Upgrading Pyrolytic Residue from End-of-Life Tires to Efficient Heterogeneous Catalysts for the Conversion of Glycerol to Acetins
Abstract
:1. Introduction
2. Results and Discussion
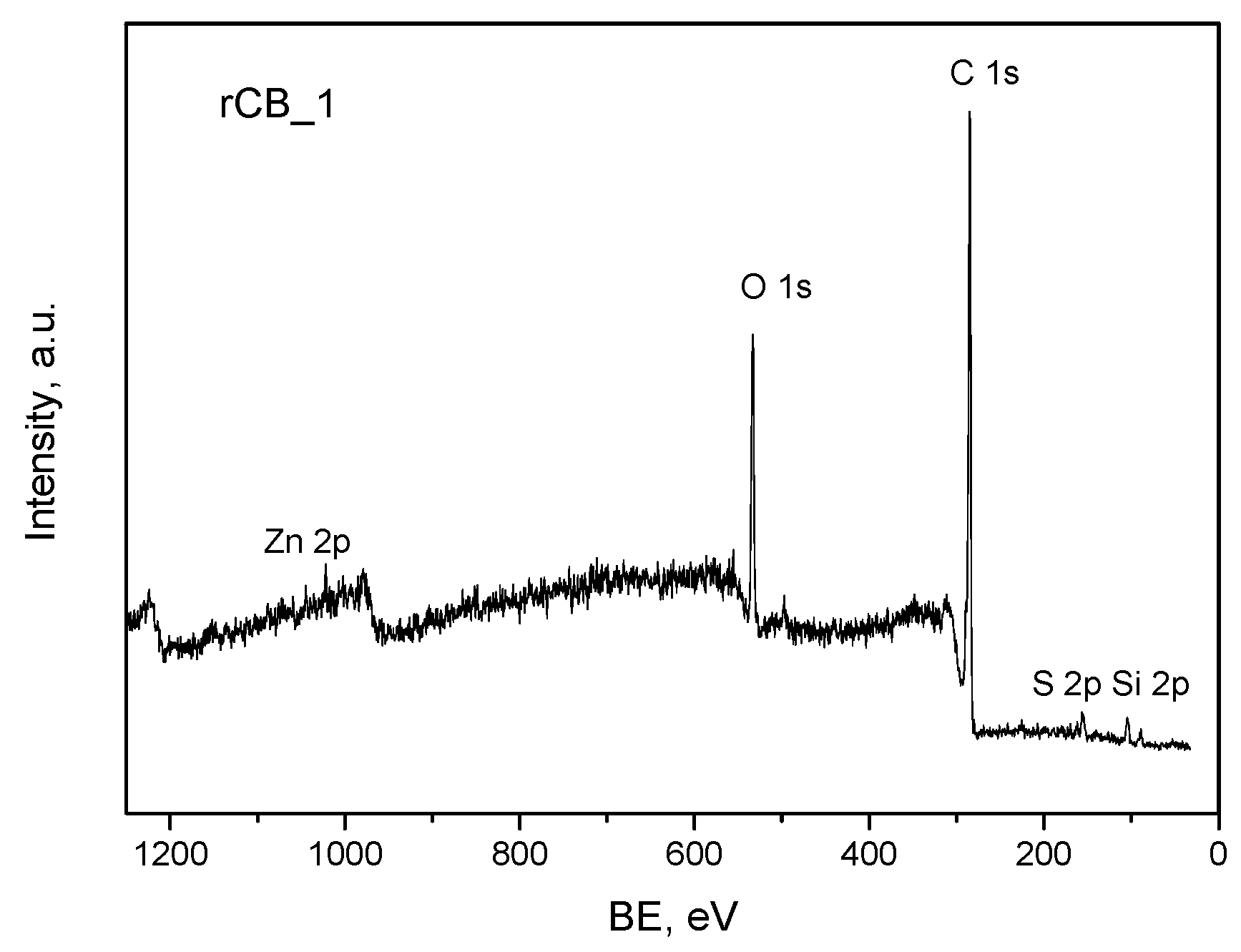
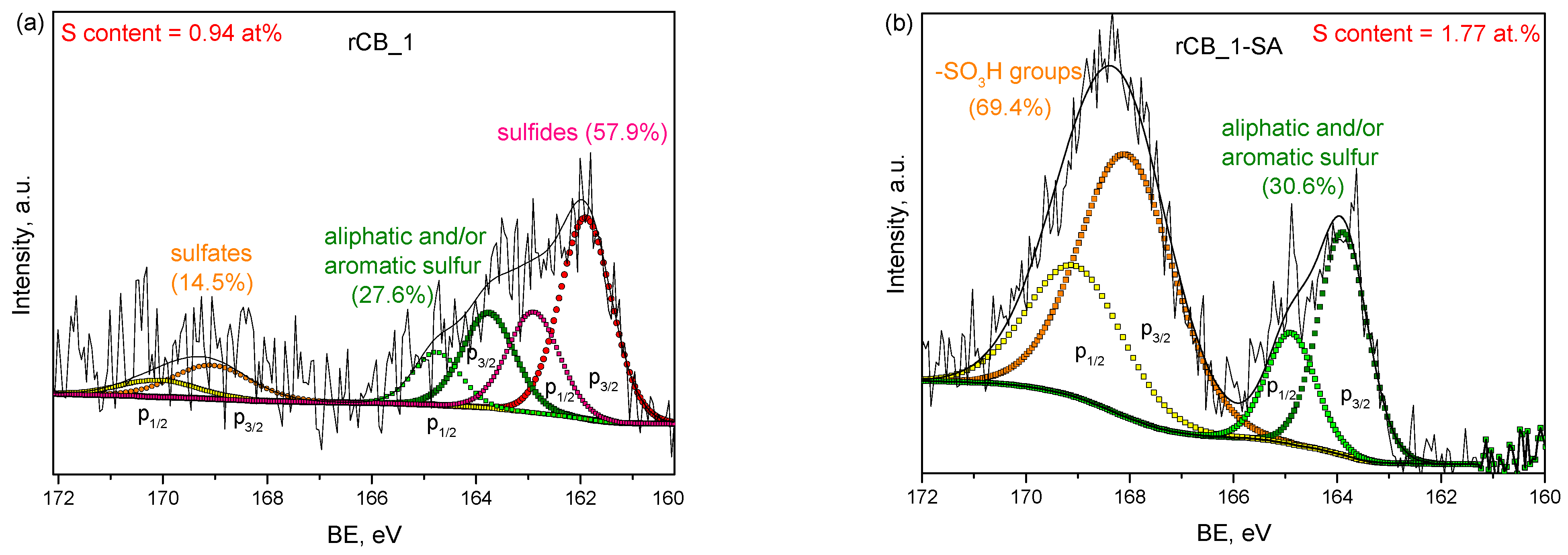
2.1. Characterization of the Samples
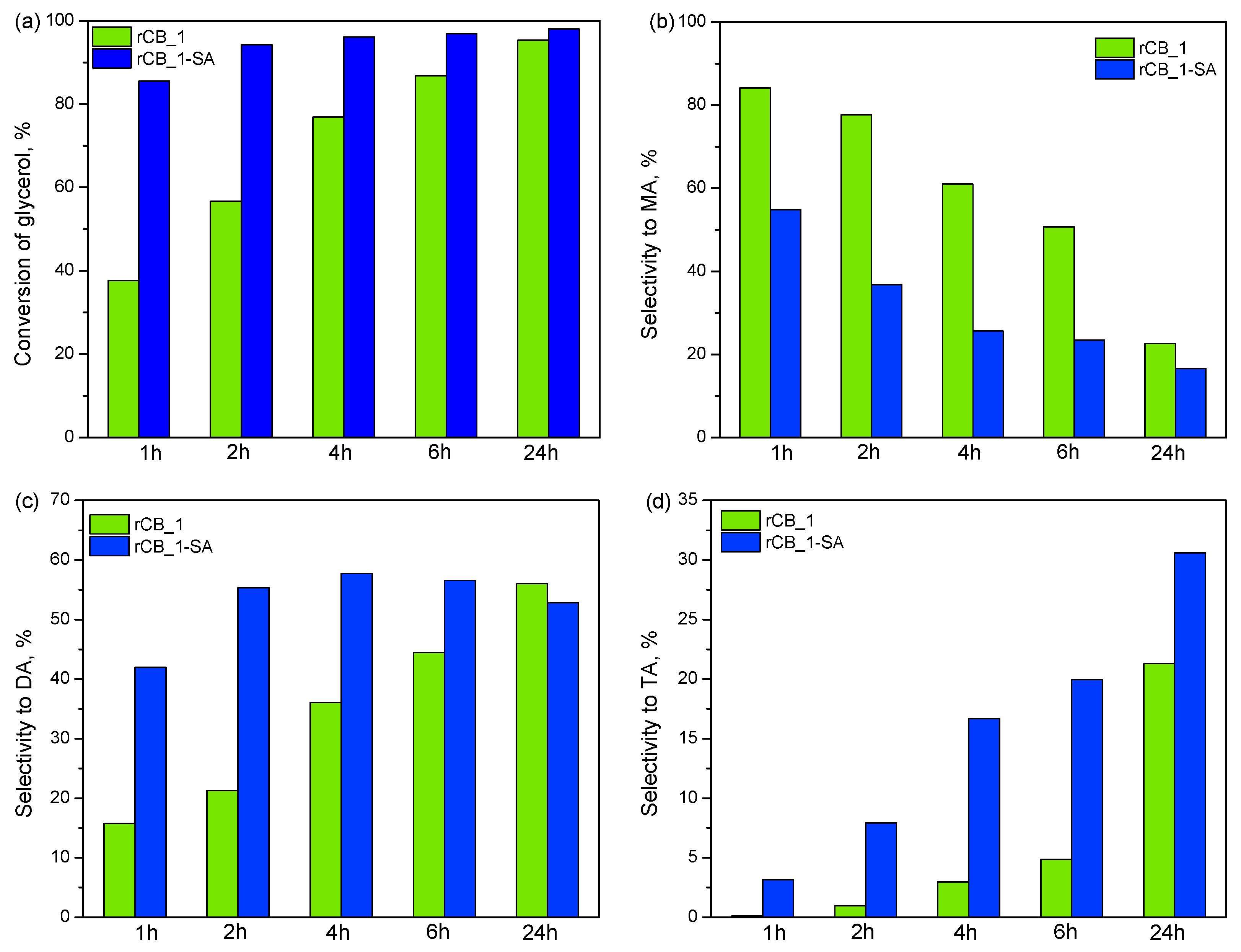
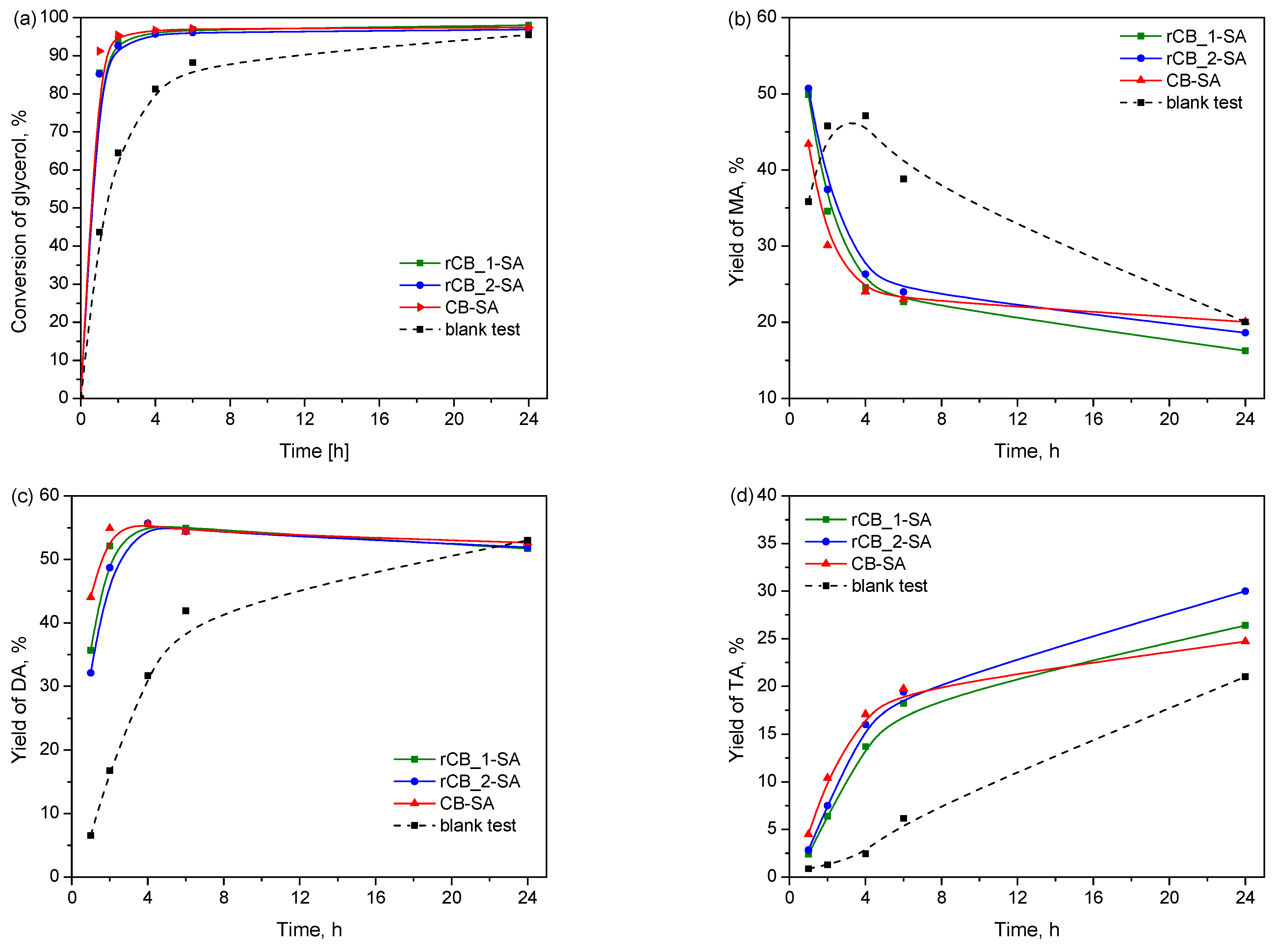
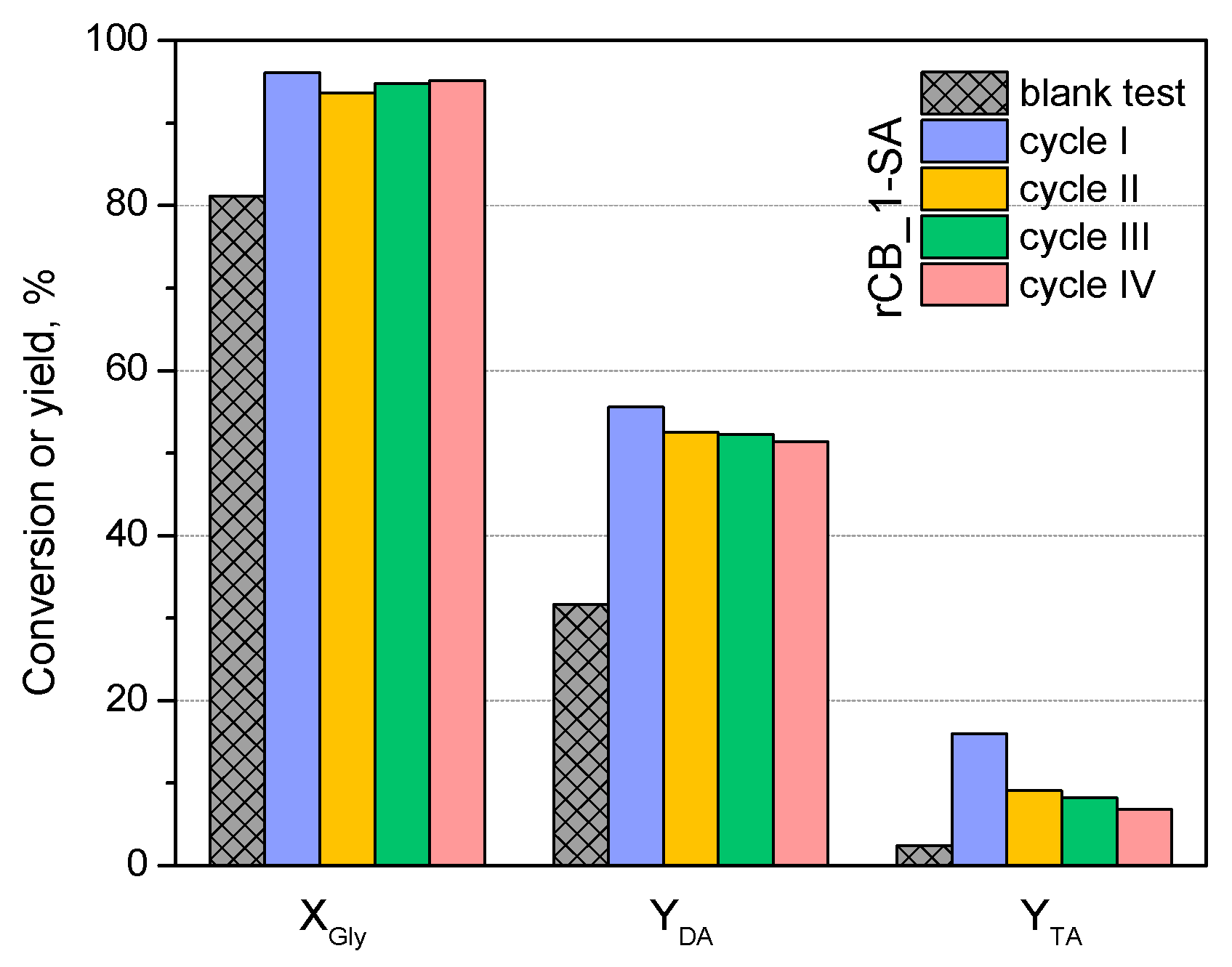
2.2. Catalytic Results
3. Materials and Methods
3.1. Recovered Carbon Black Preparation
3.2. Functionalization of the Samples
3.3. Characterization of the Samples
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhi, M.; Yang, F.; Meng, F.; Li, M.; Manivannan, A.; Wu, N. Effects of Pore Structure on Performance of An Activated-Carbon Supercapacitor Electrode Recycled from Scrap Waste Tires. ACS Sustain. Chem. Eng. 2014, 2, 1592–1598. [Google Scholar] [CrossRef]
- van Beukering, P.J.H.; Janssen, M.A. Trade and recycling of used tyres in Western and Eastern Europe. Resour. Conserv. Recycl. 2001, 33, 235–265. [Google Scholar] [CrossRef]
- Laanemäe, J.; Jäger, R.; Teppor, P.; Volobujeva, O.; Lust, E. Waste Tire Derived Carbon Support for Non-Platinum-Group Metal Catalyst Materials for Oxygen Reduction Reaction in Alkaline Medium. ECS Trans. 2023, 111, 63–72. [Google Scholar] [CrossRef]
- Irfan, M.; Li, A.; Zhang, L.; Liu, J.; Javaid, T.; Farooqi, A.; Javid, M.; Rauf, A. Waste tire derived char supported Ni-Fe catalyst for catalytic thermochemical conversion of wet municipal solid waste. Int. J. Energy Res. 2022, 46, 3634–3646. [Google Scholar] [CrossRef]
- Ayoob, A.K.; Fadhil, A.B. Valorization of waste tires in the synthesis of an effective carbon based catalyst for biodiesel production from a mixture of non-edible oils. Fuel 2020, 264, 116754. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, X.; Liu, S. Hydrogen production by catalytic dehydrogenation of methylcyclohexane over Pt catalysts supported on pyrolytic waste tire char. Int. J. Hydrogen Energy 2011, 36, 8902–8907. [Google Scholar] [CrossRef]
- Laanemäe, J.; Jäger, R.; Teppor, P.; Volobujeva, O.; Lust, E. Oxygen Reduction Reaction on Waste Tire Derived Carbon Material and Synthesized Non-Platinum Group Metal Catalysts in Alkaline Solution. ECS Trans. 2022, 108, 39–47. [Google Scholar] [CrossRef]
- Maafa, I.M. Biodiesel Synthesis from High Free-Fatty-Acid Chicken Fat using a Scrap-Tire Derived Solid Acid Catalyst and KOH. Polymers 2022, 14, 643. [Google Scholar] [CrossRef]
