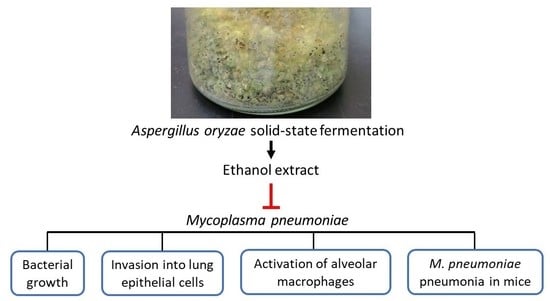
Aspergillus oryzae Fermentation Extract Alleviates Inflammation in Mycoplasma pneumoniae Pneumonia
Abstract
1. Introduction
2. Results
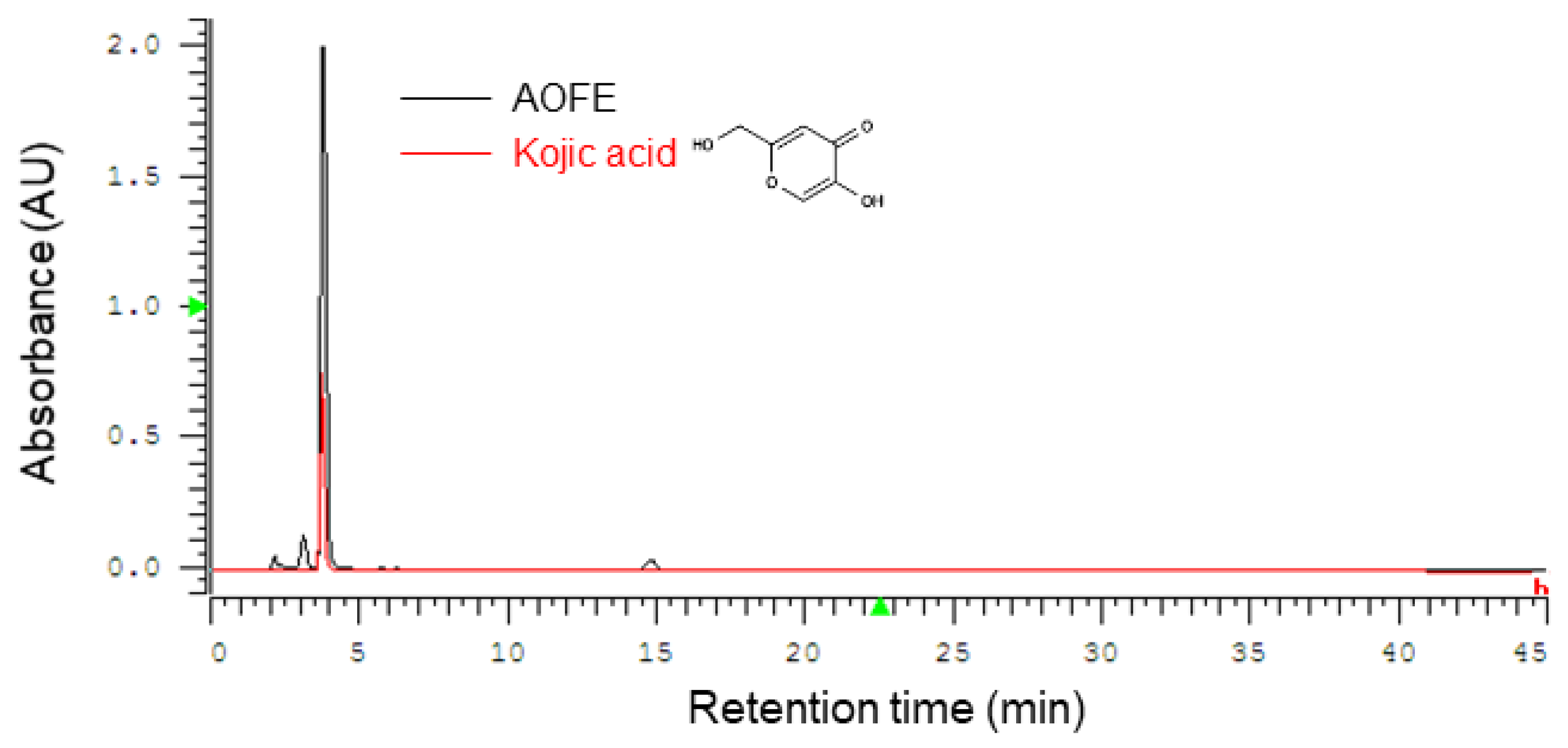
2.1. High-Performance Liquid Chromatography (HPLC) Profile of AOFE
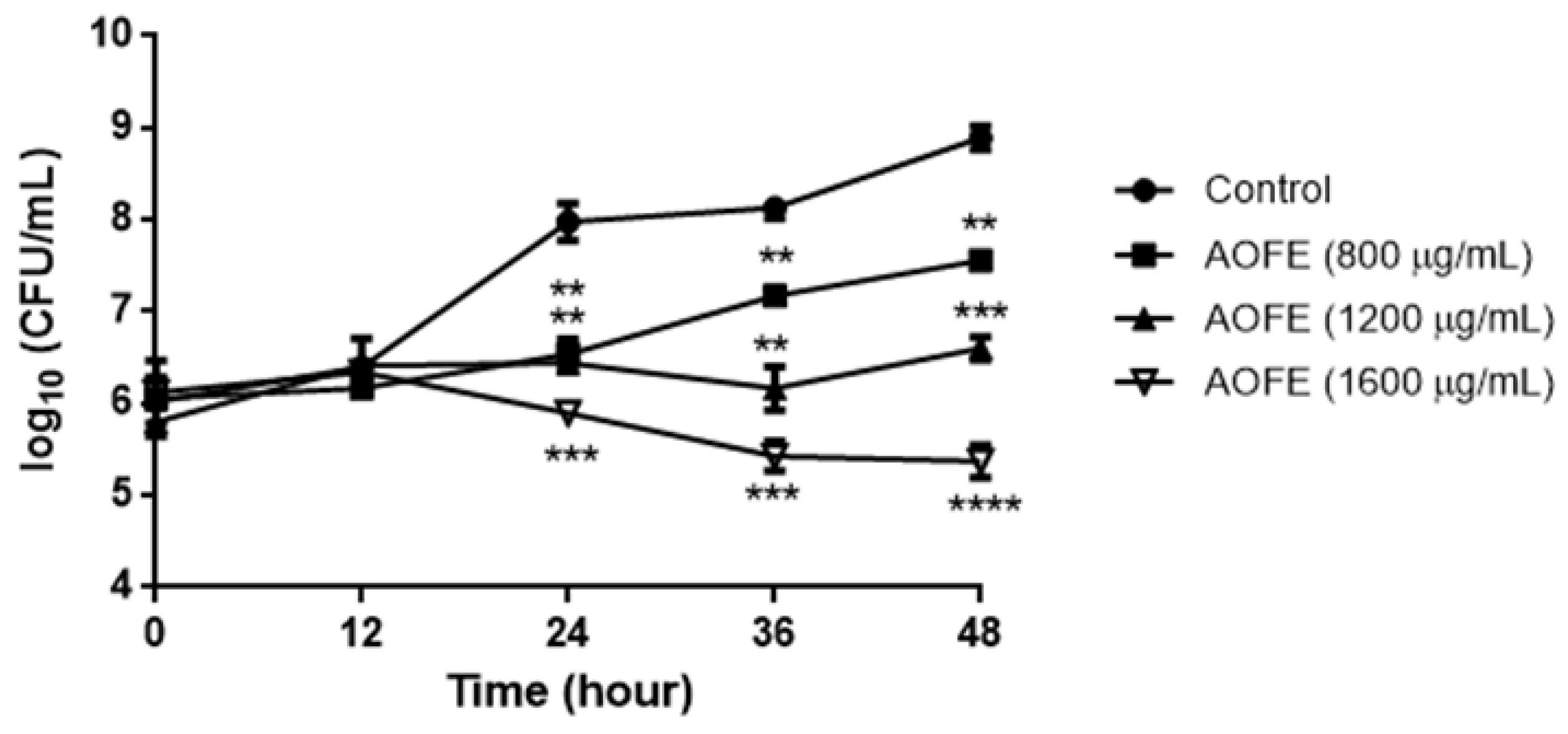
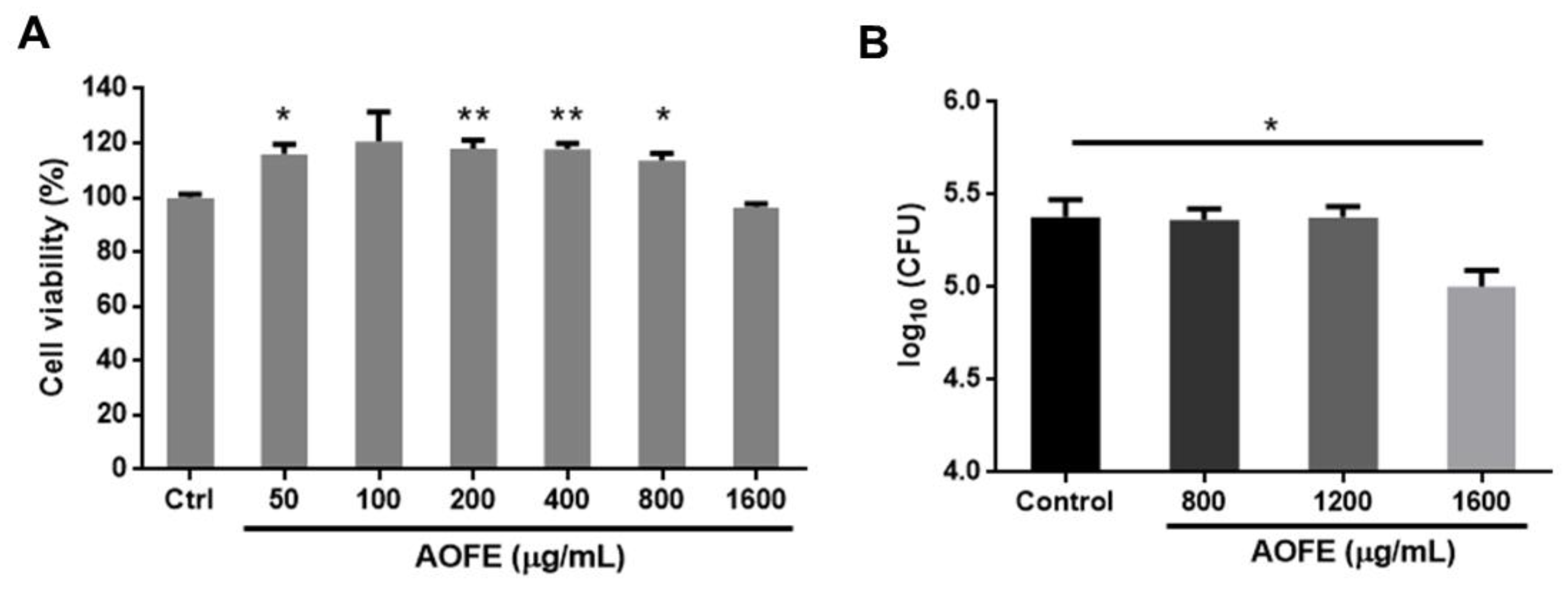
2.2. AOFE Inhibits the Growth of Mp
2.3. AOFE Reduces the Invasion of Mp into Lung Epithelial Cells
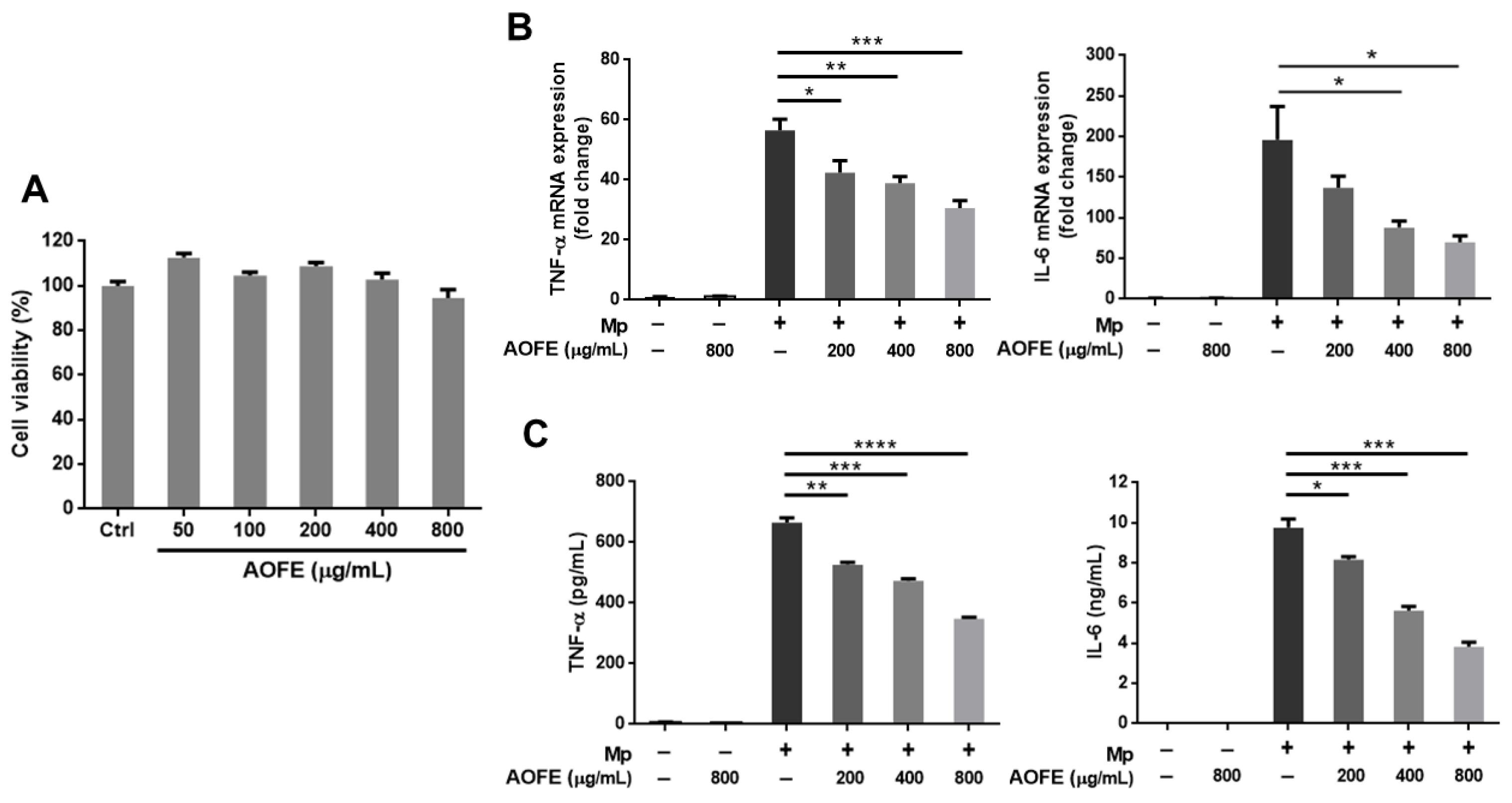
2.4. AOFE Reduces Mp-Induced Inflammatory Response in Alveolar Macrophages
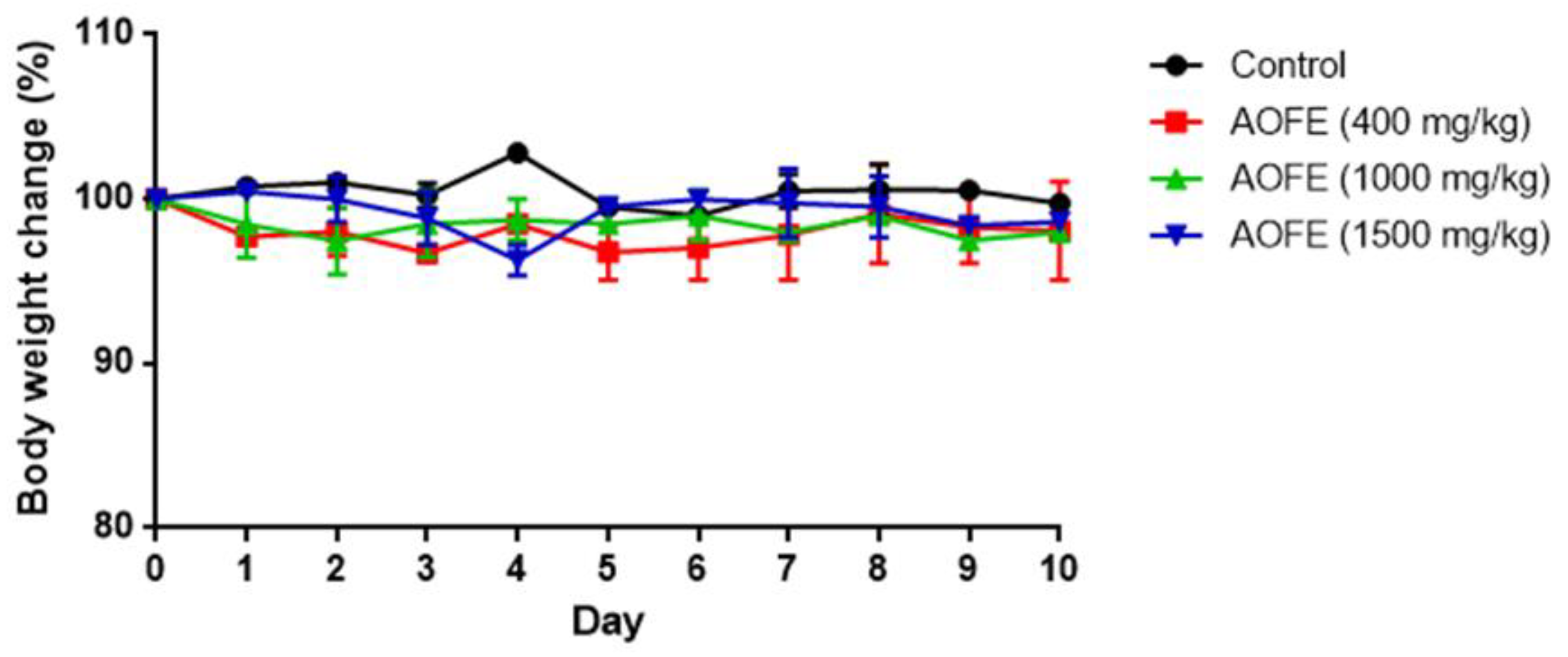
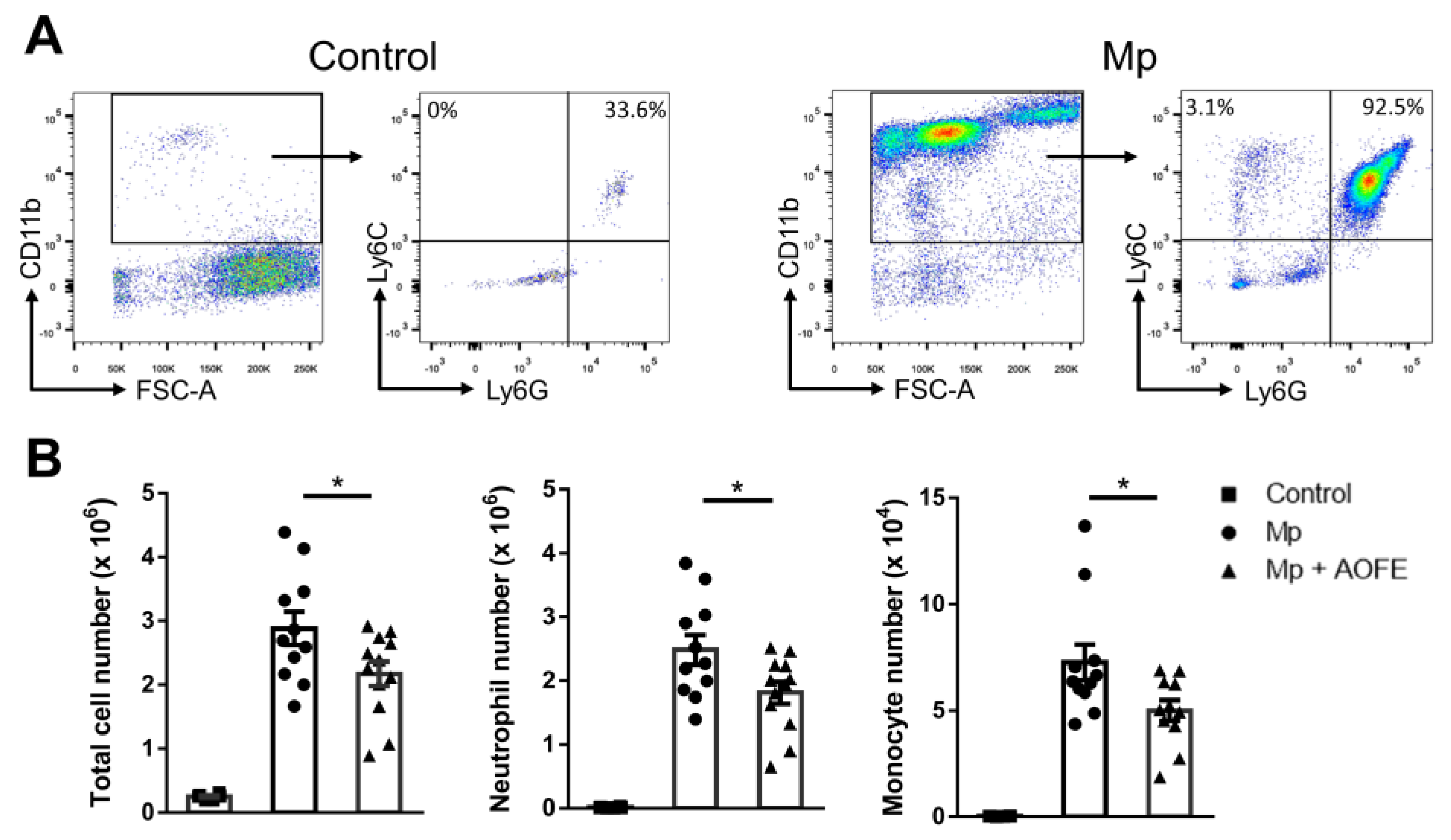
2.5. AOFE Reduces Mp-Induced Lung Inflammation in Mice
3. Discussion
4. Materials and Methods
4.1. Mp and Culture Conditions
4.2. Cell Lines
4.3. A. oryzae Fermentation and AOFE Preparation
4.4. HPLC Analysis
4.5. Extraction of Mp DNA
4.6. Detection of Mp by qPCR
4.7. Cell Viability Analysis
4.8. In Vitro Stimulation of MH-S Cells with Mp
4.9. Gentamicin Protection Assay
4.10. Mp Growth Curve
4.11. Animals and Mp Inoculation
4.12. Bronchoalveolar Lavage and BALF Analysis
4.13. Flow Cytometric Analysis
