Possibilities and Limitations of the Sono-Fenton Process Using Mid-High-Frequency Ultrasound for the Degradation of Organic Pollutants
Abstract
:1. Introduction
2. Results and Discussion
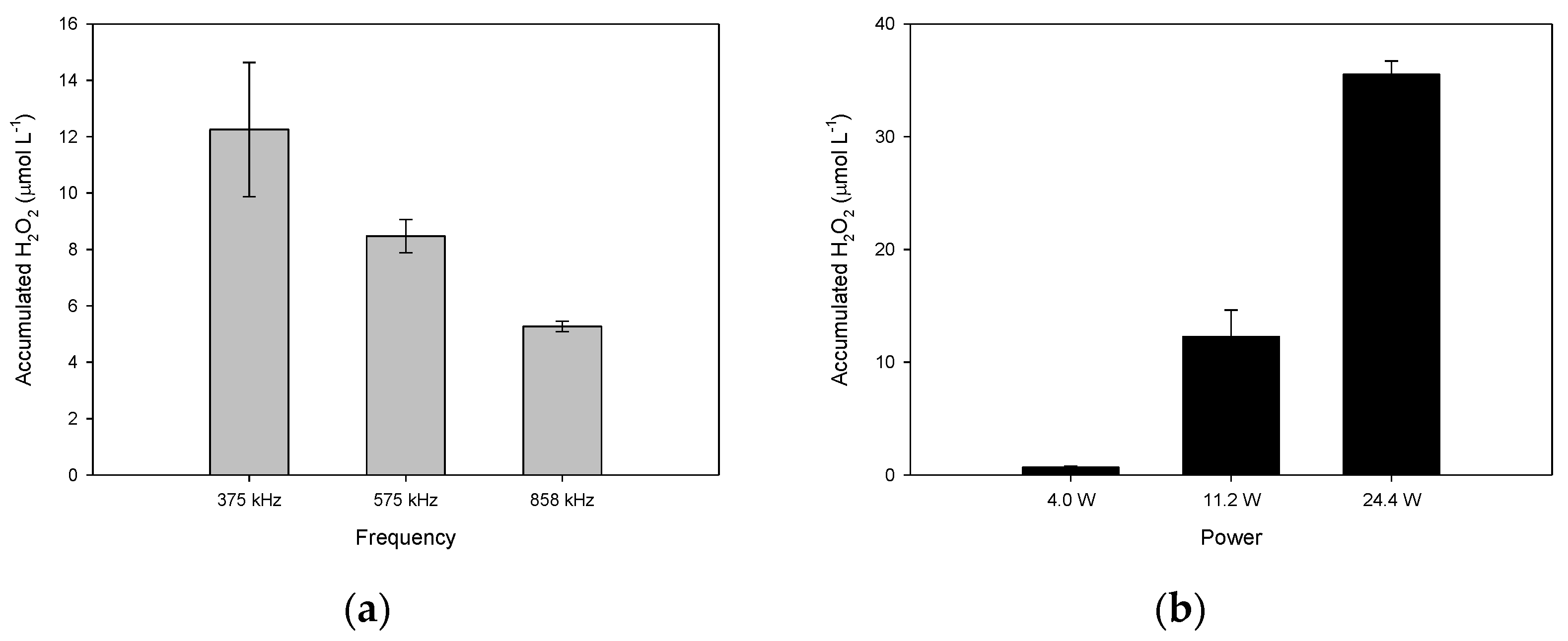
2.1. Suitable Conditions for the Operation of the Ultrasound Reactor to Produce H2O2
2.2. Heterogeneous Sono-Fenton Processes for the Elimination of Pollutants
2.3. Homogeneous Sono-Fenton to Degrade Diverse Organic Pollutants
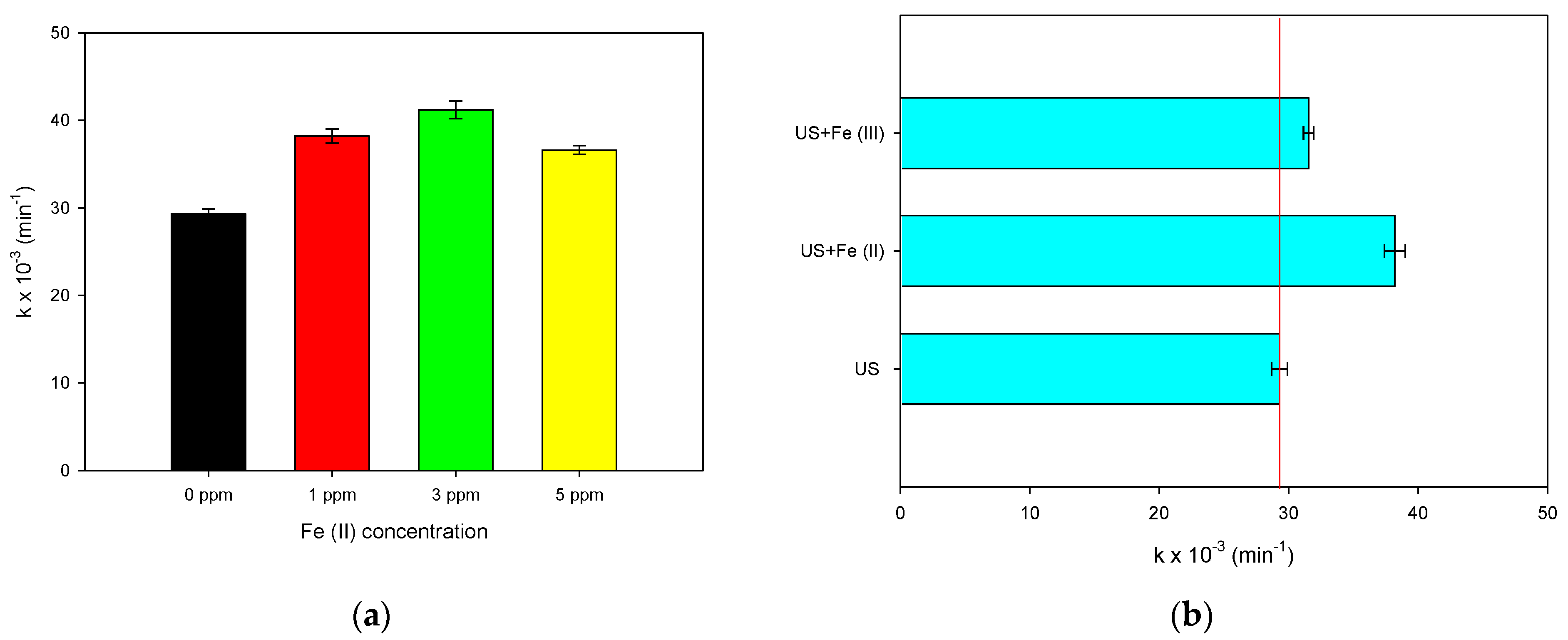
2.3.1. Effect of Iron (II) Concentration and Iron Species (II or III)
2.3.2. Degradation of Diverse Organic Pollutants by Homogeneous Sono-Fenton
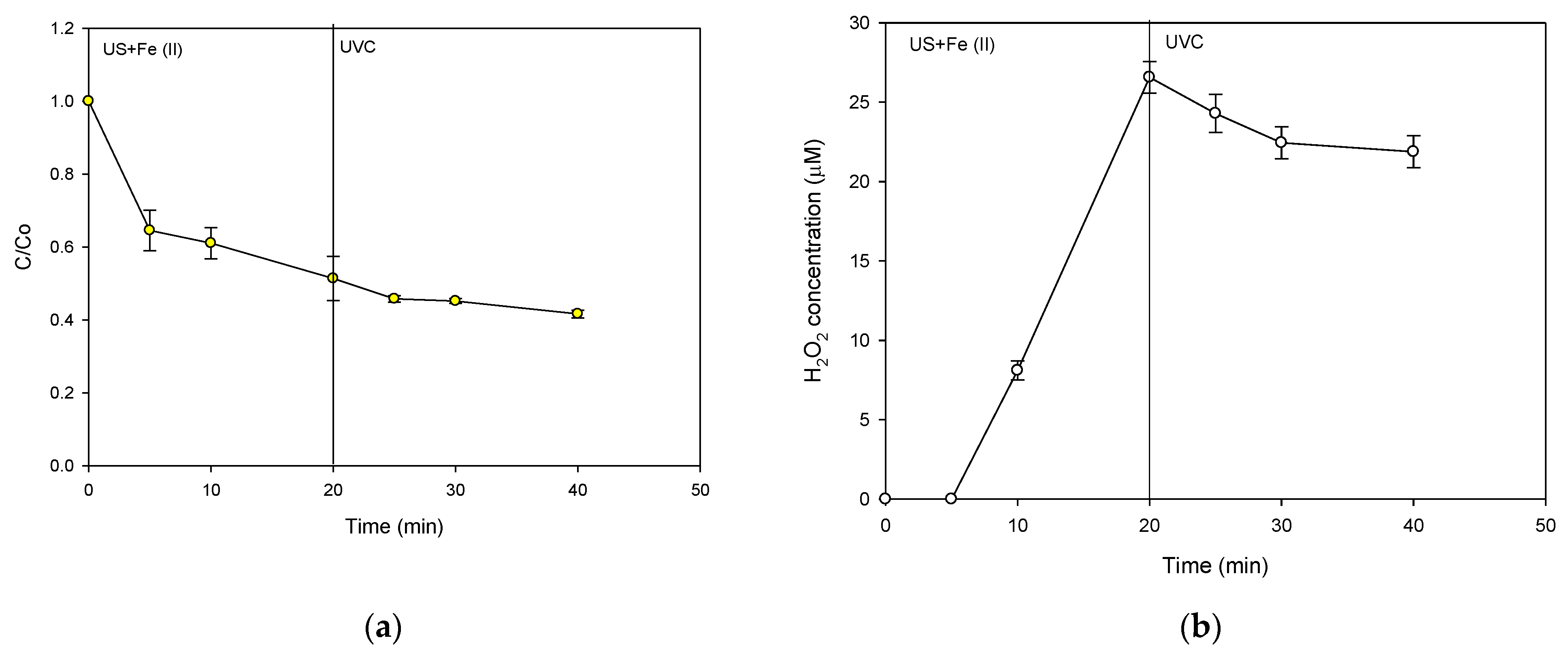
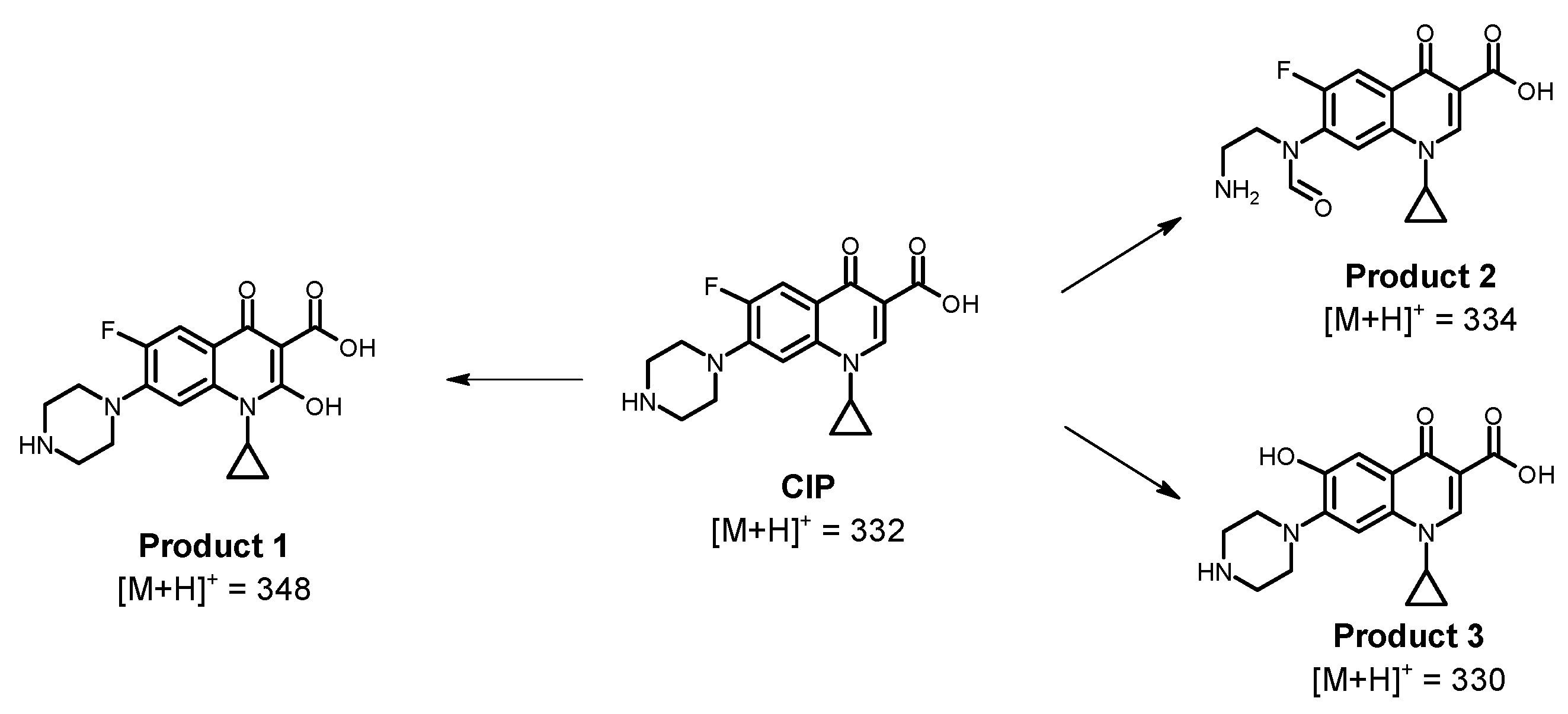
2.4. A Strategy for the Treatment of CIP
3. Materials and Methods
3.1. Reagents
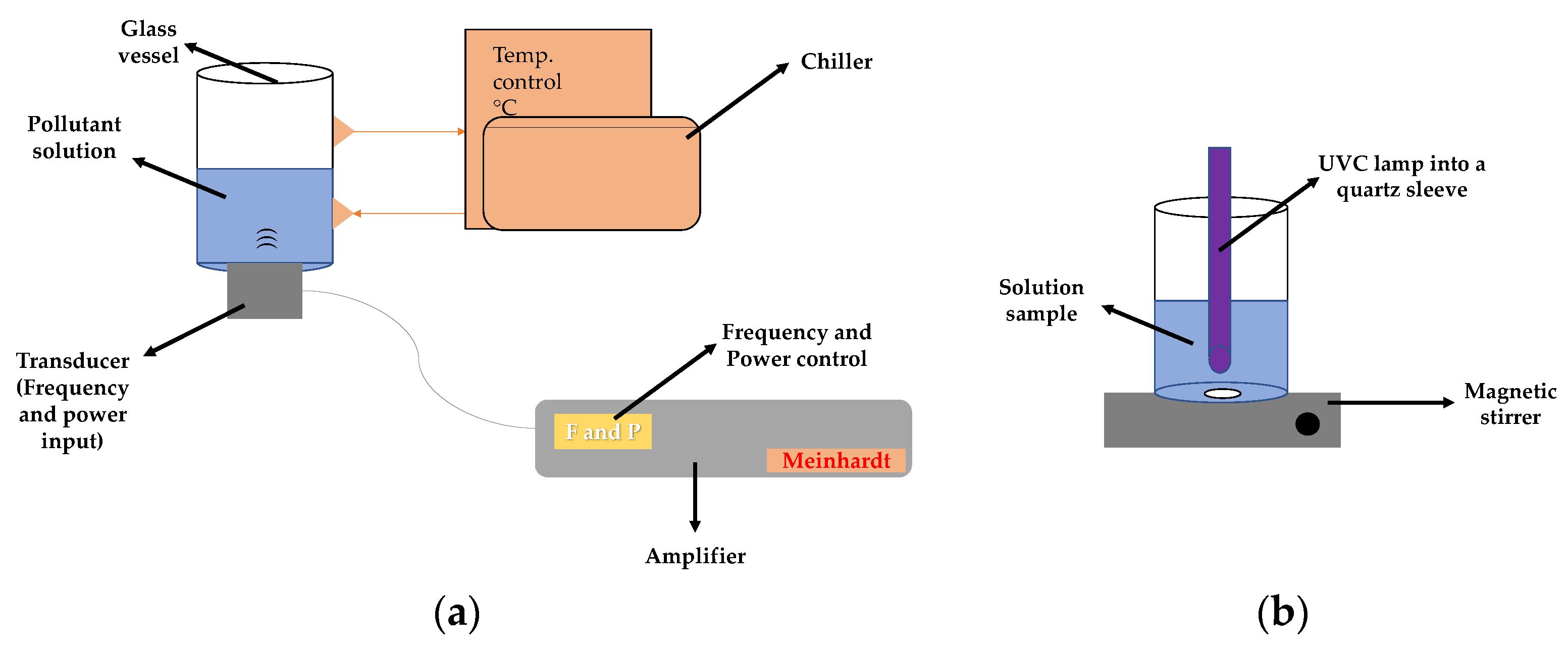
3.2. Reaction System
3.3. Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chakma, S.; Moholkar, V.S. Physical mechanism of sono-Fenton process. AIChE J. 2013, 59, 4303–4313. [Google Scholar] [CrossRef]
- Dükkanci, M.; Vinatoru, M.; Mason, T.J. The sonochemical decolourisation of textile azo dye Orange II: Effects of Fenton type reagents and UV light. Ultrason. Sonochem. 2014, 21, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rodríguez, D.M.; Serna-Galvis, E.A.; Ferraro, F.; Torres-Palma, R.A. Degradation of the emerging concern pollutant ampicillin in aqueous media by sonochemical advanced oxidation processes—Parameters effect, removal of antimicrobial activity and pollutant treatment in hydrolyzed urine. J. Environ. Manag. 2020, 261, 110224. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Papoutsakis, S.; Miralles-Cuevas, S.; Gondrexon, N.; Baup, S.; Malato, S.; Pulgarin, C. Coupling between high-frequency ultrasound and solar photo-Fenton at pilot scale for the treatment of organic contaminants: An initial approach. Ultrason. Sonochem. 2015, 22, 527–534. [Google Scholar] [CrossRef]
- Basturk, E.; Karatas, M. Advanced oxidation of Reactive Blue 181 solution: A comparison between Fenton and Sono-Fenton Process. Ultrason. Sonochem. 2014, 21, 1881–1885. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Kamani, H.; Bazrafshan, E.; Panahi, A.H.; Silva, M.F.; Abi, G. Optimization the Effects of Physicochemical Parameters on the Degradation of Cephalexin in Sono-Fenton Reactor by Using Box-Behnken Response Surface Methodology. Catal. Lett. 2019, 149, 1186–1196. [Google Scholar] [CrossRef]