- Arumugamurthi, S.S.; Sivanandi, P.; Kandasamy, S. Biodiesel production from non-edible crops using waste tyre heterogeneous acid catalyst. Energy Sources A Recovery Util. Environ. Eff. 2022, 44, 3223–3238. [Google Scholar] [CrossRef]
- Chaichana, E.; Wiwatthanodom, W.; Jongsomjit, B. Carbon-Based Catalyst from Pyrolysis of Waste Tire for Catalytic Ethanol Dehydration to Ethylene and Diethyl Ether. Int. J. Chem. Eng. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Rechnia-Gorący, P.; Malaika, A.; Kozłowski, M. Acidic activated carbons as catalysts of biodiesel formation. Diam. Relat. Mater. 2018, 87, 124–133. [Google Scholar] [CrossRef]
- Zong, M.-H.; Duan, Z.-Q.; Lou, W.-Y.; Smith, T.J.; Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 2007, 9, 434–437. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energ. Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Zhao, X.; Feng, P. Sulfonated ordered mesoporous carbon for catalytic preparation of biodiesel. Carbon 2008, 46, 1664–1669. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Goodwin, J.G.; Prasertdham, P. A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Aziz, A.M.; Al-Tamer, M.H. Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energy Conver. Manag. 2016, 108, 255–265. [Google Scholar] [CrossRef]
- Pan, H.; Sun, J.; Liu, J.; Zhang, Y.; Zhou, S. Preparation of sulfonated carbon derived from orange peel and its application in esterification. Chem. Phys. Lett. 2021, 770, 138395. [Google Scholar] [CrossRef]
- Zhong, R.; Sels, B.F. Sulfonated mesoporous carbon and silica-carbon nanocomposites for biomass conversion. Appl. Catal. B Environ. 2018, 236, 518–545. [Google Scholar] [CrossRef]
- Weerasai, K.; Champreda, V.; Sakdaronnarong, C.; Shotipruk, A.; Laosiripojana, N. Hydrolysis of eucalyptus wood chips under hot compressed water in the presence of sulfonated carbon-based catalysts. Food Bioprod. Process. 2018, 110, 136–144. [Google Scholar] [CrossRef]
- Onda, A.; Ochi, T.; Yanagisawa, K. Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem. 2008, 10, 1033–1037. [Google Scholar] [CrossRef]
- Janaun, J.; Ellis, N. Glycerol etherification by tert-butanol catalyzed by sulfonated carbon catalyst. J. Appl. Sci. 2010, 10, 2633–2637. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zhao, Y.; Shen, J. Preparation of a novel sulfonated carbon catalyst for the etherification of isopentene with methanol to produce tert-amyl methyl ether. Catal. Commun. 2010, 11, 824–828. [Google Scholar] [CrossRef]
