Design, Synthesis, Biological Evaluation, and Preliminary Mechanistic Study of a Novel Mitochondrial-Targeted Xanthone
Abstract
:1. Introduction
2. Results
2.1. Molecular Design and Chemical Synthesis
2.2. In Vitro Biological Activity
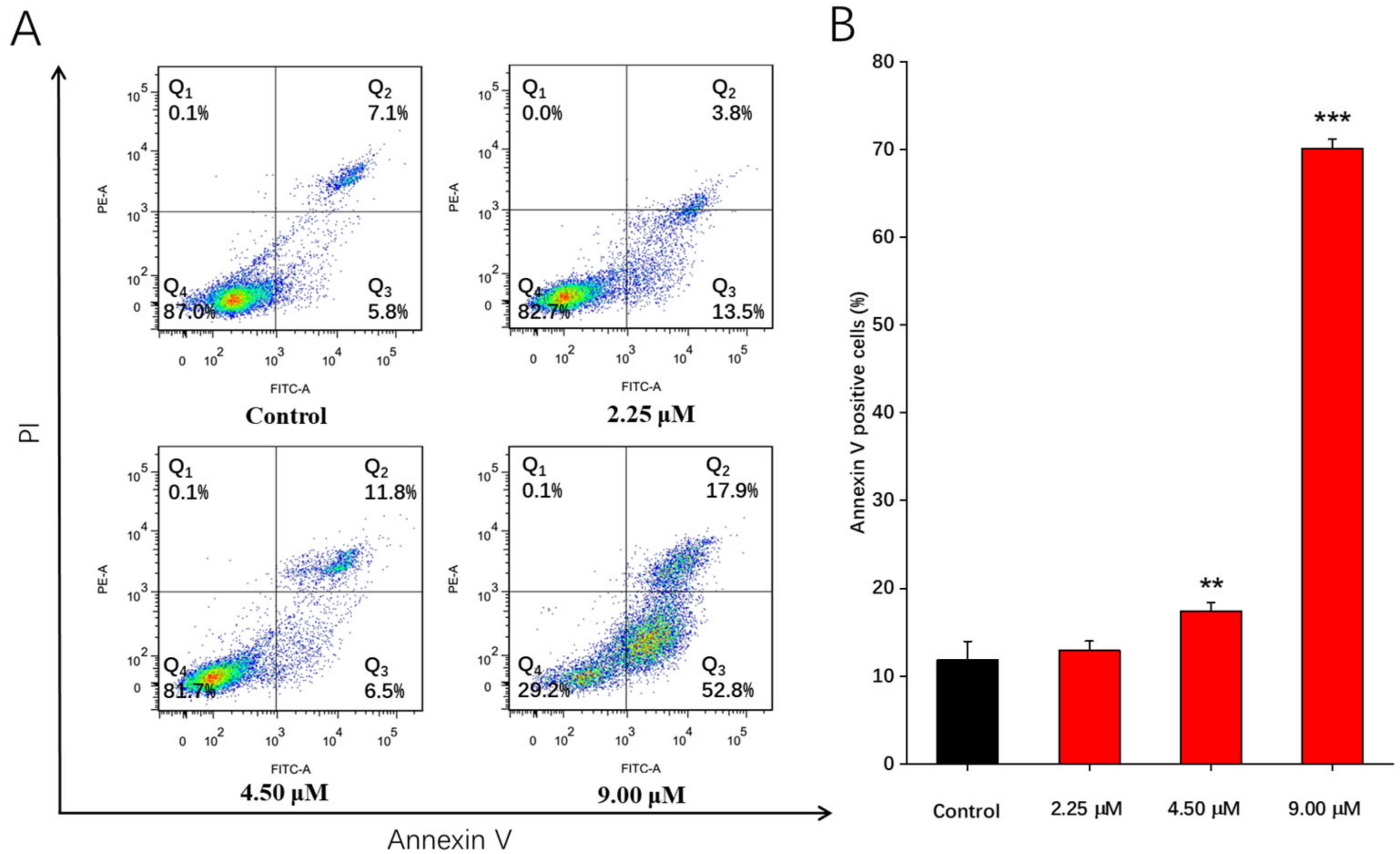
2.3. Apoptosis Effects Induced by Compound 1b in A549 Cells
2.4. Arrest Effects of Compound 1b on A549 Cell Cycle
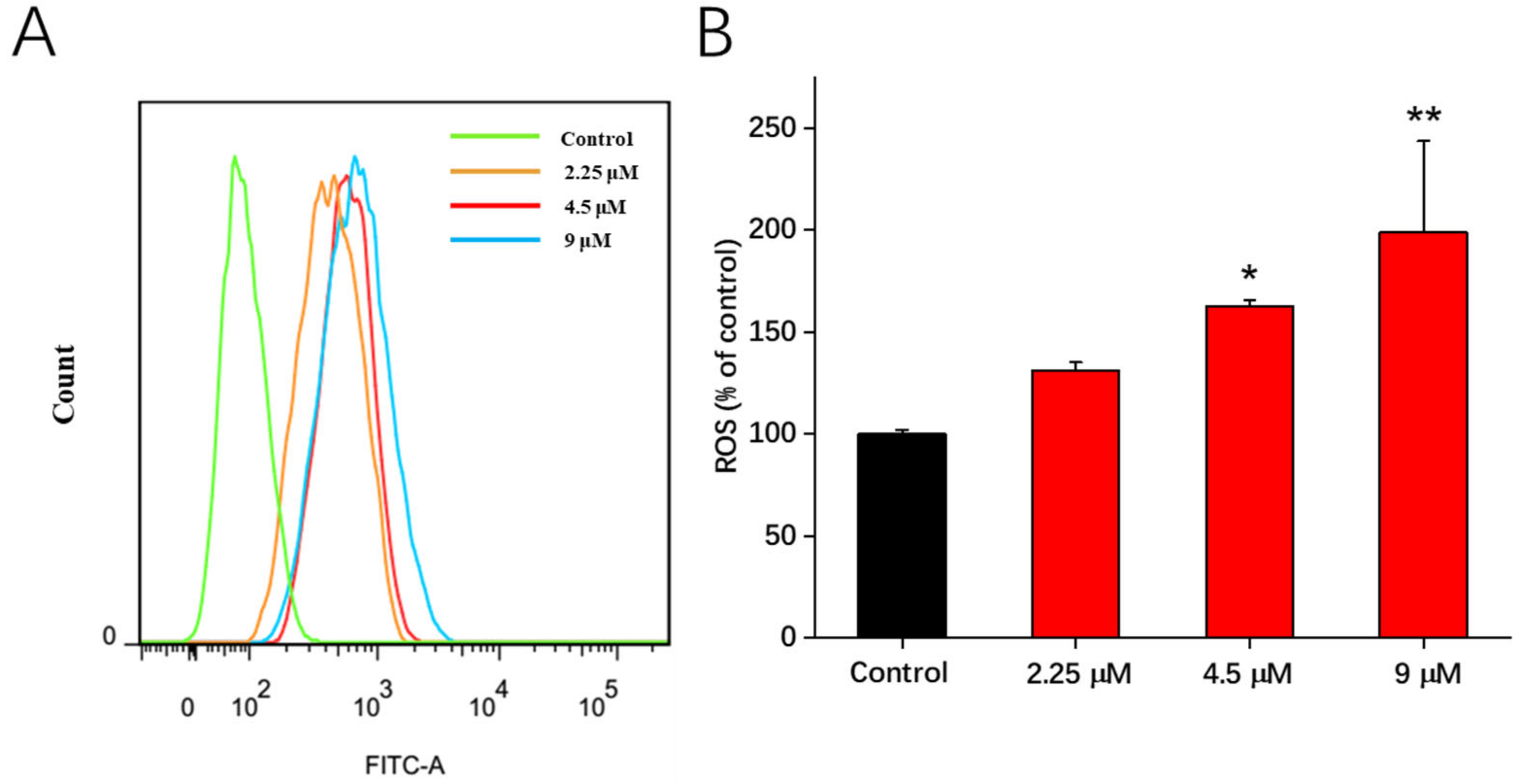
2.5. The Increase in Intracellular Reactive Oxygen Species (ROS) Production Stimulated by 1b
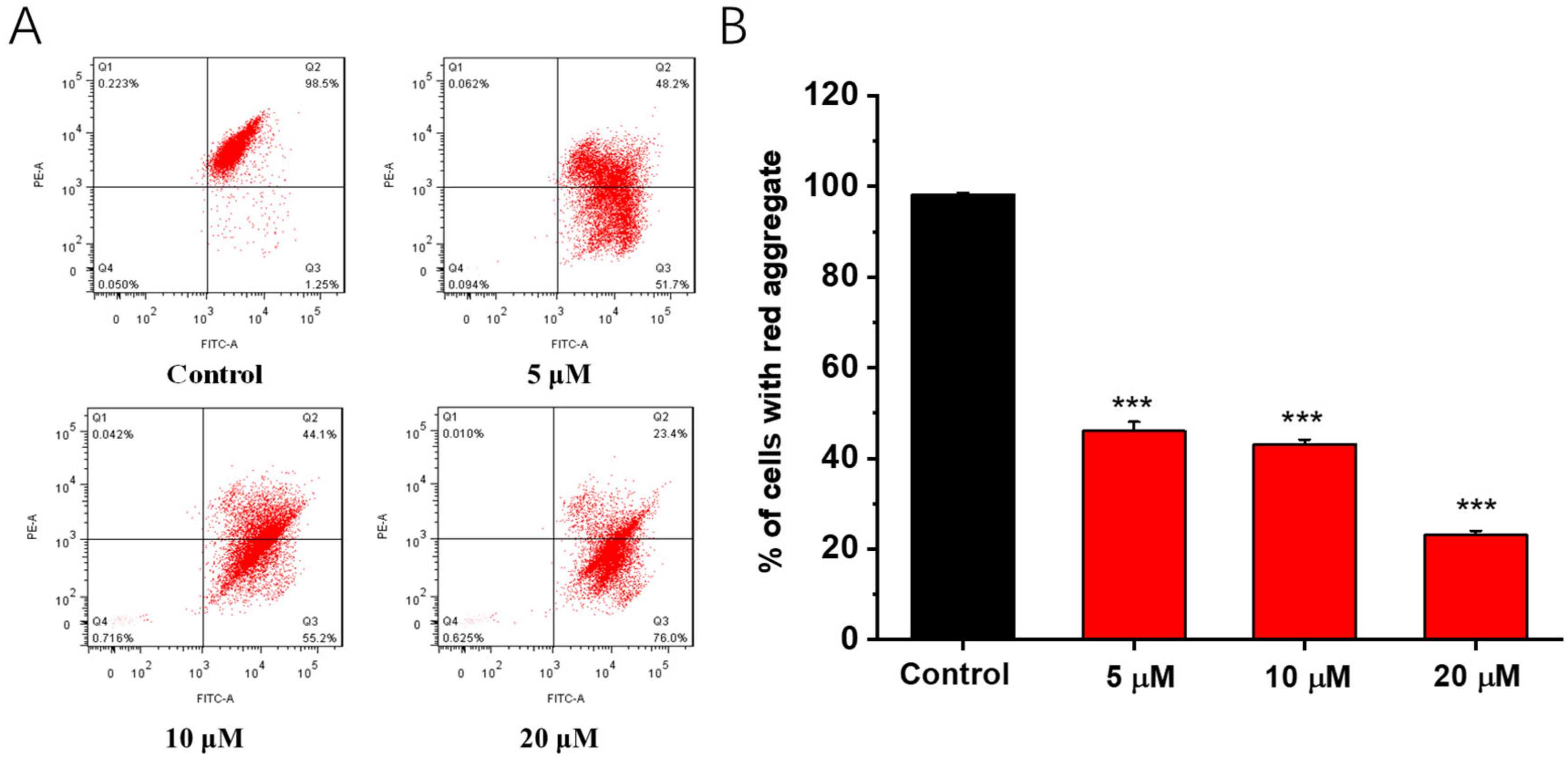
2.6. Effects of Compound 1b on the Mitochondrial Membrane Potential (Δψm)
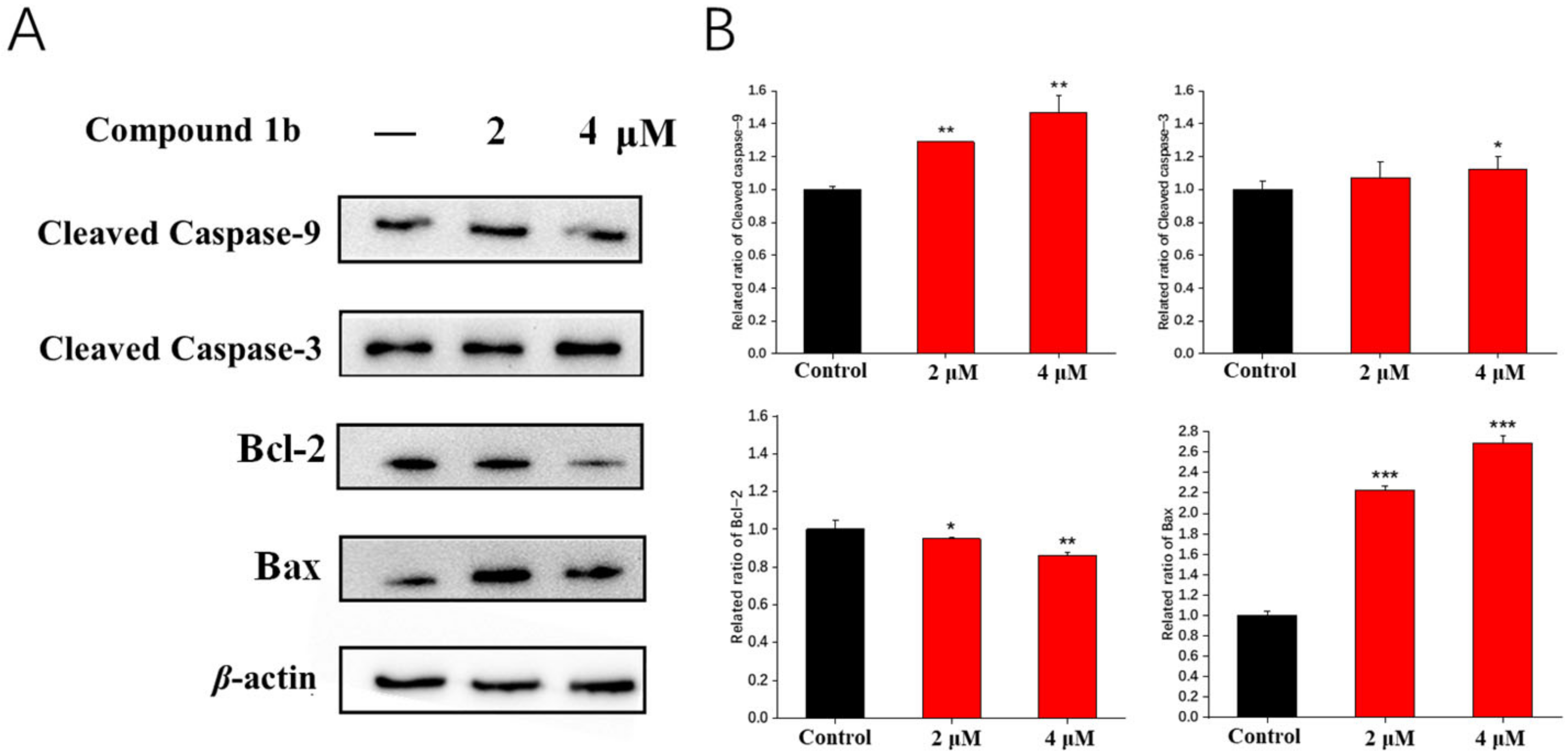
2.7. Effects on the Expression of Mitochondrial Pathway Proteins
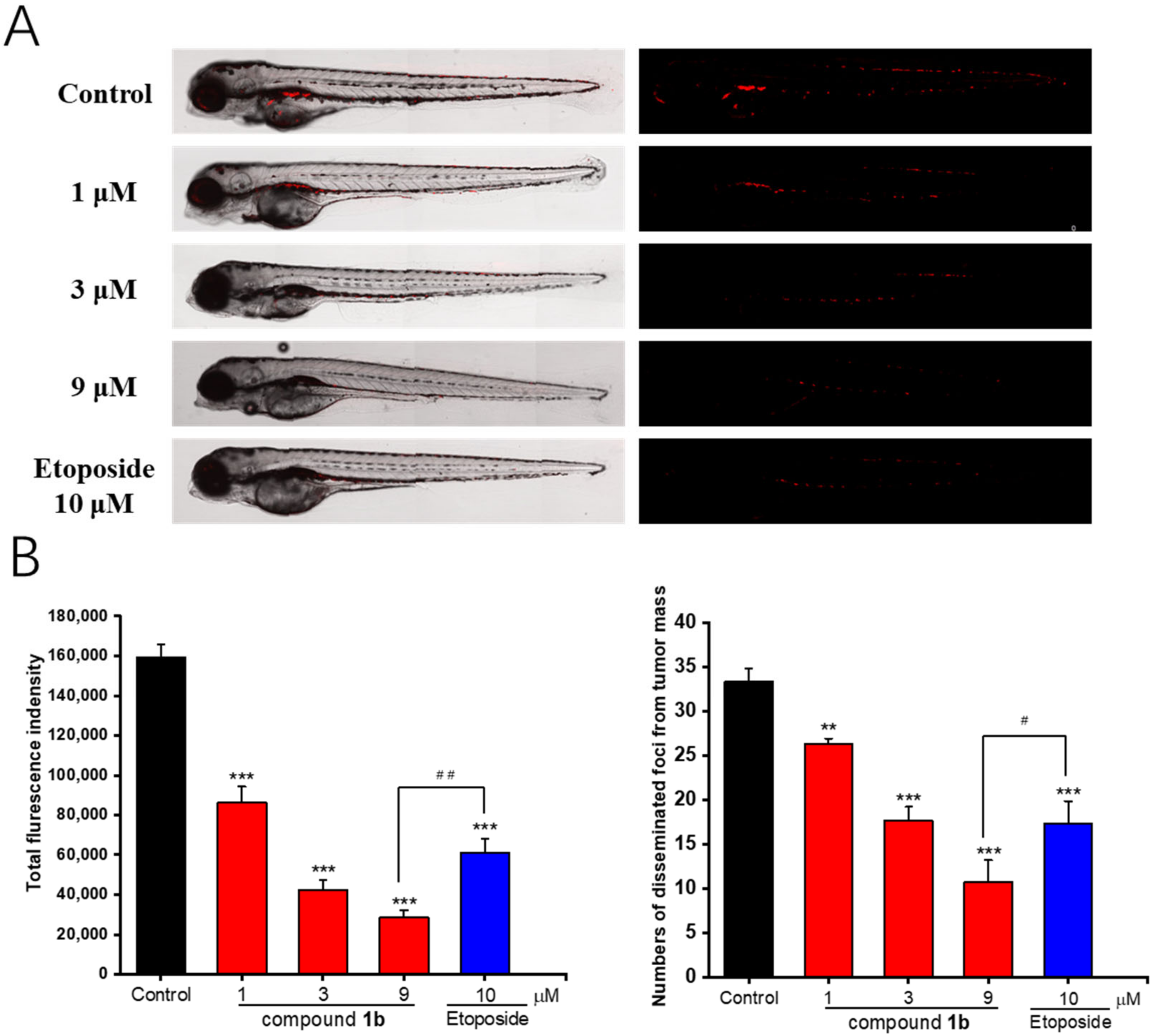
2.8. In Vivo Antitumor Effects of Compound 1b in Zebrafish Xenografts
2.9. In Vivo Antiangiogenic Activity of Compound 1b in a Transgenic Zebrafish Model
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. General Procedure for the Synthesis of Compound 1b
4.2.1. Synthesis of the Intermediate 1a
4.2.2. Synthesis of Target Compound 1b
4.3. Cell Culture
4.4. In Vitro Cell Viability Assay
4.5. Cell Apoptosis Analysis by Flow Cytometry
4.6. Cell Cycle Arrest under Flow Cytometry
4.7. Detection of Intracellular Reactive Oxygen Species (ROS)
4.8. Measurement of the Mitochondrial Membrane Potential (Δψm)
