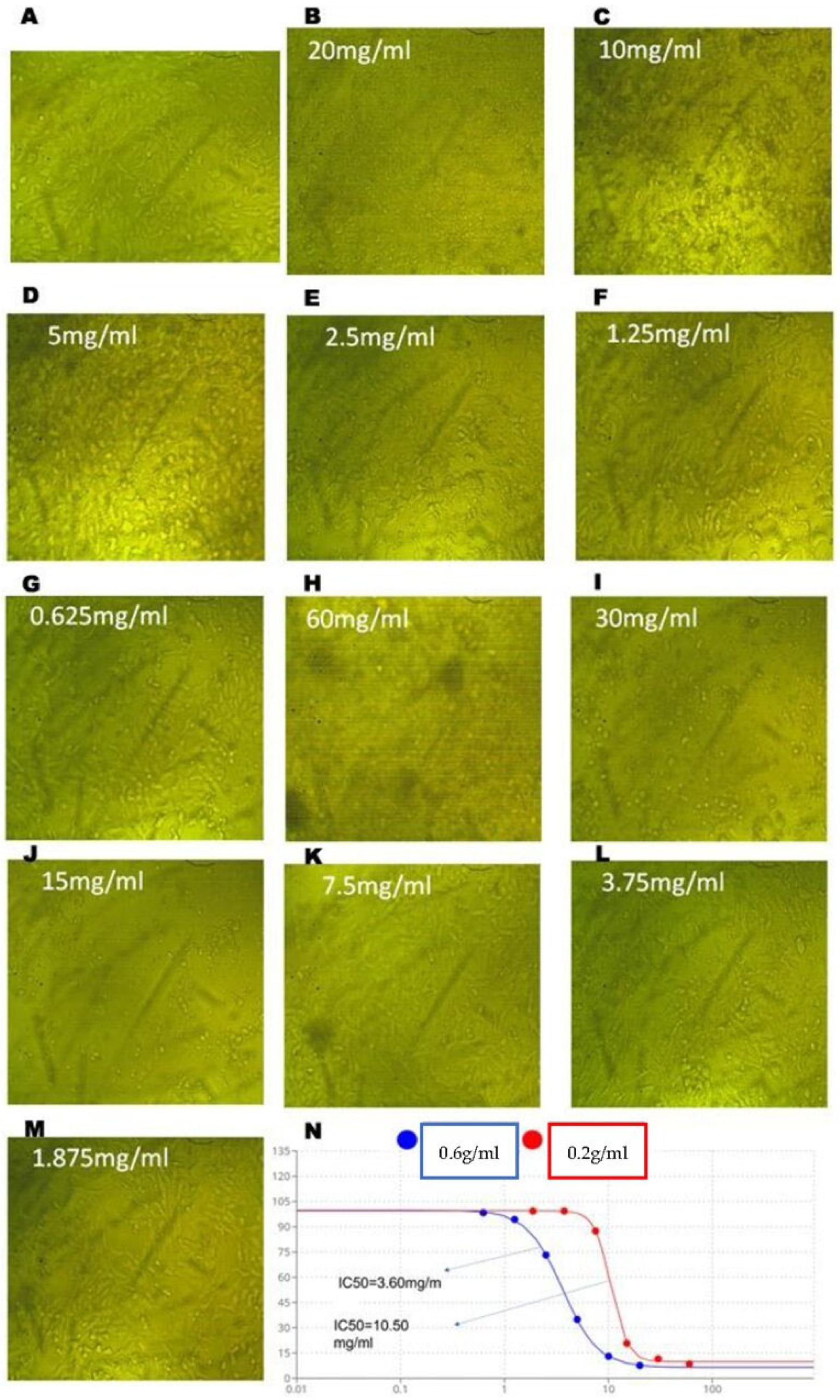
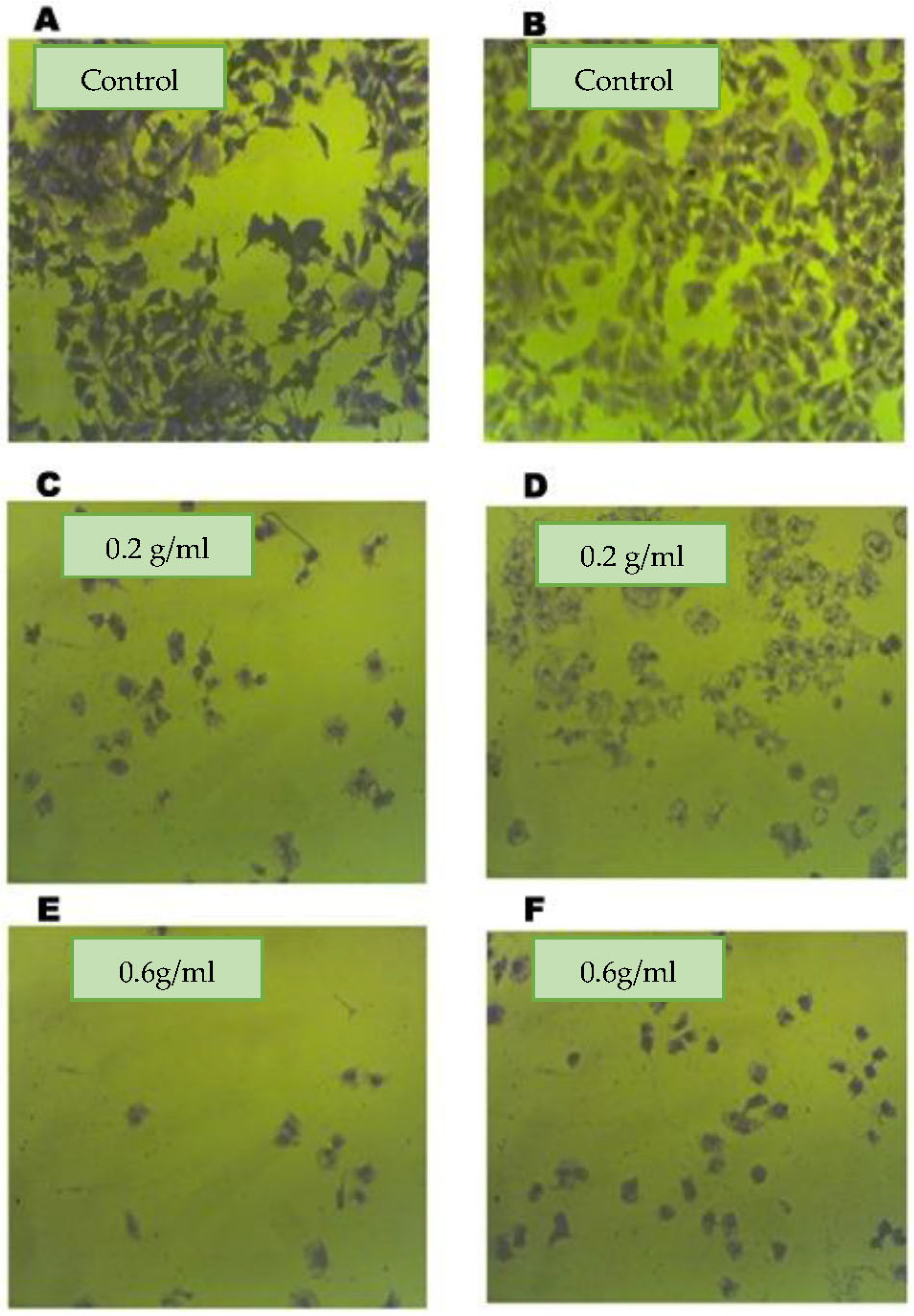
2.2. Ferula assa-foetida L. OGR Extract Inhibited the Viability of HT-29 CRC Cells and Colony Formation
2.3. Effect of Assa-foetida OGR Extract on Apoptosis and Pro-Death Proteins of CRC Cells
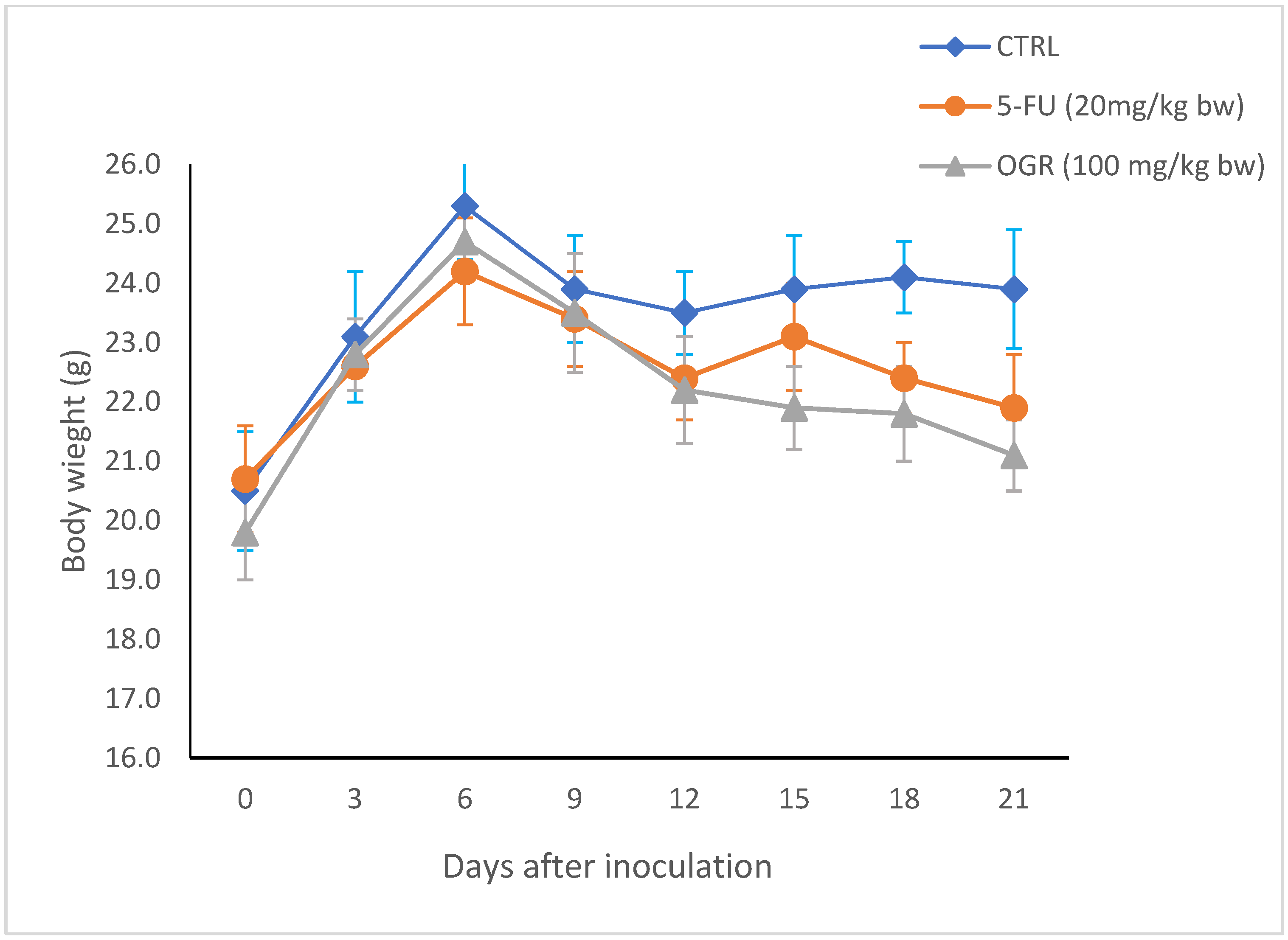
2.4. Effect of Assa-foetida OGR Extract on a HT-29 Tumor in the Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Plant Extract Preparation
4.2. Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry (LC–ESI–MS/MS) Analysis of the Extract
4.3. Cell Culture and Cytotoxicity against HT-29 CRC Tumor Cells
4.4. In Vitro Clonogenic Assay
4.5. Immunoblotting
4.6. Animals and Ethics
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and the United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M. Recent advances of tubulin inhibitors targeting the colchicine binding site for cancer therapy. Biomolecules 2022, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Jaradat, N.; Eid, A.M.; Abubaker, A.; Mufleh, O.; Al-Hroub, Q.; Sobuh, S. Synthesis of novel isoxazole–carboxamide derivatives as promising agents for melanoma and targeted nano-emulgel conjugate for improved cellular permeability. BMC Chem. 2022, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.N.; Goel, A.; Blum, H.E.; Boland, C.R. Molecular pathogenesis of colorectal cancer: Implications for molecular diagnosis. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2005, 104, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Vichitsakul, K.; Laowichuwakonnukul, K.; Soontornworajit, B.; Poomipark, N.; Itharat, A.; Rotkrua, P. Anti-proliferation and induction of mitochondria-mediated apoptosis by Garcinia hanburyi resin in colorectal cancer cells. Heliyon 2023, 9, e16411. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Ricci, A.D.; Rizzo, A.; Zanfi, C.; Tavolari, S.; Palloni, A.; De Lorenzo, S.; Ravaioli, M.; Cescon, M. Is post-transplant chemotherapy feasible in liver transplantation for colorectal cancer liver metastases? Cancer Commun. 2020, 40, 461–464. [Google Scholar] [CrossRef]
- Rizzo, A.; Nannini, M.; Novelli, M.; Ricci, A.D.; Scioscio, V.D.; Pantaleo, M.A. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936932. [Google Scholar] [CrossRef]
