Extraction Optimization, Antioxidant, Cosmeceutical and Wound Healing Potential of Echinacea purpurea Glycerolic Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Box–Behnken Design
2.2. Model Analysis
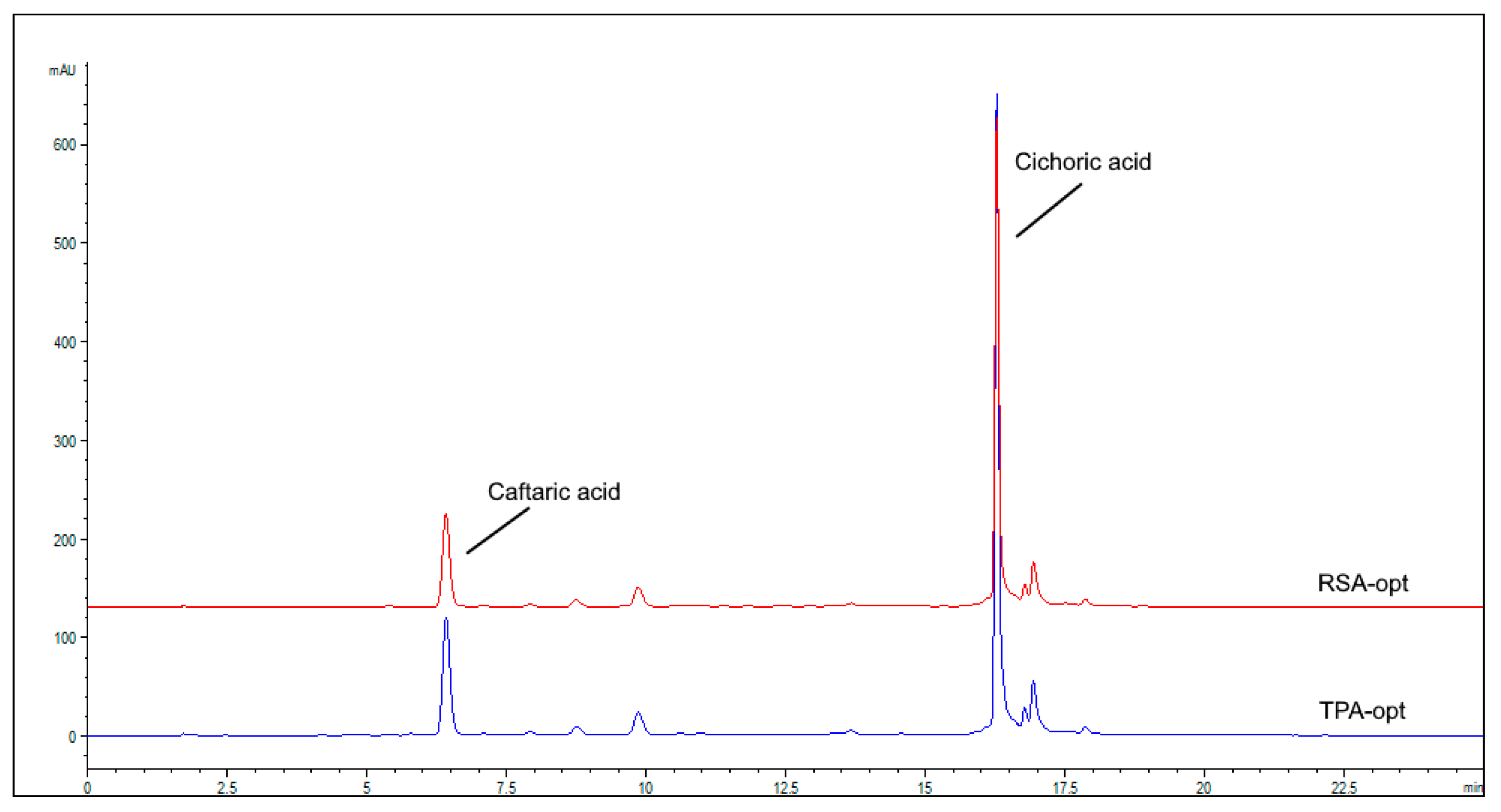
2.3. Validation of Optimal Extraction Conditions
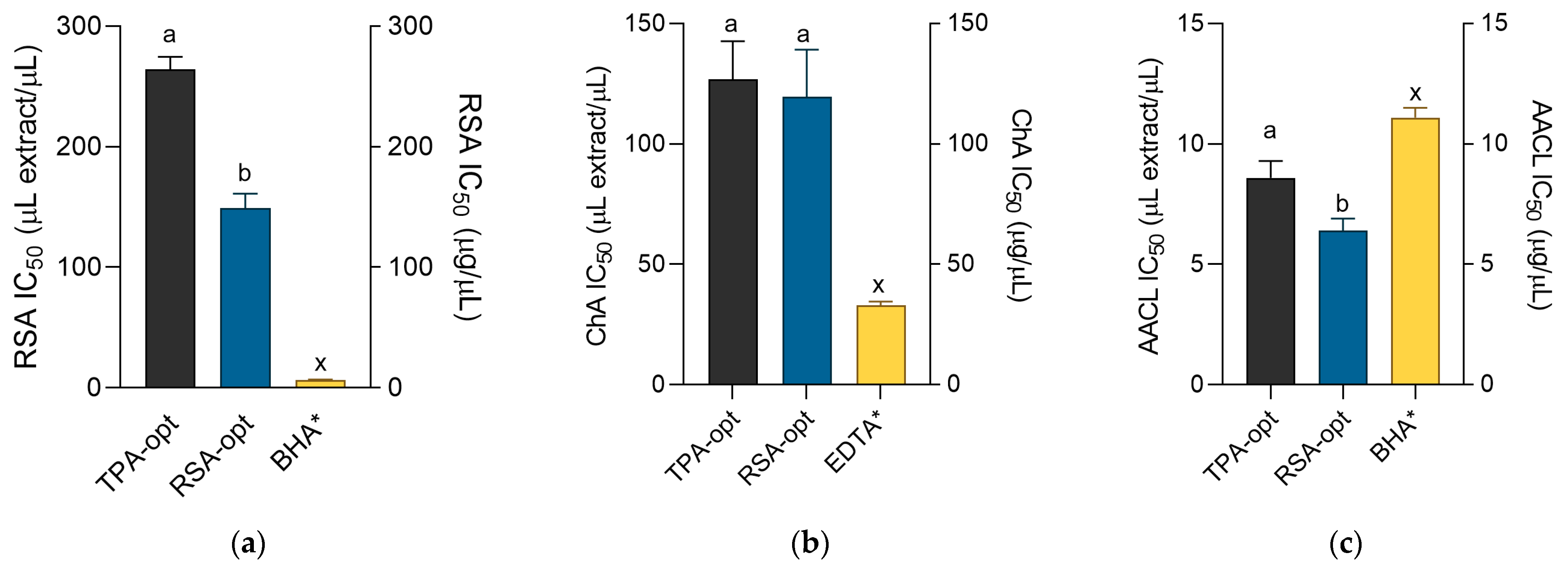
2.4. Antioxidant Activity of the Optimized Extracts
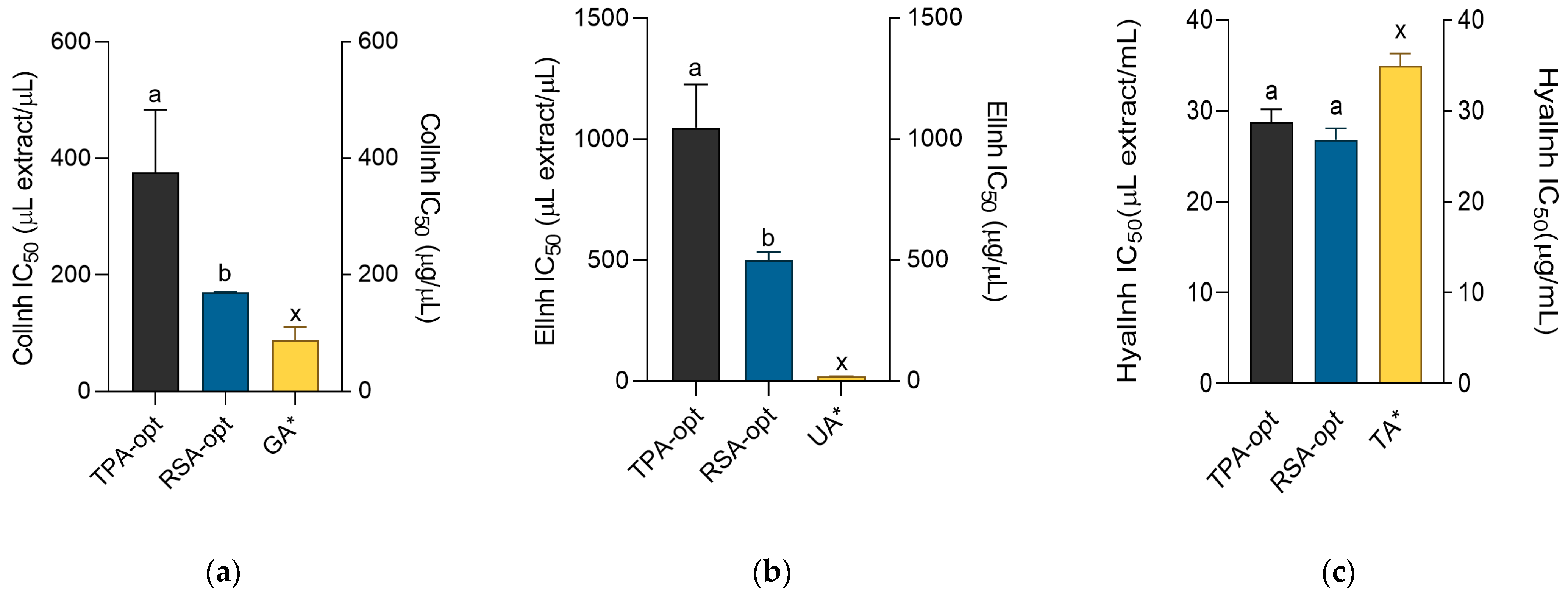
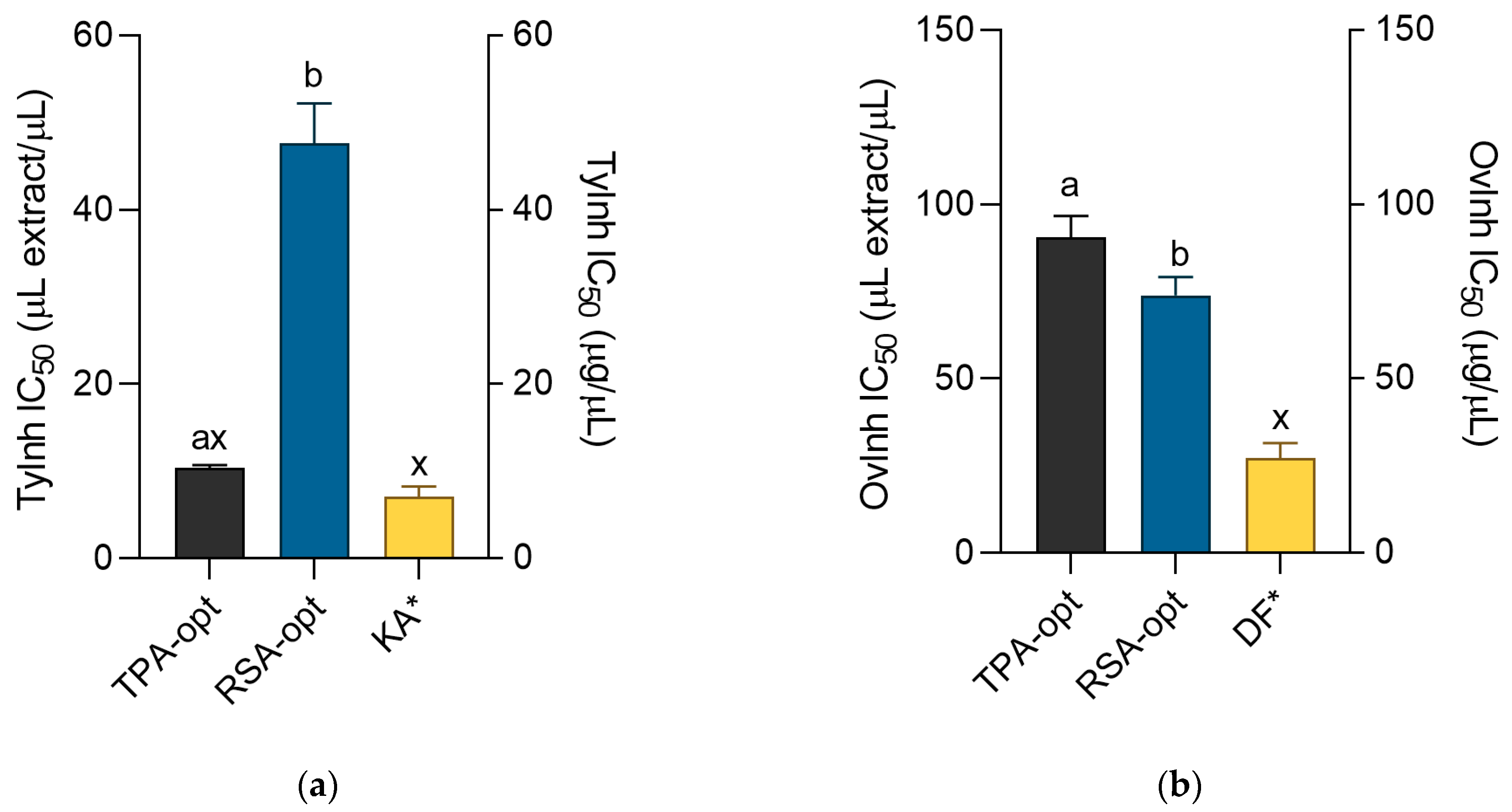