- Bremner, D.H.; Molina, R.; Martínez, F.; Melero, J.A.; Segura, Y. Degradation of phenolic aqueous solutions by high frequency sono-Fenton systems (US–Fe2O3/SBA-15–H2O2). Appl. Catal. B Environ. 2009, 90, 380–388. [Google Scholar] [CrossRef]
- Huang, R.; Fang, Z.; Yan, X.; Cheng, W. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem. Eng. J. 2012, 197, 242–249. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron(II,III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Khataee, A.; Gholami, P.; Vahid, B.; Joo, S.W. Heterogeneous sono-Fenton process using pyrite nanorods prepared by non-thermal plasma for degradation of an anthraquinone dye. Ultrason. Sonochem. 2016, 32, 357–370. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, Y.T.; Yuan, H.M. Rapid decoloration of Reactive Black 5 by an advanced Fenton process in conjunction with ultrasound. Sep. Purif. Technol. 2013, 117, 75–82. [Google Scholar] [CrossRef]
- Cai, M.; Su, J.; Lian, G.; Wei, X.; Dong, C.; Zhang, H.; Jin, M.; Wei, Z. Sono-advanced Fenton decolorization of azo dye Orange G: Analysis of synergistic effect and mechanisms. Ultrason. Sonochem. 2016, 31, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Y.; Cai, W.; Zhang, J. Preparation and sono-Fenton performance of 4A-zeolite supported α-Fe2O3. J. Hazard. Mater. 2010, 177, 743–749. [Google Scholar] [CrossRef]
- Wang, C.; Shih, Y. Degradation and detoxification of diazinon by sono-Fenton and sono-Fenton-like processes. Sep. Purif. Technol. 2015, 140, 6–12. [Google Scholar] [CrossRef]
- Siddique, M.; Farooq, R.; Price, G.J. Synergistic effects of combining ultrasound with the Fenton process in the degradation of Reactive Blue 19. Ultrason. Sonochem. 2014, 21, 1206–1212. [Google Scholar] [CrossRef] [Green Version]
- Baştürk, E.; Alver, A. Modeling azo dye removal by sono-fenton processes using response surface methodology and artificial neural network approaches. J. Environ. Manag. 2019, 248, 109300. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Jáuregui Haza, U.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste) water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Villegas-Guzman, P.; Giannakis, S.; Rtimi, S.; Grandjean, D.; Bensimon, M.; de Alencastro, L.F.; Torres-Palma, R.; Pulgarin, C. A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl. Catal. B Environ. 2017, 219, 538–549. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ. Chem. Lett. 2016, 14, 259–290. [Google Scholar] [CrossRef]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Phys. Rev. Lett. 2009, 102, 084302. [Google Scholar] [CrossRef] [PubMed]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical degradation of Coomassie Brilliant Blue: Effect of frequency, power density, pH and various additives. Chemosphere 2015, 119, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M. Sonochemical degradation of Rhodamine B in aqueous phase: Effects of additives. Chem. Eng. J. 2010, 158, 550–557. [Google Scholar] [CrossRef]
- Jojoa-Sierra, S.D.; Herrero-Albillos, J.; Ormad, M.P.; Serna-Galvis, E.A.; Torres-Palma, R.A.; Mosteo, R. Wüstite as a catalyst source for water remediation: Differentiated antimicrobial activity of by-products, action routes of the process, and transformation of fluoroquinolones. Chem. Eng. J. 2022, 435, 134850. [Google Scholar] [CrossRef]
- Liang, J.; Komarov, S.; Hayashi, N.; Kasai, E. Improvement in sonochemical degradation of 4-chlorophenol by combined use of Fenton-like reagents. Ultrason. Sonochem. 2007, 14, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Drijvers, D.; Van Langenhove, H.; Beckers, M. Decomposition of phenol and trichloroethylene by the ultrasound/H2O2/CuO process. Water Res. 1999, 33, 1187–1194. [Google Scholar] [CrossRef]
- Morosini, V.; Chave, T.; Virot, M.; Moisy, P.; Nikitenko, S.I. Sonochemical water splitting in the presence of powdered metal oxides. Ultrason. Sonochem. 2016, 29, 512–516. [Google Scholar] [CrossRef]
- Berberidou, C.; Poulios, I.; Xekoukoulotakis, N.P.; Mantzavinos, D. Sonolytic, photocatalytic and sonophotocatalytic degradation of malachite green in aqueous solutions. Appl. Catal. B Environ. 2007, 74, 63–72. [Google Scholar] [CrossRef]
- Giraldo-Aguirre, A.L.; Serna-Galvis, E.A.; Erazo-Erazo, E.D.; Silva-Agredo, J.; Giraldo-Ospina, H.; Flórez-Acosta, O.A.; Torres-Palma, R.A. Removal of β-lactam antibiotics from pharmaceutical wastewaters using photo-Fenton process at near-neutral pH. Environ. Sci. Pollut. Res. 2018, 25, 20293–20303. [Google Scholar] [CrossRef]
- Enric Brillas Electro-Fenton, UVA Photoelectro-Fenton and Solar Photoelectro-Fenton Treatments of Organics in Waters Using a Boron-Doped Diamond Anode: A Review. J. Mex. Chem. Soc. 2014, 58, 239–255.
- Sun, Y.; Pignatello, J.J. Photochemical Reactions Involved in the Total Mineralization of 2,4-D by Fe3+/H2O2/UV. Environ. Sci. Technol. 1993, 27, 304–310. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Serna-Galvis, E.A.; Martínez-Mena, Y.L.; Porras, J.; Ávila-Torres, Y.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the Role of Complexation of Fluoroquinolone and β-Lactam Antibiotics with Iron (III) on the Photodegradation under Solar Light and UVC Light. Water 2021, 13, 2603. [Google Scholar] [CrossRef]