- Hood, Z.D.; Adhikari, S.P.; Li, Y.; Naskar, A.K.; Figueroa-Cosme, L.; Xia, Y.; Chi, M.; Wright, M.W.; Lachgar, A.M.; Paranthaman, M.P. Novel Acid Catalysts from Waste-Tire-Derived Carbon: Application in Waste–to-Biofuel Conversion. Chemistry Select. 2017, 2, 4975–4982. [Google Scholar] [CrossRef]
- Hood, Z.D.; Cheng, Y.; Evans, S.F.; Adhikari, S.P.; Paranthaman, M.P. Unraveling the structural properties and dynamics of sulfonated solid acid carbon catalysts with neutron vibrational spectroscopy. Catal. Today 2020, 358, 387–393. [Google Scholar] [CrossRef]
- Malaika, A.; Ptaszyńska, K.; Kozłowski, M. Conversion of renewable feedstock to bio-carbons dedicated for the production of green fuel additives from glycerol. Fuel 2021, 288, 119609. [Google Scholar] [CrossRef]
- Bounoukta, C.E.; Megías-Sayago, C.; Ivanova, S.; Penkova, A.; Ammari, F.; Centeno, M.A.; Odriozola, J.A. Effect of the sulphonating agent on the catalytic behavior of activated carbons in the dehydration reaction of fructose in DMSO. Appl. Catal. A 2021, 617, 118108. [Google Scholar] [CrossRef]
- Ptaszyńska, K.; Malaika, A.; Kapska, M.; Kozłowski, M. SO3 H-functionalized carbon fibers for the catalytic transformation of glycerol to glycerol tert-butyl ethers. Sci. Rep. 2023, 13, 565. [Google Scholar] [CrossRef]
- Hosseini, M.-S.; Masteri-Farahani, M.; Ghahremani, M.; Forouzeshfar, N. New approach for sulfonation of carbonaceous materials: Highly efficient solid acid catalysts for benzaldehyde acetalization with ethylene glycol. J. Phys. Chem. Solids 2021, 150, 109846. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO: Rome, Italy; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Kowalska-Kuś, J.; Held, A.; Nowińska, K. A continuous-flow process for the acetalization of crude glycerol with acetone on zeolite catalysts. Chem. Eng. J. 2020, 401, 126143. [Google Scholar] [CrossRef]
- Kaura, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Dizoğlu, G.; Sert, E. Fuel additive synthesis by acetylation of glycerol using activated carbon/UiO-66 composite materials. Fuel 2020, 281, 118584. [Google Scholar] [CrossRef]
- Neto, A.B.S.; Oliveira, A.C.; Rodriguez-Castellón, E.; Campos, A.F.; Freire, P.T.C.; Souza, F.F.F.; Filho, J.M.; Araujo, J.C.S.; Lang, R. A comparative study on porous solid acid oxides as catalysts in the esterification of glycerol with acetic acid. Catal. Today 2020, 349, 57–67. [Google Scholar] [CrossRef]
- Malaika, A.; Mesjasz, D.; Kozłowski, M. Maximizing the selectivity to triacetin in glycerol acetylation through a plastic waste-derived carbon catalyst development and selection of a reaction unit. Fuel 2023, 333, 126271. [Google Scholar] [CrossRef]