2.5. Cosmeceutical Activity of the Optimized Extracts
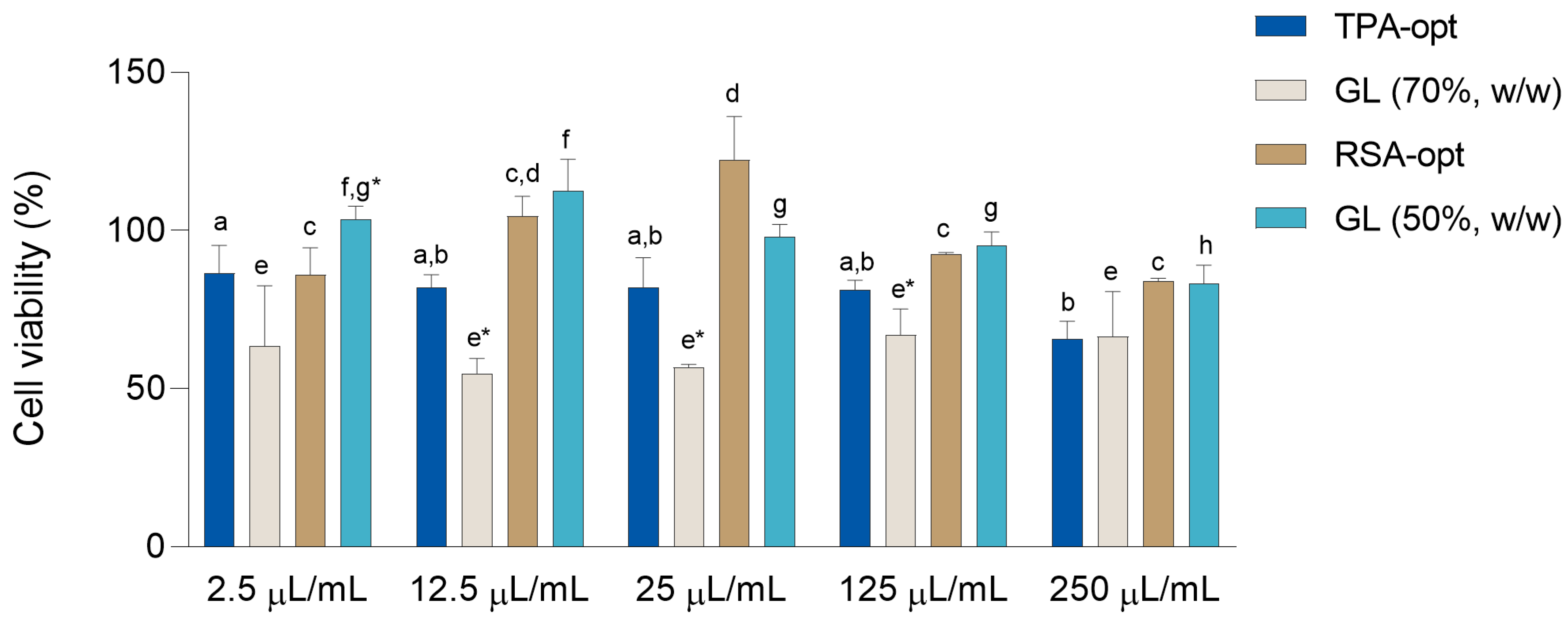
2.6. Evaluation of Cell Viability
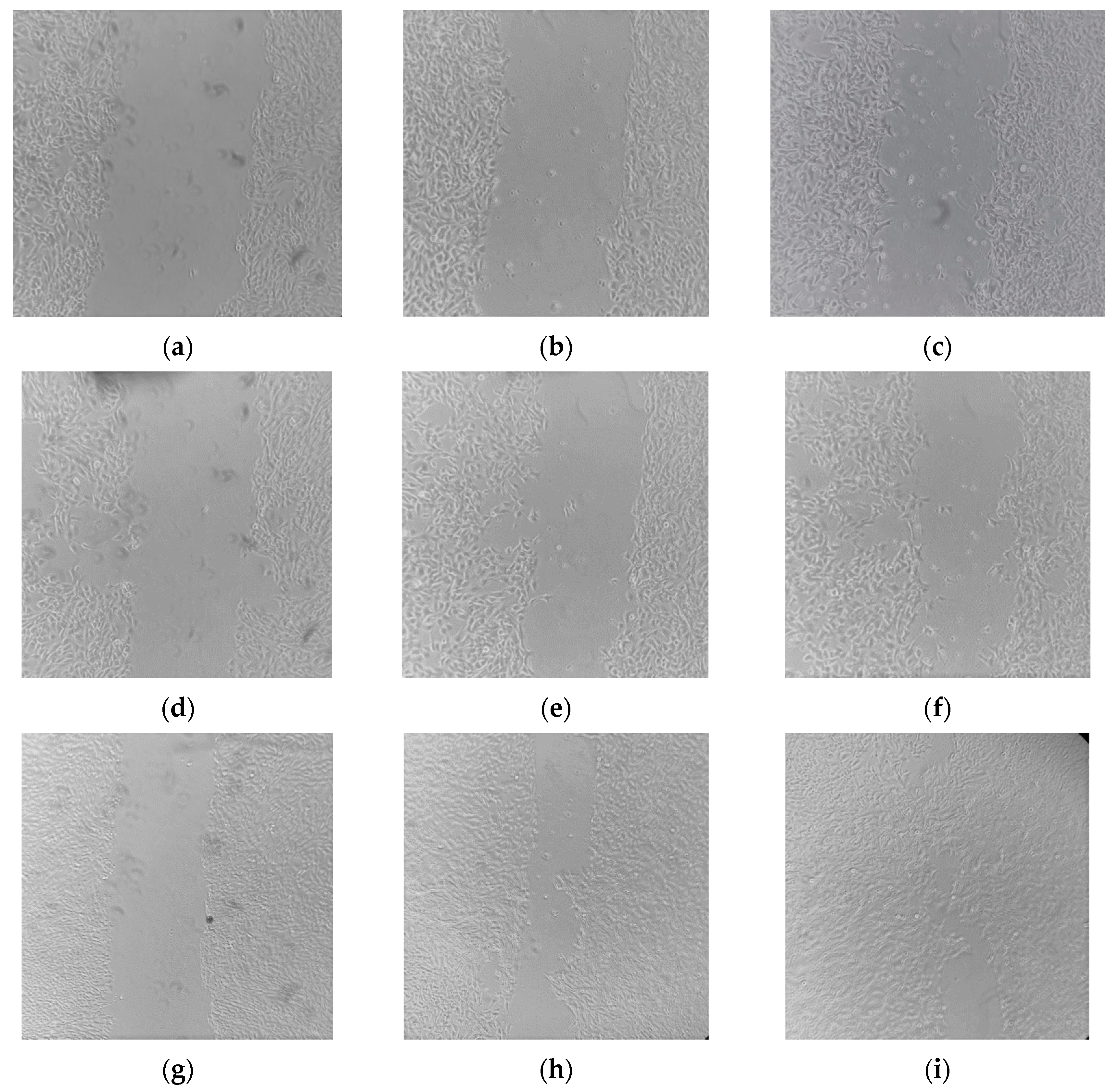
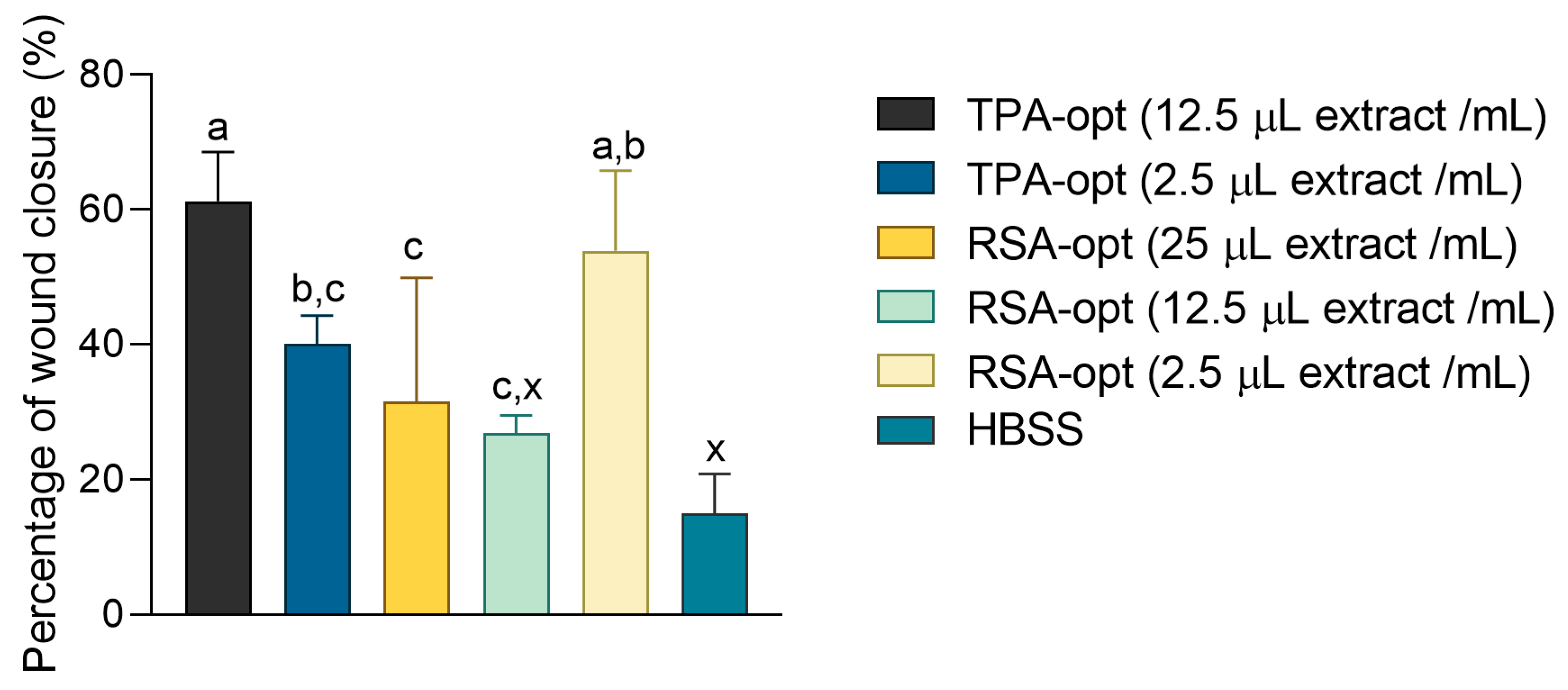
2.7. Wound Healing Effects of E. purporea
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Preparation of the Extracts According to Box–Behnken Design
3.4. HPLC Determinations of Caffeic Acid Derivatives
3.5. Radical Scavenging Activity
3.6. Fe2+ Chelating Activity
