Bode Phase Angle Signaling of a TB Disease Biomarker
Abstract
:1. Introduction
2. Results and Discussion
The Design and Fabrication of the Aptasensor
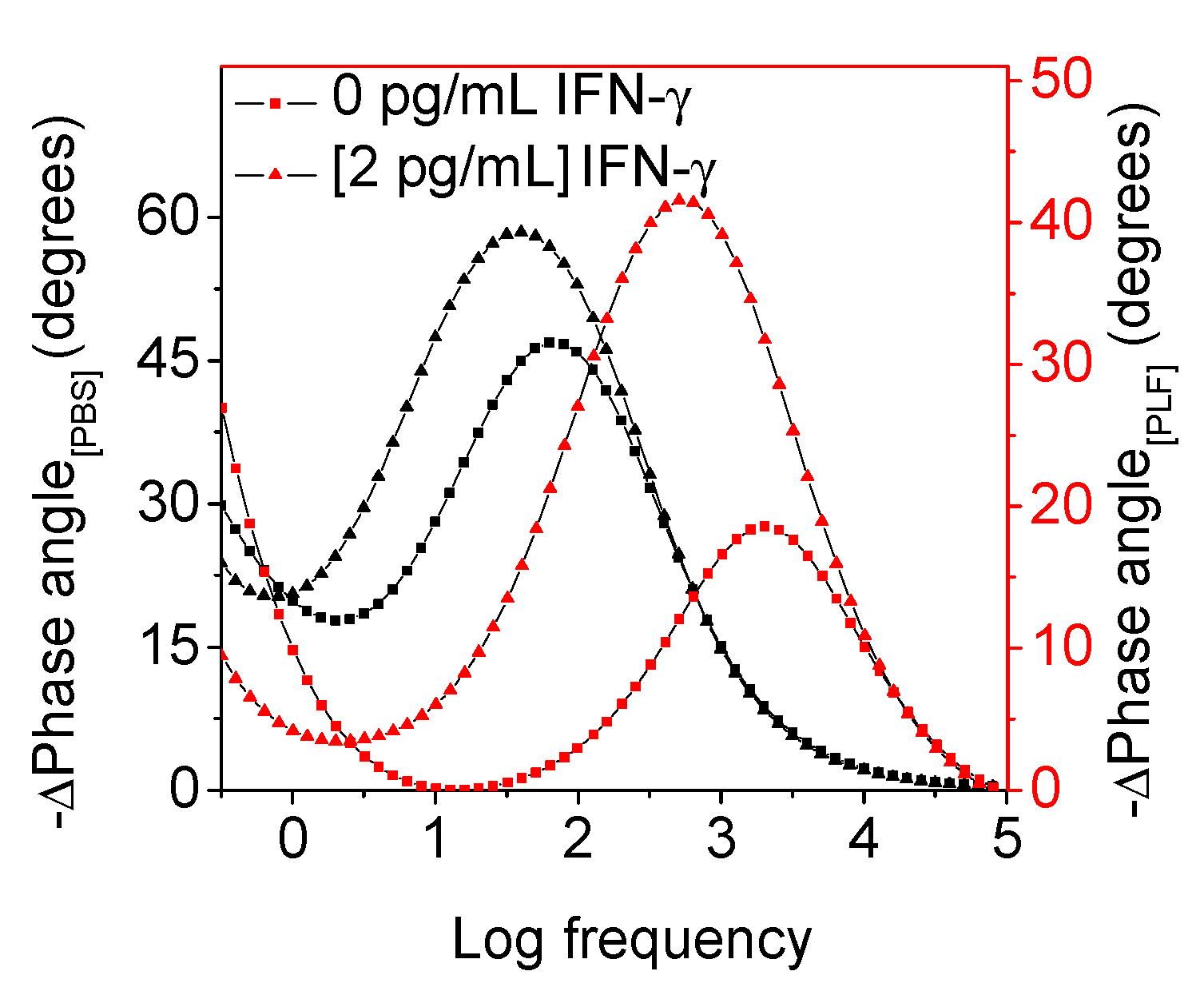
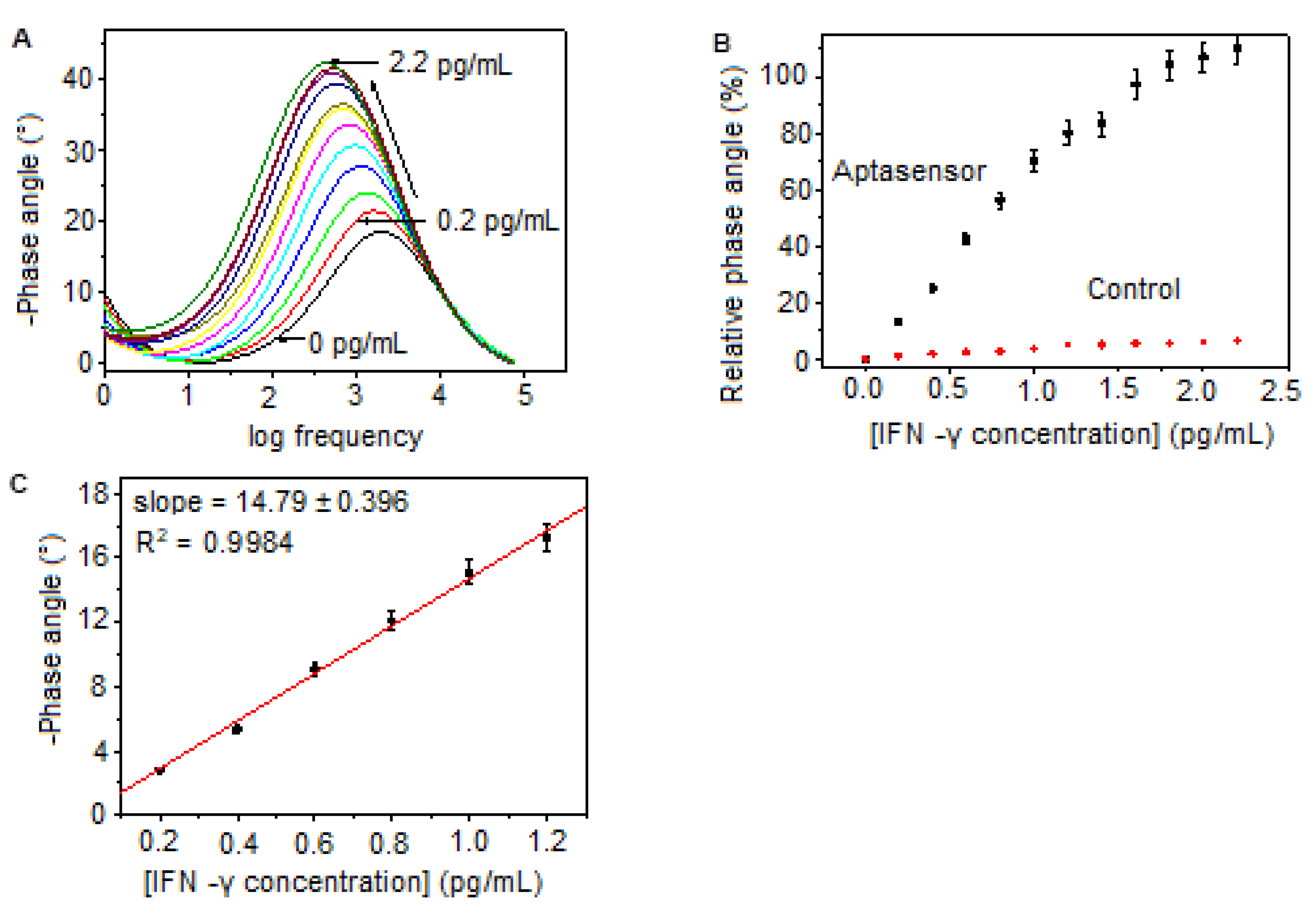
Bode Phase Angle Detection of IFN-γ
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Synthesis of PProDOT
3.4. Assembly of the Aptasensor and Detection of IFN-γ
4. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, W.; Zhang, L.; Liu, J.; Denning, D.W. Tuberculosis/cryptococcosis co-infection in China between 1965 and 2016. Emerg. Microbes Infect. 2017, 6, 73–77. [Google Scholar] [CrossRef]
- Girardi, E.; Schepis, M.S.; Goletti, D.; Bates, M.; Mwaba, P.; Yeboah-Manu, D.; Ntoumi, F.; Palmieri, F.; Maeurer, M.; Zumla, A.; et al. The global dynamics of diabetes and tuberculosis: The impact of migration and policy implications. Int. J. Infect. Dis. 2017, 56, 45–53. [Google Scholar] [CrossRef]
- Fox, G.J.; Dobler, C.C.; Marais, B.J.; Denholm, J.T. Preventive therapy for latent tuberculosis infection—The promise and the challenges. Int. J. Infect. Dis. 2017, 56, 68–76. [Google Scholar] [CrossRef]
- Gilpin, C.; Korobitsyn, A.; Weyer, K. Current tools available for the diagnosis of drug-resistant tuberculosis. Ther. Adv. Infect. Dis. 2016, 3, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.A.; Schaaf, H.S. Drug-resistant tuberculosis and advances in the treatment of childhood tuberculosis. Pneumonia 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; van Rijn, C.J.M.; Jongsma, M.A. Biosensor-based detection of tuberculosis. RCS Adv. 2016, 6, 17759. [Google Scholar] [CrossRef]
- Chang, P.C.; Wang, P.H.; Chen, K.T. Use of the QuantiFERON-TB Gold In-Tube Test in the Diagnosis and Monitoring of Treatment Efficacy in Active Pulmonary Tuberculosis. Int. J. Environ. Res. Public Health 2017, 14, 236. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Wan, S.; Cansiz, S.; Cui, C.; Liu, Y.; Cai, R.; Hong, C.; Teng, I.; Shi, M.; et al. Aptasensor with Expanded Nucleotide Using DNA Nanotetrahedra for Electrochemical Detection of Cancerous Exosomes. ACS Nano 2017, 11, 3943–3949. [Google Scholar] [CrossRef]