- Nda-Umar, U.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An overview of recent research in the conversion of glycerol into biofuels, fuel additives and other bio-based chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- Moklis, M.H.; Cheng, S.; Cross, J.S. Current and Future Trends for Crude Glycerol Upgrading to High Value-Added Products. Sustainability 2023, 15, 2979. [Google Scholar] [CrossRef]
- Keshavarzi, M.; Mohammadi, P.; Rastegari, H.; Lam, S.S.; Abas, M.A.; Chong, W.W.F.; Hajiahmad, A.; Peng, W.; Aghbashlo, M.; Tabatabaei, M. Investigation of ketal-acetin mixture synthesized from glycerol as a renewable additive for gasoline-ethanol fuel blend: Physicochemical characterization and engine combustion, performance, and emission assessment. Fuel 2023, 348, 128519. [Google Scholar] [CrossRef]
- Hernández, D.; Fernández, J.J.; Mondragón, F.; López, D. Production and utilization performance of a glycerol derived additive for diesel engines. Fuel 2012, 92, 130–136. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Acetylation of glycerol over heteropolyacids supported on activated carbon. Catal. Commun. 2011, 12, 573–576. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Esterification of glycerol with acetic acid over dodecamolybdophosphoric acid encaged in USY zeolite. Catal. Commun. 2009, 10, 481–484. [Google Scholar] [CrossRef]
- Rastegari, H.; Ghaziaskar, H.S.; Yalpani, M. Valorization of biodiesel derived glycerol to acetins by continuous esterification in acetic acid: Focusing on high selectivity to diacetin and triacetin with no byproducts. Ind. Eng. Chem. Res. 2015, 54, 3279–3284. [Google Scholar] [CrossRef]
- Meireles, B.A.; Pereira, V.L.P. Synthesis of bio-additives: Transesterification of ethyl acetate with glycerol using homogeneous or heterogeneous acid catalysts. J. Braz. Chem. Soc. 2013, 24, 17–25. [Google Scholar] [CrossRef]
- Popova, M.; Szegedi, A.; Ristić, A.; Tušar, N.N. Glycerol acetylation on mesoporous KIL-2 supported sulphated zirconia catalysts. Catal. Sci. Technol. 2014, 4, 3993–4000. [Google Scholar] [CrossRef]
- Trejda, M.; Stawicka, K.; Dubinska, A.; Ziolek, M. Development of niobium containing acidic catalysts for glycerol esterification. Catal. Today 2012, 18, 129–134. [Google Scholar] [CrossRef]
- Melero, J.A.; van Grieken, R.; Morales, G.; Paniagua, M. Acidic Mesoporous Silica for the Acetylation of Glycerol: Synthesis of Bioadditives to Petrol. Energy Fuels 2007, 21, 1782–1791. [Google Scholar] [CrossRef]