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Atkinson, T.P.; Balish, M.F.; Waites, K.B. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol. Rev. 2008, 32, 956–973. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Xiao, L.; Liu, Y.; Balish, M.F.; Atkinson, T.P. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin. Microbiol. Rev. 2017, 30, 747–809. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, M.; Ye, Z.; Tan, T.; Liu, X.; You, X.; Zeng, Y.; Wu, Y. Insights into the pathogenesis of Mycoplasma pneumoniae (Review). Mol. Med. Rep. 2016, 14, 4030–4036. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kida, Y.; Kuwano, K. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect. Immun. 2008, 76, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.W.; Jeyaseelan, S.; Rino, J.G.; Voelker, D.R.; Wexler, R.B.; Campbell, K.; Harbeck, R.J.; Martin, R.J. TLR2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J. Immunol. 2005, 174, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- Saraya, T.; Nakata, K.; Nakagaki, K.; Motoi, N.; Iihara, K.; Fujioka, Y.; Oka, T.; Kurai, D.; Wada, H.; Ishii, H.; et al. Identification of a mechanism for lung inflammation caused by Mycoplasma pneumoniae using a novel mouse model. Results Immunol. 2011, 1, 76–87. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Bredt, W. Phagocytosis by macrophages of Mycoplasma pneumoniae after opsonization by complement. Infect. Immun. 1975, 12, 694–695. [Google Scholar] [CrossRef]
- Lai, J.F.; Zindl, C.L.; Duffy, L.B.; Atkinson, T.P.; Jung, Y.W.; van Rooijen, N.; Waites, K.B.; Krause, D.C.; Chaplin, D.D. Critical role of macrophages and their activation via MyD88-NFκB signaling in lung innate immunity to Mycoplasma pneumoniae. PLoS ONE 2010, 5, e14417. [Google Scholar] [CrossRef]
- Tamiya, S.; Yoshikawa, E.; Ogura, M.; Kuroda, E.; Suzuki, K.; Yoshioka, Y. Neutrophil-mediated lung injury both via TLR2-dependent production of IL-1α and IL-12 p40, and TLR2-Independent CARDS Toxin after Mycoplasma pneumoniae Infection in Mice. Microbiol. Spectr. 2021, 9, e0158821. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, X.; Zhou, Y.; Tang, H.; Zhao, D.; Liu, F. Immunosuppression reduces lung injury caused by Mycoplasma pneumoniae infection. Sci. Rep. 2019, 9, 7147. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, K. Cell biology of the Koji mold Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2015, 79, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Ichishima, E. Development of enzyme technology for Aspergillus oryzae, A. sojae, and A. luchuensis, the national microorganisms of Japan. Biosci. Biotechnol. Biochem. 