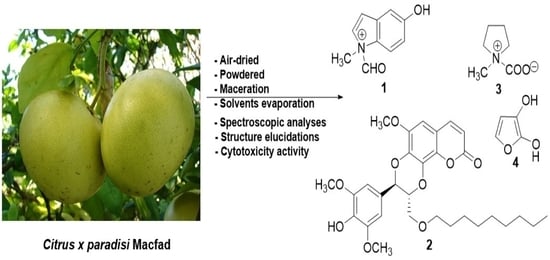
Coumarinolignoid and Indole Alkaloids from the Roots of the Hybrid Plant Citrus × paradisi Macfad (Rutaceae)
Abstract
1. Introduction
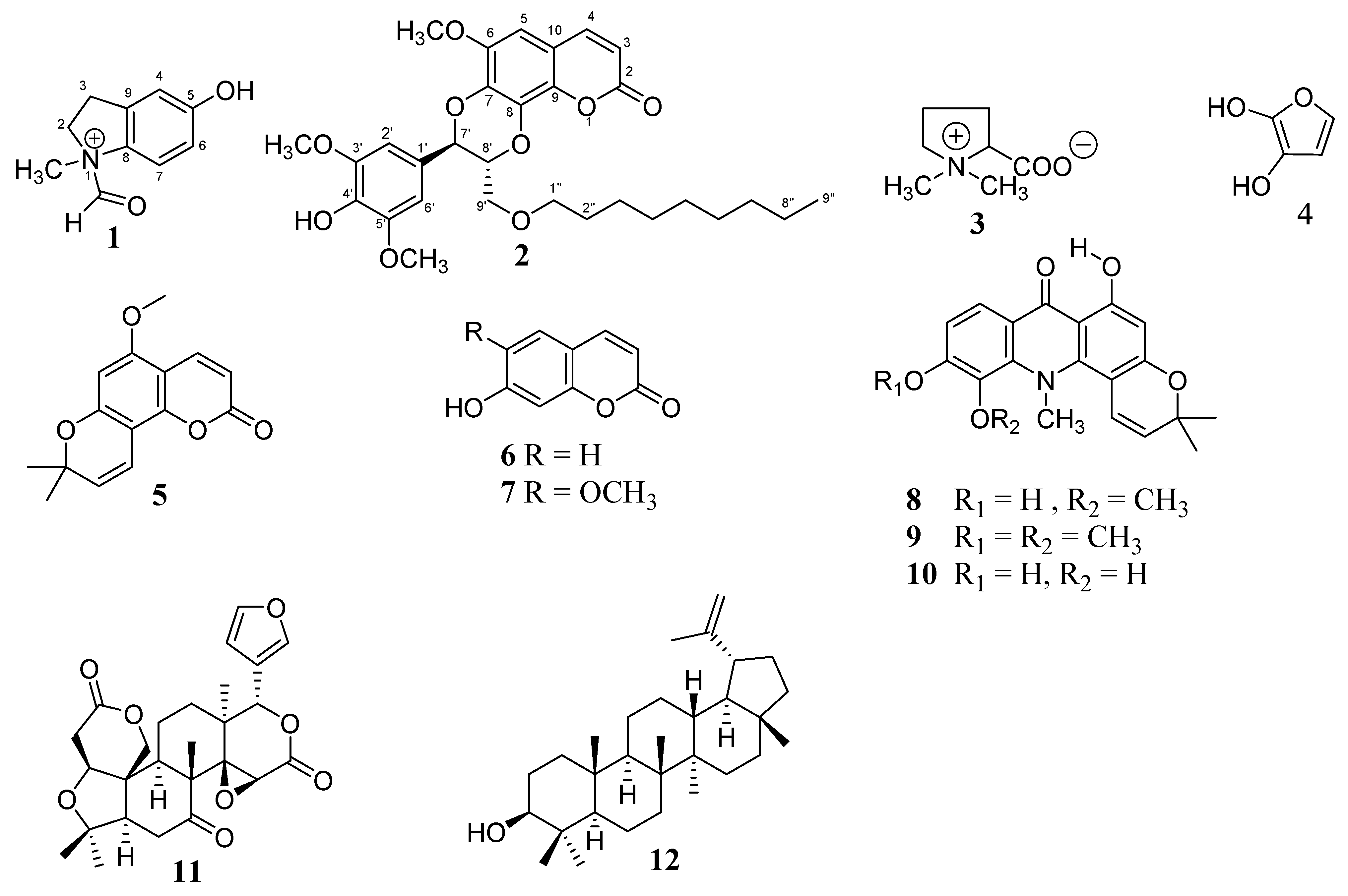
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
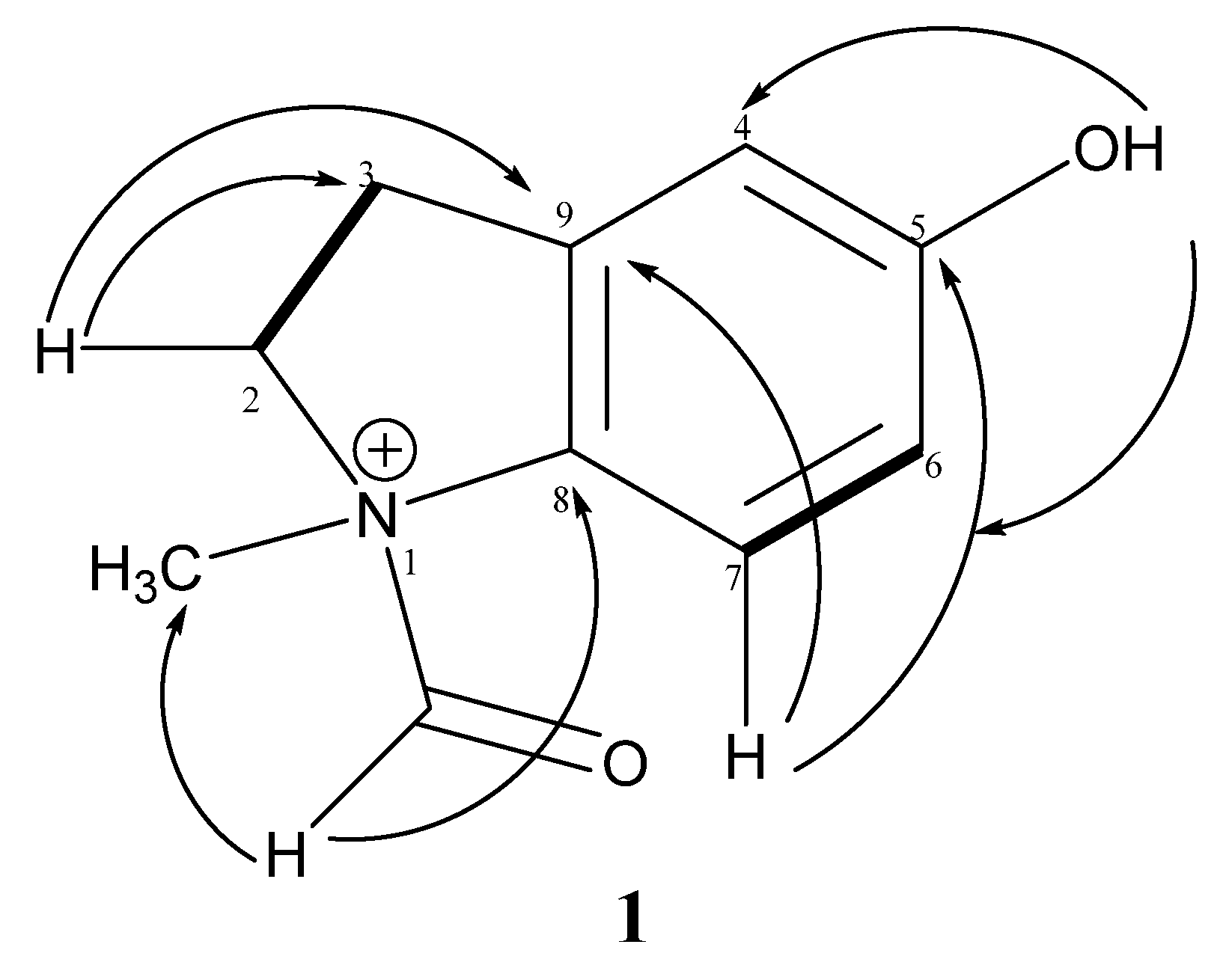
3.3.1. 1-Formyl-5-hydroxy-N-methylindolin-1-ium (1)
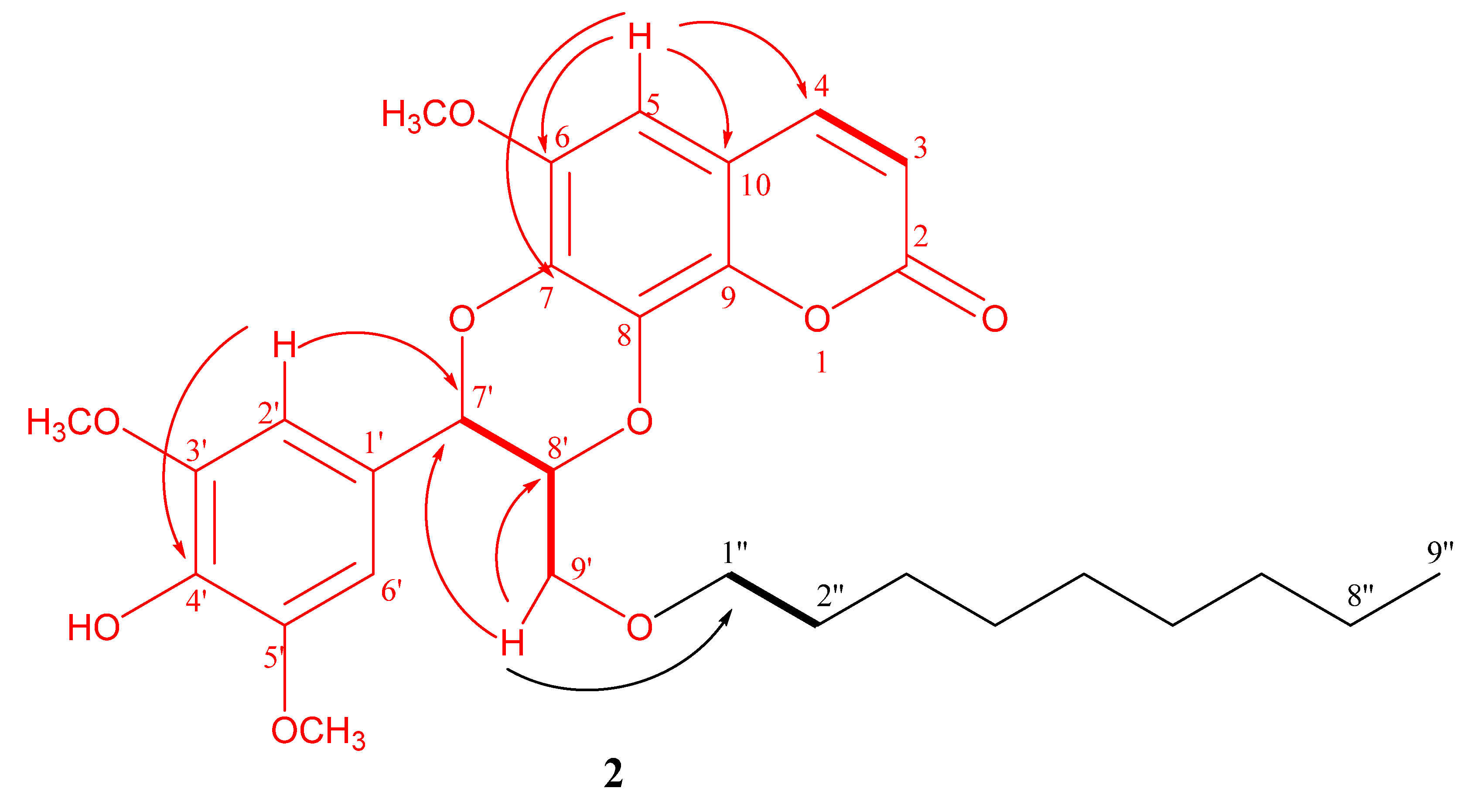
3.3.2. Decyloxycleomiscosin D (2)
3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 2000, 100, 1155–1166. [Google Scholar] [CrossRef]
- Gmitter, F.G., Jr. Origin, evolution, and breeding of the grapefruit. Plant Breed. Rev. 1995, 13, 345–363. [Google Scholar]
- Adeneye, A.A. Hypoglycemic and hypolipidemic effects of methanol seed extract of Citrus paradisi Macfad (Rutaceae) in alloxan-induced diabetic Wistar rats. Nig. Q. J. Hosp. Med. 2008, 18, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dibong, S.; Mpondo Mpondo, E.; Ngoye, A.; Kwin, M.; Betti, J. Ethnobotanique et phytomédecine des plantes médicinales de Douala, Cameroun. J. Appl. Sci. 2011, 37, 2496–2507. [Google Scholar]
