Determination of Some Heavy Metals in European and Polish Coal Samples
Abstract
:1. Introduction
2. Experimental Research Conditions
2.1. Materials
2.2. Solutions and Preparations
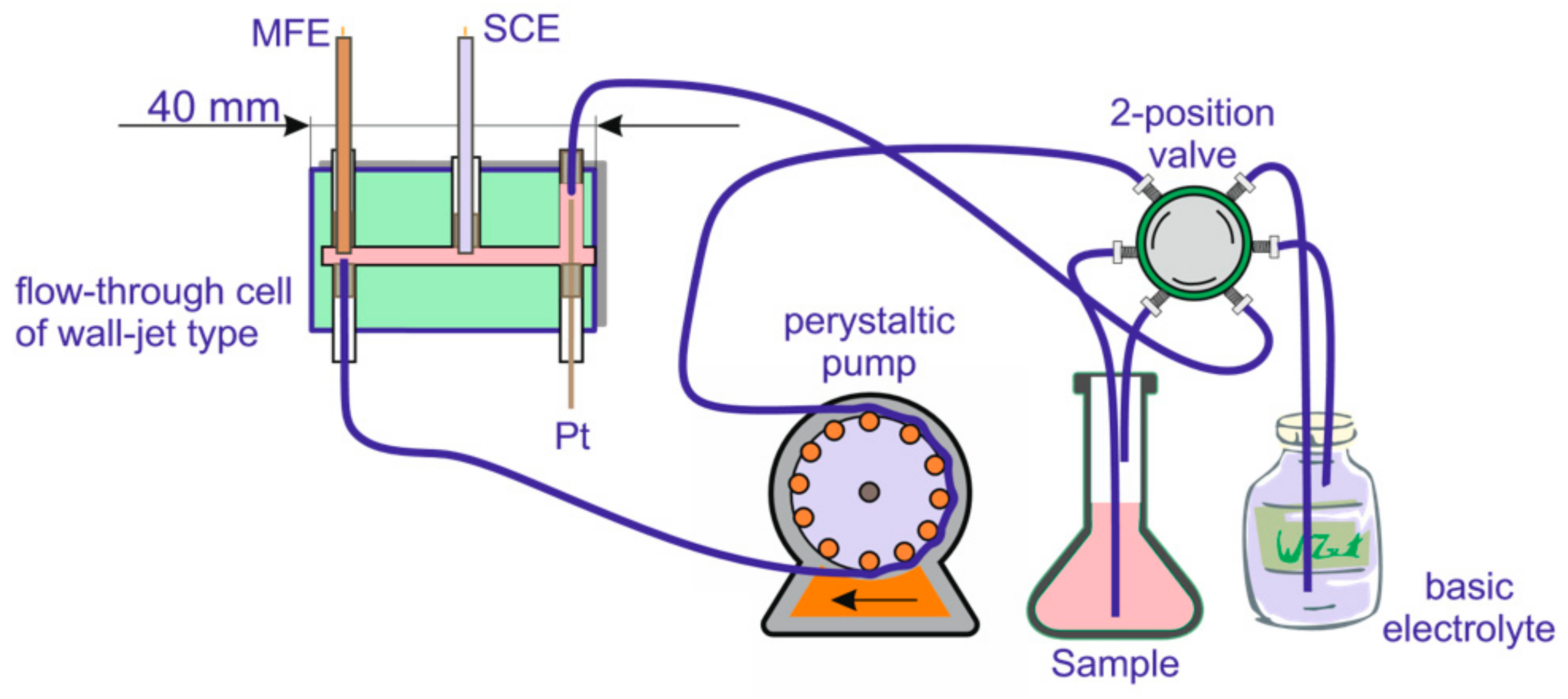
2.3. Instruments
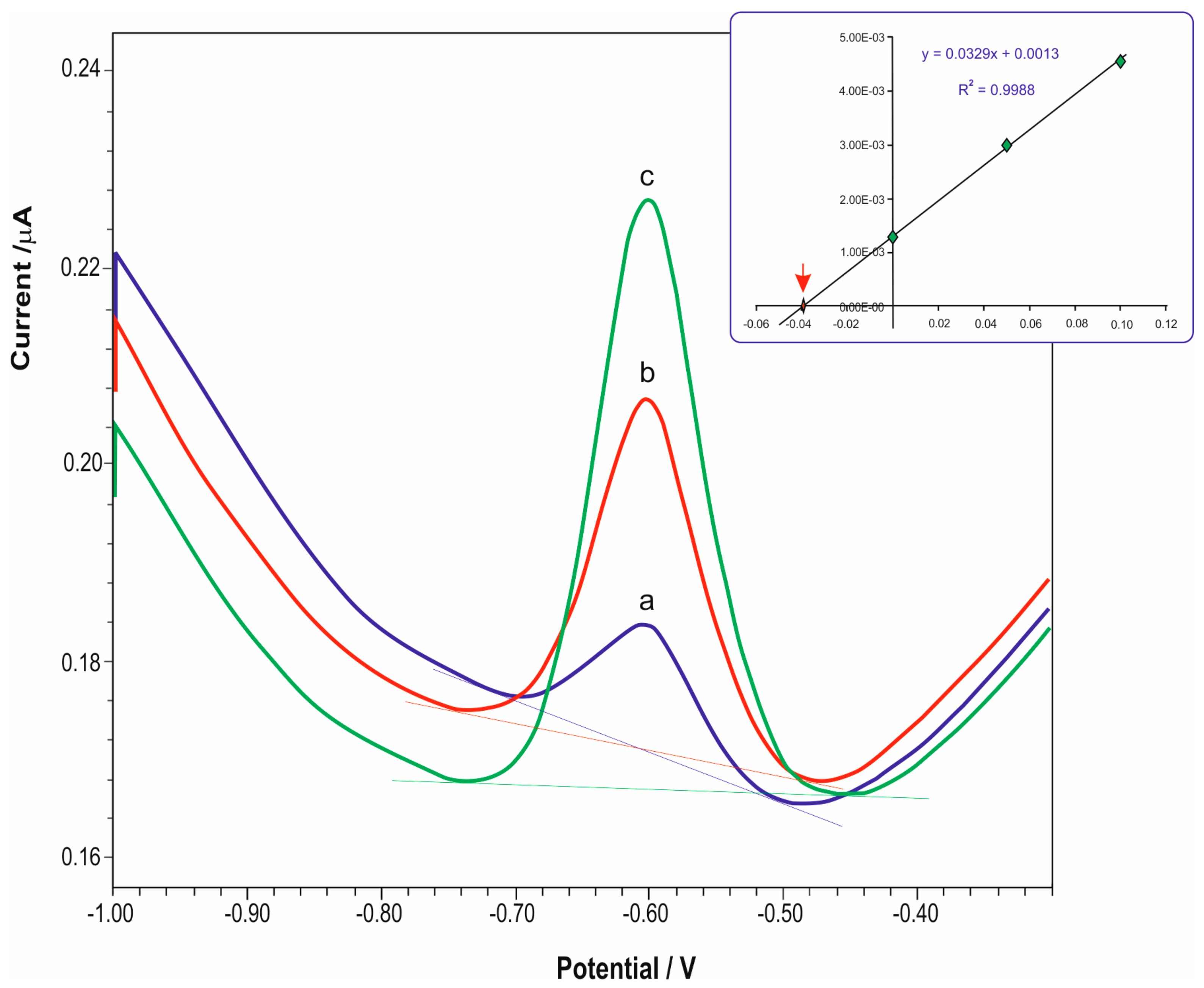
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Xu, H.; Meng, F.; Guo, Q.; He, T.; Yang, Z.; Qu, W.; Li, H. A Molten-Salt Pyrolysis Synthesis Strategy toward Sulfur-Functionalized Carbon for Elemental Mercury Removal from Coal-Combustion Flue Gas. Energies 2022, 15, 1840. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of Thallium in the Environment: Sources of Contaminations, Distribution and Monitoring Methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, R.; Trojanowska, M.; Karbowska, B.; Zembrzuski, W. Speciation and Mobility of Volatile Heavy Metals (Cd, Pb, Tl) in Fly Ashes. Environ. Monit. Assess. 2016, 188, 637. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewski, Z.; Karbowska, B.; Zembrzuski, W.; Siepak, M. Thallium in Fractions of Sediments Formed during the 2004 Tsunami in Thailand. Ecotoxicol. Environ. Saf. 2012, 80, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, L.N.; Kost, L.A.; Gorlov, E.G.; Samuilov, E.V. Thermodynamic Simulation of Transfer of Lead, Cadmium, and Zinc to the Gas Phase during Oxidative and Reductive Thermal Treatment of Coals from Some Coal Deposits of the Russian Federation. Solid Fuel Chem. 2007, 41, 58–63. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Wei, Q.; Wang, P.; Wei, J. Mineralogical and Geochemical Characteristics of Late Permian Coals from the Mahe Mine, Zhaotong Coalfield, Northeastern Yunnan, China. Minerals 2015, 5, 380–396. [Google Scholar] [CrossRef]