- Sciscenko, I.; Arques, A.; Varga, Z.; Bouchonnet, S.; Monfort, O.; Brigante, M.; Mailhot, G. Significant role of iron on the fate and photodegradation of enrofloxacin. Chemosphere 2021, 270, 129791. [Google Scholar] [CrossRef]
- Aristilde, L.; Sposito, G. Molecular modeling of metal complexation by a fluoroquinolone antibiotic. Environ. Toxicol. Chem. 2008, 27, 2304–2310. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Mononuclear metal complexes of the second-generation quinolone antibacterial agent enrofloxacin: Synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron 2008, 27, 1729–1738. [Google Scholar] [CrossRef]
- Albini, A.; Monti, S. Photophysics and photochemistry of fluoroquinolones. Chem. Soc. Rev. 2003, 32, 238. [Google Scholar] [CrossRef]
- Sciscenko, I.; Garcia-Ballesteros, S.; Sabater, C.; Castillo, M.A.; Escudero-Oñate, C.; Oller, I.; Arques, A. Monitoring photolysis and (solar photo)-Fenton of enrofloxacin by a methodology involving EEM-PARAFAC and bioassays: Role of pH and water matrix. Sci. Total Environ. 2020, 719, 137331. [Google Scholar] [CrossRef]
- Torres, R.; Pétrier, C.; Combet, E.; Moulet, F.; Pulgarin, C. Bisphenol A Mineralization by Integrated Ultrasound-UV-Iron (II) Treatment. Environ. Sci. Technol. 2007, 41, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Giraldo-Aguirre, A.L.; Torres-Palma, R.A. Sonochemical degradation of the pharmaceutical fluoxetine: Effect of parameters, organic and inorganic additives and combination with a biological system. Sci. Total Environ. 2015, 524–525, 354–360. [Google Scholar] [CrossRef]
- Giannakis, S.; Jovic, M.; Gasilova, N.; Pastor Gelabert, M.; Schindelholz, S.; Furbringer, J.-M.M.; Girault, H.; Pulgarin, C. Iohexol degradation in wastewater and urine by UV-based Advanced Oxidation Processes (AOPs): Process modeling and by-products identification. J. Environ. Manag. 2017, 195, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Hendaoui, I.; Jovic, M.; Grandjean, D.; De Alencastro, L.F.; Girault, H.; Pulgarin, C. Solar photo-Fenton and UV/H2O2 processes against the antidepressant Venlafaxine in urban wastewaters and human urine. Intermediates formation and biodegradability assessment. Chem. Eng. J. 2017, 308, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Chen, J.; Xie, Q.; Zhang, S.; Li, Y.; Zhang, Y.; Xie, H. Photochemical behavior of antibiotics impacted by complexation effects of concomitant metals: A case for ciprofloxacin and Cu(II). Environ. Sci. Process. Impacts 2015, 17, 1220–1227. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Hao, C.; Peijnenburg, W.J.G.M. DFT/TDDFT insights into effects of dissociation and metal complexation on photochemical behavior of enrofloxacin in water. Environ. Sci. Pollut. Res. 2018, 25, 30609–30616. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z. Elucidating Direct Photolysis Mechanisms of Different Dissociation Species of Norfloxacin in Water and Mg2+ Effects by Quantum Chemical Calculations. Molecules 2017, 22, 1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, T.; Yang, H.; Li, G.; Song, W.; Cooper, W.J.; Nie, X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Jia, M.; Wang, S.; Huang, Y.; Chen, C. Degradation of ciprofloxacin in aqueous bismuth oxybromide (BiOBr) suspensions under visible light irradiation: A direct hole oxidation pathway. Chem. Eng. J. 2015, 274, 290–297. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Zhang, M.; Zhang, B.; Yu, Y. The electrochemical degradation of ciprofloxacin using a SnO2-Sb/Ti anode : Influencing factors, reaction pathways and energy demand. Chem. Eng. J. 2016, 296, 79–89. [Google Scholar] [CrossRef]
- Ji, Y.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808. [Google Scholar] [CrossRef]
- Mahdi-Ahmed, M.; Chiron, S. Ciprofloxacin oxidation by UV-C activated peroxymonosulfate in wastewater. J. Hazard. Mater. 2014, 265, 41–46. [Google Scholar] [CrossRef]
- Paul, T.; Dodd, M.C.; Strathmann, T.J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin : Transformation products and residual antibacterial activity. Water Res. 2010, 44, 3121–3132. [Google Scholar] [CrossRef]
- Andersson, M.I.; MacGowan, A.P. Development of the quinolones. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Sakamoto, T.; Leveque, J.M.; Sohmiya, H.; Fujita, M.; Ikeda, S.; Ando, T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996, 3, S157–S161. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Moreno-SanSegundo, J.; Álvarez-Fernández, C.; van Grieken, R.; Marugán, J. High-performance low-cost solar collectors for water treatment fabricated with recycled materials, open-source hardware and 3d-printing technologies. Sci. Total Environ. 2021, 784, 147119. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Giraldo-Aguirre, A.L.; Flórez-Acosta, O.A.; Torres-Palma, R.A. High frequency ultrasound as a selective advanced oxidation process to remove penicillinic antibiotics and eliminate its antimicrobial activity from water. Ultrason. Sonochem. 