2016, 80, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Kitagaki, H. Medical application of substances derived from non-pathogenic fungi Aspergillus oryzae and A. luchuensis-containing koji. J. Fungi. 2021, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Zilles, J.C.; Dos Santos, F.L.; Kulkamp-Guerreiro, I.C.; Contri, R.V. Biological activities and safety data of kojic acid and its derivatives: A review. Exp. Dermatol. 2022, 31, 1500–1521. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shi, Y.G.; Zeng, L.Y.; Pan, Y.; Huang, X.Y.; Bian, L.Q.; Zhu, Y.J.; Zhang, R.R.; Zhang, J. Evaluation of antibacterial and anti-biofilm properties of kojic acid against five food-related bacteria and related subcellular mechanisms of bacterial inactivation. Food Sci. Technol. Int. 2019, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Park, T.J.; Ikram, M.; Ahmad, S.; Ahmad, R.; Jo, M.G.; Kim, M.O. Antioxidative and anti-inflammatory effects of kojic acid in Aβ-induced mouse model of Alzheimer’s disease. Mol. Neurobiol. 2021, 58, 5127–5140. [Google Scholar] [CrossRef]
- Rho, H.S.; Ahn, S.M.; Yoo, D.S.; Kim, M.K.; Cho, D.H.; Cho, J.Y. Kojyl thioether derivatives having both tyrosinase inhibitory and anti-inflammatory properties. Bioorg. Med. Chem. Lett. 2010, 20, 6569–6571. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Behjati, M.; Sadeghi, A.; Fassihi, A. Wound healing by topical application of antioxidant iron chelators: Kojic acid and deferiprone. Int. Wound J. 2013, 10, 260–264. [Google Scholar] [CrossRef]
- Onuma, K.; Kanda, Y.; Suzuki Ikeda, S.; Sakaki, R.; Nonomura, T.; Kobayashi, M.; Osaki, M.; Shikanai, M.; Kobayashi, H.; Okada, F. Fermented brown rice and rice bran with Aspergillus oryzae (FBRA) prevents inflammation-related carcinogenesis in mice, through inhibition of inflammatory cell infiltration. Nutrients 2015, 7, 10237–10250. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ota, H.; Sugiyama, A.; Ishizone, S.; Maruta, F.; Akita, N.; Okimura, Y.; Kumagai, T.; Jo, M.; Tokuyama, T. Suppressive effect of rice extract on Helicobacter pylori infection in a Mongolian gerbil model. J. Gastroenterol. 