4.9. Western Blot Analysis
4.10. In Vivo Antitumor Activity Using Zebrafish Xenografts
4.11. In Vivo Angiogenesis Activity Using a Transgenic Zebrafish Model
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, G.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Sig. Transduct. Target Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-targeted triphenylphosphonium-based compounds: Syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, J.; Lu, W. The significance of mitochondrial dysfunction in cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, T.; Yuan, H.; Li, D.; Lou, H.; Fan, P. Mitochondria-targeted lupane triterpenoid derivatives and their selective apoptosis-inducing anticancer mechanisms. J. Med. Chem. 2017, 60, 6353–6363. [Google Scholar] [CrossRef]
- Song, H.; Xing, W.; Shi, X.; Zhang, T.; Lou, H.; Fan, P. Antitumor and toxicity study of mitochondria-targeted triptolide derivatives using triphenylphosphine (TPP+) as a carrier. Bioorganic Med. Chem. 2021, 50, 116466. [Google Scholar] [CrossRef]
- Shi, L.; Gao, L.L.; Cai, S.Z.; Xiong, Q.W.; Ma, Z.R. A novel selective mitochondrial-targeted curcumin analog with remarkable cytotoxicity in glioma cells. Eur. J. Med. Chem. 2021, 221, 113528. [Google Scholar] [CrossRef]
- Kong, L.; Deng, Z.; You, D. Chemistry and biosynthesis of bacterial polycyclic xanthone natural products. Nat. Prod. Rep. 2022, 9, 2057–2095. [Google Scholar] [CrossRef]