- Pan, K.; Wang, W.X. Reconstructing the Biokinetic Processes of Oysters to Counteract the Metal Challenges: Physiological Acclimation. Environ. Sci. Technol. 2012, 46, 10765–10771. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, H.; Zong, S.; Jiang, B.; Guaughe, L.; Ejanavi, O.; Zhu, J. Magnet Bioreporter Device for Ecological Toxicity Assessment on Heavy Metal Contamination of Coal Cinder Sites. Sens. Actuators B Chem. 2016, 222, 290–299. [Google Scholar] [CrossRef]
- Dai, S.; Jiang, Y.; Ward, C.R.; Gu, L.; Seredin, V.; Liu, H.; Zhou, D.; Wang, X.; Sun, J.; Ren, D. Mineralogical and Geochemie Compositions of the Coal in the Guanbanwusu Mine, Inner Mongolia, China, Further evidence for the existence of an Al (Ga and REE) ore deposit in the Jungar Coalfield. Int. J. Coal Geol. 2012, 98, 10–40. [Google Scholar] [CrossRef]
- Spears, D.A.; Borrego, A.G.; Cox, A.; Martinez-Tarazona, R.M. Use of Laser Ablation ICP-MS to Determine Trace Element Distributions in Coals, with Special Reference to V, Ge and Al. Int. J. Coal Geol. 2007, 72, 165–176. [Google Scholar] [CrossRef]
- Taghipour, N.; Marshk, Z.S. Geochemistry and origin of elements of Upper Triassic Olang coal deposits in Northeastern Iran. Geopersia 2015, 5, 189–204. [Google Scholar]