- Viscardi, G.; Tralongo, A.C.; Massari, F.; Lambertini, M.; Mollica, V.; Rizzo, A.; Comito, F.; Di Liello, R.; Alfieri, S.; Imbimbo, M.; et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: A systematic review and meta-analysis. Eur. J. Cancer 2022, 177, 175–185. [Google Scholar] [CrossRef]
- Park, G.H.; Park, J.H.; Song, H.M.; Eo, H.J.; Kim, M.K.; Lee, J.W.; Lee, M.H.; Cho, K.-H.; Lee, J.R.; Cho, H.J. Anti-cancer activity of Ginger (Zingiber officinale) leaf through the expression of activating transcription factor 3 in human colorectal cancer cells. BMC Complement. Altern. Med. 2014, 14, 408. [Google Scholar] [CrossRef]
- Adeleye, O.A.; Bamiro, O.A.; Bakre, L.G.; Odeleye, F.O.; Adebowale, M.N.; Okunye, O.L.; Sodeinde, M.A.; Adebona, A.C.; Menaa, F. Medicinal Plants with Potential Inhibitory Bioactive Compounds against Coronaviruses. Adv. Pharm. Bull. 2022, 12, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Hawash, M.; Abualhasan, M.; Naser, S.; Dwaikat, M.; Mansour, M. Exploring the Potent Anticancer, Antimicrobial, and Anti-Inflammatory Effects of Capparis Spinosa Oil Nanoemulgel. Coatings 2023, 13, 1441. [Google Scholar] [CrossRef]
- Jiménez-Orozco, F.A.; Ranđelović, I.; Hegedüs, Z.; Vega-Lopez, A.; Martínez-Flores, F.; Tóvarí, J. In vitro anti-proliferative effect and in vivo antitumor action of daphnetin in different tumour cells. Cirugía Y Cir. 2020, 88, 765–771. [Google Scholar]
- Mondal, A.; Banerjee, S.; Bose, S.; Das, P.P.; Sandberg, E.N.; Atanasov, A.G.; Bishayee, A. Cancer preventive and therapeutic potential of banana and its bioactive constituents: A systematic, comprehensive, and mechanistic review. Front. Oncol. 2021, 11, 697143. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Hung, C.-H.; Chen, C.-C.; Kao, S.-H.; Wang, C.-J. Hibiscus sabdariffa polyphenol-enriched extract inhibits colon carcinoma metastasis associating with FAK and CD44/c-MET signalling. J. Funct. Foods 2018, 48, 542–550. [Google Scholar] [CrossRef]
- Lima, A.; Batista-Santos, P.; Veríssimo, E.; Rebelo, P.; Ferreira, R.B. Differential inhibition of gelatinase activity in human colon adenocarcinoma cells by Aloe vera and Aloe arborescens extracts. BMC Complement. Med. Ther. 2020, 20, 379. [Google Scholar] [CrossRef] [PubMed]