- Nauman, M.C.; Johnson, J.J. The purple mangosteen (Garcinia mangostana): Defining the anticancer potential of selected xanthones. Pharmacol. Res. 2022, 175, 106032. [Google Scholar] [CrossRef]
- Ansori, A.N.M.; Fadholly, A.; Hayaza, S.; Susilo, R.J.K.; Inayatillah, B.; Winarni, D.; Husen, S.A. A review on medicinal properties of mangosteen (Garcinia mangostana L.). Res. J. Pharm. Tech. Technol. 2020, 13, 974–982. [Google Scholar] [CrossRef]
- Narasimhan, S.; Maheshwaran, S.; Abu-Yousef, I.A.; Majdalawieh, A.F.; Rethavathi, J.; Das, P.E.; Poltronieri, P. Anti-bacterial and anti-fungal activity of xanthones obtained via semi-synthetic modification of α-mangostin from Garcinia mangostana. Molecules 2017, 22, 275. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Yang, Y.; Gupta, P.; Wang, A.; Zhao, M.; Zhao, Y.; Qu, M.; Ke, Y.; Liu, Y.; Liu, H.M.; et al. A small molecule inhibitor, OGP46, is effective against imatinib-resistant BCR-ABL mutations via the BCR-ABL/JAK-STAT pathway. Mol. Ther.-Oncolytics 2020, 18, 137–148. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Z.; Lin, M.; Shang, Y.; Wang, F.; Zhou, J.; Wang, F.; Zhang, X.; Luo, X.; Huang, W. In vitro and in vivo antitumor activity of Cucurbitacin C, a novel natural product from cucumber. Front. Pharmacol. 2019, 10, 1287. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liang, Z.; Zhang, J.; Wang, W.P.; Zhang, H.; Lu, Q. Zinc oxide nanoparticle synthesized from Euphorbia fischeriana root inhibits the cancer cell growth through modulation of apoptotic signaling pathways in lung cancer cells. Arab. J. Chem. 2020, 13, 6174–6183. [Google Scholar] [CrossRef]
- Kang, F.; Ai, Y.; Zhang, Y.; Huang, Z. Design and synthesis of new hybrids from 2-cyano-3, 12-dioxooleana-9-dien-28-oic acid and O2-(2, 4-dinitrophenyl) diazeniumdiolate for intervention of drug-resistant lung cancer. Eur. J. Med. Chem. 2018, 149, 269–280. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Li, Y.; Wang, H.; Zhang, S.; Reid, A.M.; Lall, N.; Zhang, J.; Wang, C.; Lee, D.; et al. Cytotoxic and antiangiogenetic xanthones inhibiting tumor proliferation and metastasis from Garcinia xipshuanbannaensis. J. Nat. Prod. 2021, 84, 1515–1523. [Google Scholar] [CrossRef]
- Han, D.; Wu, X.; Liu, L.; Shu, W.; Huang, Z. Sodium tanshinone IIA sulfonate protects ARPE-19 cells against oxidative stress by inhibiting autophagy and apoptosis. Sci. Rep. 2018, 8, 15137. [Google Scholar] [CrossRef] [Green Version]
- Rao, N.S.; Nayak, V.L.; Rao, A.S.; Hussaini, S.A.; Sunkari, S.; Alarifi, A.; Kamal, A. Arylcinnamido-propionone conjugates as tubulin polymerization inhibitors and apoptotic inducers. Arab. J. Chem. 2019, 12, 4740–4755. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef]