- Tao, L.; Guan, H.Y.; Wang, X.H.; Liu, Y.C.; Louh, R.F. Fabrication of sulfonated carbon catalyst from biomass waste and its use for glycerol esterification. Fuel Process. Technol. 2015, 138, 355–360. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Hernández, D.L.; Moreno, J.A.; Modragón, F.; Fernández, J.J. Alternative carbon based acid catalyst for selective esterification of glycerol to acetyl glycerols. Appl. Catal. A General 2011, 405, 55–60. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel 2017, 209, 538–544. [Google Scholar] [CrossRef]
- Urrego-Yepes, W.; Cardona-Uribe, N.; Vargas-Isaza, C.A.; Martínez, J.D. Incorporating the recovered carbon black produced in an industrial-scale waste tire pyrolysis plant into a natural rubber formulation. J. Environ. Manag. 2021, 287, 112292. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Siron, A. Functionalized sp2 carbon allotropes as fillers for rubber nanocomposites. In High-Performance Elastomeric Materials Reinforced by Nano-Carbons; Valentini, L., Lopez Manchado, M.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Chapter 3; pp. 43–92. [Google Scholar]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Li, S.-Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Chen, K.F. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Bielowicz, B. Ash Characteristics and Selected Critical Elements (Ga, Sc, V) in Coal and Ash in Polish Deposits. Resources 2020, 9, 115. [Google Scholar] [CrossRef]
- Rechnia, P.; Malaika, A.; Kozłowski, M. Synthesis of tert-amyl methyl ether (TAME) over modified activated carbon catalysts. Fuel 2015, 154, 338–345. [Google Scholar] [CrossRef]
- Malaika, A.; Heinrich, M.; Goscianska, J.; Kozłowski, M. Synergistic effect of functional groups in carbonaceous spheres on the formation of fuel enhancers from glycerol. Fuel 2020, 280, 118523. [Google Scholar] [CrossRef]
- Malaika, A.; Ptaszyńska, K.; Kapska, M.; Kozłowski, M. The role of surface chemistry of carbons in the catalytic production of fuel additives by glycerol etherification. Fuel 2024, 358, 130147. [Google Scholar] [CrossRef]
- Cardona-Uribe, N.; Betancur, M.; Martínez, J.D. Towards the chemical upgrading of the recovered carbon black derived from pyrolysis of end-of-life tires. Sus. Mater. Technol. 2021, 28, e00287. [Google Scholar] [CrossRef]
- Smith, Y.R.; Bhattacharyya, D.; Willhard, T.; Misra, M. Adsorption of aqueous rare earth elements using carbon black derived from recycled tires. Chem. Eng. J. 2016, 296, 102–111. [Google Scholar] [CrossRef]