- Sirizi, M.A.G.; Ghalenoei, J.A.; Allahtavakoli, M.; Forouzanfar, H.; Bagheri, S.M. Anticancer potential of Ferula assa-foetida and its constituents, a powerful plant for cancer therapy. World J. Biol. Chem. 2023, 14, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.N.M.; Raghad, J.F.; Nuaman, R.S.; Mahmod, Z.S.; Alamery, N.D. Anticancer Potentiality of Ferula assa-foetida Gum Extract against CT-26, HT-29, SW742 and WiDr Colorectal Cancer Cell Lines. South Asian Res. J. Bio. Appl. Biosci. 2023, 5, 1–6. [Google Scholar] [CrossRef]
- Mahendra, P.; Bisht, S. Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacogn. Rev. 2012, 6, 141. [Google Scholar] [CrossRef]
- Yatham, P.; Shukla, D.; Srivastava, A.K.; Pragadheesh, V.S.; Kumar, D. Purification and identification of anticancer organosulfides from Ferula assa-foetida gum: Integrative analysis employing GC/GC-MS/RP-HPLC/NMR. Nat. Prod. Res. 2022, 36, 2869–2874. [Google Scholar] [CrossRef]
- Azani, H.; Tabrizi, M.H.; Neamati, A.; Khadem, F.; Khatamian, N. The Ferula assa-foetida Essential Oil Nanoemulsion (FAEO-NE) as the Selective, Apoptotic, and Anti-Angiogenic Anticancer Compound in Human MCF-7 Breast Cancer Cells and Murine Mammary Tumor Models. Nutr. Cancer 2022, 74, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Boskabadi, S.H.; Balanezhad, S.Z.; Neamati, A.; Tabrizi, M.H. The green-synthesized zinc oxide nanoparticle as a novel natural apoptosis inducer in human breast (MCF7 and MDA-MB231) and colon (HT-29) cancer cells. Inorg. Nano-Metal. Chem. 2020, 51, 733–743. [Google Scholar] [CrossRef]
- Asghari, J.; Atabaki, V.; Baher, E.; Mazaheritehrani, M. Identification of sesquiterpene coumarins of oleo-gum resin of Ferula assa-foetida L. from the Yasuj region. Nat. Prod. Res. 2016, 30, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Earle-Payne, K.; Kidd, M.T. Taurine as a natural antioxidant: From direct antioxidant effects to protective action in various toxicological models. Antioxidants 2021, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Kürbitz, C.; Heise, D.; Redmer, T.; Goumas, F.; Arlt, A.; Lemke, J.; Rimbach, G.; Kalthoff, H.; Trauzold, A. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumour cells. Cancer Sci. 2011, 102, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.A.; Sharma, N.; An, S.S.A. Phyto-carbazole alkaloids from the Rutaceae family as potential protective agents against neurodegenerative diseases. Antioxidants 2022, 11, 493. [Google Scholar] [CrossRef]