- Cicioglu, E.S.; Karayigit, A.I. Mineral matter, major and trace element content of the Afşin–Elbistan coals, Kahramanmaraş, Turkey. Int. J. Coal Geol. 2015, 144–145, 111–129. [Google Scholar] [CrossRef]
- Duarte, A.T.; Dessuy, M.B.; Goreti, R.; Vale, M.; Weiz, B.; Andrade, J. Sequential Determination of Cd and Cr In Biomass Samples and Their Ashes Using High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry and Direct Solid Sample Analysis. Talanta 2013, 115, 55–60. [Google Scholar] [CrossRef]
- Das, T.B.; Pal, S.K.; Gouricharan, T.; Sharma, K.K.; Choudhury, A. Evaluation of Reduction Potential of Selected Heavy Metals from an Indian Coal by Conventional Coal Cleaning. Int. J. Coal Prep. Util. 2013, 33, 300–312. [Google Scholar] [CrossRef]
- Berndt, H.; Messerschmidt, J.; Alt, F.; Sommer, D. Determination of Thallium in Minerals and Coal by AAS and ICP-AES. Fresenius’ Z. Anal. Chem. 1981, 306, 385–393. [Google Scholar] [CrossRef]
- Paulo, A.; Bojanowska, I.; Piaseczna, A. Thallium in Brown and Hard Coals of Poland. Geochemica et Cosmochemica Acta. In Proceedings of the 17th Annual Goldschmidt Conference, Cologne, Germany, 19–24 August 2007; p. 767. Available online: https://www.academia.edu/18288510 (accessed on 7 December 2023).
- Gawlik, L. Węgiel dla Polskiej Energetyki w Perspektywie 2050 Roku—Analizy Scenariuszowe; Górnicza Izba Przemysłowo-Handlowa, Wydawnictwo Instytutu GSMiE PAN: Katowice, Poland, 2013; p. 299. Available online: http://www.giph.com.pl/giph/attachments/article/278/Wegiel_dla_polskiej_energetyki_2050_GIPH_MINPAN.pdf (accessed on 7 December 2023).