3.7. Antioxidant Activity in β-Carotene–Linoleic Acid Assay
3.8. Collagenase Inhibitory Activity
3.9. Elastase Inhibitory Activity
3.10. Hyaluronidase Inhibitory Activity
3.11. Tyrosinase Inhibitory Activity
3.12. Inhibition of Heat-Induced Ovalbumin Coagulation
3.13. Cell Culture Conditions
3.14. Cell Viability Study
3.15. In Vitro Scratch Wound Healing Assay
3.16. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, A.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Costa, R.; Santos, L. Delivery Systems for Cosmetics—From Manufacturing to the Skin of Natural Antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Martins, C.V.B.; Fariña, L.O.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a Green Solvent for High Product Yields and Selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Karsch-Völk, M.; Barrett, B.; Kiefer, D.; Bauer, R.; Ardjomand-Woelkart, K.; Linde, K. Echinacea for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2014, 2014, CD000530. [Google Scholar] [CrossRef]
- Echinaceae purpureae Herba. Available online: https://www.ema.europa.eu/en/medicines/herbal/echinaceae-purpureae-herba (accessed on 13 February 2020).
- Senica, M.; Mlinsek, G.; Veberic, R.; Mikulic-Petkovsek, M. Which Plant Part of Purple Coneflower (Echinacea purpurea (L.) Moench) Should Be Used for Tea and Which for Tincture? J. Med. Food 2019, 22, 102–108. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2013.