- Salehi, A.; Ghanadian, M.; Zolfaghari, B.; Jassbi, A.R.; Fattahian, M.; Reisi, P.; Csupor, D.; Khan, I.A.; Ali, Z. Neuropharmacological Potential of Diterpenoid Alkaloids. Pharmaceuticals 2023, 16, 747. [Google Scholar] [CrossRef]
- Iranshahy, M.; Iranshahi, M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)—A review. J. Ethnopharmacol. 2011, 134, 1–10. [Google Scholar] [CrossRef]
- Iranshahi, M.; Sahebkar, A.; Takasaki, M.; Konoshima, T.; Tokuda, H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur. J. Cancer Prev. 2009, 18, 412–415. [Google Scholar] [CrossRef]
- Abroudi, M.; Fard, A.G.; Dadashizadeh, G.; Gholami, O.; Mahdian, D. Antiproliferative effects of Ferula assafoetida extract on PC12 and MCF7 cancer cells. Int. J. Biomed. Eng. Clin. Sci. 2020, 6, 60–67. [Google Scholar] [CrossRef]
- Bagheri, S.M.; Sahebkar, A.; Gohari, A.R.; Saeidnia, S.; Malmir, M.; Iranshahi, M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm. Biol. 2010, 48, 242–246. [Google Scholar] [CrossRef]
- Valiahdi, S.M.; Iranshahi, M.; Sahebkar, A. Cytotoxic activities of phytochemicals from Ferula species. Daru 2013, 21, 39. [Google Scholar] [CrossRef]
- Soltani, F.; Mosaffa, F.; Iranshahi, M.; Karimi, G.; Malekaneh, M.; Haghighi, F.; Behravan, J. Evaluation of antigenotoxicity effects of umbelliprenin on human peripheral lymphocytes exposed to oxidative stress. Cell Biol. Toxicol. 2009, 25, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.H.; Davari, A.S.; Iranshahi, M.; Sabouri-Rad, S.; Najaran, Z.T. Comparative analysis of the cytotoxic effect of 7-prenyloxycoumarin compounds and herniarin on MCF-7 cell line. Avicenna J. Phytomed. 2015, 5, 520–530. [Google Scholar] [PubMed]
- Saleem, M.; Alam, A.; Sultana, S. Asafoetida inhibits early events of carcinogenesis: A chemopreventive study. Life Sci. 2001, 68, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, G.U.; Dhanalakshmi, S.; Raisuddin, S.; Rao, A.R. Chemomodulatory influence of Ferula asafoetida on mammary epithelial differentiation, hepatic drug-metabolizing enzymes, antioxidant profiles and N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Breast Cancer Res. Treat. 2003, 81, 1–10. [Google Scholar] [CrossRef]