- Al-Abd, A.M.; Alamoudi, A.J.; Abdel-Naim, A.B.; Neamatallah, T.A.; Ashour, O.M. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies–A review. J. Adv. Res. 2017, 8, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Gallagher, J.D.; Olenyuk, B.Z.; Neamati, N. A selective mitochondrial-targeted chlorambucil with remarkable cytotoxicity in breast and pancreatic cancers. J. Med. Chem. 2013, 56, 9170–9179. [Google Scholar] [CrossRef] [PubMed]
- Blaikie, F.H.; Brown, S.E.; Samuelsson, L.M.; Brand, M.D.; Smith, R.A.; Murphy, M.P. Targeting dinitrophenol to mitochondria: Limitations to the development of a self-limiting mitochondrial protonophore. Biosci. Rep. 2006, 26, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zielonka, J.; Ouari, O.; Lopez, M.; McAllister, D.; Boyle, K.; Barrios, C.S.; Weber, J.J.; Johnson, B.D.; Hardy, M.; et al. Mitochondria-targeted analogues of metformin exhibit enhanced antiproliferative and radiosensitizing effects in pancreatic cancer cells. Cancer Res. 2016, 76, 3904–3915. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, A.R.; Zielonka, J.; Kalyanaraman, B.; Hartley, R.C.; Murphy, M.P.; Avadhani, N.G. Mitochondria-targeted paraquat and metformin mediate ROS production to induce multiple pathways of retrograde signaling: A dose-dependent phenomenon. Redox Biol. 2020, 36, 101606. [Google Scholar] [CrossRef]
