Paeoniflorin Alleviates Cisplatin-Induced Diminished Ovarian Reserve by Restoring the Function of Ovarian Granulosa Cells via Activating FSHR/cAMP/PKA/CREB Signaling Pathway
Abstract
:1. Introduction
2. Results
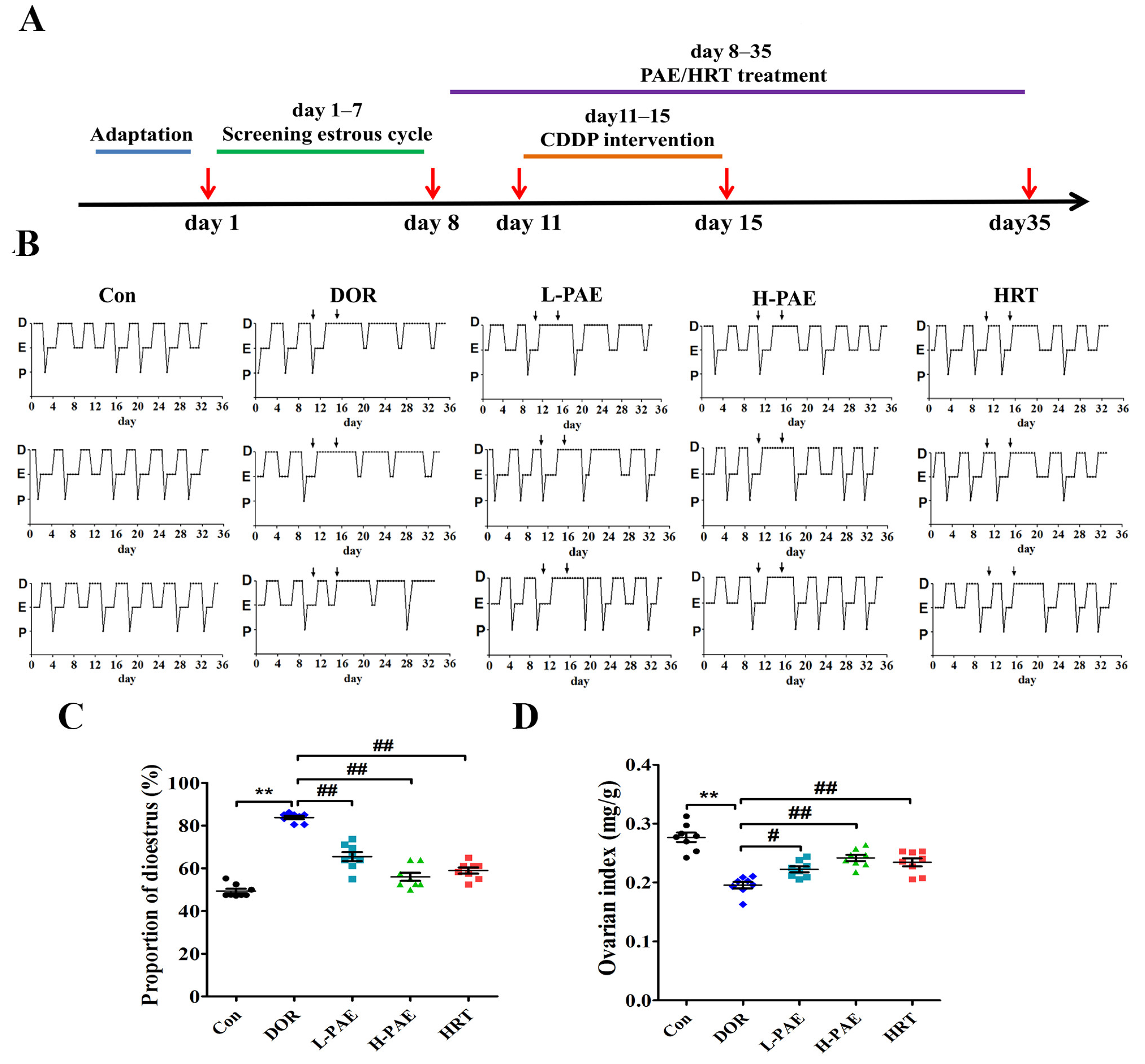
2.1. PAE Supplementation Improved Estrous Cycle and Ovarian Index in DOR Mice
2.2. PAE Supplementation Upregulated the Number of Antral Follicles and Corpora Lutea in DOR Mice to Varying Degrees
2.3. PAE Supplementation Restored the Balance of Serum Hormones in DOR Mice
2.4. PAE Supplementation Upregulated FSHR/cAMP/PKA/CREB Signaling Pathway in the Ovaries of DOR Mice
2.5. PAE Restored the Function of E2 Synthesis in Ovarian Granulosa Cells by Activating FSHR/cAMP/PKA/CREB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Treatment
4.3. Determinations of Estrous Cycle, Body Weight, and Ovarian Index
4.4. Histological Analysis and Follicle Classification
4.5. Serum Hormones and Ovarian cAMP Assay in Mice
4.6. Cell Viability Assay
4.7. Measurement of E2 and cAMP in KGN Cells
4.8. Knockdown of FSHR by siRNA Transfection
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.H.; Liu, Y.R.; Jiang, D.H.; Tang, Z.S.; Qian, D.W.; Song, Z.X. Research on the mechanism of Chinese herbal medicine Radix Paeoniae Rubra in improving chronic pelvic inflammation disease by regulating PTGS2 in the arachidonic acid pathway. Biomed. Pharmacother. 2020, 129, 110052. [Google Scholar] [CrossRef]
- Jia, W.; Wang, X.; Xu, D.; Zhao, A.; Zhang, Y. Common traditional Chinese medicinal herbs for dysmenorrhea. Phytother. Res. 2006, 20, 819–824. [Google Scholar] [CrossRef]
- Gao, D.; Bernie, B. The Encyclopedia of Chinese Medicine; Carlton: London, UK, 1997. [Google Scholar]
- Park, M.J.; Han, S.E.; Kim, H.J.; Heo, J.D.; Choi, H.J.; Ha, K.T.; Yang, S.W.; Lee, K.S.; Kim, S.C.; Kim, C.W.; et al. Paeonia lactiflora improves ovarian function and oocyte quality in aged female mice. Anim. Reprod. 2020, 17, e20200013. [Google Scholar] [CrossRef]
- Li, X.; Shi, F.; Zhang, R.; Sun, C.; Gong, C.; Jian, L.; Ding, L. Pharmacokinetics, Safety, and Tolerability of Amygdalin and Paeoniflorin After Single and Multiple Intravenous Infusions of Huoxue-Tongluo Lyophilized Powder for Injection in Healthy Chinese Volunteers. Clin. Ther. 2016, 38, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Feng, S.T.; Wang, Y.T.; Chen, N.H.; Wang, Z.Z.; Zhang, Y. Paeoniflorin: A neuroprotective monoterpenoid gly coside with promising anti-depressive properties. Phytomedicine 2021, 90, 153669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, X.; Kuang, G.; Jiang, R.; Guo, X.; Wu, S. Paeoniflorin modulates oxidative stress, inflammation and hepatic stellate cells activation to alleviate CCl4-induced hepatic fibrosis by upregulation of heme oxygenase-1 in mice. J. Pharm. Pharmacol. 