- Peng, Y.; Sun, Q.; Park, Y. The Bioactive Effects of Chicoric Acid as a Functional Food Ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.M. Caftaric Acid: An Overview on Its Structure, Daily Consumption, Bioavailability and Pharmacological Effects. Biointerface Res. Appl. Chem. 2020, 10, 5616–5623. [Google Scholar] [CrossRef]
- Yotsawimonwat, S.; Rattanadechsakul, J.; Rattanadechsakul, P.; Okonogi, S. Skin Improvement and Stability of Echinacea purpurea Dermatological Formulations. Int. J. Cosmet. Sci. 2010, 32, 340–346. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-de-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of Ultrasound-Assisted Extraction of Phenolic Compounds from Industrial Potato by-Products Using Response Surface Methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef]
- Fumić, B.; Jug, M.; Končić, M.Z. Optimization of Ultrasound-Assisted Extraction of Phenolic Antioxidants from Lotus Corniculatus. Croat. Chem. Acta 2019, 92, 369–377. [Google Scholar] [CrossRef]
- Pandey, A.; Belwal, T.; Sekar, K.C.; Bhatt, I.D.; Rawal, R.S. Optimization of Ultrasonic-Assisted Extraction (UAE) of Phenolics and Antioxidant Compounds from Rhizomes of Rheum moorcroftianum Using Response Surface Methodology (RSM). Ind. Crop. Prod. 2018, 119, 218–225. [Google Scholar] [CrossRef]
- Momchev, P.; Ciganović, P.; Jug, M.; Marguí, E.; Jablan, J.; Končić, M.Z. Comparison of Maceration and Ultrasonication for Green Extraction of Phenolic Acids from Echinacea purpurea Aerial Parts. Molecules 2020, 25, 5142. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In Vitro Methods to Determine the Antioxidant Activity of Caffeic Acid. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Main Cosmetic Vehicles. In Handbook of Cosmetic Science and Technology; Barel, A., Paye, M., Maibach, H., Eds.; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Mlakar, A.; Batna, A.; Dudda, A.; Spiteller, G. Iron (II) Ions Induced Oxidation of Ascorbic Acid and Glucose. Free Radic. Res. 1996, 25, 525–539. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an Active Ingredient for Cosmetic and Dermatological Applications: A Review. J. Cosmet. Laser Ther. Off. Publ. Eur. Soc. Laser Dermatol. 2019, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Elsner, P. Oxidants and Antioxidants in Cutaneous Biology; Current Problems in Dermatology; Karger: Basel, Switzerland, 2001; Volume 29. [Google Scholar]
- Zhu, X.; Huang, F.; Xiang, X.; Fan, M.; Chen, T. Evaluation of the Potential of Chicoric Acid as a Natural Food Antioxidant. Exp. Ther. Med. 2018, 16, 3651–3657. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin Anti-Aging Strategies. Dermato-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Aziz, J.; Shezali, H.; Radzi, Z.; Yahya, N.A.; Kassim, N.H.A.; Czernuszka, J.; Rahman, M.T. Molecular Mechanisms of Stress-Responsive Changes in Collagen and Elastin Networks in Skin. Skin Pharmacol. Physiol. 2016, 29, 190–203. [Google Scholar] [CrossRef]
- Uitto, J. Connective Tissue Biochemistry of the Aging Dermis: Age-Associated Alterations in Collagen and Elastin. Clin. Geriatr. Med. 1989, 5, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-Aging Potential and Anti-Tyrosinase Activity of Three Hypericum Species with Focus on Phytochemical Composition by LC–MS/MS. Ind. Crop. Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Imokawa, G. Recent Advances in Characterizing Biological Mechanisms Underlying UV-Induced Wrinkles: A Pivotal Role of Fibrobrast-Derived Elastase. Arch. Dermatol. Res. 2008, 300 (Suppl. 1), S7–S20. [Google Scholar] [CrossRef]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal Extracts as Potential Antioxidant, Anti-Aging, Anti-Inflammatory, and Whitening Cosmeceutical Ingredients. Chem. Biodivers. 2021, 18, e2100245. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory Effects of Polyphenols from Grape Pomace Extract on Collagenase and Elastase Activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.-H.; Lin, W.-C.; Lue, S.-C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a Natural Source of Skin Anti-Ageing Compounds. Ind. Crop. Prod. 2019, 127, 217–224. [Google Scholar] [CrossRef]