- Wu, S.; Cao, Q.; Wang, X.; Cheng, K.; Cheng, Z. Design, synthesis and biological evaluation of mitochondria targeting theranostic agents. Chem. Commun. 2014, 50, 8919–8922. [Google Scholar] [CrossRef]
- Han, M.; Vakili, M.R.; Soleymani Abyaneh, H.; Molavi, O.; Lai, R.; Lavasanifar, A. Mitochondrial delivery of doxorubicin via triphenylphosphine modification for overcoming drug resistance in MDA-MB-435/DOX cells. Mol. Pharm. 2014, 11, 2640–2649. [Google Scholar] [CrossRef]
- Smith, R.A.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef]
- Jara, J.A.; Castro-Castillo, V.; Saavedra-Olavarría, J.; Peredo, L.; Pavanni, M.; Jana, F.; Letelier, M.E.; Parra, E.; Becker, M.I.; Morello, A.; et al. Antiproliferative and uncoupling effects of delocalized, lipophilic, cationic gallic acid derivatives on cancer cell lines. Validation in vivo in singenic mice. J. Med. Chem. 2014, 57, 2440–2454. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Q.; Yang, X.; Li, Y.; Zhang, X.; Li, Y.; Du, Q.; Jin, D.Q.; Cui, J.; Lall, N.; et al. Diterpenoids from the leaves of Casearia kurzii showing cytotoxic activities. Bioorganic Chem. 2020, 98, 103741. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Song, Z.; Zhao, Y.; Zhang, H.; Li, Y.; Xu, J.; Guo, Y. The antitumor activity and mechanism of a natural diterpenoid from Casearia graveolens. Front. Oncol. 