- Güner, A.; Çevik, E.; Şenel, M.; Alpsoy, L. An electrochemical immunosensor for sensitive detection of Escherichia coli O157: H7 by using Chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chem. 2017, 229, 358–365. [Google Scholar] [CrossRef]
- Teng, J.; Yuan, F.; Ye, Y.; Zheng, L.; Yao, L.; Chen, W.; Li, B. Aptamer-Based Technologies in Foodborne Pathogen Detection. Front. Microbiol. 2016, 7, 1426. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lee, C.; Kwon, O.S. Conducting Polymer Based Nanobiosensors. Polymers 2016, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Xu, T.; Walter, E.C.; Agrawal, A.; Bohn, C.; Velmurugan, J.; Zhu, W.; Lezec, H.J.; Talin, A. High-contrast and fast electrochromic switching enabled by plasmonics. Nat. Commun. 2016, 7, 10479. [Google Scholar] [CrossRef]
- Otley, M.T.; Alamer, F.A.; Zhu, Y.; Singhaviranon, A.; Zhang, X.; Li, M.; Kumar, A.; Sotzing, G.A. Acrylated Poly(3,4-propylenedioxythiophene) for Enhancement of Lifetime and Optical Properties for Single-Layer Electrochromic Devices. ACS Appl. Mater. Interfaces 2014, 6, 1734–1739. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chen, H.-W.; Lee, K.-M.; Hu, C.-W.; Ho, K.-C. A dye-sensitized photo-supercapacitor based on PProDOT-Et2 thick films. J. Power Sources 2010, 195, 6232–6238. [Google Scholar] [CrossRef]
- Chen, X. Colorimetric sensing of non-ionic and cationic surfactants using a versatile anionic poly(3{,}4-propylenedioxythiophene) derivative. Anal. Methods 2015, 7, 2800–2805. [Google Scholar] [CrossRef]
- Guler, F. Electrochemical synthesis of Poly[3,4-Propylenedioxythiophene-co-N-Phenylsulfonyl Pyrrole]: Morphological, electrochemical and spectroscopic characterization. Express Polym. Lett. 2011, 5, 493–505. [Google Scholar] [CrossRef]
- Barreda-garcía, S.; González-álvarez, M.J. Attomolar quantitation of Mycobacterium tuberculosis by asymmetric helicase-dependent isothermal DNA-amplification and electrochemical detection. Biosens. Bioelectron 2015, 68, 122–128. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Huang, C.-Y.; Chen, C.-A.; Shen, S.-W.; Wang, M.-C.; Cheng, C.-M.; Chen, C.-F. Diagnosis of Tuberculosis Using Colorimetric Gold Nanoparticles on a Paper-Based Analytical Device. ACS Sens. 2017, 2, 1345–1354. [Google Scholar] [CrossRef]