- Facino, R.M.; Carini, M.; Aldini, G.; Marinello, C.; Arlandini, E.; Franzoi, L.; Colombo, M.; Pietta, P.; Mauri, P. Direct Characterization of Caffeoyl Esters with Antihyaluronidase Activity in Crude Extracts from Echinacea angustifolia Roots by Fast Atom Bombardment Tandem Mass Spectrometry. Farm. Soc. Chim. Ital. 1993, 48, 1447–1461. [Google Scholar]
- Lengers, I.; Herrmann, F.; Le Borgne, M.; Jose, J. Improved Surface Display of Human Hyal1 and Identification of Testosterone Propionate and Chicoric Acid as New Inhibitors. Pharmaceuticals 2020, 13, 54. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin Wound Healing and Phytomedicine: A Review. Skin Pharmacol. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef]
- Chang, T.-S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Honisch, C.; Osto, A.; de Matos, A.D.; Vincenzi, S.; Ruzza, P. Isolation of a Tyrosinase Inhibitor from Unripe Grapes Juice: A Spectrophotometric Study. Food Chem. 2020, 305, 125506. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in Vitro Anti-Inflammatory Activity of Coffee against the Denaturation of Protein. Asian Pac. J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]
- Penkova, R.; Goshev, I.; Gorinstein, S.; Nedkov, P. Stability of Collagen during Denaturation. J. Protein Chem. 1999, 18, 397–401. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable in Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Blažević, F.; Milekić, T.; Romić, M.D.; Juretić, M.; Pepić, I.; Filipović-Grčić, J.; Lovrić, J.; Hafner, A. Nanoparticle-Mediated Interplay of Chitosan and Melatonin for Improved Wound Epithelialisation. Carbohydr. Polym. 2016, 146, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound Healing Promotion by Hyaluronic Acid: Effect of Molecular Weight on Gene Expression and in Vivo Wound Closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Ibezim, E.C.; Momoh, M.A.; Onyishi, V.I.; Nwabunike, I.; Odimegwu, D.C.; Ibezim, N.E.; Nzekwe, N.J. Evaluating the Ethyl-Acetate Fraction of Crude Methanol Leaf Extract of Ocimum Gratissimum Formulated as Ointments for Wound Healing Properties Using the Excision Wound Model. J. Pharm. Allied Sci. 2018, 15. [Google Scholar] [CrossRef]
- Jug, M.; Končić, M.Z.; Kosalec, I. Modulation of Antioxidant, Chelating and Antimicrobial Activity of Poplar Chemo-Type Propolis by Extraction Procures. LWT-Food Sci. Technol. 2014, 57, 530–537. [Google Scholar] [CrossRef]
- Končić, M.Z.; Barbarić, M.; Perković, I.; Zorc, B. Antiradical, Chelating and Antioxidant Activities of Hydroxamic Acids and Hydroxyureas. Molecules 2011, 16, 6232–6242. [Google Scholar] [CrossRef] [PubMed]
- Rajić, Z.; Končić, M.; Miloloža, K.; Perković, I.; Butula, I.; Bucar, F.; Zorc, B. Primaquine-NSAID Twin Drugs: Synthesis, Radical Scavenging, Antioxidant and Fe2+ Chelating Activity. Acta Pharm. 2010, 60, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Y.; Zhou, S.; Kang, L.; Li, C. A Straightforward Ninhydrin-Based Method for Collagenase Activity and Inhibitor Screening of Collagenase Using Spectrophotometry. Anal. Biochem. 2013, 437, 46–48. [Google Scholar] [CrossRef]
- Jabłonowska, M.; Ciganović, P.; Jablan, J.; Marguí, E.; Tomczyk, M.; Končić, M.Z. Silybum Marianum Glycerol Extraction for the Preparation of High-Value Anti-Ageing Extracts. Ind. Crop. Prod. 2021, 168, 113613. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In Vitro Screening of Elastase, Collagenase, Hyaluronidase, and Tyrosinase Inhibitory and Antioxidant Activities of 22 Halophyte Plant Extracts for Novel Cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
| Run | Std | X1 | X2 | X3 | X4 | Caf | Cic | TPA | RSA IC50 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | 70 | 70 | 72 | 40 | 28.17 | 91.39 | 119.56 | 14.53 |
| 2 | 19 | 50 | 55 | 360 | 40 | 27.58 | 88.74 | 116.32 | 8.17 |
| 3 | 6 | 70 | 55 | 360 | 20 | 28.68 | 92.21 | 120.89 | 8.65 |
| 4 | 2 | 90 | 40 | 216 | 40 | 13.70 | 61.11 | 74.81 | 19.9 |
| 5 | 5 | 70 | 55 | 72 | 20 | 31.55 | 103.26 | 134.81 | 18.29 |
| 6 | 1 | 50 | 40 | 216 | 40 | 21.23 | 65.28 | 86.51 | 19.2 |
| 7 | 21 | 70 | 40 | 216 | 20 | 23.19 | 79.58 | 102.77 | 41.58 |
| 8 | 26 | 70 | 55 | 216 | 40 | 29.24 | 96.86 | 126.1 | 14.9 |
| 9 | 13 | 70 | 40 | 72 | 40 | 25.82 | 85.41 | 111.23 | 15.51 |
| 10 | 11 | 50 | 55 | 216 | 60 | 29.25 | 94.15 | 123.4 | 10.75 |
| 11 | 3 | 50 | 70 | 216 | 40 | 23.93 | 77.59 | 101.52 | 11.07 |