2021, 11, 688195. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Wang, Y.; Yang, D.; Tan, H.; Zhan, Y.; Yang, Y.; Luo, Y.; Chen, G. Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds. Int. J. Biol. Macromol. 2019, 135, 1151–1161. [Google Scholar] [CrossRef]
- Wu, X.; Gao, H.; Hou, Y.; Yu, J.; Sun, W.; Wang, Y.; Chen, X.; Feng, Y.; Xu, Q.M.; Chen, X. Dihydronortanshinone, a natural product, alleviates LPS-induced inflammatory response through NF-κB, mitochondrial ROS, and MAPK pathways. Toxicol. Appl. Pharmacol. 2018, 355, 1–8. [Google Scholar] [CrossRef]
- Ni, W.; Yao, S.; Zhou, Y.; Liu, Y.; Huang, P.; Zhou, A.; Liu, J.; Che, L.; Li, J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol. Cancer 2019, 18, 143. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Gong, X.; Li, Z.; Wang, H.; Ma, L.; Tuerhong, M.; Abudukeremu, M.; Ohizumi, Y.; Xu, J.; et al. Preparation, characterization, and antitumor activity of Chaenomeles speciosa polysaccharide-based selenium nanoparticles. Arab. J. Chem. 2022, 15, 103943. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Shi, L.; Li, Y.; Tuerhong, M.; Abudukeremu, M.; Cui, J.; Li, Y.; Jin, D.Q.; Xu, J.; et al. Structure features, selenylation modification, and improved anti-tumor activity of a polysaccharide from Eriobotrya japonica. Carbohydr. Polym. 2021, 273, 118496. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch VMSupuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]