2016, 31, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Cáceres-Peña, A.C.; Torres-Palma, R.A. Elimination of representative fluoroquinolones, penicillins, and cephalosporins by solar photo-Fenton: Degradation routes, primary transformations, degradation improvement by citric acid addition, and antimicrobial activity evolution. Environ. Sci. Pollut. Res. 2020, 27, 41381–41393. [Google Scholar] [CrossRef]


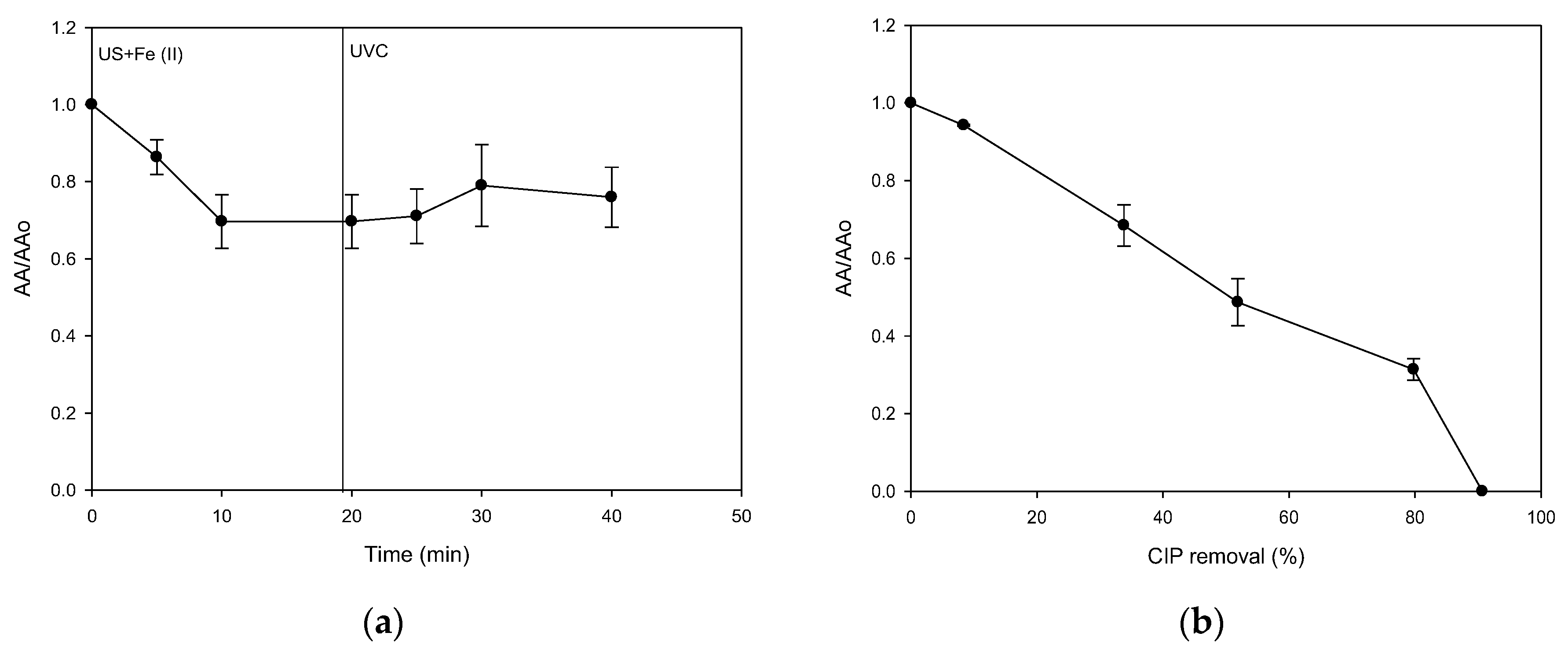
| Heterogeneous Iron Source | Ultrasound System | Target Pollutant | Main Results | Reference |
|---|---|---|---|---|
| Fe2O3/SBA-15 (0.6 g L−1) | 20, 382, 584, and 1142 kHz, with external addition of H2O2 (1.19 g L−1) | Phenol | Highest elimination of aromatic compounds and mineralization at 584 kHz due to its highest acoustic power and elevated production of degrading radicals. Iron ions are leached from the solid catalyst. | [8] |
| ZSM-5 zeolite containing iron (0.1 mmol L−1 of iron) | 850 kHz, with the external addition of H2O2 (5.0 mmol L−1) | Orange II | The degradation of Orange II induced by the sono-Fenton system was very similar to that obtained under the combination of ultrasound with hydrogen peroxide. | [2] |
| 4A-zeolite supported α-Fe2O3 (0.5 g L−1) | 40 kHz | Orange II | The removal of the pollutant is related to adsorption on the catalyst, heterogeneous, and homogeneous (iron dissolved into the solution) Fenton reaction. | [14] |
| Zero valent iron-ZVI (0.5 g L−1) | 28 kHz, without and with external addition of H2O2 (30–100 µmol L−1) | Orange G | Ultrasound promotes the leaching of iron ions, which enhances the dye degradation regarding ultrasound alone or adsorption on the ZVI. Moreover, the external addition of H2O2 increases the degradation and mineralization. However, an excess of H2O2 induces scavenging effects. | [13] |
| Zero valent iron-ZVI (1.0 g L−1) | 60 kHz, with the external addition of H2O2 (10.3 mmol L−1) | Reactive Black 5 | Synergistic effects for the dye degradation by the ZVI/H2O2/ultrasound combination. Hydrogen peroxide produced from sonolysis in contact with Fe (II), coming from ZVI corrosion, triggers the Fenton reaction. | [12] |