- Bagheri, S.M.; Abdian-Asl, A.; Moghadam, M.T.; Yadegari, M.; Mirjalili, A.; Zare-Mohazabieh, F.; Momeni, H. Antitumor effect of Ferula assa foetida oleo gum resin against breast cancer induced by 4T1 cells in BALB/c mice. J. Ayurveda Integr. Med. 2017, 8, 152–158. [Google Scholar] [CrossRef]
- Efati, Z.; Shahangian, S.S.; Darroudi, M.; Amiri, H.; Hashemy, S.I.; Aghamaali, M.R. Green chemistry synthesized zinc oxide nanoparticles in Lepidium sativum L. seed extract and evaluation of their anticancer activity in human colorectal cancer cells. Ceramics Int. 2023, 49, 32568–32576. [Google Scholar] [CrossRef]
- Govaerts, R.; Lughadha, E.N.; Black, N.; Turner, R.; Paton, A. The World Checklist of Vascular Plants, a continuously updated resource for exploring global plant diversity. Sci. Data 2021, 8, 215. [Google Scholar] [CrossRef]
- Freshney, R.I. Culture of Animal Cell, 6th ed.; Wily-Liss: New York, NY, USA, 2012. [Google Scholar]
- Bliss, C.I. The calculation of microbial assays. Bacteriol. Rev. 1956, 20, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Hao, E.; Tan, D.; Wei, W.; Xie, J.; Feng, X.; Du, Z.; Huang, C.; Bai, G.; Hou, Y.; et al. Apoptosis effect of Aegiceras corniculatum on human colorectal cancer via activation of FoxO signalling pathway. Food Chem. Toxicol. 2019, 134, 110861. [Google Scholar] [CrossRef] [PubMed]
- Yazan, L.S.; Ong, Y.S.; Zaaba, N.E.; Ali, R.M.; Foo, J.B.; Tor, Y.S. Anti-breast cancer properties and toxicity of Dillenia suffruticosa root aqueous extract in BALB/c mice. Asian Pac. J. Trop. Biomed. 2015, 5, 1018–1026. [Google Scholar] [CrossRef]
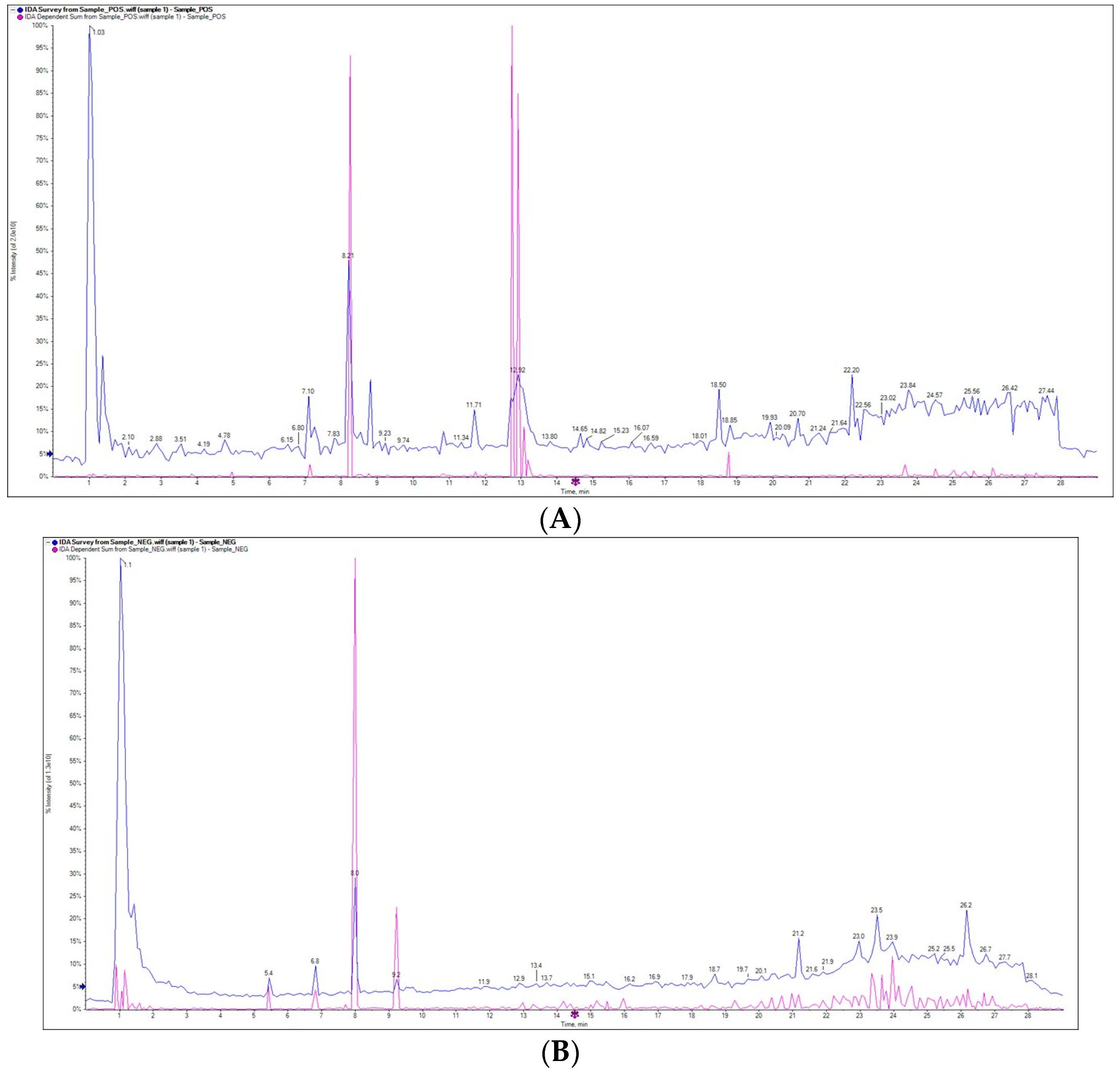


| Metabolite Name | RT (min) | m/z | Type | MS/MS | Level (%) |
|---|---|---|---|---|---|
| Taurine | 1.00 | 126.0 | Positive | 241.1 | 4.47 |
| 5-Aminosalicylic acid | 1.03 | 151.9 | Negative | 31.5 | 0.81 |
| Melamine | 1.07 | 127.1 | Positive | 56.0 | 1.58 |
| N,n-dimethylaniline | 1.13 | 122.2 | Positive | 63.2 | 1.39 |
| 2,4,6-Trimethylbenzoic acid | 1.03 | 163.1 | Negative | 58.4 | 1.10 |