- Gupta, V.; Bansal, P.; Kumar, P.; Shri, R. Anxiolytic and antidepressant activities of different extracts from Citrus × paradisi var. Duncan. Asian J. Pharm. Clin. Res. 2010, 3, 98–100. [Google Scholar]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya. J. Agric. Food Chem. 2005, 53, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Okunowo, W.O.; Oyedeji, O.; Afolabi, L.O.; Matanmi, E. Essential oil of grape fruit (Citrus paradisi) peels and its antimicrobial activities. Am. J. Plant Sci. 2013, 4, 34556. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, C.; Zang, Y.; Huang, Z.; Liu, G. The flavonoid composition of flavedo and juice from the pummelo cultivar (Citrus grandis (L.) Osbeck) and the grapefruit cultivar (Citrus × paradisi) from China. Food Chem. 2011, 129, 1530–1536. [Google Scholar] [CrossRef]
- Malolo, F.-A.E.; Tabekoueng, G.B.; Tsopgni, W.D.T.; Chimeze, V.W.N.; Kouam, A.K.; Mas-Claret, E.; Langat, M.K.; Ndom, J.C.; Frese, M.; Sewald, N.; et al. Chemical constituents of the stem bark of the hybrid plant Citrus × paradisi Macfad. (Rutaceae). Chem. Biodivers. 2022, 19, e202101033. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Basha, A.; Atta-ur-Rahman. Identification and C-13 NMR spectrum of stachydrine from Cadaba fruticosa. Phytochemistry 1975, 14, 292–293. [Google Scholar]