- Larcheri, S.; Armellini, C.; Rocca, F.; Kuzmin, A.; Kalendarev, R.; Dalba, G.; Graziola, R.; Purans, J.; Pailharey, D.; Jandard, F. X-ray studies on optical and structural properties of ZnO nanostructured thin films. Superlattices Microstruct. 2006, 39, 267–274. [Google Scholar] [CrossRef]
- Mis-Fernandez, R.; Azamar-Barrios, J.A.; Rios-Soberanis, C.R. Characterization of the powder obtained from wasted tires reduced by pyrolysis and thermal shock process. J. Appl. Res. Technol. 2008, 6, 95–104. [Google Scholar] [CrossRef]
- Kang, G.S.; Lee, G.; Youn, S.; Cho, S.Y.; Joh, H.I.; Lee, D.C.; Lee, S. Recycling of waste tires by synthesizing N-doped carbon-based catalysts for oxygen reduction reaction. Appl. Surf. Sci. 2021, 548, 149027. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; Rodríguez, O.; Alguacil, F.J. Preparation and characterization of activated carbon from the char produced in the thermolysis of granulated scrap tyres. J. Air Waste Manag. Assoc. 2013, 63, 534–544. [Google Scholar] [CrossRef]
- Shilpa; Kumar, R.; ·Sharma, A. Morphologically tailored activated carbon derived from waste tires as high-performance anode for Li-ion battery. J. Appl. Electrochem. 2018, 48, 1–13. [Google Scholar] [CrossRef]
- Choi, D.S.; Yoo, S.H.; Lee, S. Safer and more effective route for polyethylene-derived carbon fiber fabrication using electron beam irradiation. Carbon 2019, 146, 9–16. [Google Scholar] [CrossRef]
- Hu, H.; Fang, Y.; Liu, H.; Yu, R.; Luo, G.; Liu, W.; Li, A.; Yao, H. The fate of sulfur during rapid pyrolysis of scrap tires. Chemosphere 2014, 97, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, P.; Chen, Y.; He, J.; Zhang, W.; Schmid, O.G.; Li, Y. Pure thiophene–sulfur doped reduced graphene oxide: Synthesis, structure, and electrical properties. Nanoscale 2014, 6, 7281. [Google Scholar] [CrossRef] [PubMed]
- Malaika, A.; Ptaszyńska, K.; Morawa Eblagon, K.; Pereira, M.F.R.; Figueiredo, J.L.; Kozłowski, M. Solid acid carbon catalysts for sustainable production of biofuel enhancers via transesterification of glycerol with ethyl acetate. Fuel 2021, 304, 121381. [Google Scholar] [CrossRef]
- Varodi, C.; Pogăcean, F.; Cioriţă, A.; Pană, O.; Leoştean, C.; Cozar, B.; Radu, T.; Coroş, M.; Ştefan-van Staden, R.I.; Pruneanu, S.-M. Nitrogen and sulfur co-doped graphene as efficient electrode material for L-cysteine detection. Chemosensors 2021, 9, 146. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Wang, J.; Dong, F.; Chu, P.K.; Zhang, T.; Zhang, Y. Anionic group self-doping as a promising strategy: Band-gap engineering and multi-functional applications of high-performance CO32−—Doped Bi2O2CO3. ACS Catal. 2015, 5, 4094–4103. [Google Scholar] [CrossRef]