- Adhi, B.; Alom, A.; Khari, M. Graphene-based portable SPR sensor for the detection of Mycobacterium tuberculosis DNA strain. Procedia Eng. 2016, 168, 541–545. [Google Scholar]
- Gupta-wright, A.; Peters, J.A.; Flach, C.; Lawn, S.D. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: A systematic review and meta-analysis. BMC Med. 2016, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, D.; Aroca, M.A.; Osma, J.F. Bioelectrochemical Detection of Mycobacterium tuberculosis ESAT-6 in an Antibody-Based Biomicrosystem. Sensors 2017, 17, 2178. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, H.; Nguyen, D.T.; Lyon, C.J.; Mitchell, C.D.; Zhao, Z. Rapid diagnosis of new and relapse tuberculosis by quantification of a circulating antigen in HIV-infected adults in the Greater Houston metropolitan area. BMC Med. 2017, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ryndak, M.B.; Aggarwal, A.N.; Yadav, R.; Sethi, S.; Laal, S.; Verma, I. Transcriptome analysis of mycobacteria in sputum samples of pulmonary tuberculosis patients. PLoS ONE 2017, 12, 0173508. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Shi, S.; Yan, Y.; Zhang, M. Detection of Interferon-Gamma for Latent Tuberculosis Diagnosis Using an Immunosensor Based on CdS Quantum Dots Coupled to Magnetic Beads as Labels. Int. J. Electrochem. Sci. 2015, 10, 2580–2593. [Google Scholar] [CrossRef]
- Ding, S.; Mosher, C.L.; Lee, X.Y.; Das, S.R.; Cargill, A.A.; Tang, X.; Chen, B.; McLamore, E.S.; Gomes, C.; Hostetter, J.M.; et al. Rapid and Label-Free Detection of Interferon Gamma via an Electrochemical Aptasensor Comprising a Ternary Surface Monolayer on a Gold Interdigitated Electrode Array. ACS Sens. 2017, 2, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.N.; Singh, P.; Chavan, R.M. Gold nanoparticle: Synthesis and characterization. Vet. World 2014, 7, 72–77. [Google Scholar] [CrossRef]
- Sankar, K.V.; Selvan, R.K. The preparation of MnFe2O4 decorated flexible graphene wrapped with PANI and its electrochemical performances for hybrid supercapacitors. RSC Adv. 2014, 4, 17555–17566. [Google Scholar] [CrossRef]
- Bai, L.; Chai, Y.; Yuan, R.; Yuan, Y.; Xie, S.; Jiang, L. Amperometric aptasensor for thrombin detection using enzyme-mediated direct electrochemistry and DNA-based signal amplification strategy. Biosens. Bioelectron. 2013, 50, 325–330. [Google Scholar] [CrossRef]