- Bissim, S.; Kenmogne, S.B.; Tadjong, T.A.; Mehreen, L.; Ayaz, A.; Ngeufa, E.H.; Wansi, J.D.; Muhammad, S.A.; Kamdem, A.F.W. Bioactive acridone alkaloids and their derivatives from Citrus aurantium (Rutaceae). Phytochem. Lett. 2009, 29, 148–153. [Google Scholar] [CrossRef]
- Fomani, M.; Ngeufa, E.H.; Nouga, A.B.; Ndom, J.C.; Kamdem, A.F.W.; Sewald, N.; Wansi, J.D. Oxidative burst inhibition, cytotoxicity and antibacterial acriquinoline alkaloids from Citrus reticulata (Blanco). Bioorg. Med. Chem. Lett. 2016, 26, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Menichini, F.; Loizzo, M.R.; Bonesi, M.; Conforti, F.; De Luca, D.; Statti, G.A.; De Cindio, B.; Menichini, F.; Tundis, R. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. Diamante flowers, leaves and fruits at two maturity stages. Food. Chem. Toxicol. 2011, 49, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Chodvadiya, V.D.; Pambhar, K.D.; Parmar, N.D.; Ashish, P.D.; Shahrukh, K.A.S.; Pratiksha, V.C.; Hemal, N.R.; Ranjan, C.K.; Patel, P.K. Synthesis and characterization of n-methyl indole derivatives via desulfitative displacement by various amines and its antimicrobial activity. World Sci. News 2019, 120, 181–191. [Google Scholar]
- Son, Y.K.; Lee, M.H.; Han, Y.N. A new antipsychotic effective neolignan from Firmiana simplex. Arch. Pharm. Res. 2005, 28, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.B.; Chattopadhyay, S.K.; Kumar, S.; Chohachi, K.; Yoshnobu, K.; Hiroshi, H. Structures of cleomiscosins, coumarinolignoids of Cleome viscosa seeds. Tetrahedron 1985, 41, 209–214. [Google Scholar] [CrossRef]
- Kumar, S.; Ray, A.B.; Konno, C.; Oshima, Y.; Hikino, H. Cleomiscosin D, a coumarino-lignan from seeds of Cleome viscosa. Phytochemistry 1988, 27, 636–638. [Google Scholar] [CrossRef]
- Guetchueng, T.S.; Lutfun, N.; Kenneth, J.R.; Fyaz, M.D.; I Andrew, R.E.; Sarker, S.D. Ent-clerodane diterpenes from the bark of Croton oligandrus Pierre ex Hutch. and assessment of their cytotoxicity against human cancer cell lines. Molecules 2018, 23, 410. [Google Scholar] [CrossRef] [PubMed]
 COSY
COSY  .
.
 COSY
COSY  .