| Sample Number (-) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Tl | ||||||||||
| Cav(mg·kg−1) | 0.284 | 0.268 | 0.636 | 0.591 | 0.558 | 0.531 | 0.328 | 0.297 | 0.055 | 0.053 |
| S (mg·kg−1) | 0.282 | 0.5723 | 0.006 | 0.011 | 0.0377 | 0.0366 | 0.0046 | 0.0044 | 0.0017 | 0.0017 |
| RSD (%) | 9.93 | 21.37 | 0.94 | 1.86 | 6.7 | 6.9 | 1.39 | 1.460 | 3.1 | 3.2 |
| Cd | ||||||||||
| Cav (mg·kg−1) | 1.99 | 2.002 | 1.99 | 2.0 | 1.99 | 2.0 | 1.99 | 2.0 | 1.4 | 1.0 |
| S (mg·kg−1) | 0.0054 | 0.0 | 0.0176 | 0.0 | 0.0 | 0.0 | 0.01 | 0.0 | 0.1 | 0.0 |
| RSD (%) | 0.27 | 0.0 | 0.88 | 0.0 | 0.0 | 0.0 | 5.02 | 0.0 | 7.44 | 0.0 |
| Zn | ||||||||||
| Cav (mg·kg−1) | 7.98 | 8.01 | 6.99 | 6.23 | 24.97 | 26.0 | 4.99 | 5.0 | 6.990 | 6.990 |
| S (mg·kg−1) | 0.0 | 0.024 | 0.01 | 0.2 | 0.043 | 0.017 | 0.017 | 0.0 | 0.020 | 0.010 |
| RSD (%) | 0.0 | 0.29 | 0.14 | 3.21 | 0.17 | 0.06 | 0.34 | 0.0 | 0.280 | 0.140 |
| Cu | ||||||||||
| Cav (mg·kg−1) | 4.98 | 5.0 | 1.99 | 2.0 | 4.99 | 6.0 | 2.99 | 3.02 | - | - |
| S (mg·kg−1) | 0.072 | 0.01 | 0.01 | 0 | 0.017 | 0.0 | 0.01 | 0.026 | - | - |
| RSD (%) | 1.4 | 0.2 | 0.5 | 0 | 0.34 | 0.0 | 0.33 | 0.86 | - | - |
| Fe | ||||||||||
| Cav (mg·kg−1) | 6.9 | 8.01 | 22.0 | 23.0 | 23.8 | 26.0 | 4.98 | 5.0 | - | - |
| S (mg·kg−1) | 0.8 | 0.0 | 1.0 | 0.86 | 0.26 | 1.73 | 0.01 | 0.0 | - | - |
| RSD (%) | 11.59 | 0.0 | 4.5 | 3.7 | 1.09 | 6.65 | 0.2 | 0.0 | - | - |
| Metal | Place of Sample Collection | Concentration (mg·g−1) | Source |
|---|---|---|---|
| Zinc | Kańsk-Achińsk, Russia | 9.0 | Lebedeva L. et al., 2007 [5] |
| Zinc | Kuźnieck, Russia | 30 | Lebedeva L. et al., 2007 [5] |
| Zinc | Yunnan, China | 1.4 | Wang X. et al., 2015 [6] |
| Zinc | Fujian, China | 65–135 | Ke P.; Wen—Xiong W. 2012 [7] |
| Zinc | Bohaibai, China | 25–32 | Ke P.; Wen—Xiong W. 2012 [7] |
| Zinc | Yulin, China | 8.55 | Jia J. et al., 2016 [8] |
| Zinc | Guanbauwushire, Mongolia, China | 7–40 | Dai S. et al., 2012 [9] |
| Zinc | Nottinghamshire, UK, coalfield | 33 | Spears D.A. 2007 et al. [10] |
| Zinc | Alborzagan, Olang, Iran | 62 | Taghipour N.; Marshk K. 2015 [11] |
| Zinc | Razi, Olang, Iran | 193 | Taghipour N.; Marshk K. 2015 [11] |
| Zinc | Melech Aram, Olang, Iran | 84 | Taghipour N.; Marshk K. 2015 [11] |
| Zinc | Elbistein, Turkey | 17.28 | Sutan Cicioglu E.; Karayigit A.I. 2015 [12] |
| Cadmium | Kańsk-Achińsk, Russia | 0.3 | Lebedeva L.; et al., 2007 [5] |
| Cadmium | Kuźnieck, Russia | 0.5 | Lebedeva L.; et al., 2007 [5] |
| Cadmium | Fujian, China | 0.1–1 | Pan, K.; Wang, W.X. 2012 [7] |
| Cadmium | Bohaibai, China | 0.07–0.2 | Pan, K.; Wang, W.X. 2012 [7] |
| Cadmium | Guanbauwushire, Mongolia, China | 0.085–0.2 | Cicioglu, E.S.; Karayigit, A.I. 2015 [12] |