| Compounds | IC50 (μM) a | |||
|---|---|---|---|---|
| A549 | K562 | HepG2 | Hela | |
| 1 | 16.6 ± 0.6 | 25.3 ± 0.3 | 37.3 ± 0.8 | 27.5 ± 0.1 |
| 1b | 3.0 ± 0.3 | 4.5 ± 0.5 | 5.0 ± 0.1 | 4.1 ± 0.1 |
| Etoposide | 27.4 ± 0.9 | 17.9 ± 1.0 | 21.0 ± 2.7 | 25.8 ± 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, Q.; Peng, M.; Xu, J.; Guo, Y. Design, Synthesis, Biological Evaluation, and Preliminary Mechanistic Study of a Novel Mitochondrial-Targeted Xanthone. Molecules 2023, 28, 1016. https://doi.org/10.3390/molecules28031016
Wang S, Zhang Q, Peng M, Xu J, Guo Y. Design, Synthesis, Biological Evaluation, and Preliminary Mechanistic Study of a Novel Mitochondrial-Targeted Xanthone. Molecules. 2023; 28(3):1016. https://doi.org/10.3390/molecules28031016
Chicago/Turabian StyleWang, Sibei, Qi Zhang, Maoqin Peng, Jing Xu, and Yuanqiang Guo. 2023. "Design, Synthesis, Biological Evaluation, and Preliminary Mechanistic Study of a Novel Mitochondrial-Targeted Xanthone" Molecules 28, no. 3: 1016. https://doi.org/10.3390/molecules28031016
APA StyleWang, S., Zhang, Q., Peng, M., Xu, J., & Guo, Y. (2023). Design, Synthesis, Biological Evaluation, and Preliminary Mechanistic Study of a Novel Mitochondrial-Targeted Xanthone. Molecules, 28(3), 1016. https://doi.org/10.3390/molecules28031016