| 12 | 12 | 90 | 55 | 216 | 60 | 21.37 | 75.9 | 97.27 | 16.33 |
| 13 | 25 | 70 | 55 | 216 | 40 | 27.38 | 90.33 | 117.71 | 8.33 |
| 14 | 17 | 50 | 55 | 72 | 40 | 29.03 | 90.58 | 119.61 | 8.32 |
| 15 | 7 | 70 | 55 | 72 | 60 | 29.62 | 98.63 | 128.25 | 9.16 |
| 16 | 20 | 90 | 55 | 360 | 40 | 22.36 | 78.6 | 100.96 | 23.53 |
| 17 | 29 | 70 | 55 | 216 | 40 | 27.41 | 86.64 | 114.05 | 14.3 |
| 18 | 10 | 90 | 55 | 216 | 20 | 20.37 | 73.95 | 94.32 | 54.03 |
| 19 | 16 | 70 | 70 | 360 | 40 | 28.39 | 92.58 | 120.97 | 9.76 |
| 20 | 8 | 70 | 55 | 360 | 60 | 30.82 | 103.02 | 133.84 | 8.27 |
| 21 | 24 | 70 | 70 | 216 | 60 | 28.46 | 94.37 | 122.83 | 9.43 |
| 22 | 23 | 70 | 40 | 216 | 60 | 29.13 | 97.92 | 127.05 | 23.53 |
| 23 | 15 | 70 | 40 | 360 | 40 | 22.72 | 78.13 | 100.85 | 23.52 |
| 24 | 28 | 70 | 55 | 216 | 40 | 21.91 | 74.26 | 96.17 | 8.67 |
| 25 | 9 | 50 | 55 | 216 | 20 | 26.76 | 84.36 | 111.12 | 19.66 |
| 26 | 22 | 70 | 70 | 216 | 20 | 27.4 | 89.79 | 117.19 | 8.71 |
| 27 | 4 | 90 | 70 | 216 | 40 | 20.06 | 65.24 | 85.3 | 17.28 |
| 28 | 18 | 90 | 55 | 72 | 40 | 20.74 | 76.8 | 97.54 | 19.1 |
| 29 | 27 | 70 | 55 | 216 | 40 | 28.7 | 91.82 | 120.52 | 12.79 |
| Response | Unit | The Equation Coefficients | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | k | l | m | n | o | ||
| Caf | µg/mL | −4.3 * | −2.4 * | 1.8 * | 1.9 * | 0.9 | 0.8 | −0.4 | 0.8 | −1.2 | 1.0 | −3.3 * | 1.7 * | −0.4 | 0.9 | 26.9 |
| Cic | µg/mL | −12.4 * | −6.6 * | 6.0 * | 7.0 * | −2.0 | 0.9 | −2.0 | 2.1 | −3.4 | 3.9 | −5.8 * | 3.6 * | −1.1 | 3.4 | 88.0 |
| TPA | µg/mL | −18.4 * | −10.7 * | 8.6 * | 9.7 * | 3.9 | 1.7 | −2.3 | 2.9 | −4.7 | 4.9 | −10.7 * | 7.0 * | −1.4 | 4.3 | 114.9 |
| (RSA IC50)−1/2 | µL extract/µL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.7 * | −0.7 * | −0.1 | −0.6 * | 3.9 |
| Caf | Cic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 = 0.8958; Ra2 = 0.79158; Rp2 = 0.6933 | R2 = 0.8765; Ra2 = 0.7531; Rp2 = 0.6634 | |||||||||
| Source | SS | df | MS | F Value | p-value | SS | df | MS | F Value | p-value |
| Model | 436.6 | 14 | 31.2 | 8.59 | 0.0001 | 4264.7 | 14 | 304.6 | 6.85 | 0.0005 |
| LoF | 16.7 | 10 | 1.6 | 0.20 | 0.9833 | 333.5 | 10 | 33.3 | 0.46 | 0.8535 |
| PE | 34.1 | 4 | 8.5 | 289.1 | 4 | 72.3 | ||||
| TPA | IC50 RSA | |||||||||
| R2 = 0.8823; Ra2 = 0.7647; Rp2 = 0.5814 | R2 = 0.4928; Ra2 = 0.4083; Rp2 = 0.2382 | |||||||||
| Source | SS | df | MS | F Value | p-value | SS | df | MS | F Value | p-value |
| Model | 7380.8 | 14 | 527.2 | 7.50 | 0.0003 | 15.9 | 4 | 4.0 | 5.83 | 0.0020 |
| LoF | 467. 9 | 10 | 46.8 | 0.36 | 0.9121 | 15.5 | 20 | 0.8 | 3.60 | 0.1111 |
| PE | 516.4 | 4 | 129.1 | 0.9 | 4 | 0.2 | ||||
| Extract | Measured Response | X1 | X2 | X3 | X3 | Resppred | Respms | RD |
|---|---|---|---|---|---|---|---|---|
| (%. w/w) | (°C) | (W) | (min) | (%) | ||||
| TPA-opt | Caf | 70 | 60 | 360 | 60 | 32.37 | 31.82 | -1.7 |
| TPA-opt | Cic | 70 | 60 | 360 | 60 | 107.16 | 113.11 | 5.6 |
| TPA-opt | TPA | 70 | 60 | 360 | 60 | 139.53 | 144.93 | 3.9 |
| RSA-opt | RSA IC50 | 50 | 70 | 144 | 55 | 4.90 | 5.32 | 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciganović, P.; Jakupović, L.; Momchev, P.; Nižić Nodilo, L.; Hafner, A.; Zovko Končić, M. Extraction Optimization, Antioxidant, Cosmeceutical and Wound Healing Potential of Echinacea purpurea Glycerolic Extracts. Molecules 2023, 28, 1177. https://doi.org/10.3390/molecules28031177
Ciganović P, Jakupović L, Momchev P, Nižić Nodilo L, Hafner A, Zovko Končić M. Extraction Optimization, Antioxidant, Cosmeceutical and Wound Healing Potential of Echinacea purpurea Glycerolic Extracts. Molecules. 2023; 28(3):1177. https://doi.org/10.3390/molecules28031177
Chicago/Turabian StyleCiganović, Petar, Lejsa Jakupović, Plamen Momchev, Laura Nižić Nodilo, Anita Hafner, and Marijana Zovko Končić. 2023. "Extraction Optimization, Antioxidant, Cosmeceutical and Wound Healing Potential of Echinacea purpurea Glycerolic Extracts" Molecules 28, no. 3: 1177. https://doi.org/10.3390/molecules28031177
APA StyleCiganović, P., Jakupović, L., Momchev, P., Nižić Nodilo, L., Hafner, A., & Zovko Končić, M. (2023). Extraction Optimization, Antioxidant, Cosmeceutical and Wound Healing Potential of Echinacea purpurea Glycerolic Extracts. Molecules, 28(3), 1177. https://doi.org/10.3390/molecules28031177