| Cadmium | Yulin, Chiny | 0.05 | Jia J.; et al., 2016 [8] |
| Cadmium | Brazil | 0.01–7.99 | Duarte A.T.; et al., 2013 [13] |
| Cadmium | Indie | 0.4 | Das T.B.; et al., 2013 [14] |
| Cadmium | Elbistein, Turkey | 0.39 | Cicioglu, E.S.; Karayigit, A.I. 2015 [12] |
| Copper | Yunnan, China | 70.4 | Wang X.; et al., 2015 [6] |
| Copper | Fujian, China | 19–97 | Pan, K.; Wang, W.X. 2012 [7] |
| Copper | Bohaibai, China | 25–32 | Pan, K.; Wang, W.X. 2012 [7] |
| Copper | Yulin, China | 6.62 | Jia J.; Li H.; et al., 2016 [8] |
| Copper | Yulin, China | 6.62 | Jia J.; et al., 2016 [8] |
| Copper | Guanbauwushire, Mongolia, China | 10–19 | Dai S.; et al., 2012 [9] |
| Copper | Nottinghamshire, UK, coalfield | 47 | Spears D.A.; et al., 2007 [10] |
| Copper | India | 14.5 | Das T.B.; et al., 2013 [14] |
| Copper | Elbistein, Turkey | 9.97 | Cicioglu, E.S.; Karayigit, A.I. 2015 [12] |
| Thallium | Yunnan, China | 0.47 | Wang X.; et al., 2015 [6] |
| Thallium | Guanbauwushire, Mongolia, China | 0.06–0.2 | Dai S.; et al., 2012 [9] |
| Thallium | Alborzegan, Olang, Iran | 0.8 | Taghipour N.; Marshk K. 2015 [11] |
| Thallium | Razi, Olang, Iran | 1 | Taghipour N.; Marshk K. 2015 [11] |
| Thallium | Melech Aram, Olang, Iran | 1 | Taghipour N.; Marshk K. 2015 [11] |
| Thallium | Elbistein, Turkey | 0.71 | Cicioglu, E.S.; Karayigit, A.I. 2015 [12] |
| Thallium | Germany | 2 | Berndt H.; et al., 1981 [15] |
| Thallium | Belchatow, Polend | 0.2–5.3 | Paulo A.; et al., 2007 [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbowska, B.; Włódarzewska, E.; Zembrzuski, W.; Zembrzuska, J.; Janeba-Bartoszewicz, E.; Bartoszewicz, J.; Selech, J. Determination of Some Heavy Metals in European and Polish Coal Samples. Molecules 2023, 28, 8055. https://doi.org/10.3390/molecules28248055
Karbowska B, Włódarzewska E, Zembrzuski W, Zembrzuska J, Janeba-Bartoszewicz E, Bartoszewicz J, Selech J. Determination of Some Heavy Metals in European and Polish Coal Samples. Molecules. 2023; 28(24):8055. https://doi.org/10.3390/molecules28248055
Chicago/Turabian StyleKarbowska, Bożena, Ewelina Włódarzewska, Włodzimierz Zembrzuski, Joanna Zembrzuska, Edyta Janeba-Bartoszewicz, Jarosław Bartoszewicz, and Jarosław Selech. 2023. "Determination of Some Heavy Metals in European and Polish Coal Samples" Molecules 28, no. 24: 8055. https://doi.org/10.3390/molecules28248055
APA StyleKarbowska, B., Włódarzewska, E., Zembrzuski, W., Zembrzuska, J., Janeba-Bartoszewicz, E., Bartoszewicz, J., & Selech, J. (2023). Determination of Some Heavy Metals in European and Polish Coal Samples. Molecules, 28(24), 8055. https://doi.org/10.3390/molecules28248055