2005, 40, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Yavlovich, A.; Tarshis, M.; Rottem, S. Internalization and intracellular survival of Mycoplasma pneumoniae by non-phagocytic cells. FEMS Microbiol. Lett. 2004, 233, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Xu, X.; Wang, Y.; Shen, X.; Chen, Z.; Yang, J. Mycoplasma pneumoniae infection induces reactive oxygen species and DNA damage in A549 human lung carcinoma cells. Infect. Immun. 2008, 76, 4405–4413. [Google Scholar] [CrossRef]
- Chen, Z.; Shao, X.; Dou, X.; Zhang, X.; Wang, Y.; Zhu, C.; Hao, C.; Fan, M.; Ji, W.; Yan, Y. Role of the Mycoplasma pneumoniae/interleukin-8/neutrophil axis in the pathogenesis of pneumonia. PLoS ONE 2016, 11, e0146377. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Qu, J.X.; Yin, Y.D.; Eldere, J.V. Overview of antimicrobial options for Mycoplasma pneumoniae pneumonia: Focus on macrolide resistance. Clin. Respir. J. 2017, 11, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Bebear, C.M.; Pereyre, S. Mechanisms of drug resistance in Mycoplasma pneumoniae. Curr. Drug Targets Infect. Disord. 2005, 5, 263–271. [Google Scholar] [CrossRef]
- Hu, P.C.; Collier, A.M.; Baseman, J.B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J. Exp. Med. 1977, 145, 1328–1343. [Google Scholar] [CrossRef]