- Pilehvar, S.; Dierckx, T.; Blust, R.; Breugelmans, T.; Wael, K.D. An Electrochemical Impedimetric Aptasensing Platform for Sensitive and Selective Detection of Small Molecules Such as Chloramphenicol. Sensors 2014, 14, 12059–12069. [Google Scholar] [CrossRef]
- Shahdost-fard, F.; Roushani, M. The use of a signal amplification strategy for the fabrication of a TNT impedimetric nanoaptasensor based on electrodeposited NiONPs immobilized onto a GCE surface. Sens. Act. B Chem. 2017, 246, 848–853. [Google Scholar] [CrossRef]
- Zehani, N.; Dzyadevych, S.V.; Kherrat, R.; Jaffrezic-Renault, N.J. Sensitive impedimetric biosensor for direct detection of diazinon based on lipases. Front. Chem. 2014, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abnous, K.; Danesh, N.M.; Ramezani, M. A triple-helix molecular switch-based electrochemical aptasensor for interferon-gamma using a gold electrode and Methylene Blue as a redox probe. Microchim. Acta 2017, 184, 4151–4157. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Ma, A.; Chen, L.; Liang, H.; Litifu, A.; Xue, F. Fabrication of Electrochemical Immunosensor for Interferon-γ Determination and Its Application of Tuberculosis Diagnosis. Int. J. Electrochem. Sci. 2017, 12, 7262–7271. [Google Scholar] [CrossRef]
- Januarie, K.C.; Oranzie, M.; Feleni, U.; Iwuoha, E. Quantum dot amplified impedimetric aptasensor for interferon-gamma. Electrochim. Acta 2023, 436, 142825. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidwaba, U.; Januarie, K.C.; Mini, S.; Mokwebo, K.V.; Iwuoha, E.; Feleni, U. Bode Phase Angle Signaling of a TB Disease Biomarker. Molecules 2023, 28, 8100. https://doi.org/10.3390/molecules28248100
Sidwaba U, Januarie KC, Mini S, Mokwebo KV, Iwuoha E, Feleni U. Bode Phase Angle Signaling of a TB Disease Biomarker. Molecules. 2023; 28(24):8100. https://doi.org/10.3390/molecules28248100
Chicago/Turabian StyleSidwaba, Unathi, Kaylin Cleo Januarie, Sixolile Mini, Kefilwe Vanessa Mokwebo, Emmanuel Iwuoha, and Usisipho Feleni. 2023. "Bode Phase Angle Signaling of a TB Disease Biomarker" Molecules 28, no. 24: 8100. https://doi.org/10.3390/molecules28248100