| 1,5-anhydroglucitol | 1.03 | 163.2 | Negative | 58.2 | 0.50 |
| 2-mercaptobenzothiazole | 1.03 | 165.8 | Negative | 55.6 | 0.41 |
| N-acetylglutamate | 1.03 | 187.9 | Negative | 332.8 | 2.67 |
| Kynurenic acid | 1.03 | 188.0 | Negative | 301.2 | 2.28 |
| Isoquinoline | 1.13 | 130.0 | Positive | 61.2 | 0.75 |
| Gluconate | 1.03 | 195.0 | Negative | 81.6 | 1.00 |
| Indole-3-carbinol | 1.13 | 130.1 | Positive | 67.1 | 0.92 |
| Metformin | 1.13 | 130.2 | Positive | 305.2 | 5.92 |
| 5-hydroxymethylcytosine | 1.11 | 140.0 | Negative | 452.0 | 5.88 |
| Threo-b-hydroxyaspartate | 1.11 | 147.8 | Negative | 300.4 | 5.44 |
| Methionine | 1.11 | 148.1 | Negative | 313.6 | 2.57 |
| L-histidine | 1.11 | 154.2 | Negative | 262.6 | 2.53 |
| Shikimate | 1.11 | 155.0 | Negative | 906.1 | 3.54 |
| Menthol(-) | 1.11 | 155.2 | Negative | 655.3 | 2.64 |
| 10-methylacridone | 2.87 | 210.0 | Positive | 1102.8 | 6.03 |
| Indole-3-ethanol | 1.11 | 160.1 | Negative | 452.4 | 3.83 |
| Trichloroacetic acid | 1.11 | 160.8 | Negative | 189.1 | 1.12 |
| Methionine sulfoxide | 1.11 | 164.0 | Negative | 162.3 | 1.02 |
| 2-(4-chlorophenyl)-1-(2,4,6-trihydroxyphenyl) ethenone | 1.18 | 277.0 | Negative | 277.7 | 0.73 |
| 4-aminophenylsulfone | 1.27 | 246.9 | Negative | 26.7 | 0.29 |
| 2-Aminoethylphosphonic acid | 1.43 | 124.1 | Negative | 25.5 | 0.28 |
| S-Adenosyl-L-methionine | 7.10 | 226.9 | Positive | 53.9 | 0.38 |
| Brugierol | 2.46 | 136.9 | Negative | 75.6 | 1.42 |
| Urocanate | 2.46 | 137.0 | Negative | 68.4 | 1.00 |
| Crotetamide | 7.10 | 227.2 | Positive | 22.8 | 0.37 |
| Umbelliferone | 5.44 | 161.2 | Negative | 39.7 | 1.44 |
| (3as,3a1s,10br,13S)-3a-ethyl-5-(methoxycarbonyl)-1,2,3,3a,3a1,4,6,11,12,13-decahydroindolizino [8,1-cd]carbazol-13-ium iodide | 8.28 | 340.2 | Positive | 27.6 | 0.35 |
| N-acetyl-L-cysteine | 5.44 | 162.0 | Negative | 48.3 | 0.46 |
| 6-hydroxyoctadec-4-enoic acid | 8.28 | 340.3 | Positive | 18.3 | 0.40 |
| Sphingomyelin | 8.02 | 713.2 | Negative | 65.6 | 0.12 |
| Verminoside | 8.02 | 713.3 | Negative | 34.3 | 0.10 |
| Quinovin | 8.07 | 677.4 | Negative | 41.9 | 0.27 |
| Ononin | 8.81 | 453.1 | Positive | 11.3 | 0.13 |
| Carnosic acid diacetate methyl ester | 8.81 | 453.2 | Positive | 306.5 | 2.32 |
