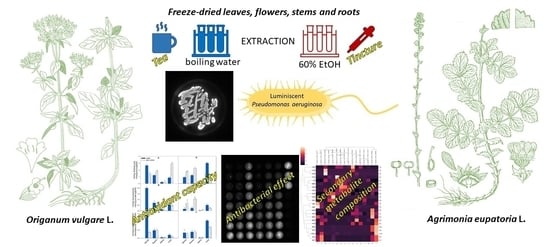
Effect of Agrimonia eupatoria L. and Origanum vulgare L. Leaf, Flower, Stem, and Root Extracts on the Survival of Pseudomonas aeruginosa
Abstract
Share and Cite
Bělonožníková, K.; Sladkovská, E.; Kavan, D.; Hýsková, V.; Hodek, P.; Šmíd, D.; Ryšlavá, H. Effect of Agrimonia eupatoria L. and Origanum vulgare L. Leaf, Flower, Stem, and Root Extracts on the Survival of Pseudomonas aeruginosa. Molecules 2023, 28, 1019. https://doi.org/10.3390/molecules28031019
Bělonožníková K, Sladkovská E, Kavan D, Hýsková V, Hodek P, Šmíd D, Ryšlavá H. Effect of Agrimonia eupatoria L. and Origanum vulgare L. Leaf, Flower, Stem, and Root Extracts on the Survival of Pseudomonas aeruginosa. Molecules. 2023; 28(3):1019. https://doi.org/10.3390/molecules28031019
Chicago/Turabian StyleBělonožníková, Kateřina, Eliška Sladkovská, Daniel Kavan, Veronika Hýsková, Petr Hodek, Daniel Šmíd, and Helena Ryšlavá. 2023. "Effect of Agrimonia eupatoria L. and Origanum vulgare L. Leaf, Flower, Stem, and Root Extracts on the Survival of Pseudomonas aeruginosa" Molecules 28, no. 3: 1019. https://doi.org/10.3390/molecules28031019
APA StyleBělonožníková, K., Sladkovská, E., Kavan, D., Hýsková, V., Hodek, P., Šmíd, D., & Ryšlavá, H. (2023). Effect of Agrimonia eupatoria L. and Origanum vulgare L. Leaf, Flower, Stem, and Root Extracts on the Survival of Pseudomonas aeruginosa. Molecules, 28(3), 1019. https://doi.org/10.3390/molecules28031019