- Han, I.; Rhee, C.; Kim, D. Investigations on potential applications of CaMg(CO3)2 nanoparticles. Molecules 2023, 28, 316. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Nahil, M.A.; Williams, P. Pyrolysis−Catalytic Reforming/Gasification of Waste Tires for Production of Carbon Nanotubes and Hydrogen. Energy Fuels 2015, 29, 3328–3334. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A.; Darmstadt, H. The vacuum pyrolysis of used tires End-uses for oil and carbon black products. J. Anal. Appl. Pyrol. 1999, 51, 201–221. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Mufrodi, Z.; Rochmadi, R.; Sutijan, S.; Budiman, A. Synthesis acetylation of glycerol using batch reactor and continuous reactive distillation column. Eng. J. 2014, 18, 29–40. [Google Scholar] [CrossRef]
- de la Calle, C.; Fraile, J.M.; García-Bordejé, E.; Pires, E.; Roldánc, L. Biobased catalyst in biorefinery processes: Sulphonated hydrothermal carbon for glycerol esterification. Catal. Sci. Technol. 2015, 5, 2897–2903. [Google Scholar] [CrossRef]
- Malaika, A.; Ptaszyńska, K.; Gaidukevic, J.; Kozłowski, M. The impact of surface groups of functionalized graphene on glycerol acetylation. Fuel 2022, 313, 122987. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Acetylation of glycerol to biofuel additives over sulfated activated carbon catalyst. Bioresour. Technol. 2011, 102, 9229–9235. [Google Scholar] [CrossRef]
- Mahammad Rafi, J.; Rajashekar, A.; Srinivas, M.; Rao, B.V.S.K.; Prasad, R.B.N.; Lingaiah, N. Esterification of glycerol over a solid acid biochar catalyst derived from waste biomass. RSC Adv. 2015, 5, 44550–44556. [Google Scholar] [CrossRef]
- Available online: https://contec.tech/product/recovered-carbon-black/ (accessed on 30 October 2023).
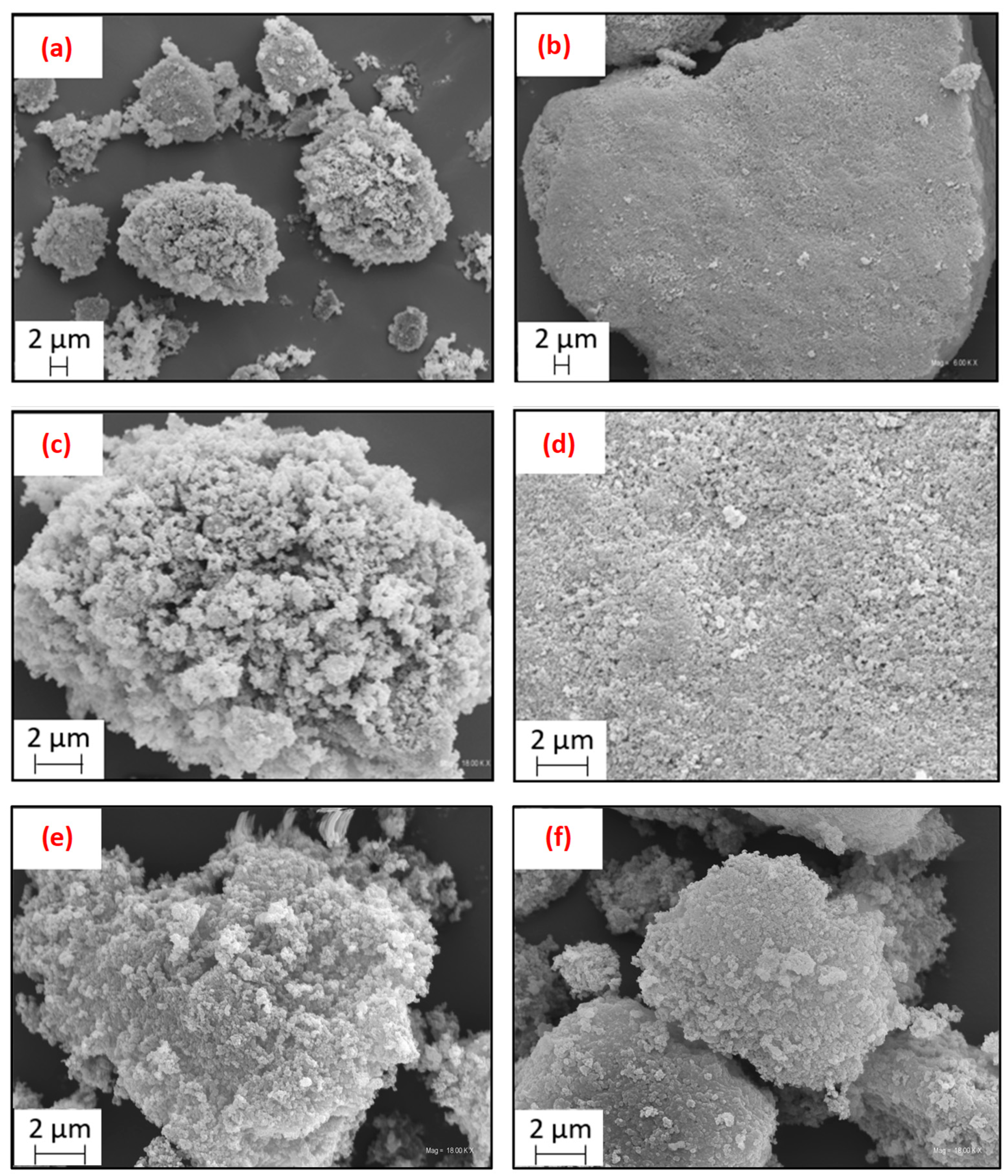
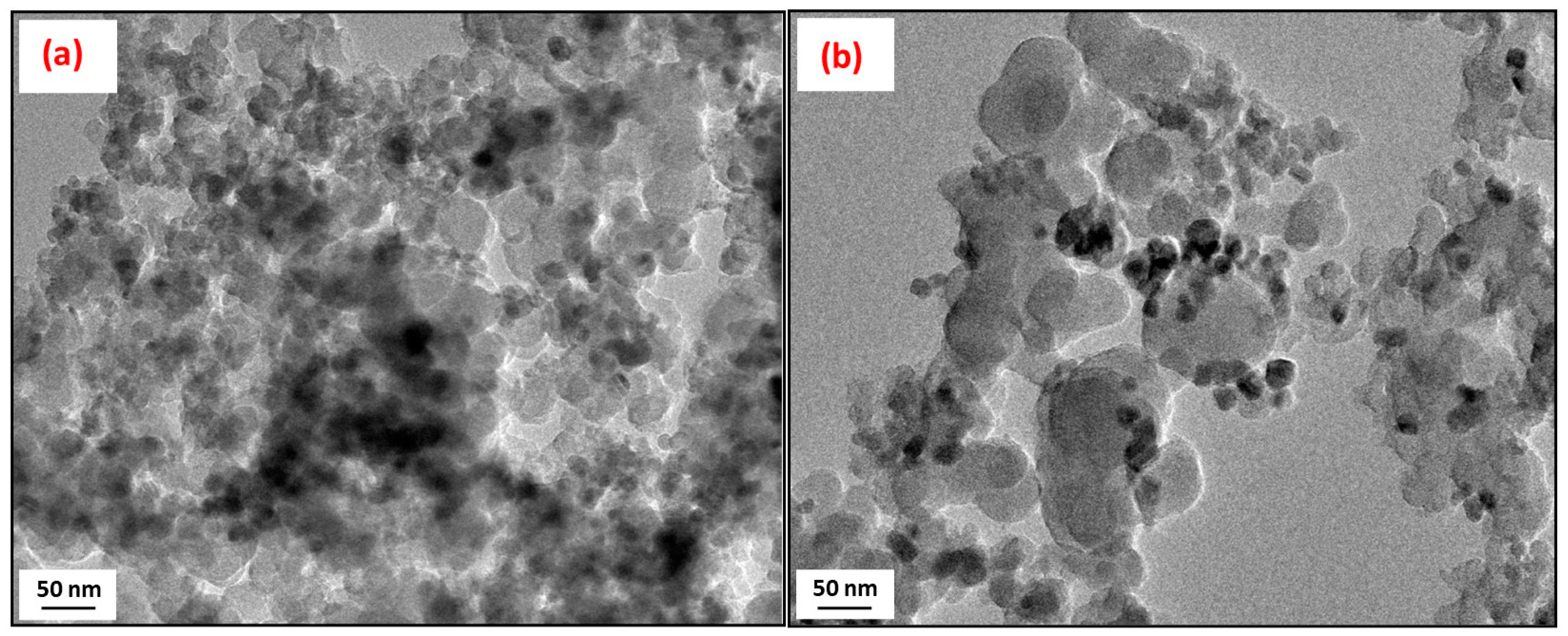
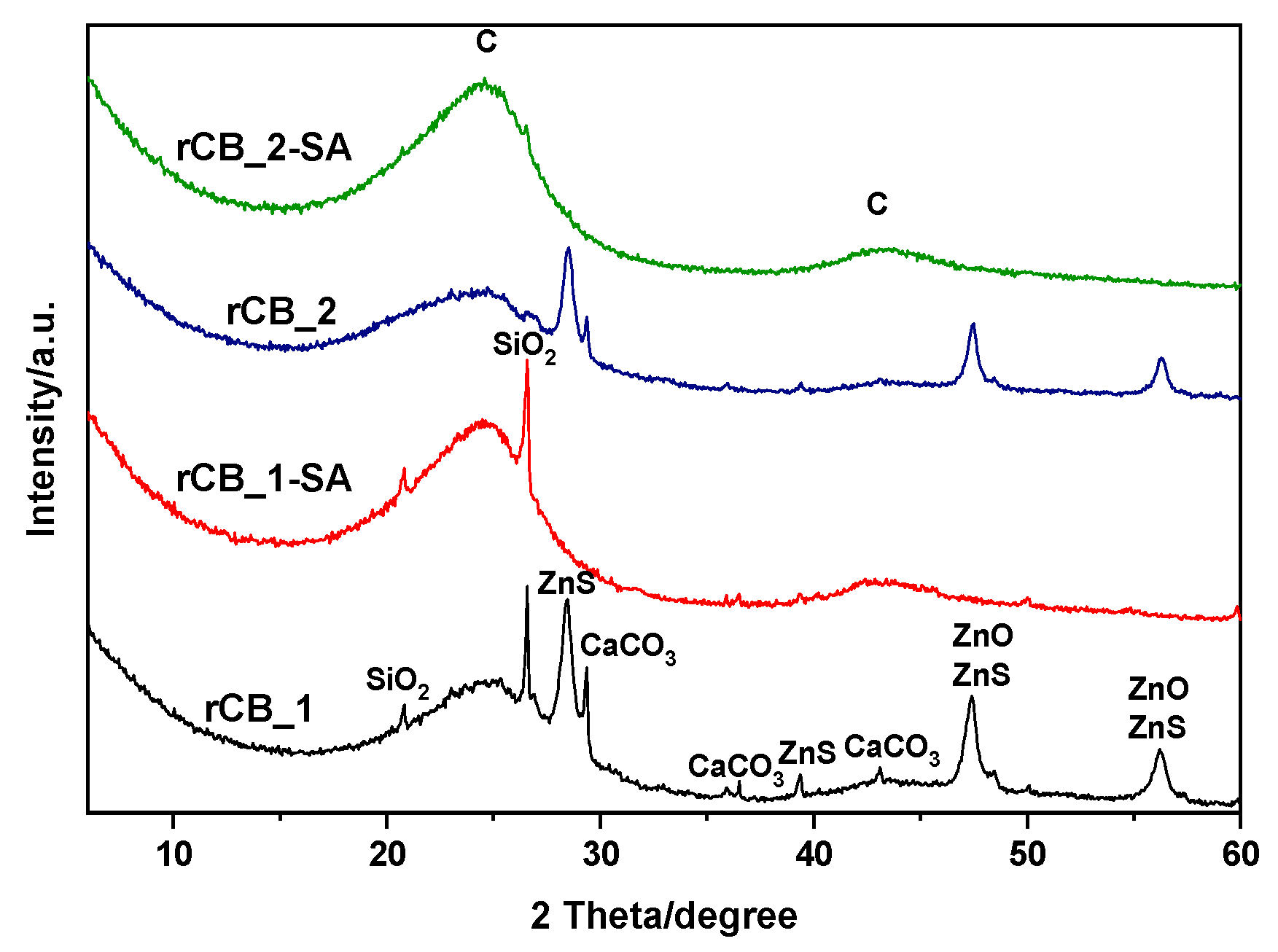
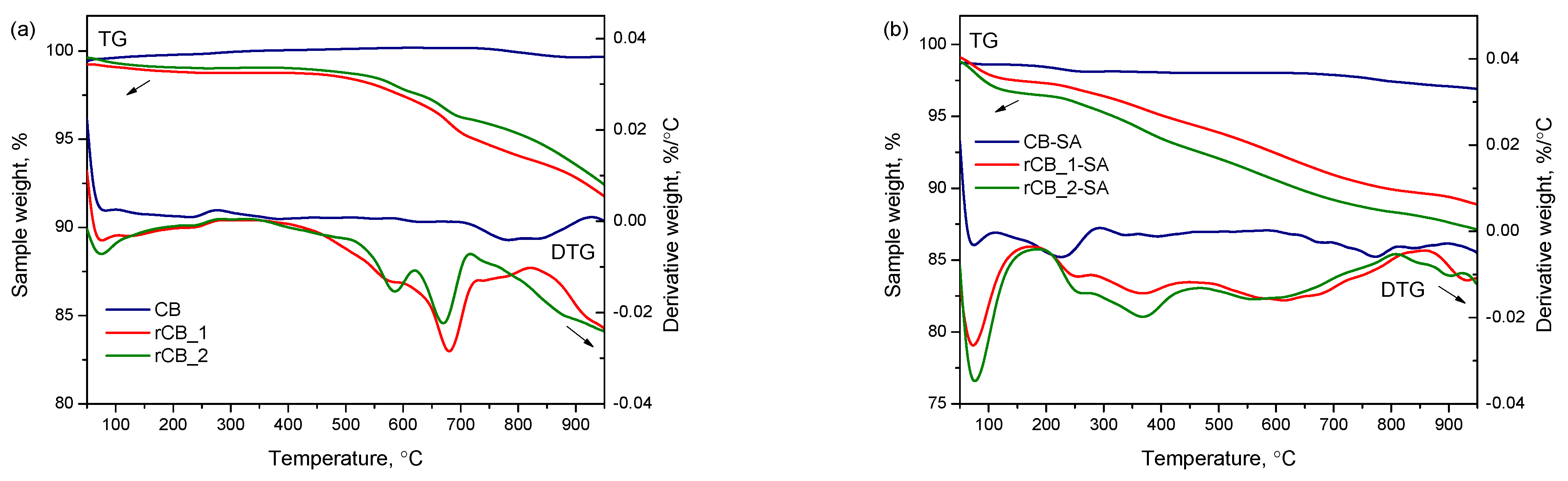
| Sample | Ash | C | H | N | S | O * | Atot |
|---|---|---|---|---|---|---|---|
| CB | 0.0 | 99.6 | 0.1 | 0.0 | 0.0 | 0.2 | 0.03 |
| CB-SA | 0.0 | 97.4 | 0.1 | 0.0 | 1.0 | 0.8 | 0.30 |
| rCB_1 | 20.7 | 79.6 | 0.6 | 0.4 | 2.8 | ns # | 0.04 |
| rCB_1-SA | 11.3 | 76.1 | 0.5 | 0.4 | 3.8 | 7.9 | 0.61 |
| rCB_2 | 26.8 | 74.7 | 0.9 | 0.3 | 2.4 | ns # | 0.09 |
| rCB_2-SA | 12.6 | 73.5 | 0.4 | 0.3 | 3.7 | 9.5 | 0.76 |
| Sample | SBET (m2/g) | Vtot (cm3/g) | D (nm) |
|---|---|---|---|
| CB | 68 | 0.40 | 23.5 |
| CB-SA | 78 | 0.55 | 27.9 |
| rCB_1 | 66 | 0.30 | 22.4 |