| (+)-A-tocopherol | 8.81 | 453.4 | Positive | 238.3 | 1.45 |
| 4-(4-Decanyl)benzenesulfonic acid | 13.29 | 297.0 | Negative | 20.4 | 0.24 |
| 2-[4-(Diethylamino)-2-hydroxybenzoyl] benzoic acid | 14.19 | 312.1 | Negative | 26.2 | 0.29 |
| Sinomenine | 15.45 | 328.2 | Negative | 192.4 | 1.18 |
| Armexifolin | 17.39 | 247.2 | Negative | 105.5 | 0.85 |
| Indinavir | 21.48 | 612.2 | Negative | 60.8 | 0.28 |
| Oregonoyl A | 21.48 | 623.2 | Negative | 382.8 | 1.99 |
| N-Butyryl coenzyme A lithium salt hydrate | 22.78 | 836.7 | Negative | 42.3 | 0.34 |
| 3-(5-phenylthiophen-2-yl)prop-2-vinyl Acetate | 22.97 | 255.0 | Negative | 44.4 | 0.35 |
| Palmitic acid | 22.97 | 255.2 | Negative | 15.1 | 0.94 |
| 3-cyanopyridine | 11.70 | 105.0 | Positive | 24.1 | 1.23 |
| 2-methoxy-5-methyl-3-(2-methylbut-3-en-2-yl)chromen-4-one | 22.97 | 257.2 | Negative | 125.5 | 0.46 |
| Estrone | 23.96 | 268.8 | Negative | 162.7 | 0.51 |
| Homatropine | 12.77 | 276.1 | Positive | 322.4 | 0.70 |
| 1-(3,4-Dimethoxycinnamoyl) piperidine | 12.77 | 276.2 | Positive | 317,664.9 | 0.73 |
| Canrenone | 26.17 | 339.2 | Negative | 119.9 | 0.87 |
| Mgmg 18:3 | 27.22 | 559.3 | Negative | 15.5 | 0.14 |
| (2R)-1-[2-(hydroxymethyl)-5,5,8a-trimethyl-1,4,4a,6,7,8-hexahydronaphthalen-1-yl]-2-methylbut-3-en-2-ol | 28.59 | 236.9 | Negative | 22.4 | 0.19 |
| Atropine | 13.07 | 290.2 | Positive | 6.5 | 0.02 |
| N-acetylgalactosamine | 13.19 | 244.1 | Positive | 19.0 | 0.07 |
| 3,3’,4’,7-tetrahydroxyflavylium chloride | 13.27 | 272.0 | Positive | 29.6 | 0.25 |
| (E)-8-(1-(hydroxyimino)ethyl)-4,9-dimethyl-2H-furo[2,3-h]chromen-2-one | 13.27 | 272.2 | Positive | 40.1 | 0.13 |
| Myristoyl ethanolamide | 13.27 | 272.3 | Positive | 403.8 | 2.65 |
| (Z)-2-(3-hydroxy-4-methoxybenzylidene)-9-(quinolin-4-yl)-8,9-dihydro-2H-furo[2,3-f]chromene-3,7-dione | 13.89 | 466.1 | Positive | 568.3 | 4.49 |
| Deltamine | 13.89 | 466.2 | Positive | 474.3 | 5.30 |
| Epicatechin gallate | 14.82 | 460.1 | Positive | 779.1 | 4.54 |
| Apixaban | 14.82 | 460.2 | Positive | 22.1 | 0.15 |
| Hesperidin | 20.09 | 645.1 | Positive | 29.3 | 0.09 |
| Propanoic acid | 21.76 | 959.4 | Positive | 75.6 | 0.91 |
| A-D-Glucopyranoside, β-D-glucopyranosyl | 26.35 | 853.3 | Positive | 30.1 | 0.12 |