| Pyrite nanorods (0.6 g L−1) | 40 kHz, with the external addition of H2O2 (1.0 mmol L−1) | Reactive Blue 69 | The sono-elimination of the target dye is significantly improved by the addition of pyrite nanorods and H2O2, reporting synergy for the combination of ultrasound with pyrite and hydrogen peroxide. Synergy is explained considering that ultrasound waves increase the turbulence and mass transfer and also promote particle disaggregation by augmenting the active sites on the catalyst surface. In turn, the crevices of the solid particles act as cavitation nuclei. | [11] |
| Fe3O4/ZnO/graphene composites | 40 kHz is added to a Fenton process | Methylene blue and Congo-red | The addition of ultrasound irradiation to the Fenton process improves the degradation of both dyes. | [10] |
| Fe3O4 magnetic nanoparticles (0.585 g L−1) | 40 kHz, with the external addition of H2O2 (160 mmol L−1) | Bisphenol-A | No adsorption of the pollutant on the catalyst. The decomposition of H2O2 into radicals promoted by ultrasound plus disaggregation of particles favors the Fenton reaction, leading to synergistic effects on the degradation of bisphenol-A. | [9] |
| Natural mineral containing iron oxides, mainly hematite (Fe2O3) (0.20 g L−1) | 375 kHz | MO and ACE | Degradation of the pollutants by sono-Fenton was very close to that obtained by ultrasound alone | This work |
| Homogeneous Iron Source | Ultrasound System | Target Pollutant | Main Results | Reference |
|---|---|---|---|---|
| Fe2+ (10 mg L−1) | 35 and 53 kHz, with the external addition of H2O2 (50 mg L−1) | Reactive Blue 181 | The sono-Fenton process has superior performance compared to the Fenton system in terms of degrading the target pollutant because of the production of some oxidizing agents as a result of sonication. | [6] |
| Fe2+ (3.0 mg L−1) | 20 kHz, with the external addition of H2O2 (0.5 mmol L−1) | Reactive Blue 19 | The combination of ultrasound with Fe2+ and H2O2 leads to a higher degradation of the dye than the individual components (even more than the Fenton system) of the sono-Fenton process. | [16] |
| Fe2+ (0.134 mmol L−1) | 20 kHz, with the external addition of H2O2 (6.4 mmol L−1) | Ibuprofen | The addition of Fe2+ and H2O2 to the ultrasound reactor increases both the degradation and mineralization of the pharmaceutical. | [18] |
| Fe2+ (Different concentrations) | 850 kHz, without and with the external addition of H2O2 (Diverse concentrations) | Orange II | Acceleration of the pollutant degradation by adding Fe2+, taking advantage of the sono-generated H2O2. The external addition of both Fe2+ and H2O2 lead to the best dye degradation. However, an excess of Fe2+ and H2O2 leads to scavenging effects. | [2] |