| rCB_1-SA | 75 | 0.35 | 19.3 |
| rCB_2 | 63 | 0.40 | 31.1 |
| rCB_2-SA | 81 | 0.38 | 25.9 |
| Sample | Ash | C | H | N | S | O * | Atot |
|---|---|---|---|---|---|---|---|
| rCB_1-SA after 4th cycle | 11.7 | 78.6 | 0.8 | 0.7 | 2.2 | 6.0 | 0.52 |
| Catalyst | Operating Conditions | Catalytic Performance | Ref. |
|---|---|---|---|
| C_glycerol | 110 °C, Gly:AA molar ratio = 1:6, t = 2 h | XGly = 97%, SDA = 56%, STA = 23% | [25] |
| SHTC | 115 °C, Gly:AA molar ratio = 1:9, t = 4 h | XGly ≈ 100%, SDA ≈ 60%, STA ≈ 30% | [76] |
| TRGO_BDS | 110 °C, Gly:AA molar ratio = 1:6, t = 2 h | XGly = 97%, SDA = 59%, STA = 20% | [77] |
| ACmicro_BDS | 110 °C, Gly:AA molar ratio = 1:6, t = 4 h | XGly = 97.5%, SDA = 55%, STA = 22.5% | [35] |
| AC-SA5 | 120 °C, Gly:AA molar ratio = 1:8, t = 3 h, pressurized reactor | XGly = 91%, SDA = 28%, STA = 34% | [78] |
| KJ-400 | 120 °C, Gly:AA molar ratio = 1:5, t = 4 h | XGly = 88.5%, SDA = 40%, STA = 4% | [79] |
| C-SA | 120 °C, Gly:AA molar ratio = 1:5, t = 2 h | XGly = 98.4%, SDA ≈ 54.5%, STA ≈ 13% | [47] |
| rCB_2-SA | 110 °C, Gly:AA molar ratio = 1:6, t = 4 h | XGly = 95%, YDA = 55% (SDA = 58%) YTA = 16.5% (STA = 17.4%) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaika, A.; Kowalska-Kuś, J.; Końska, K.; Ptaszyńska, K.; Jankowska, A.; Held, A.; Wróblewski, K.; Kozłowski, M. Upgrading Pyrolytic Residue from End-of-Life Tires to Efficient Heterogeneous Catalysts for the Conversion of Glycerol to Acetins. Molecules 2023, 28, 8137. https://doi.org/10.3390/molecules28248137
Malaika A, Kowalska-Kuś J, Końska K, Ptaszyńska K, Jankowska A, Held A, Wróblewski K, Kozłowski M. Upgrading Pyrolytic Residue from End-of-Life Tires to Efficient Heterogeneous Catalysts for the Conversion of Glycerol to Acetins. Molecules. 2023; 28(24):8137. https://doi.org/10.3390/molecules28248137
Chicago/Turabian StyleMalaika, Anna, Jolanta Kowalska-Kuś, Klaudia Końska, Karolina Ptaszyńska, Aldona Jankowska, Agnieszka Held, Krzysztof Wróblewski, and Mieczysław Kozłowski. 2023. "Upgrading Pyrolytic Residue from End-of-Life Tires to Efficient Heterogeneous Catalysts for the Conversion of Glycerol to Acetins" Molecules 28, no. 24: 8137. https://doi.org/10.3390/molecules28248137
APA StyleMalaika, A., Kowalska-Kuś, J., Końska, K., Ptaszyńska, K., Jankowska, A., Held, A., Wróblewski, K., & Kozłowski, M. (2023). Upgrading Pyrolytic Residue from End-of-Life Tires to Efficient Heterogeneous Catalysts for the Conversion of Glycerol to Acetins. Molecules, 28(24), 8137. https://doi.org/10.3390/molecules28248137