| Methoxy-mca-albicidin | 26.44 | 857.2 | Positive | 7.6 | 0.08 |
| Variable | mg/mL | Mean O.D. | SEM | Viability % | Toxicity % | IC50 ± SD |
|---|---|---|---|---|---|---|
| HT-29 | — | 0.779 | 0.004 | 100 | 0 | Mg |
| 0.6 g/mL | 20 | 0.059 | 0.001 | 7.62 | 92.38 | 3.60 ± 0.02 mg/mL |
| 10 | 0.102 | 0.005 | 13.10 | 86.91 | ||
| 5 | 0.272 | 0.006 | 34.92 | 65.08 | ||
| 2.5 | 0.570 | 0.005 | 73.21 | 26.79 | ||
| 1.25 | 0.735 | 0.006 | 94.35 | 5.65 | ||
| 0.625 | 0.766 | 0.007 | 98.33 | 1.67 | ||
| 0.2 g/mL | 60 | 0.066 | 0.006 | 8.47 | 91.53 | 10.50 ± 0.1 mg/mL |
| 30 | 0.09 | 0.005 | 11.55 | 88.45 | ||
| 15 | 0.162 | 0.005 | 20.75 | 79.25 | ||
| 7.5 | 0.681 | 0.004 | 87.46 | 12.54 | ||
| 3.75 | 0.774 | 0.003 | 99.32 | 0.68 | ||
| 1.875 | 0.774 | 0.003 | 99.32 | 0.68 |
| Groups (n = 10) | Tumor Volume (mm3) | Tumor Weight (g) | Tumor Burden (%) |
|---|---|---|---|
| Control | 1325 ± 112 | 1.8 ± 0.4 | 72.6 ± 8.3 |
| 5-FU (20 mg/kg bw) | 399 ± 24 * | 0.7 ± 0.2 * | 9.2 ± 1.9 * |
| Assa-foetida OGR (100 mg/kg bw) | 550 ± 32 * | 1.2 ± 0.3 * | 16.3 ± 3.6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elarabany, N.; Hamad, A.; Alzamel, N.M. Antitumor and Phytochemical Properties of Ferula assa-foetida L. Oleo-Gum–Resin against HT-29 Colorectal Cancer Cells In Vitro and in a Xenograft Mouse Model. Molecules 2023, 28, 8012. https://doi.org/10.3390/molecules28248012
Elarabany N, Hamad A, Alzamel NM. Antitumor and Phytochemical Properties of Ferula assa-foetida L. Oleo-Gum–Resin against HT-29 Colorectal Cancer Cells In Vitro and in a Xenograft Mouse Model. Molecules. 2023; 28(24):8012. https://doi.org/10.3390/molecules28248012
Chicago/Turabian StyleElarabany, Naglaa, Abeer Hamad, and Nurah M. Alzamel. 2023. "Antitumor and Phytochemical Properties of Ferula assa-foetida L. Oleo-Gum–Resin against HT-29 Colorectal Cancer Cells In Vitro and in a Xenograft Mouse Model" Molecules 28, no. 24: 8012. https://doi.org/10.3390/molecules28248012
APA StyleElarabany, N., Hamad, A., & Alzamel, N. M. (2023). Antitumor and Phytochemical Properties of Ferula assa-foetida L. Oleo-Gum–Resin against HT-29 Colorectal Cancer Cells In Vitro and in a Xenograft Mouse Model. Molecules, 28(24), 8012. https://doi.org/10.3390/molecules28248012