2021, 73, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Bayram, P.; Karamese, S.A.; Erol, H.S.; Ozdemir, B.; Toktay, E.; Salum, C. Protective effects of a natural product, paeoniflorin, on ischemia reperfusion injury on rat ovary tissue: Histopathological, immunohistochemical, and biochemical study. J. Histotechnol. 2023, 46, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, Y.; Wang, X.; Zhu, M. Paeoniflorin attenuates DHEA-induced polycystic ovary syndrome via inactivation of TGF-β1/Smads signaling pathway in vivo. Aging 2021, 13, 7084–7095. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Choi, H.J.; Kim, B.S.; Chung, T.W.; Kim, K.J.; Joo, J.K. Paeoniflorin enhances endometrial receptivity through leukemia inhibitory factor. Biomolecules 2021, 11, 439. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, X. Advancement in the treatment of diminished ovarian reserve by traditional Chinese and Western medicine. Exp. Ther. Med. 2016, 11, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Jaswa, E.G.; McCulloch, C.E.; Simbulan, R.; Cedars, M.I.; Rosen, M.P. Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: Evidence for concomitant reduction in oocyte quality with quantity. Fertil. Steril. 2021, 115, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Devine, K.; Mumford, S.L.; Wu, M.; DeCherney, A.H.; Hill, M.J.; Propst, A. Diminished ovarian reserve in the United States assisted reproductive technology population: Diagnostic trends among 181,536 cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil. Steril. 2015, 104, 612–619.e3. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.D.; Patounakis, G.; Segars, J.H. Genetic associations with diminished ovarian reserve: A systematic review of the literature. J. Assist. Reprod. Genet. 2014, 31, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Y. Obstetrics and Gynaecology, 2nd ed.; People’s Medical Publishing House: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Farquhar, C.; Marjoribanks, J.; Lethaby, A.; Suckling, J.A.; Lamberts, Q. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2009, 15, CD004143. [Google Scholar]
- Nakagawa, K.; Kaneyama, M.; Nishi, Y.; Sugiyama, R.; Motoyama, H.; Sugiyama, R. Clomiphene citrate affects the receptivity of the uterine endometrium. Reprod. Med. Biol. 2014, 14, 73–78. [Google Scholar] [CrossRef]
- Annalisa, R.; Dominic, S.; Nikolaos, P.P. Editorial: Diminished Ovarian Reserve and Poor Ovarian Response: Diagnostic and Therapeutic Management. Front. Physiol. 2022, 13, 827678. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fan, Q.; Zhu, Q.; He, R.; Li, Y.; Liu, C. Decreased fatty acids induced granulosa cell apoptosis in patients with diminished ovarian reserve. J. Assist. Reprod. Genet. 2022, 39, 1105–1114. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Mu, H.; Mei, Q.; Liu, Y.; Min, Z. Mir-484 contributes to diminished ovarian reserve by regulating granulosa cell function via YAP1-mediated mitochondrial function and apoptosis. Int. J. Biol. Sci. 2022, 18, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Bulgurcuoglu-Kuran, S.; Altun, A.; Karamustafaoglu-Balci, B.; Keskin, I.; Hocaoglu, M. Expression of pro-apoptotic and anti-apoptotic proteins in granulosa cells of women with diminished ovarian reserve. J. Assist. Reprod. Genet. 2022, 39, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Kubo, N.; Cayo-Colca, I.S.; Miyano, T. Effect of estradiol-17β during in vitro growth culture on the growth, maturation, cu mulus expansion and development of porcine oocytes from early antral follicles. Anim. Sci. J. 2015, 86, 251–259. [Google Scholar] [CrossRef]
- Duan, J.; Chen, H.; Xu, D.; Li, Y.; Li, X.; Cheng, J. 17β-estradiol improves the developmental ability, inhibits reactive oxygen species levels and apoptosis of porcine oocytes by regulating autophagy events. J. Steroid. Biochem. Mol. Biol. 2021, 209, 105826. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G.; Whitelaw, P.F.; Smyth, C.D. Follicular oestrogen synthesis: The ‘two-cell, two-gonadotrophin’ model revisited. Mol. Cell. Endocrinol. 1994, 100, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Palermo, R. Differential actions of FSH and LH during folliculogenesis. Reprod. Biomed. Online 2007, 15, 326–337. [Google Scholar] [CrossRef]
- Dierich, A.; Sairam, M.R.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; LeMeur, M. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar] [CrossRef]
- Danilovich, N.; Javeshghani, D.; Xing, W.; Sairam, M.R. Endocrine alterations and signaling changes associated with declining ovarian function and advanced biological aging in follicle-stimulating hormone receptor haploinsufficient mice. Biol. Reprod. 2002, 67, 370–378. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.M.; Mohamed, A.S.M.; Abdelazem, O.; Okasha, A.M.M.; Kamel, M.Y. Cilostazol protects against cyclophos phamide-induced ovarian toxicity in female rats: Role of cAMP and HO-1. Toxicol. Mech. Methods 2020, 30, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Lispi, M.; Longobardi, S.; Mattei, M.; Di-Rella, F.; Salustri, A. LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell Death. Differ. 2017, 24, 72–82. [Google Scholar] [CrossRef]
- Pellatt, L.; Rice, S.; Dilaver, N.; Heshri, A.; Galea, R.; Brincat, M. Anti-Müllerian hormone reduces follicle sensitivity to foll cle-stimulating hormone in human granulosa cells. Fertil. Steril. 2011, 96, 1246–1251.e1. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Yoon, J.A.; Park, M. Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice. Stem. Cell Res. Ther. 2020, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Biyik, I.; Ozatik, F.Y.; Albayrak, M.; Biyik, I.; Ozatik, F.Y.; Albayrak, M.; Ozatik, O.; Teksen, Y.; Ari, N.S.; Soysal, C. The effects of recombinant klotho in cisplatin-induced ovarian failure in mice. J. Obstet. Gynaecol. Res. 2021, 47, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Tano, M.; Minegishi, T.; Nakamura, K.; Karino, S.; Ibuki, Y.; Miyamoto, K. Transcriptional and post-transcriptional regulation of FSH receptor in rat granulosa cells by cyclic AMP and activin. J. Endocrinol. 1997, 153, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, H.C. Amplification of FSH signalling by CFTR and nuclear soluble adenylyl cyclase in the ovary. Clin. Exp. Pharmacol. Physiol. 2017, 44, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Z.; Fan, Q.; Li, H.; Zhao, L.; Liu, C. Cholesterol metabolism is decreased in patients with diminished ovarian reserve. Reprod. Biomed. Online 2022, 44, 185–192. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; Liu, Z.; Weng, Z.; Nguyen, T.N.; Feng, J. Zihuai recipe alleviates cyclophosphamide-induced diminished ovarian reserve via suppressing PI3K/AKT-mediated apoptosis. J. Ethnopharmacol. 2021, 277, 113789. [Google Scholar] [CrossRef]
- Huang, C.; Song, K.; Ma, W.; Ding, J.; Chen, Z.; Zhang, M. Immunomodulatory mechanism of Bushen Huoxue Recipe alleviates cyclophosphamide-induced diminished ovarian reserve in mouse model. J. Ethnopharmacol. 2017, 208, 44–56. [Google Scholar] [CrossRef]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968, 17, 555–557. [Google Scholar] [CrossRef]
- Nishi, Y.; Yanase, T.; Mu, Y.; Oba, K.; Ichino, I.; Saito, M.; Nomura, M.; Mukasa, C.; Okabe, T.; Goto, K.; et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001, 142, 437–445. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Chen, M.; Li, Y.; Zhao, X.; Fan, C.; Dai, Y. Paeoniflorin Alleviates Cisplatin-Induced Diminished Ovarian Reserve by Restoring the Function of Ovarian Granulosa Cells via Activating FSHR/cAMP/PKA/CREB Signaling Pathway. Molecules 2023, 28, 8123. https://doi.org/10.3390/molecules28248123
Wu Q, Chen M, Li Y, Zhao X, Fan C, Dai Y. Paeoniflorin Alleviates Cisplatin-Induced Diminished Ovarian Reserve by Restoring the Function of Ovarian Granulosa Cells via Activating FSHR/cAMP/PKA/CREB Signaling Pathway. Molecules. 2023; 28(24):8123. https://doi.org/10.3390/molecules28248123
Chicago/Turabian StyleWu, Qingchang, Miao Chen, Yao Li, Xiangyun Zhao, Cailian Fan, and Yi Dai. 2023. "Paeoniflorin Alleviates Cisplatin-Induced Diminished Ovarian Reserve by Restoring the Function of Ovarian Granulosa Cells via Activating FSHR/cAMP/PKA/CREB Signaling Pathway" Molecules 28, no. 24: 8123. https://doi.org/10.3390/molecules28248123
APA StyleWu, Q., Chen, M., Li, Y., Zhao, X., Fan, C., & Dai, Y. (2023). Paeoniflorin Alleviates Cisplatin-Induced Diminished Ovarian Reserve by Restoring the Function of Ovarian Granulosa Cells via Activating FSHR/cAMP/PKA/CREB Signaling Pathway. Molecules, 28(24), 8123. https://doi.org/10.3390/molecules28248123