.
| Attribution | 1H [m, J (Hz)] | 13C |
|---|---|---|
| 1 | - | - |
| 2 | 3.98 (d, 4.4) | 57.6 |
| 3 | 3.19 (d, 4.4) | 26.2 |
| 4 | 7.45 (brs) | 126.8 |
| 5 | - | 160.7 |
| 6 | 7.76 (d, 8.6) | 125.1 |
| 7 | 6.90 (d, 8.6) | 110.8 |
| 8 | - | 105.7 |
| 9 | - | 130.0 |
| N-CH3 | 3.82 (s) | 50.9 |
| N-CHO | 9.16 (s) | 163.7 |
| OH | 11.60 (s) | - |
| Attribution | 1H [m, J (Hz)] | 13C |
|---|---|---|
| 1 | - | - |
| 2 | - | 161.4 |
| 3 | 6.31 (d, 9.5) | 113.6 |
| 4 | 7.67 (d, 9.5) | 144.4 |
| 5 | 6.53 (s) | 100.2 |
| 6 | 146.1 | |
| 7 | 137.7 | |
| 8 | 132.1 | |
| 9 | 138.6 | |
| 10 | 111.6 | |
| 1′ | 125.9 | |
| 2′ | 6.64 (s) | 113.6 |
| 3′ | 147.6 | |
| 4′ | 135.8 | |
| 5′ | 147.6 | |
| 6′ | 6.64 (s) | 104.6 |
| 7′ | 4.99 (d,13.7) | 75.6 |
| 8′ | 4.10 (m) | 78.8 |
| 9′ | 3.66 (dd, 12.5; 6.9) 3.76 (dd, 12.5; 7.0) | 61.2 |
| 1″ | 3.60 (m) | 60.7 |
| 2″ | 2.00 (m) | 31.8 |
| 3″–8″ | 1.21 (brs) | 22.6–29.6 |
| 9″ | 0.83 (t; 7.5) | 14.0 |
| MeO-6 | 3.86 (s) | 56.4 |
| MeO-3′ | 3.84 (s) | 56.3 |
| MeO-5′ | 3.84 (s) | 56.3 |
| Sample | A549 | PC-3 |
|---|---|---|
| 1 | 112.5 ± 11.5 µM | 99.5 ± 11.5 µM |
| 2 | 250.2 ± 10.2 µM | 192.7 ± 12.3 µM |
| 3 | 99.2 ± 9.5 µM | 100.2 ± 12.7 µM |
| 4 | 152.5 ± 11.3 µM | 147.3 ± 21.5 µM |
| 5 | 201.3 ± 13.5 µM | 180.2 ± 14.3 µM |
| 6 | 156.2 ± 18.2 µM | 150.2 ± 21.3 µM |
| 7 | 135.6 ± 21.8 µM | 1335 ± 22.6 µM |
| root extract | 35.2 ± 2.3 µg/mL | 38.1 ± 2.5 µg/mL |
| doxorubicin | 0.9 ± 0.1 µM | 1.6 ± 0.2 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essombe Malolo, F.-A.; Kouam, A.D.K.; Nyobe, J.C.N.; Ngah, L.; Frese, M.; Ndom, J.C.; Langat, M.K.; Lenta, B.N.; Mulholland, D.A.; Sewald, N.; et al. Coumarinolignoid and Indole Alkaloids from the Roots of the Hybrid Plant Citrus × paradisi Macfad (Rutaceae). Molecules 2023, 28, 1078. https://doi.org/10.3390/molecules28031078
Essombe Malolo F-A, Kouam ADK, Nyobe JCN, Ngah L, Frese M, Ndom JC, Langat MK, Lenta BN, Mulholland DA, Sewald N, et al. Coumarinolignoid and Indole Alkaloids from the Roots of the Hybrid Plant Citrus × paradisi Macfad (Rutaceae). Molecules. 2023; 28(3):1078. https://doi.org/10.3390/molecules28031078
Chicago/Turabian StyleEssombe Malolo, Fanny-Aimée, Ariane Dolly Kenmogne Kouam, Judith Caroline Ngo Nyobe, Lidwine Ngah, Marcel Frese, Jean Claude Ndom, Moses K. Langat, Bruno Ndjakou Lenta, Dulcie A. Mulholland, Norbert Sewald, and et al. 2023. "Coumarinolignoid and Indole Alkaloids from the Roots of the Hybrid Plant Citrus × paradisi Macfad (Rutaceae)" Molecules 28, no. 3: 1078. https://doi.org/10.3390/molecules28031078
APA StyleEssombe Malolo, F.-A., Kouam, A. D. K., Nyobe, J. C. N., Ngah, L., Frese, M., Ndom, J. C., Langat, M. K., Lenta, B. N., Mulholland, D. A., Sewald, N., & Wansi, J. D. (2023). Coumarinolignoid and Indole Alkaloids from the Roots of the Hybrid Plant Citrus × paradisi Macfad (Rutaceae). Molecules, 28(3), 1078. https://doi.org/10.3390/molecules28031078