- Krause, D.C.; Leith, D.K.; Wilson, R.M.; Baseman, J.B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect. Immun. 1982, 35, 809–817. [Google Scholar] [CrossRef]
- Craig, A.; Mai, J.; Cai, S.; Jeyaseelan, S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 2009, 77, 568–575. [Google Scholar] [CrossRef]
- Guo, L.; Liu, F.; Lu, M.P.; Zheng, Q.; Chen, Z.M. Increased T cell activation in BALF from children with Mycoplasma pneumoniae pneumonia. Pediatr. Pulmonol. 2015, 50, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Meng, F.; Gao, M.; Cheng, G.; Wang, X. Cytokine signatures associate with disease severity in children with Mycoplasma pneumoniae pneumonia. Sci. Rep. 2019, 9, 17853. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Yu, J.E.; Guo, A.H.; Li, X.; Lin, Y.; Jiang, Z.Y.; Xiao, Z. Ameliorative effects of Qingfei Tongluo formula on experimental mycoplasmal pneumonia in mice. J. Nat. Med. 2016, 70, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.; Chen, J.; Zhou, L.; Wang, H.; Chen, J.; Xu, Z.; Zhu, S.; Liu, W.; Yu, R.; et al. Morusin alleviates mycoplasma pneumonia via the inhibition of Wnt/β-catenin and NF-κB signaling. Biosci. Rep. 2019, 39, BSR20190190. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Sharma, S.; Javed, S.; Passi, K.; Dey, A.B.; Malhotra, P. Molecular detection of Mycoplasma pneumoniae by quantitative real-time PCR in patients with community acquired pneumonia. Indian J. Med. Res. 2013, 138, 244–251. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Chen, C.-C.; Pi, C.-C.; Chen, C.-J. Aspergillus oryzae Fermentation Extract Alleviates Inflammation in Mycoplasma pneumoniae Pneumonia. Molecules 2023, 28, 1127. https://doi.org/10.3390/molecules28031127
Lee H-Y, Chen C-C, Pi C-C, Chen C-J. Aspergillus oryzae Fermentation Extract Alleviates Inflammation in Mycoplasma pneumoniae Pneumonia. Molecules. 2023; 28(3):1127. https://doi.org/10.3390/molecules28031127
Chicago/Turabian StyleLee, Hui-Yu, Chun-Chia Chen, Chia-Chen Pi, and Chun-Jen Chen. 2023. "Aspergillus oryzae Fermentation Extract Alleviates Inflammation in Mycoplasma pneumoniae Pneumonia" Molecules 28, no. 3: 1127. https://doi.org/10.3390/molecules28031127
APA StyleLee, H.-Y., Chen, C.-C., Pi, C.-C., & Chen, C.-J. (2023). Aspergillus oryzae Fermentation Extract Alleviates Inflammation in Mycoplasma pneumoniae Pneumonia. Molecules, 28(3), 1127. https://doi.org/10.3390/molecules28031127