| Fe2+ (0.1 mmol L−1) | 300 kHz | Bisphenol-A | The degradation and mineralization of bisphenol-A are enhanced by the presence of ferrous ions due to the Fenton reaction using the H2O2 coming from the sonochemical system. | [39] |
| Fe2+ (90 µmol L−1) | 600 kHz | Fluoxetine | The degradation of fluoxetine is enhanced by the presence of ferrous ions that react with the sonogenerated H2O2. | [40] |
| Fe2+ (90 µmol L−1) | 375 kHz | Ampicillin | The degradation and mineralization of ampicillin are enhanced by the presence of ferrous ions due to the Fenton reaction using the H2O2 coming from the sonochemical system. | [3] |
| Fe2+ (1.0, 3.0, and 5.0 mg L−1) and Fe3+ (1.0 mg L−1) | 375 kHz | MO, ACE, and CIP | Acceleration of the MO and ACE degradation by adding Fe2+ by taking advantage of the sono-generated H2O2. However, an excess of Fe2+ leads to scavenging effects. Ferrous ions are more efficient than Ferric ions at accelerating the degradation of pollutants. Furthermore, CIP is complexed by Fe3+, limiting the performance of the sono-Fenton process. | This work |
| Pharmaceutical | Acetonitrile/Formic Acid (%/%) | Detection Wavelength (nm) | Flow (mL min−1) |
|---|---|---|---|
| Ciprofloxacin (CIP) | 15/85 | 278 | 1.0 |
| Acetaminophen (ACE) | 15/85 | 243 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Galvis, E.A.; Silva-Agredo, J.; Lee, J.; Echavarría-Isaza, A.; Torres-Palma, R.A. Possibilities and Limitations of the Sono-Fenton Process Using Mid-High-Frequency Ultrasound for the Degradation of Organic Pollutants. Molecules 2023, 28, 1113. https://doi.org/10.3390/molecules28031113
Serna-Galvis EA, Silva-Agredo J, Lee J, Echavarría-Isaza A, Torres-Palma RA. Possibilities and Limitations of the Sono-Fenton Process Using Mid-High-Frequency Ultrasound for the Degradation of Organic Pollutants. Molecules. 2023; 28(3):1113. https://doi.org/10.3390/molecules28031113
Chicago/Turabian StyleSerna-Galvis, Efraím A., Javier Silva-Agredo, Judy Lee, Adriana Echavarría-Isaza, and Ricardo A. Torres-Palma. 2023. "Possibilities and Limitations of the Sono-Fenton Process Using Mid-High-Frequency Ultrasound for the Degradation of Organic Pollutants" Molecules 28, no. 3: 1113. https://doi.org/10.3390/molecules28031113
APA StyleSerna-Galvis, E. A., Silva-Agredo, J., Lee, J., Echavarría-Isaza, A., & Torres-Palma, R. A. (2023). Possibilities and Limitations of the Sono-Fenton Process Using Mid-High-Frequency Ultrasound for the Degradation of Organic Pollutants. Molecules, 28(3), 1113. https://doi.org/10.3390/molecules28031113








